Abstract
Esophageal squamous cell cancer is a highly aggressive cancer with a dismal 5-year survival rate. CD47 is a cell transmembrane protein that is involved in cell apoptosis, proliferation, adhesion, migration, and antigen presentation in the immune system. By interacting with signal regulatory protein-α expressed in antigen-presenting cells (APCs), CD47 acts as an antiphagocytic mechanism to inhibit APC-dependent antigen presentation. Overexpression of CD47 was found in various types of cancer. However, its role in esophageal squamous cell cancer is not yet clear. Anti-CD47 is an antagonist of CD47 signaling pathways by competing with its ligand. In the current study, we investigated the effects of anti-CD47 treatment on the antitumor immune response in an esophageal squamous cell cancer preclinical model. We found that anti-CD47 treatment enhanced proinflammatory responses and increased CD8+ T-cell infiltration in tumor tissue in the animal model. T cells in anti-CD47-treated tumors showed higher PD-1 and CTLA-4 expression, indicating T-cell activation and the rationale of combining anti-CD47 with anti-PD-1 and CLTA-4. The combinatory treatment showed the best antitumor response, implying a novel treatment strategy. The effects of anti-CD47 depended on dendritic cell function. In patient samples, expression of CD47 was negatively correlated with CD8+ T-cell infiltration in esophageal squamous cell cancer patients. Taken together, CD47 might be a novel target to enhance anti-PD-1 and CLTA-4 efficacy in esophageal squamous cell cancer.
Key words: CD47, Dendritic cells, PD-1, CTLA-4, Esophageal squamous cell cancer
INTRODUCTION
Cluster of differentiation 47 (CD47) is a ubiquitously expressed membrane protein of the immunoglobulin superfamily1. It is known that expression of CD47 is upregulated in various types of tumors including bladder cancer, breast cancer, and acute myeloid leukemia2–4. CD47 interacts with thrombospondin-1 and signal regulatory protein-α (SIRPα), which is expressed in myeloid-lineage hematopoietic cells such as dendritic cells (DCs) and macrophages5. The ligation of SIRPα on phagocytes by CD47-expressed tumor cells results in phosphorylation of SIRPα cytoplasmic immunoreceptor tyrosine-based inhibition (ITIM) motifs, leading to a negative regulation of the phagocytosis of tumor cells and, consequently, impairment of antigen presentation5. However, the clinical potential of anti-CD47 in cancer treatment is still under debate5,6.
Immunotherapy in cancer treatment has long been proposed and showed promising effects in recent clinical trials7. By blocking the inhibitory immune checkpoint pathways such as programmed cell death protein (PD-1)/programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), obvious improvement of clinical outcomes was observed in late stage melanoma, lung cancer, and renal cancer patients8. However, like all cancer treatments, resistance to immune checkpoint blockades (ICBs) occurred in a large proportion of treated patients9. Both innate and adaptive resistance happened in clinical trials, leading to treatment failure9,10. Thus, identifying novel targets to improve the sensitivity of current ICB is highly meaningful.
Esophageal cancer is a highly malignant disease with 16,910 estimated new cases and 15,690 estimated deaths in the US11. In P.R. China, esophageal cancer is one of the most common cancer types with more than 477,000 incidences each year12. Among histological subtypes, squamous cell cancer is the major one. However, the current treatment for esophageal squamous cell cancer has remained unchanged for decades, and novel therapies are urgently needed13. CD47 and immune checkpoint pathways have shown potential targeting values in esophageal cancer14,15. Based on these facts, we aimed to investigate whether anti-CD47 could synergize with current ICBs, therefore being a novel target for esophageal squamous cell cancer treatment. The mechanisms by which anti-C47 synergizes in cancer immunotherapy were also investigated.
MATERIALS AND METHODS
Preclinical Models
The widely used 4-nitroquinoline 1-oxide (4-NQO; 4-NQO in drinking water for 16 weeks) induced the esophageal squamous cell cancer model that was established in C57BL/6 mice. Primary tumor cells were then isolated from the chemically induced tumors by following a previously reported procedure16 and cultured in complete DMEM for three passages for cell expansion. Mouse primary esophageal squamous cell cancer cells (106) were then used to establish a syngeneic subcutaneous tumor model in either wild-type C57BL/6 mice (20–22 g; Shanghai SLAC Laboratory Animal Center of Chinese Academy of Sciences, P.R. China) or CD11c-DTR C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA). Diphtheria toxin (DT; 150 ng) was administrated via intraperitoneal injection 1 day before subcutaneous tumor inoculation and repeated every 5 days to deplete DCs in CD11c-DTR mice. In wild-type mice, the same amount of DT was injected to control the baseline between groups. All mice were raised in a specific pathogen-free environment with free access to water and standard food. Tumor growth was monitored every 7 days after inoculation and was calculated based on the following formula: tumor volume = length × width2 × π/6. Then 20 μg/mouse anti-PD-1 antibody, 20 μg/mouse anti-CTLA-4 antibody, and 400 μg/mouse anti-CD47 antibody (CD47 antagonist; clone: MIAP410; Cat. No. BE0283; Bio X Cell, West Lebanon, NH, USA; antibody was diluted by sterilized PBS buffer) were used in cancer treatment. The detailed treatment plans are included in the corresponding figures. The animal experiment was approved by the Jiangsu Cancer Hospital.
Patient Samples
In order to evaluate the expression of CD47 in esophageal squamous cell cancer tissue, we collected 60 formalin-fixed, paraffin-embedded (FFPE) tumor tissues from 60 esophageal squamous cell cancer patients. These patients were diagnosed between 2007 and 2013 at Jiangsu Cancer Hospital, Jiangsu Province, P.R. China. The cohort contained 60% males and 40% females, 58.3% early stage (I–II) and 41.7% advanced stage (III–IV). All tissues were collected before chemotherapy or radiotherapy. Written informed consent was signed by each patient. This study was approved by the ethics committee of Jiangsu Cancer Hospital.
Immunohistochemistry
Standard immunohistochemistry procedures were performed to evaluate the CD47 expression and the tumor-infiltrating CD8+ cells in tumor tissues. The FFPE sections were deparaffinized by heating in a dry oven and submerging in xylene and were then rehydrated using ethanol. The slides were then steamed with Reveal Decloaker (Biocare Medical, Pacheco, CA, USA) for 10 min for antigen retrieval. Unspecific staining was blocked by incubating the slides with 5% bovine serum albumin buffer for 30 min at room temperature. Primary antibodies (diluted by 1:100; Abcam, Cambridge, MA, USA) were then applied to the slides and incubated for 6 h at 37°C. After washing with PBS, the slides were incubated with 0.3% H2O2 in TBS for 15 min. Horseradish peroxidase-conjugated secondary antibodies were then applied to incubate for 1 h at room temperature. The color was developed using DAB. Hematoxylin was used for counterstaining. In the negative control sections, the primary antibodies were omitted. Finally, the slides were dehydrated and mounted for observation with light microscopy. High CD47 expression was defined as >60% dark brown area in tumor cells per field (magnification: 400×). The number of CD8+ cells was counted in each field.
Flow Cytometry
Flow cytometry was conducted to measure the number of multiple cells and protein expression in tumor tissues from the mouse model. Single-cell suspensions were prepared from fresh tumor tissues. Tumors were first digested with collagenase I (10 mg/ml) for 60 min at 37°C. The cells were then washed with cold PBS and cleaned by filtering with cell strainers (40 μm). Fluorescence-conjugated primary antibodies (all 1:100 dilution; eBioscience, CA, USA) were applied to the cell pallets for 15 min of incubation at room temperature, followed by washing with PBS. Cells were then analyzed on the flow cytometry machine (FACSCelest). Data were analyzed using FlowJo software. Mean fluorescence intensity (MFI) was calculated to show the protein expression level. Gating strategy for each cell type is reported in figure legends.
Cytokine Measurement
TH1 and TH2 cytokines (IL-2, IL-4, IL-6, IL-10, IL-12, TNF-β, and IFN-γ) were measured in fresh tumor tissues by the ELISA method. All ELISA kits were purchased from eBioscience. The standard ELISA procedure provided by the vendor was followed. The data (relative high or low expression) were shown with a heat map. Clustering of tumor tissue groups (x-axis) and cytokine expression was made based on the Euclidian distance metric. Samples having similar features were grouped together. The color of each cube represented the z-score of the cytokine expression in a specific tumor tissue. Details about z-score calculation are included in https://en.wikipedia.org/wiki/Standard_score. White color indicated high expression (high z-score), while black color indicated low expression (low z-score).
Statistical Analysis
GraphPad Prism 6.0 software and R studio software were used for statistical analysis and data visualization. Differences in means were analyzed by t-test or one-way ANOVA. Kaplan–Meier method was used to perform survival analysis of animals; log-rank test was used to compare the survival curves of different groups of esophageal cancer patients. A value of p < 0.05 was considered as the indication of significant difference.
RESULTS
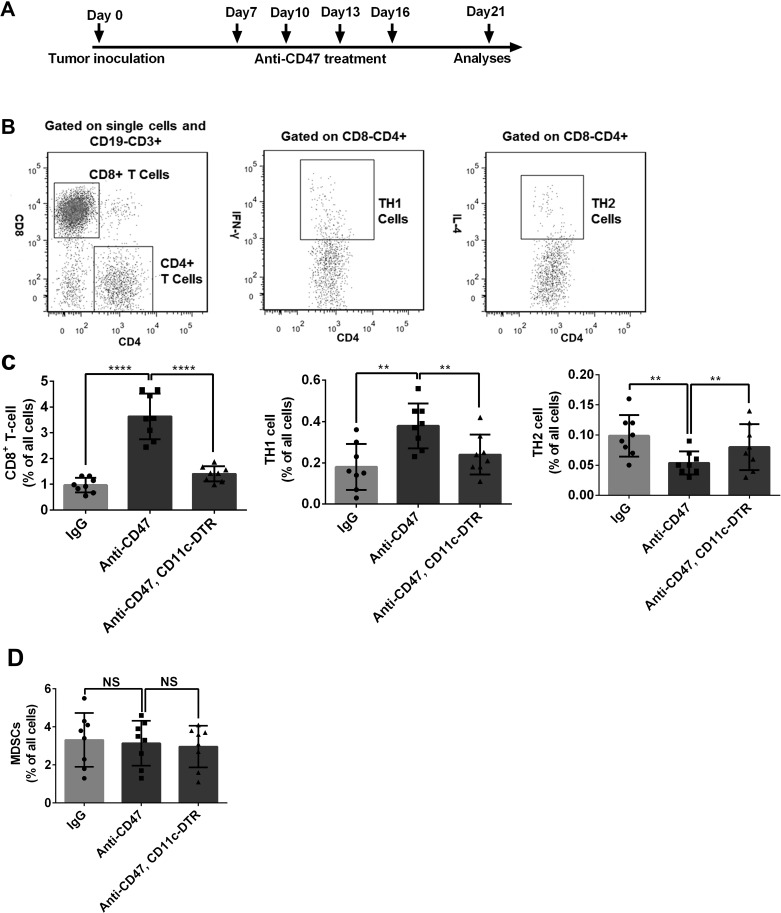
Anti-CD47 Treatment Enhanced the Number of Tumor-Infiltrating CD8+ T Cells and TH1 Cells
We established a subcutaneous esophageal squamous cell cancer mouse model to explore the role of CD47 in the cancer immune response. The wild-type mice and CD11c-DTR mice (DC deficiency) were treated with anti-CD47 antibody. Tumors treated with anti-CD47 antibody had an increased number of CD8+ T and TH1 cells, but a decreased number of TH2 cells when compared with the control group (treated with IgG) (Fig. 1C). Interestingly, no such phenomenon was observed in the tumors treated with anti-CD47 antibody in the CD11c-DTR background mice (Fig. 1C). There was no significant difference in the number of MDSCs between these groups (Fig. 1D). This evidence suggested that anti-CD47 treatment might enhance the antitumor immunity in a DC-dependent way in esophageal squamous cell cancer.
Figure 1.
Anti-cluster of differentiation 47 (CD47) treatment enhanced the number of TH1 and CD8+ T cells in an esophageal squamous cell cancer mouse model. (A) Treatment schedule of the in vivo experiment. (B) Gating strategies of the studied cell populations. CD8+ T cells were gated as CD19−CD3+CD4−CD8+. TH1 cells were gated as CD19−CD3+CD4+CD8−IFN-γ+. TH2 cells were gated as CD19−CD3+CD4+CD8−IL-4+. (C) CD8+ T cell and TH1 cell were increased by the anti-CD47 treatment in the wild-type mice, while the number of TH2 cells was decreased. However, in the DC-deficient mice, anti-CD47 did not show any obvious effect. (D) The number of MDSCs (CD19−CD3−CD11b+Gr1+) was not changed by anti-CD47 treatment. Eight mice were included in each group for this experiment. **p < 0.01, ****p < 0.0001.
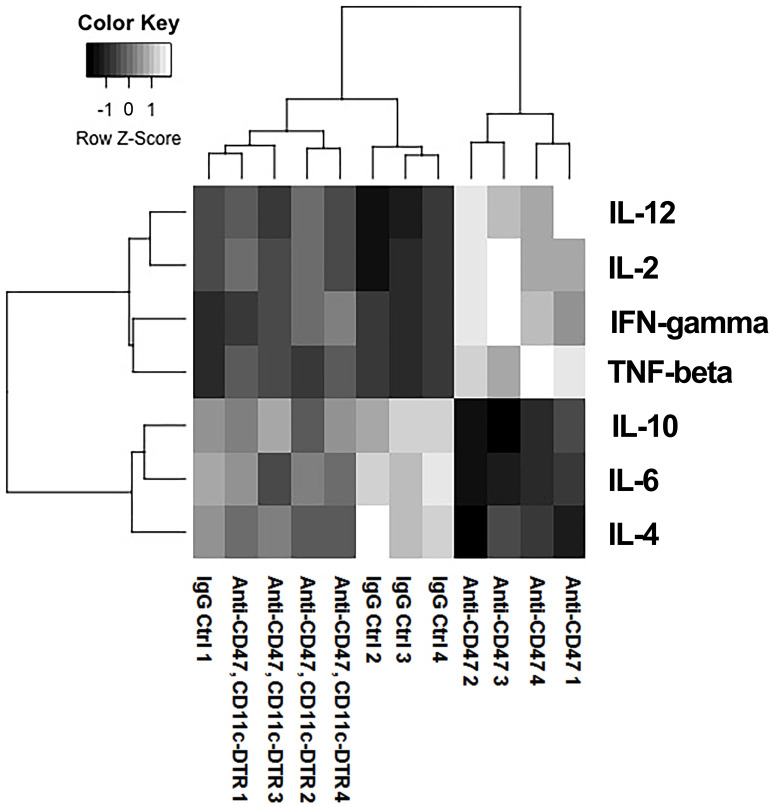
Anti-CD47 Treatment Enhanced Proinflammatory Cytokine Expression
We next evaluated the expression of multiple important inflammation regulatory cytokines expressed in the tumor tissues of the mouse model. We found that the tumors treated with anti-CD47 antibody had an increased level of proinflammatory cytokines, including IL-2, IFN-γ, TNF-β, and IL-12 (Fig. 2), but had a decreased level of some anti-inflammatory cytokines, such as IL-4, IL-6, and IL-10. However, anti-CD47 did not change the cytokine expression in the CD11c-DTR background mice. Taken together with the data that anti-CD47 increased the number of TH1 cells, we believed that anti-CD47 could trigger a strong antitumor immune response by enhancing inflammation in cancer tissues and that this effect depended on DCs.
Figure 2.
Proinflammatory cytokines were increased by anti-CD47 treatment. Heatmap showing that the level of inflammatory cytokines in each group. Samples with similar cytokine expression features were grouped together by the clustering line. White color indicates high expression while black color indicates low expression. Based on the clustering, the cytokine phenotype in anti-CD47 treatment (wild-type mice) group was different from the other two groups, meaning that anti-CD47 treatment significantly enhanced proinflammatory cytokines (IL-2, IFN-γ, TNF-β, and IL-12) while decreased the anti-inflammatory cytokines (IL-4, IL-6, and IL-10) in the wild type tumor tissues. 4 mice were included in each group for this experiment.
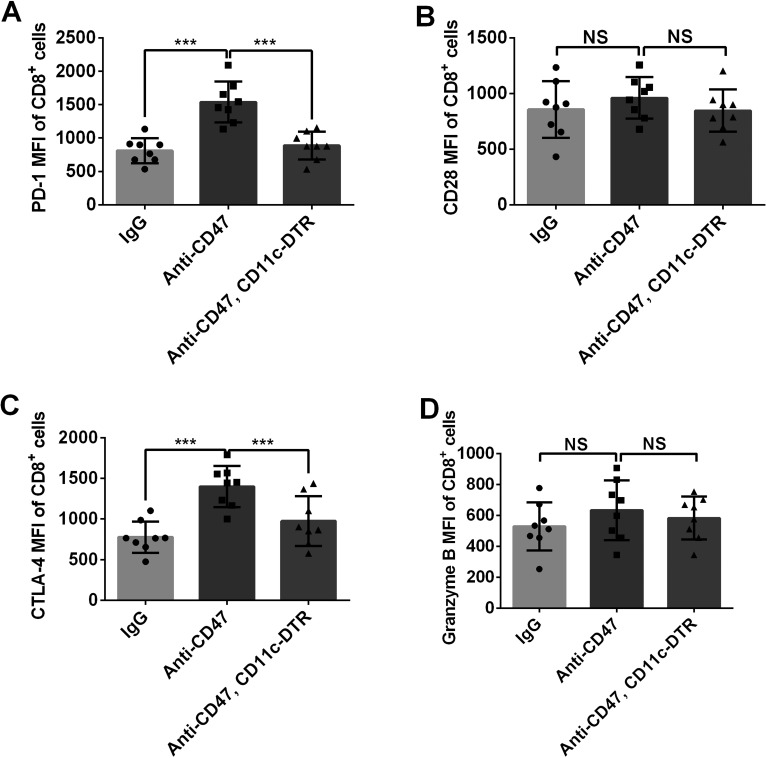
Anti-CD47 Treatment Induced Expression of Immune Checkpoint Proteins
Immune checkpoints regulate T-cell function and activation. We further measured the expression of the key immune checkpoint proteins PD-1, CTLA-4, and CD28 in the mouse model. The MFIs of PD-1 and CTLA-4 on CD8+ T cells were significantly increased in the tumors treated with anti-CD47 antibody (Fig. 3). Meanwhile, no significant difference between CD28 and granzyme B expression was observed among these treatment groups. These data suggested that PD-1 and CTLA-4 are negative regulators of anti-CD47-induced antitumor immune response.
Figure 3.
Expression of PD-1 and CTLA-4 was upregulated in CD8+ T cells with anti-CD47 treatment. (A, C) Inhibitory immune checkpoint proteins PD-L1 and CTLA-4 expressed by CD8+ T cells were upregulated by anti-CD47 treatment. This phenomenon was not observed in mice with DC deficiency. (B, D) No obvious change in CD28 and granzyme B expression was observed after anti-CD47 treatment. Eight mice were included in each group for this experiment. ***p < 0.001; NS, no significance.
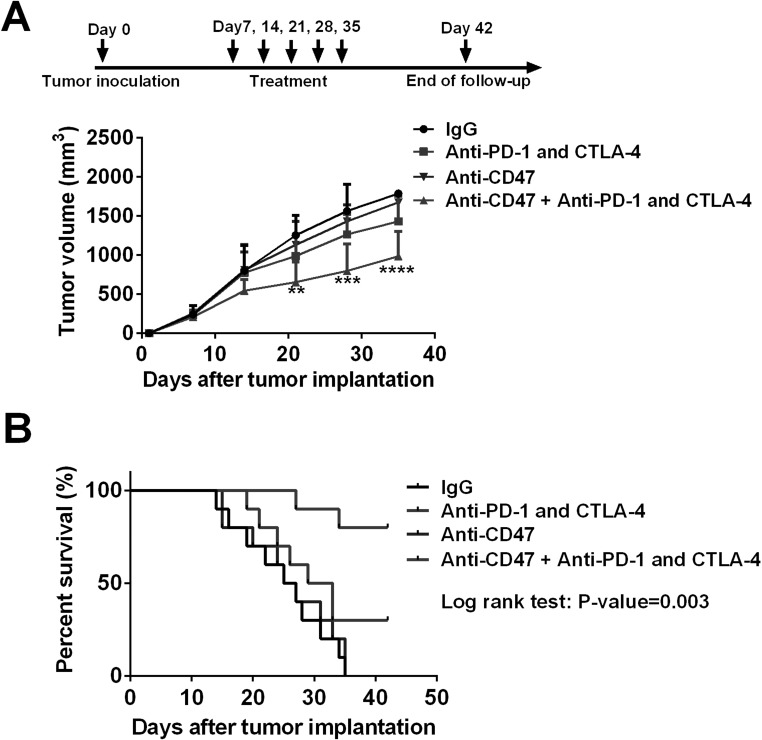
Anti-CD47 Treatment Enhanced the Effects of Anti-PD-1 and CTLA-4 in the Esophageal Squamous Cell Cancer Mouse Model
Considering that anti-CD47 treatment stimulated antitumor immune response and promoted the expression of immune checkpoint proteins, we hypothesized that anti-CD47 treatment might synergize with the anti-PD-1 and anti-CTLA-4 therapy in esophageal squamous cell cancer. Interestingly, the data from the mouse model did confirm our hypothesis. The tumors accepting anti-CD47 antibody treatment plus ICB therapy (anti-CTLA-4 + anti-PD-1 antibodies) had the slowest tumor growth when compared with the tumors treated with either anti-CD47 antibody or ICB antibodies alone (Fig. 4A). Consistently, the mice treated with anti-CD47 antibody plus ICB antibodies had a better overall survival than the other groups (Fig. 4B).
Figure 4.
Anti-CD47 treatment enhanced the effect of immune checkpoint blockade therapy in vivo. (A) The mice were given different treatments at days 7, 14, 21, 28, and 35 after tumor inoculation. Anti-CD47 treatment in combination with anti-PD-1 and CTLA-4 treatment significantly suppressed tumor growth. (B) Survival analysis of the mice. The mice treated with anti-CD47, anti-PD-1, and CTLA-4 antibodies had a longer overall survival time than other mice (p = 0.003). Ten mice were included in each group for this experiment. **p < 0.01, ***p < 0.001, ****p < 0.0001.
CD47 Expression Was Negatively Associated With the Number of CD8+ Cells in Esophageal Squamous Cell Cancer Patients’ Tissues
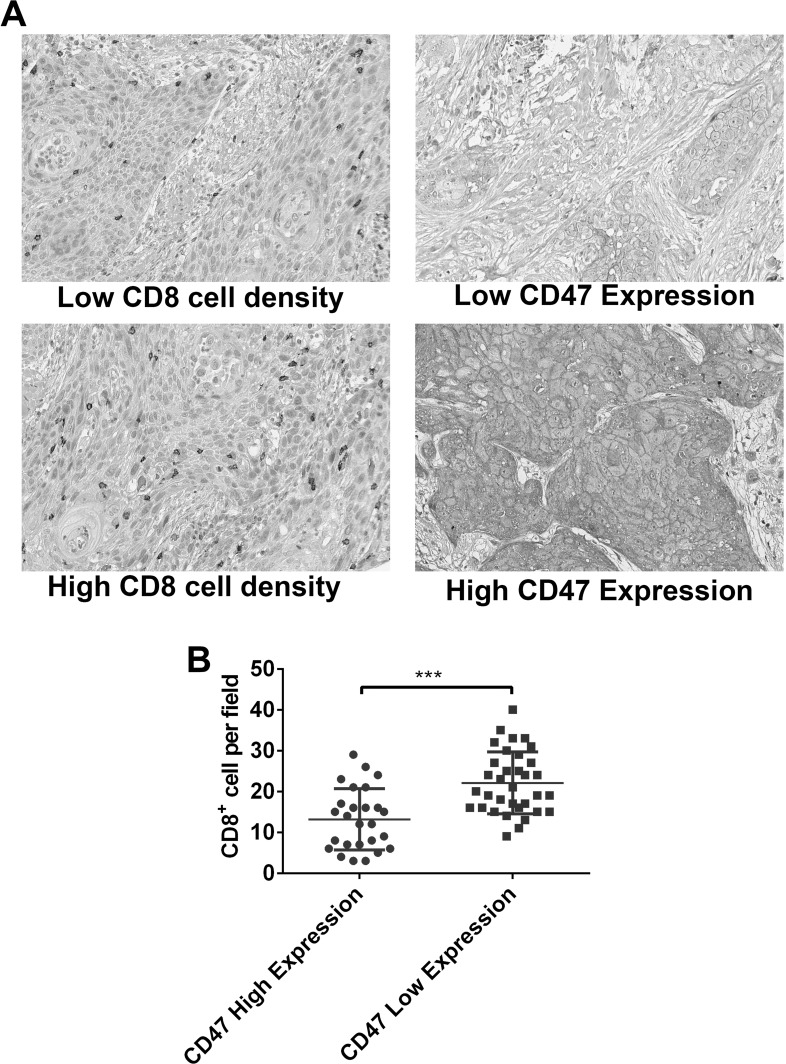
Aiming to further confirm the effects of CD47 on tumor immunity of esophageal squamous cell cancer, we collected tumor tissues from 60 esophageal squamous cell cancer patients and evaluated their CD47 expression and tumor-infiltrating CD8+ cell density. We noticed that CD47 was primarily expressed in tumor cells but not stromal cells (Fig. 5A). Patients with a high CD47 expression had lower average CD8+ cell density in tumor tissues, validating that high expression of CD47 is a negative immune regulator in esophageal squamous cell cancer patients.
Figure 5.
The relationship between CD47 expression and number of CD8+ cells in the tumor tissue of esophageal squamous cell cancer patients. (A) Representative images showing low/high CD8+ cell density and high/low CD47 expression in human esophageal squamous cell cancer tissues stained by IHC (magnification: 400×). CD47 was expressed on the membrane of tumor cells. (B) Quantitative data showed that patients with low CD47 expression had a higher mean density of CD8+ cells (***p < 0.001).
DISCUSSION
Esophageal cancer is one of the most common cancers in P.R. China, but it still lacks an effective treatment, especially for late stage patients12. ICBs as a novel cancer treatment strategy have been widely studied in different types of cancer. CD47, a membrane protein highly expressed in cancer cells, has shown to have a critical impact on regulating antigen presentation in cancer tissues17. CD47 can interact with SIRPα expression in antigen-presenting cells (APCs), leading to phosphorylation of the SIRPα cytoplasmic ITIMs and recruitment of Src homology 2 domain-containing tyrosine phosphatases. The ultimate consequence of the interaction between the tumor cells’ CD47 and APCs’ SIRPα is delivering an antiphagocytic “don’t eat me” signal to the APCs, thereby considerably impairing the antigen presentation process and following immune response in tumor tissues17. The commercialized anti-CD47 antibody used in previous studies6,18 is a blockade of the CD47 signaling pathway by competing with SIRPαs without stimulating the downstream pathways. In the present study, we investigated the potential synergistic effects of anti-CD47 and the currently used ICBs, anti-PD-1, and CTLA-4 in an esophageal squamous cell cancer preclinical model. Mechanisms of the synergistic effects were also studied.
We first evaluated the effects of anti-CD47 on immune infiltration and antitumor inflammatory response in animal models. Anti-CD47 significantly increased the number of CD8+ T cells, a major indicator of antitumor immune response, in tumor tissues in wild-type host mice. TH cells have a critical role in determining the intensity of cell-mediated immune response and interact with APCs19. High TH1 cell number and function were considered to enhance antigen presentation and the killing of T cells19. Here we found that anti-CD47 increased the number of TH1 cells while decreasing the number of TH2 cells in tumors in wild-type host mice. The expression of proinflammatory cytokines, such as IL-2, IL-12, TNF-β, and IFN-γ, was also stimulated by anti-CD47 treatment. However, in the CD11c-DTR mouse model, which has a small number of DCs, the anti-CD47 treatment could only raise a weak response. These observations implied that anti-CD47 could promote antitumor inflammation and immune response by enhancing the function of DCs.
Appropriate antigen presentation is the initial step of the antitumor immune response, while the number and function of CD8+ T cells determine the intensity of immunity20,21. Thus, we measured the function of CD8+ T cells in tumors treated with anti-CD47. It is worth noting that CD8+ T cells in anti-CD47-treated tumors showed a high expression of PD-1 and CTLA-4, two important inhibitory immune checkpoint proteins. However, CD28 and granzyme B expression remained unchanged. These data indicate that CD8+ T cells in anti-CD47-treated tumors were well activated22,23, but because of upregulation of the inhibitory immune checkpoint, their tumor killing effect was impaired, suggesting that combining anti-CD47 with anti-PD-1 and CTLA-4 may induce a strong tumor-eliminating response. In the subcutaneous tumor model, anti-CD47 monotherapy did not increase the survival time compared with the IgG control, while ICB blockade (anti-PD-1 and CTLA-4) showed a slight antitumor effect. However, when these two kinds of treatments are combined, tumors are well controlled, and animal survival was prolonged.
After we clarified the effect of CD47 on antitumor immune response in mouse models, we further validated the conclusions via analyzing the relationship between CD47 expression and immune response in esophageal squamous cell cancer patients. CD47 belongs to the immunoglobulin superfamily and is found in the tumor cells of breast cancer, bladder cancer, and leukemia1–4. In esophageal squamous cell cancer patient samples, we found that CD47 was mainly expressed in tumor cells rather than in the surrounding tumor stromal cells. Because CD47 was identified as a “don’t eat me” signal in the antigen-presentation process, the initial step of antitumor immunity1,17, we tried to correlate CD47 expression with CD8+ T-cell infiltration, the best marker for antitumor immune response19, in cancer tissue. Interestingly, the cases with high CD47 expression in tumor cells tend to have low CD8+ T-cell infiltration and vice versa. This observation suggests that CD47 expression might negatively influence the antitumor immune response in esophageal squamous cell cancer patients and supports the findings of the animal studies.
In conclusion, our study indicates that high expression of CD47 is a protective factor for esophageal squamous cell cancer to escape immune killing. Anti-CD47 could enhance antitumor inflammation and T-cell recruitment in a DC-dependent manner. However, the function of T cells was impaired due to immune checkpoint expression. Based on these facts, combinatory administration of anti-CD47, anti-PD-1, and CTLA-4 achieves a better outcome in the preclinical model.
ACKNOWLEDGMENT
The authors thank the funding support of the Jiangsu Cancer Hospital General Program (ZM201207).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA 2009;106(33):14016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baccelli I, Stenzinger A, Vogel V, Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M, Holland-Letz T, Sinn HP, Schneeweiss A, Denkert C, Weichert W, Trumpp A. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 2014;5(18):8147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138(2):286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao MP, Weissman IL, Majeti R. The CD47-SIRP alpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horrigan SK. Replication study: The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. eLife 2017;6:e18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 8. Webster RM. The immune checkpoint inhibitors: Where are we now? Nat Rev Drug Discov. 2014;13(12):883–4. [DOI] [PubMed] [Google Scholar]
- 9. Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G, Zitvogel L. Resistance mechanisms to immune-checkpoint blockade in cancer: Tumor-intrinsic and-extrinsic factors. Immunity 2016;44(6):1255–69. [DOI] [PubMed] [Google Scholar]
- 10. Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res. 2017;77(4):817–22. [DOI] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 12. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 13. Sohda M, Kuwano H. Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg. 2017;23(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki S, Yokobori T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28(2):465–72. [DOI] [PubMed] [Google Scholar]
- 15. Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53. [DOI] [PubMed] [Google Scholar]
- 16. Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10(1 Pt 1):301–13. [DOI] [PubMed] [Google Scholar]
- 17. Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–64. [DOI] [PubMed] [Google Scholar]
- 18. Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt E, Quertermous T, Betancu P, Maegdefessel L, Perisic L, Hedin U, Weissman I, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536(7614):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 21. Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1(5):405–13. [DOI] [PubMed] [Google Scholar]
- 23. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]