Highlights
-
•
Penile cancer is a rare tumor.
-
•
The organ preservation perspective makes the treatment challenging.
-
•
For early stage, conservative brachytherapy achieved excellent oncological outcome.
-
•
Conservative brachytherapy reported encouraging functional results.
-
•
HDR brachytherapy represents an attractive therapeutic option.
Keywords: Penile cancer, Brachytherapy, Conservative treatment, Penectomy
Abbreviations: ABS, American Brachytherapy Society; CCAFU, Cancer Committee of the French Association of Urology; CT, computerized tomography; CTCAE, common terminology criteria for adverse events; CTV, clinical target volume; DFS, disease-free survival; DNR, dose non-homogenity ratio; EAU, European Association of Urology; EBRT, external beam radiotherapy; EQD2, equivalent dose in 2Gy fractions; GC-SFRO, Groupe Curiethérapie/Société Française de Radiothérapie Oncologique; GEC-ESTRO, Groupe Européen de Curiethérapie/European Society for Therapeutic Radiation and Oncology; HDB, high-dose brachytherapy; IIEF, international index of erectile function; IPSS, international prostate symptom score; LC, local control; LDR, low-dose rate; MDFS, metastatic disease-free survival; MFU, median follow-up; MHB, multicatheter interstitial high-dose rate brachytherapy; MMS, Mohs micrographic surgery; MRI, magnetic resonance imaging; NCCN, national comprehensive cancer network; OS, overall survival; PDR, pulse-dose rate; PET, positron emission tomography; PP, penile preservation; RC, regional control; SCC, squamous cell carcinoma; SFRO, Société Française de Radiothérapie Oncologique; SS, specific survival; TNM, tumor node metastasis
Abstract
Purpose
To analyze the oncological outcome and toxicity profile after conservative treatment based on multicatheter interstitial high-dose rate brachytherapy (MHB) for patients presenting a localized penile cancer.
Materials and methods
Patients with histologically proven, non-metastatic (T1-T2 N0-N2 M0) localized penile cancer were treated with MHB. Needles were placed under general anesthesia into the target volume using a dedicated template. Treatment planning was performed using a post-implant CT-scan to deliver 35 Gy or 39 Gy (9f, 5d) for adjuvant or definitive treatment respectively. Five-year oncological outcome was evaluated with local relapse-free (LRFS), regional relapse-free (RRFS), and metastasis-free survival (MFS), specific (SS) and overall survival (OS). In pre-treatment and follow-up consultations, skin, urinary and sexual toxicities were investigated using CTCAEv4.0 classification, International Prostate Symptom Score (IPSS) and International Index of Erectile Function 5-items (IIEF-5). Dosimetry data were also analyzed.
Results
From 03/2006 to 05/2020, with a median follow-up of 72.4 months [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], 29 pts, mainly T1 (75.9%) and N0 (89.7%), underwent MHB. Eleven (38%) and 18 pts (62%) received MHB as adjuvant or definitive treatment respectively. Five-year LRFS, RRFS, MFS, SS and OS were 82%, 82%, 89%, 88% and 73% respectively. Six patients (20.7%) experienced local relapse and underwent salvage penectomy leading to a penile preservation rate of 79.3%. Acute skin toxicity was reported 1 month after MHB, with 28% G1, 66% G2 and 6% G3. Late skin complications were telangiectasia for 5 pts (17%) and necrosis for 3 pts (10.3% requiring hyperbaric oxygen therapy). Comparing pre- and post-treatment status, no significant change was observed for skin appearance, IPSS and IIEF-5.
Conclusion
MHB represents an efficient first line conservative treatment option for early penile cancers. Oncological outcome and late toxicity profile appear encouraging. However, larger-scale cohorts with longer follow-up are needed to more accurately precise the features of the best candidate to MHB.
1. Introduction
With a global incidence of 26,000 cases/year, penile cancer is a rare tumor [1]. In developed countries, the estimated incidence is approximately 1/100,000 men per year, most of them being squamous cell carcinomas (SCC) [2], [3].
Given the rarity of the disease and the organ preservation challenge, there are currently no high-proof level guidelines for treatment recommendations. Penile cancer management is currently based only on retrospective and single institution studies while there is no recruiting prospective trial concurrently open (Clinicaltrials.gov access 10/22/20). Partial penectomy has mostly been the historical procedure. It is often the first and only treatment performed. It allows good local control (>90% at 5 years) [4] at the expense of urinary and significant psychosexual side effects [3], [5], [6]. Consequently, therapeutic strategies have been proposed towards organ preservation. A consensus between the American Brachytherapy Society (ABS) and Groupe Européen de Curiethérapie/European Society for Therapeutic Radiation and Oncology (GEC-ESTRO) has been proposed for the use of brachytherapy as first line management for early stage penile cancers [7]. Historically, low-dose rate (LDR) brachytherapy has been the standard treatment and provided excellent oncological outcome and toxicity profile [8], [9]. However, since 2014, this technique has no longer been available while pulsed (PDR) and high-dose rate (HDR) brachytherapy techniques keep being used [10], [11].
In this study, we updated the clinical results of a cohort of patients presenting a localized stage penile cancer who underwent multicatheter interstitial high-dose rate brachytherapy (MHB) [12].
2. Materials and methods
This is a single-institution, retrospective, observational study which evaluated the oncological outcome and long-term toxicities after conservative treatment consisting in MHB for patients with a localized penile cancer. Data were collected from patients’ files. This study, as well as the ethical aspect of the protocol, were approved by the Urologic Institutional Review Board of Antoine Lacassagne Cancer Centre (n°MR-3616170920). The board waived the requirement for informed consents because of this study’s retrospective design.
2.1. Patient and tumor characteristics
Patients with histologically proven non-metastatic penile cancer were offered conservative treatment based on MHB. Patients were treated in the Antoine Lacassagne Cancer Center (Nice, France) in collaboration with the Urology department of the Nice Academic Hospital. All the patients were offered partial penectomy or conservative treatment with extensive information in regard to oncological results and side effects of each procedure. MHB was considered either in an adjuvant setting after surgical procedure or as a definitive approach after biopsy. Each patient underwent a complete physical examination (tumor depth extension, inguinal lymph node involvement). In some cases, a penile Magnetic Resonance Imaging (MRI) was performed in order to distinguish contact/bulge from corpus carvernosum true invasion. As recommended by the European Association of Urology (EAU) guidelines, inguinal lymph node involvement and metastatic status were investigated by inguinal ultrasound (+/-biopsy) and abdomino-pelvic computerized tomography (CT), respectively [3]. Before MHB, all patients underwent circumcision.
2.2. Brachytherapy procedures
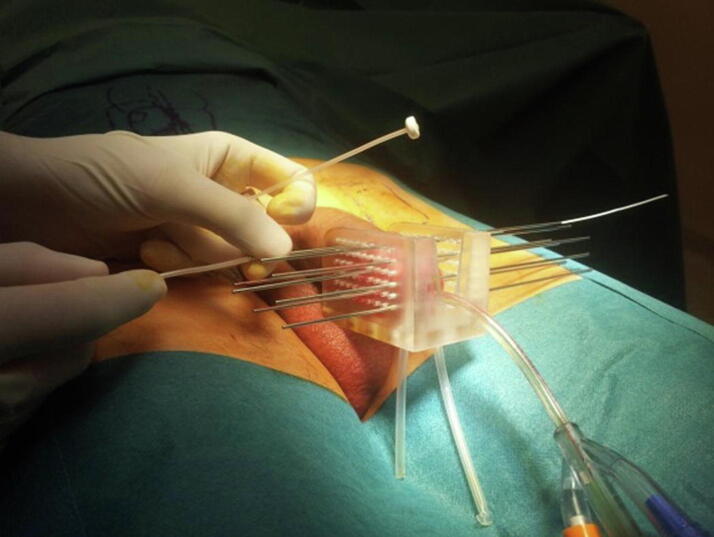
Implant procedure, planning and dose delivery were already described [12]. Briefly, after urethral catheterization, the penile was placed in a dedicated applicator (Fig. 1). Needle insertion allowed plastic catheters placement (Sharp Needles™; Elekta company, Stockholm, Sweden; Flexible Catheter Leader™ Eckert&Ziegler BEBIG, Berlin, Germany) through the templates in regard to the tumor volume in 1 to 3 plans (depending on the clinical target volume – CTV).
Fig. 1.
Penile cancer multicatheter interstitial high-dose rate brachytherapy implant.
After patient recovery, CT-scan planning was performed for the dose distribution analysis and optimization. CTV included the macroscopic tumor plus a safety margin ranging from 5 to 10 mm. For patients without gross residual at the time of implant, CTV was based on imaging, surgical reports, and/or photographs. The prescribed dose was established according to MHB indication. For adjuvant MHB, the total dose was 35 Gy in 9 fractions over 5 consecutive days (7 Gy at day 1 then 3.5 Gy/f twice daily from day 2 to day 5), while for definitive treatment the prescribed dose was 39 Gy with the same fractionation (7 Gy at day 1 then 4 Gy/f twice daily from day 2 to day 5). Dose-volume adaptation was manually achieved by dwell location and time variation (graphical optimization) (Microselectron™; Elekta company, Stockholm, Sweden; Saginova™, Eckert&Ziegler BEBIG, Berlin, Germany). CTV dose constraints were: V100% > 90%, V150% < 35%. Confluence of two V200% isodoses and V200% > 10 mm in diameter were avoided. For the urethra, dose constraints were V115% < 1%. Dose non-homogeneity ratio (DNR), D10u and D30u were also reported.
The first fraction was delivered on the day of the implant (on Monday) then the remaining dose was delivered twice daily 6 h apart from Tuesday to Friday [13], [14]. After the last irradiation session, catheters were removed, and the patient was discharge from the hospital with a medical prescription for acute radiodermatitis.
2.3. Oncological outcome and toxicities
After MHB, patients were systematically examined at 1, 3, 6 and 12 months then every 6 months during the 5 first years of the follow-up, then annually. Penile and inguinal areas clinical examinations were conducted and, if necessary, combined with an inguinal ultrasound exam, penile MRI or positron emission tomography (PET) using fluorine 18-labeled fluorodeoxyglucose. The analysis of the oncological outcome was based on local (LRFS) and regional lymph node (inguinal and/or iliac) recurrence-free survival (RRFS) rates and metastasis-free (MFS), disease-free (DFS), specific (SS) and overall survival (OS) rates.
In pre-treatment and follow-up consultations, urinary (International Prostate Symptom Score-IPSS), and sexual (International Index of Erectile Function 5-items-IIEF-5) functions as well as skin status were analyzed. IPSS was systematically rated in 3 grades according to the score: grade 1 for light (1 to 7), grade 2 for moderate (8 to 19) and grade 3 for severe urinary symptoms (20 to 35). IIEF-5 was rated in 4 grades: 1 for normal (21 to 25), 2 for light (16 to 20), 3 for moderate (11 to 15) and 4 for severe erectile dysfunction (5 to 10). Post-treatment skin toxicities were scored according to CTCAEv4.0 classification [15]. Because organ conservation represents a composite factor depending on local relapse and side effects, penile preservation rate at the end of follow-up was reported.
2.4. Statistical analysis
Qualitative data were presented as absolute frequencies, relative frequencies, 95% confidence intervals and percentages of missing data. Quantitative data were presented as medians, extremes, means, standard deviations and percentages of missing data. The normality of these parameters was assessed using the Shapiro test. Quantitative data were compared using Student's T-test or Mann-Withney's test in case of non-compliance with the conditions of Student's test. The censored data (survival data) were defined between the date of treatment start and the date of occurrence of the event: local relapse for LRFS, regional lymph node (inguinal and/or iliac) relapse for RRFS, metastasis for MFS, any oncological events for DFS, deaths due to penile cancer for SS and deaths due to any cause for OS. Patients lost to follow-up were censored at the date of last news. These data were graphically presented with Kaplan-Meier curves. The significance level was a p-value < 0.05. The median time to onset of relapse was calculated from the treatment date and the onset recurrence date. The penile preservation rate at the end of follow-up was calculated.
3. Results
3.1. Patient, tumor and treatment characteristics
From 2006 to 2020, 29 pts who underwent MHB for non-metastatic localized penile cancer were retrospectively analyzed. With a median age of 70 years [46–84], patients were mainly classified T1 (75.9%) and N0 (89.7%). Histological type was mainly SCC (93%) with a median tumor size of 15 mm [5.4–32] (Table 1).
Table 1.
Patient, tumor and treatment characteristics.
| Features | # | [min–max]/% |
|---|---|---|
| Median age (years) | 70 | [46–84] |
| Median Karnofsky Index (%) | 90 | [80–100] |
| Cardio-vascular comorbidities* | ||
| yes | 18 | 62.1 |
| no | 11 | 37.9 |
| Histological type | ||
| Squamous cell carcinoma | 27 | 93.1 |
| Bowen | 2 | 6.9 |
| Median tumor size (mm) | 15 | [5–32] |
| Clinical stages | ||
| Tis | 1 | 3.4 |
| T1 | 22 | 75.9 |
| T2 | 6 | 20.7 |
| Lymph node status | ||
| N0 | 26 | 89.7 |
| N1 | 1 | 3.4 |
| N2 | 2 | 6.9 |
| Localization | ||
| Glans/Coronal sulcus | 17 | 58,6 |
| Peri-urethral meatus | 12 | 41.4 |
| Brachytherapy indication | ||
| Definitive treatment | 18 | 62 |
| Adjuvant | 11 | 38 |
| Median time interval between surgery/MHB (days) | 76 | [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] |
| Median total dose of brachytherapy (Gy) | 36 | [31], [32], [33], [34], [35], [36] |
| Median number of fractions | 9 | [7–10] |
| Median number of needles | 12 | [3–19] |
| Median number of plans | 3 | [1–4] |
| Dosimetry Data | ||
| CTV (cc) | 16 | [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] |
| D90 (%) | 107 | [73–118] |
| V100 (%) | 95 | [78–100] |
| V150 (%) | 32 | [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] |
| V200 (%) | 12 | [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] |
| DNR | 0.35 | [0.22–0.58] |
| Urethra | ||
| D0.1u (cc) | 132 | [78–230] |
| D1u (cc) | 103 | [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] |
| D10u (%) | 127 | [59–217] |
| D30u (%) | 113 | [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] |
Cardio-vascular comorbidities*: smoking, alcohol, diabetes, high blood pressure, obesity and dyslipidemia
CTV: Clinical Target Volume; D90: dose delivered to 90% of CTV expressed in percentage of the prescribed dose; V100: CTV receiving 100% of the prescribed dose expressed in percentage; V150: CTV receiving 150% of the prescribed dose expressed in percentage; V200: CTV receiving 200% of the prescribed dose expressed in percentage; DNR: Dose Non-homogeneity Ratio = 1-[V100–V150]/V100; D0.1: dose delivered to 0.1 cc of the urethral volume; D1: dose delivered to 1 cc of the urethral volume; D10: dose delivered to 10 cc of the urethral volume expressed in percentage of the prescribed dose; D30: dose delivered to 30 cc of the urethral volume expressed in percentage of the prescribed dose.
The imaging work-up for disease extension before brachytherapy evolved upon time. From 2006 to 2013 patients were mostly explored with ultrasonography and CT scan (86%), while since 2014, MRI and PET scan were the standard work-up (73%).
MHB was performed as definitive treatment for 18 pts (62.1%: primary disease = 12 pts; local relapse after surgery = 6 pts) or as adjuvant treatment for 11 pts (37.9%). Median total dose was 36 Gy [31], [32], [33], [34], [35], [36] with a median number of fractions of 9 [7], [8], [9], [10] (EQD2αβ10 = 43 Gy [38–53] and EQD2αβ3 = 53 Gy [47–68]). Median EQD2αβ10/EQD2αβ3 were 41/50 Gy and 47/59 Gy for adjuvant and definitive MHB respectively.
4. Dosimetry characteristics
The median CTV was 16 cc [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. The median D90 was 107% [73–118]. Median V100 and DNR were 95% [78–100] and 0.35 [0.22–0.58] respectively. For urethra, the median D10u was 127% [59–217] and D30u was 113% [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] (Table 1).
4.1. Oncological outcome and toxicities
4.1.1. Oncological outcome
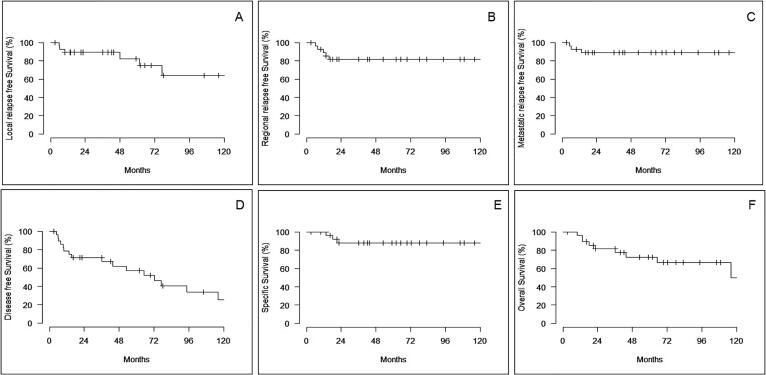
The median follow-up (MFU) was 72 months [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Six pts (20.7%) experienced local relapse leading to a 5-year LRFS rate of 82%. The median local recurrence time was 29 months [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Salvage penectomy was performed only in case of local relapse resulting in a penile preservation (PP) rate of 79.3%. Fifty-percent of the local recurrences occurred within the first 24 months (Fig. 2A). Five-year RRFS and MFS rates were 82 and 89% respectively. No regional or distant recurrences was detected after the first two years of follow-up (Fig. 2B and 2C). Five-year DFS rate was 57%, while 5-year SS and OS rates were 88% and 73% respectively (Fig. 2D-2F).
Fig. 2.
Kaplan-Meier survival curves for local recurrence-free survival (A), regional recurrence free survival (B), Metastatic disease-free survival (C), Disease-free survival (D), Specific survival (E), Overall survival (F).
4.2. Toxicities
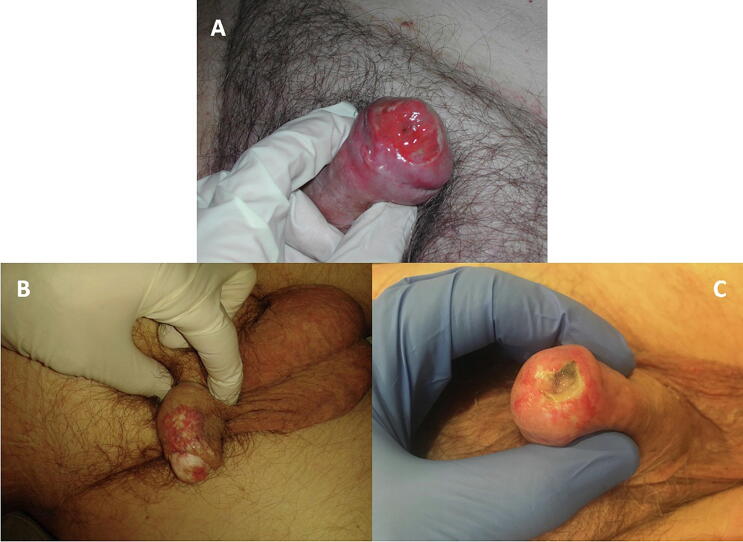
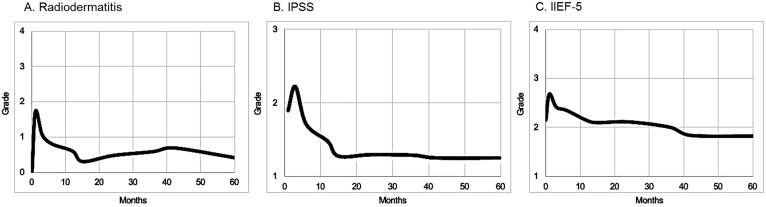
Acute skin toxicity observed at 1 month were mainly grade 2 radiodermatitis (83%) (Fig. 3A). Regarding late skin toxicity, 5 pts (17%) presented telangiectasia (Fig. 3B) and 3 pts (10%) presented grade 3 necrosis requiring hyperbaric oxygen therapy sessions allowing complete skin recover (Fig. 3C, Table 2). The skin appearance difference between pre- and post-treatment assessments were statistically significant at 1 (p < 0.01), 3 (p = 0.01), 6 (p < 0.01) and 12 months (p = 0.01) (Fig. 4A).
Fig. 3.
Post MHB skin toxicities: acute radiodermatitis (A), telangiectasia (B), necrosis (C).
Table 2.
Skin, urinary and sexual late complications.
| Toxicities | Acute |
Late |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Total | G1 | G2 | G3 | G4 | Total | |
| Skin | 2 (7%) | 24 (83%) | 1 (3%) | 0 | 27 (93%) | 7 (24%) | 5 (17%) | 3 (10%) | 0 | 15 (51%) |
| Urinary | 11 (38%) | 0 | 1 (3%) | NA | 12 (41%) | 7 (24%) | 3 (10%) | 0 | NA | 10 (34%) |
| Sexual | 5 (17%) | 0 | 0 | 0 | 5 (17%) | 3 (10%) | 0 | 2 (7%) | 0 | 5 (17%) |
NA: non-applicable for IPPS.
Fig. 4.
Toxicity analysis evolution from pre-treatment (MHB) status for skin (A; radiodermatitis according to CTCAEv4.0 classification), urinary (B; IPSS: rated in 3 grades according to the score: grade 1 for light (1 to 7), grade 2 for moderate (8 to 19) and grade 3 for severe urinary symptoms (20 to 35)) and sexual (C; IIEF-5: rated in 4 grades: 1 = normal (21 to 25), 2 = light (16 to 20), 3 = moderate (11 to 15) and 4 = severe erectile dysfunction (5 to 10)) functions.
Urinary function evaluation compared pre versus post-therapeutic IPSS calculated at each post-treatment evaluation. Assuming that mild urinary symptoms were already observed before brachytherapy in 25 pts (86%), no statistical difference was observed (pNS) (Fig. 4B). However, 2 pts (7%) presented late urethral meatus stenosis requiring iterative dilatations. Ten pts (34%) presented late urinary complications without any grade 3 (Table 2). Mean V150 was statistically correlated with the risk of G2/3 acute urinary toxicity (G1 V150: 30% versus G2/3 V150: 42%; p = 0.038) but not with late urinary toxicity (G1 V150: 32% versus G2/3 V150: 35%; p = 0.58).
Regarding sexual function, while normal erectile activity was observed in 14 pts (54%) before brachytherapy, no statistical difference was found (pNS) between pre versus post-therapeutic period (Fig. 4C). Five pts (17%) presented sexual complications with 7% of grade ≥ 3 (Table 2).
5. Discussion
The psychological consequences of total penectomy, as well as urinary and sexual deleterious impact, have progressively oriented the management of patients towards conservative treatment.
The surgical alternative to penile preservation is Mohs micrographic surgery (MMS), which consists in making intraoperative cross-sections, examined in real time by the surgeon until a negative plane appears. NCCN suggests that MMS may be useful for superficial low-risk penile cancers of the proximal diaphysis, with 5-year local control ranging from 68 to 89% [16], [17], [18], [19], [20]. None of the patients had a urinary or sexual functional deficit [16]. Because of technical difficulties in implementation and the need of qualified surgeons, MMS has not achieved broad consensus.
Studies investigating the efficacy of external beam radiation therapy (EBRT) for penile cancer conservative treatment reported high relapse rates. Zouhair et al. presented the results of 41 pts treated with exclusive EBRT (56%) or surgery (+adjuvant radiotherapy 44%) for T1-T2 penile cancers. The authors reported a 5-year penile preservation rate of 36% [21]. Compared to EBRT, brachytherapy allows a significant improvement of local control, mainly due to a more accurate and precise dose delivery combined with a dose escalation. Consequently, brachytherapy appears as the best irradiation technique in order to avoid deleterious consequences of radical surgery.
The 5-year LRFS (82%) and PP (79.3%) rates reported in our cohort are consistent with those published in LDR/PDR brachytherapy series with 5-year LRFS rates about 80% [66–100] and PP rates around 76% [69–100] (Table 3). HDB clinical data are still limited. Petera et al. reported the results of a cohort of 10 pts with penile SCC. The total delivered dose was 54 Gy (3 Gy/Fr, twice daily over 9 days). With a MFU of 20 months, LRFS rate was 100% [22]. More recently, with a MFU was 76 months, Kellas-Sleczka et al. analyzed 76 pts treated with HDB (42.8 Gy or 48.2 Gy for adjuvant or definitive treatments respectively). Five-year LRFS rate was 66% with PP rate of 67% [11]. In our study, the median time to local recurrence onset was 29 months. Other studies report similar results, leading to consider that 50% of recurrences occur within the first two years [10], [23]. It also suggests that the other half of relapses occur later and mostly during the five first years leading to consider a long surveillance [24].
Table 3.
Comparative clinical outcome analysis from brachytherapy series.
| Authors | n | MFU (months) |
Type | Dose (Gy) |
5y-LRFS (%) |
5y-OS (%) |
Necrosis (%) |
Stenosis (%) |
PP (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mazeron et al.[34] | 50 | 36-96 | LDR | 60-70 | 78 | 63 | 6 | 19 | 74 |
| Delannes et al.[28] | 51 | 65 | LDR | 50-65 | 86 | 72 | 23 | 45 | 75 |
| Rozan et al.[8] | 184 | 139 | LDR | 63 | 86 | 66 | 21 | 45 | 78 |
| Soria et al.[23] | 102 | 111 | LDR | 61-70 | 77 | 63 | 1 | 1 | 72 |
| Chaudhary et al.[27] | 23 | 21 | LDR | 50 | 70 | 66 | 0 | 9 | 70 |
| Kiltie et al.[33] | 31 | 62 | LDR | 63.5 | 81 | 69 | 8 | 44 | 75 |
| De Crevoisier et al.[9] | 144 | 68 | LDR | 65 | 80 | 26 | 29 | 72 | |
| Cordoba et al.[25] | 73 | 52 | LDR | 60 | 88 | 82 | 6.8 | 6.8 | 69.1 |
| Crook et al.[26] | 67 | 48 | PDR/LDR | 60 | 87 | 12 | 9 | 88 | |
| Escande et al.[10] | 201 | 128 | PDR/LDR | 65 | 82 | 79 | 21.4 | 24.8 | 77.1 |
| Makarewicz et al.[35] | 33 | 60 | PDR/HDR | 51 | 78.8 | 85 | 9 | - | 84.8 |
| Petera et al.[22] | 10 | 20 | HDR | 54(a) | 100 | 0 | 0 | 100 | |
| Rouscoff et al.[12] | 12 | 27 | HDR | 36/39(c) | 83 | 78 | 9 | 9 | 92 |
| Sharma et al.[29] | 14 | 22 | HDR | 42-45 | 86 | 0 | 0 | 93 | |
| Kellas-Sleczka et al. (11) | 76 | 76 | HDR | 42.8/48.2(b) | 66 | 77 | 2.6 | 1.3 | 69.5 |
| Pohankova et al.[36] | 26 | 85 | HDR | 51 | 83 | 92 | 4 | 4 | 73 |
| Marban-Orejas et al.[30] | 7 | 90 | HDR | 38.4/53(d) | 86 | 100 | 43 | 43 | 86 |
| Present study | 29 | 72 | HDR | 35/38(e) | 86 | 73 | 10 | 7 | 79 |
Type: modality of radiation therapy; LDR: Low-dose rate brachytherapy; PDR: Pulse-dose rate brachytherapy; HDR: High-dose rate brachytherapy; n: number of patients; LRFS: local relapse free survival; OS: overall survival; MFU: median follow-up in months; PP: Penile preservation
(a)54 Gy in 18 fractions over 9 days.
(b)42.8 Gy for adjuvant setting and 48.2 Gy in sole therapy, with a median fractionation dose of 3.2 Gy.
(c)36 Gy in 9 fractions over 5 days (in the adjuvant setting: 6 Gy day 1 + 2 x 3.75 Gy from day 2 to day 5) or 39 Gy in 9 fractions over 5 days (in sole therapy: 7 Gy day 1 + 2 x 4 Gy from day 2 to day 5).
(d)Prescribed dose ranged from 38.4 Gy in 6 days (3.2 Gy in 12 fractions) to 53 Gy in 9 days (3.12 Gy in 17 fractions).
(e)Median total dose of 35 Gy in 9 fractions over 5 days in the adjuvant setting or 38 Gy in 9 fractions over 5 days in sole therapy.
In this study, 5-y RRFS rate was 82%. In LDR and PDR brachytherapy series, estimated RRFS rates were estimated at 87% [84–91] [9], [25], [26], [27], [28]. Sharma et al. presented a series of 14 pts treated with MHB (42–45 Gy in 14–15 fractions) (29). With a MFU of 22 months, the regional relapse rate was 14.3%, whereas Kellas-Sleczka et al. described only one patient (1.3%) with inguinal nodal metastases 64 months after MHB [11]. As we reported, Sharma et al. described 100% of events occurring within the first 24 months. Lymph node relapse could be considered as a progression of micrometastatic disease at the time of brachytherapy, highlighting the potential place of PET-CT in the initial work-up.
Five-year actuarial SS and OS rates were 88% and 73% respectively. Our results are superimposed on those described in the literature (Table 3). However, Petera et al. and Rouscoff et al. described 5-year actuarial SS rates of 100% [12], [22]. This can be explained by the low sample size of those cohorts and a shorter follow-up.
After penile brachytherapy, acute skin complications are frequently described. In our series, 93% of patients had radiodermatitis (grade 2: 83%). Radiodermatitis is a well-documented acute complication after MHB which takes about 8 weeks to recover from [22]. The most serious late skin complication is necrosis. In our series, the rate of necrosis was 10.3%. In the literature, this rate varied from 0 to 26%. Kellas-Sleczka et al. did not find any post-therapeutic necrosis [11]. This could be explained by a median CTV 8.4 cc versus 15.1 cc in our series. Other late toxicities observed in the treated area were hyperpigmentation and telangiectasia. Kellas-Sleczka et al. described pigmentation changes in 35.5% and telangiectasias in 21% of cases [11]. Dose distribution must be homogeneous to limit the occurrence of acute and late skin toxicities. A spacing of 9–12 mm of the needles is recommended for obtaining optimal homogeneity and also limiting side effects [30].
Considering urinary function, urethra is the main organ to be considered for the evaluation of urinary function. At various follow-up visits, the IPSS measurement did not find any significant deterioration in urinary function after HDB. Various studies on LDR, PDR and HDR brachytherapy evaluated post-treatment urinary status as a function of the percentage of urethral stricture. In our cohort, 2 pts (7%) had urinary stenosis, corresponding to the interval ranged in the literature from 0 to 45% (Table 3). Stenosis is usually treated by dilatation or endoscopy. However, no significant correlation was observed between dosimetric parameters and the risk of self-reported urinary toxicity according to Gambachidze et al [31]. The challenge of brachytherapy is to limit the impact on urinary function while preserving the oncological outcome, by using dose distribution optimization to the urethra.
In our study, there was no significant deterioration in sexual function using IIEF-5 score evaluation. The treatment impact on quality of life is becoming a major issue in patient management. After radical penectomy, sexual function damage represents one of the main concerns, leading to higher anxiety level and depression [32]. Escande et al. shown that, after penile brachytherapy, 67% of patients declared having maintained a sexual activity after 3 years of follow-up [10]. Petera et al. reported a rate of 90% of patients who declared having maintained sexual function [22]. The impact of brachytherapy on QoL is thereby limited [31].
The main limitation of our retrospective observational study is represented by the small number of patients (29 pts) and a still short MFU (72 months). A low proof level is currently being observed from retrospective studies, while those aiming to randomize surgery versus brachytherapy seem ethically difficult to set up. Recently, the Groupe de Curiethérapie of the Société Française de Radiothérapie Oncologique (GC-SFRO) created a national database gathering all patients who underwent brachytherapy for penile cancer, in order to provide more consistent results.
6. Conclusion
For localized cancers penile (T1-2), brachytherapy after circumcision represents the treatment of choice aiming to offer both efficient and conservative approach. Because of its ability to optimize the dose distribution and its low constraints in terms of radiation protection, HDR brachytherapy gradually gains in popularity and respectability. For promoting penile cancer conservative treatment, MHB provides encouraging results in terms of oncological and functional results, while presenting a consistent alternative to the LDR/PDR brachytherapy series. Larger series with extended follow-up are warranted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bleeker M.C.G., Heideman D.A.M., Snijders P.J.F., Horenblas S., Dillner J. Meijer CJLM. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27(2):141–150. doi: 10.1007/s00345-008-0302-z. [DOI] [PubMed] [Google Scholar]
- 2.Heideman D.A.M., Waterboer T., Pawlita M., Delis-van Diemen P., Nindl I., Leijte J.A. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(29):4550–4556. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 3.Hakenberg O.W., Compérat E.M., Minhas S., Necchi A., Protzel C., Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015 Jan;67(1):142–150. doi: 10.1016/j.eururo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Horenblas S., Van Tinteren H., Delemarre J.F., Moonen L.M., Lustig V., Kröger R. Squamous cell carcinoma of the penis: accuracy of tumor, nodes and metastasis classification system, and role of lymphangiography, computerized tomography scan and fine needle aspiration cytology. J Urol. 1991;146(5):1279–1283. doi: 10.1016/s0022-5347(17)38068-0. [DOI] [PubMed] [Google Scholar]
- 5.Maddineni S.B., Lau M.M., Sangar V.K. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;8(9):8. doi: 10.1186/1471-2490-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaunay B., Soh P.N., Delannes M., Riou O., Malavaud B., Moreno F. Brachytherapy for penile cancer: efficacy and impact on sexual function. Brachytherapy. 2014;13(4):380–387. doi: 10.1016/j.brachy.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Crook J.M., Haie-Meder C., Demanes D.J., Mazeron J.-J., Martinez A.A., Rivard M.J. American brachytherapy Society-Groupe Européen de Curiethérapie-European Society of Therapeutic Radiation Oncology (ABS-GEC-ESTRO) consensus statement for penile brachytherapy. Brachytherapy. 2013;12(3):191–198. doi: 10.1016/j.brachy.2013.01.167. [DOI] [PubMed] [Google Scholar]
- 8.Rozan R., Albuisson E., Giraud B., Donnarieix D., Delannes M., Pigneux J. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients) Radiother Oncol J Eur Soc Ther Radiol Oncol. 1995;36(2):83–93. doi: 10.1016/0167-8140(95)01574-z. [DOI] [PubMed] [Google Scholar]
- 9.de Crevoisier R., Slimane K., Sanfilippo N., Bossi A., Albano M., Dumas I. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX) Int J Radiat Oncol Biol Phys. 2009;74(4):1150–1156. doi: 10.1016/j.ijrobp.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 10.Escande A., Haie-Meder C., Mazeron R., Maroun P., Cavalcanti A., de Crevoisier R. Brachytherapy for conservative treatment of invasive penile carcinoma: prognostic factors and long-term analysis of outcome. Int J Radiat Oncol Biol Phys. 2017;99(3):563–570. doi: 10.1016/j.ijrobp.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 11.Kellas-Ślęczka S., Białas B., Fijałkowski M., Wojcieszek P., Szlag M., Cholewka A. Nineteen-year single-center experience in 76 patients with penile cancer treated with high-dose-rate brachytherapy. Brachytherapy. 2019;18(4):493–502. doi: 10.1016/j.brachy.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Rouscoff Y., Falk A.T., Durand M., Gal J., Chand M.-E., Gautier M. High-dose rate brachytherapy in localized penile cancer: short-term clinical outcome analysis. Radiat Oncol Lond Engl. 2014;19(9):142. doi: 10.1186/1748-717X-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinot J.-L., Arribas L., Tortajada M.I., Crispín V., Carrascosa M., Santos M. From low-dose-rate to high-dose-rate brachytherapy in lip carcinoma: Equivalent results but fewer complications. Brachytherapy. 2013;12(6):528–534. doi: 10.1016/j.brachy.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Hannoun-Levi J.-M., Chand-Fouche M.-E., Gautier M., Dejean C., Marcy M., Fouche Y. Interstitial preoperative high-dose-rate brachytherapy for early stage cervical cancer: dose-volume histogram parameters, pathologic response and early clinical outcome. Brachytherapy. 2013;12(2):148–155. doi: 10.1016/j.brachy.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010) http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Access on line 10/20/2020.
- 16.Mohs F.E., Snow S.N., Messing E.M., Kuglitsch M.E. Microscopically controlled surgery in the treatment of carcinoma of the penis. J Urol. 1985;133(6):961–966. doi: 10.1016/s0022-5347(17)49334-7. [DOI] [PubMed] [Google Scholar]
- 17.Brown M.D., Zachary C.B., Grekin R.C., Swanson N.A. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13(11):1163–1167. doi: 10.1111/j.1524-4725.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 18.Shindel A.W., Mann M.W., Lev R.Y., Sengelmann R., Petersen J., Hruza G.J. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178(5):1980–1985. doi: 10.1016/j.juro.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Machan M., Brodland D., Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with mohs micrographic surgery. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2016;42(8):936–944. doi: 10.1097/DSS.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 20.Network, N.C.C. Penile Cancer Version 1; 2017. Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf. Access on line 12/17/2020.
- 21.Zouhair A., Coucke P.A., Jeanneret W., Douglas P., Do H.P., Jichlinski P. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer Oxf Engl. 1990;37(2):198–203. doi: 10.1016/s0959-8049(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 22.Petera J., Sirák I., Kašaová L., Mačingová Z., Paluska P., Zouhar M. High-dose rate brachytherapy in the treatment of penile carcinoma–first experience. Brachytherapy. 2011;10(2):136–140. doi: 10.1016/j.brachy.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Soria J.C., Fizazi K., Piron D., Kramar A., Gerbaulet A., Haie-Meder C. Squamous cell carcinoma of the penis: multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol Off J Eur Soc Med Oncol. 1997;8(11):1089–1098. doi: 10.1023/a:1008248319036. [DOI] [PubMed] [Google Scholar]
- 24.Solsona E., Bahl A., Brandes S.B., Dickerson D., Puras-Baez A., van Poppel H. New developments in the treatment of localized penile cancer. Urology. 2010;76(2 Suppl 1):S36–S42. doi: 10.1016/j.urology.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Cordoba A., Escande A., Lopez S., Mortier L., Mirabel X., Coche-Déqueant B. Low-dose brachytherapy for early stage penile cancer: a 20-year single-institution study (73 patients) Radiat Oncol Lond Engl. 2016;27(11):96. doi: 10.1186/s13014-016-0676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook J., Ma C., Grimard L. Radiation therapy in the management of the primary penile tumor: an update. World J Urol. 2009;27(2):189–196. doi: 10.1007/s00345-008-0309-5. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary A.J., Ghosh S., Bhalavat R.L., Kulkarni J.N., Sequeira B.V. Interstitial brachytherapy in carcinoma of the penis. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 1999;175(1):17–20. doi: 10.1007/BF02743456. [DOI] [PubMed] [Google Scholar]
- 28.Delannes M., Malavaud B., Douchez J., Bonnet J., Daly N.J. Iridium-192 interstitial therapy for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1992;24(3):479–483. doi: 10.1016/0360-3016(92)91062-r. [DOI] [PubMed] [Google Scholar]
- 29.Sharma D.N., Joshi N.P., Gandhi A.K., Haresh K.P., Gupta S., Julka P.K. High-dose-rate interstitial brachytherapy for T1-T2-stage penile carcinoma: short-term results. Brachytherapy. 2014;13(5):481–487. doi: 10.1016/j.brachy.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Marbán M., Crook J., Keyes M., Dubash R., Batchelar D. High-dose-rate brachytherapy for localized penile cancer: Evolution of a technique. Brachytherapy. 2020;19(2):201–209. doi: 10.1016/j.brachy.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Gambachidze D., Lebacle C., Maroun P., Escande A., Bossi A., Blanchard P. Long-term evaluation of urinary, sexual, and quality of life outcomes after brachytherapy for penile carcinoma. Brachytherapy. 2018;17(1):221–226. doi: 10.1016/j.brachy.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Troiano G., Nante N. Quality of life after surgical treatment for penile carcinoma. Int J Sex Health. 2018;30(2):141–148. [Google Scholar]
- 33.Kiltie A.E., Elwell C., Close H.J., Ash D.V. Iridium-192 implantation for node-negative carcinoma of the penis: the Cookridge Hospital experience. Clin Oncol R Coll Radiol G B. 2000;12(1):25–31. doi: 10.1053/clon.2000.9106. [DOI] [PubMed] [Google Scholar]
- 34.Mazeron J.J., Langlois D., Lobo P.A., Huart J.A., Calitchi E., Lusinchi A. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires: the Henri Mondor experience (1970–1979) Int J Radiat Oncol Biol Phys. 1984;10(10):1891–1895. doi: 10.1016/0360-3016(84)90268-2. [DOI] [PubMed] [Google Scholar]
- 35.Makarewicz R., Lebioda A., Terlikiewicz J., Kabacińska R. Interstitial brachytherapy for penile cancer: the experience of Oncology Centre in Bydgoszcz. J Contemp Brachytherapy. 2010;2(4):157–159. doi: 10.5114/jcb.2010.19495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pohanková D., Sirák I., Kašaová L., Grepl J., Paluska P., Louda M. High-dose rate brachyther-apy in the treatment of early stages of penile carcinoma. Klin Onkol Cas Ceske Slov Onkol Spolecnosti. 2019;32(1):52–57. doi: 10.14735/amko201952. [DOI] [PubMed] [Google Scholar]