Abstract
Signal peptide CUB EGF-like domain-containing protein 2 (SCUBE2), a member of the SCUBE family of proteins, was recently found to play an important role in cancer development. However, little is known regarding its biological function in glioma. In the present study, we investigated the effect of SCUBE2 on glioma and explored its relevant mechanisms. The study showed that SCUBE2 had a low expression in glioma tissue and cell lines. SCUBE2 overexpression inhibited glioma cell proliferation in vitro and in vivo as well as suppressed glioma cell migration and invasion in vitro. Furthermore, we found that the Sonic hedgehog (Shh) signaling pathway was involved in the inhibitory effect of SCUBE2 overexpression on glioma cells. In light of the results obtained from our study, SCUBE2 may be regarded as a potential therapeutic target for glioma.
Key words: Signal peptide CUB EGF-like domain-containing protein 2 (SCUBE2), Proliferation, Migration, Invasion, Glioma
INTRODUCTION
Glioma, a common type of brain tumor, is characterized by aggressiveness1,2. According to statistics, the incidence of glioma is 5 in 100,000, and this high rate makes glioma a great threat to public health3. Glioma often causes a high mortality rate because of its high incidence of malignancy4,5. A majority of patients with glioma are diagnosed at an advanced stage and suffer from a poor outcome3,6. What makes the situation worse are such obstacles as radioresistance, multidrug resistance, insufficient preclinical models, impermeable blood–brain barriers, and an incomplete understanding of the pathogenesis7. Therefore, we desperately need to explore new mechanisms underlying glioma progression, thus realizing more effective diagnosis and treatment of glioma.
The signal peptide CUB EGF-like domain-containing protein (SCUBE) family comprises three members, which are SCUBE1, SCUBE2, and SCUBE38–11. All the family members are conserved evolutionarily from zebrafish to humans and encode secreted proteins associated with cell surface12–14. Recently, several reports demonstrated a significant role of the SCUBE family in cancer development. For example, SCUBE1 was found to be highly expressed in gastric cancer patients and was suggested as a new biological marker for gastric cancer15. Additionally, SCUBE2 was reported to be linked with progression of several kinds of cancers. Cheng et al. proved the tumor-suppression effect of SCUBE2 on breast cancer cells16. Lin et al. drew a similar conclusion that SCUBE2 served as a tumor suppressor in breast cancer by inhibiting cell migration and invasion17. In colorectal cancer, SCUBE2 was suggested to be a potential therapeutic target for its inhibitory effect on colorectal cancer cell proliferation, migration, and invasion18. Despite these studies on the role of SCUBE2 in cancer progression, there have been no reports on its biological function in glioma.
In the present study, we investigated the effect of SCUBE2 on glioma and explored its relevant mechanisms. The study showed that SCUBE2 had a low expression in glioma tissue and cell lines. SCUBE2 overexpression inhibited glioma cell proliferation, migration, and invasion. Furthermore, we found that SCUBE2 overexpression exerted an inhibitory effect on glioma cells via regulating the Sonic hedgehog (Shh) signaling pathway.
MATERIALS AND METHODS
Tissue Specimen Collection
Glioma tissue and normal brain tissue were collected from patients of The Second Hospital of Hebei Medical University (P.R. China). Tissue was frozen in liquid nitrogen directly after collection and then stored at −80°C for future use. All patients provided written consent before their participation in the study. All experiments were carried out with approval of the ethics committee of The Second Hospital of Hebei Medical University.
Cell Culture
Human glioma cell lines (U87 and A172) and human astrocytes (HA1800) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). After culturing in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), streptomycin (100 μg/ml), and penicillin (100 U/ml), all cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Quantitative RT-PCR
Isolation of total RNA from tissue or cells was performed with the TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA). Reverse transcription of RNA into cDNA was conducted with the Gene Amp PCR System 9700 (Life Technologies, Carlsbad, CA, USA). Quantitative RT-PCR was performed using the Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) with the following primers: SCUBE2, 5′-CCCCCAAGCGCCGCATCCTGA-3′ (forward) and 5′-TATTGAGTGGCACGTGGGCTGAGT-3′ (reverse); GAPDH, 5′-GCCAAAAGGGTCATCATCTC-3′ (forward) and 5′-ACCACCTGGTGCTCAGTGTA-3′ (reverse). The conditions for PCR amplification were as follows: 5 min of initial denaturation at 95°C, 30 s of denaturation for 35 cycles at 56°C, 60 s of annealing at 60°C, 60 s of elongation at 72°C, and 7 min of final elongation at 72°C. The relative mRNA levels were normalized to GAPDH, and fold change was calculated using the comparative CT method (2−ΔΔCT)19.
Western Blot
Lysis buffer was used to extract total protein from tissue or cells. The BCA Protein Assay Kit (Pierce Chemical, Rockford, IL, USA) was applied to determine the protein concentration. Lysates were separated by 10% SDS-PAGE and then transferred to PVDF membranes. After blocking in 5% skim milk for 1 h, membranes were incubated overnight at 4°C with primary antibodies against SCUBE2, Gli1, Ptch1, or GAPDH. After washing three times with PBST, the membranes were incubated with HRP-conjugated secondary antibody. Protein bands were detected using ECL detection reagents (Bio-Rad, Hercules, CA, USA). The relative protein expression was analyzed using the Image-Pro plus software 6.0.
Plasmids and Transfection
The pcDNA3.1-SCUBE2 expression vector and the empty pcDNA3.1 vector were purchased from Gene Pharma Co., Ltd. (Shanghai, P.R. China). U87 and A172 cells were transfected with pcDNA3.1-SCUBE2 or the empty vector using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instruction. After 48 h, the transfection results were checked via Western blot analysis.
MTT Assay
MTT assay was performed to examine cell proliferation ability. Cells were placed into 96-well plates at a density of 5 × 103 cells/well and then cultured for 48 h. After 20 μl of MTT (5 mg/ml; Sigma-Aldrich) was added to each well, cells were cultured for an additional 4 h. Following removal of the culture medium, 150 μl of dimethyl sulfoxide (Sigma-Aldrich) was added. The optical density was determined at 450 nm using a microplate reader (Molecular Devices, Menlo Park, CA, USA).
Colony Formation Assay
Cells were seeded in a six-well plate at a density of 200 cells per well and then cultured at 37°C with 5% CO2 in a humidified incubator for 12 days. Subsequently, cells were stained with 1% crystal violet, and the number of colonies (>50 cells/colony) was counted.
Transwell Assay
Transwell chambers with polycarbonate membranes (8-μm pore size) were used to test cell migration and invasion. For the migration assay, 5 × 103 cells were resuspended in serum-free medium and placed in the upper chamber. DMEM containing 20% FBS was added to the lower chamber. Following 24 h of incubation at 37°C, cells on the upper surface of the membrane were wiped off by cotton swabs, and cells that migrated to the lower surface of the membrane were fixed with methanol and stained with 0.05% Giemsa. The number of migrated cells was counted under a microscope (200×). For the invasion assay, the same procedure as that for the migration assay was followed, except that polycarbonate membranes were coated with Matrigel.
Tumor Xenograft Formation Assay
Four-week-old male BALB/c nude mice were obtained from the National Laboratory Animal Center (Beijing, P.R. China). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Hebei Medical University. U87 cells (5 × 106) transfected with pcDNA3.1-SCUBE2 or the empty vector were subcutaneously injected into the left flank of each mouse. Tumor volume was measured every 5 days. After 30 days, mice were euthanized, and tumors were weighed.
Statistical Analysis
Experimental data were shown as means ± standard deviation (SD). The GraphPad Prism V5.0 software was used for graphing. Student’s t-tests were used to analyze the differences between groups. A value of p < 0.05 was considered statistically significant.
RESULTS
SCUBE2 Was Lowly Expressed in Glioma Tissue and Cell Lines
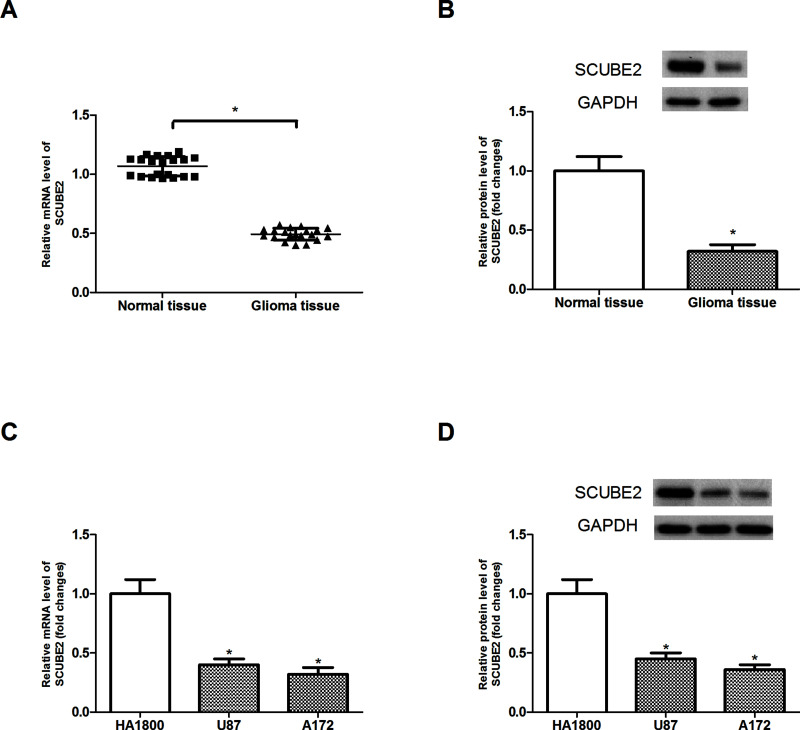
To define the role of SCUBE2 in glioma, we performed both RT-PCR and Western blot to measure the expression levels of SCUBE2 in glioma tissue. The results showed that SCUBE2 had a much lower expression level in glioma tissue than in normal brain tissue (Fig. 1A and B). We further assessed the expression levels of SCUBE2 in glioma cell lines (U87 and A172) and human astrocytes (HA1800). SCUBE2 was also lowly expressed in glioma cell lines, compared to the HA1800 cells (Fig. 1C and D).
Figure 1.
SCUBE2 was lowly expressed in glioma tissue and cell lines. (A, B) RT-PCR and Western blot analysis of SCUBE2 expression in glioma tissues (n = 20) and normal brain tissues (n = 20). (C, D) RT-PCR and Western blot analysis of SCUBE2 expression in glioma cell lines and astrocytes (HA1800). *p < 0.05.
Overexpression of SCUBE2 Inhibited Glioma Cell Proliferation In Vitro and In Vivo
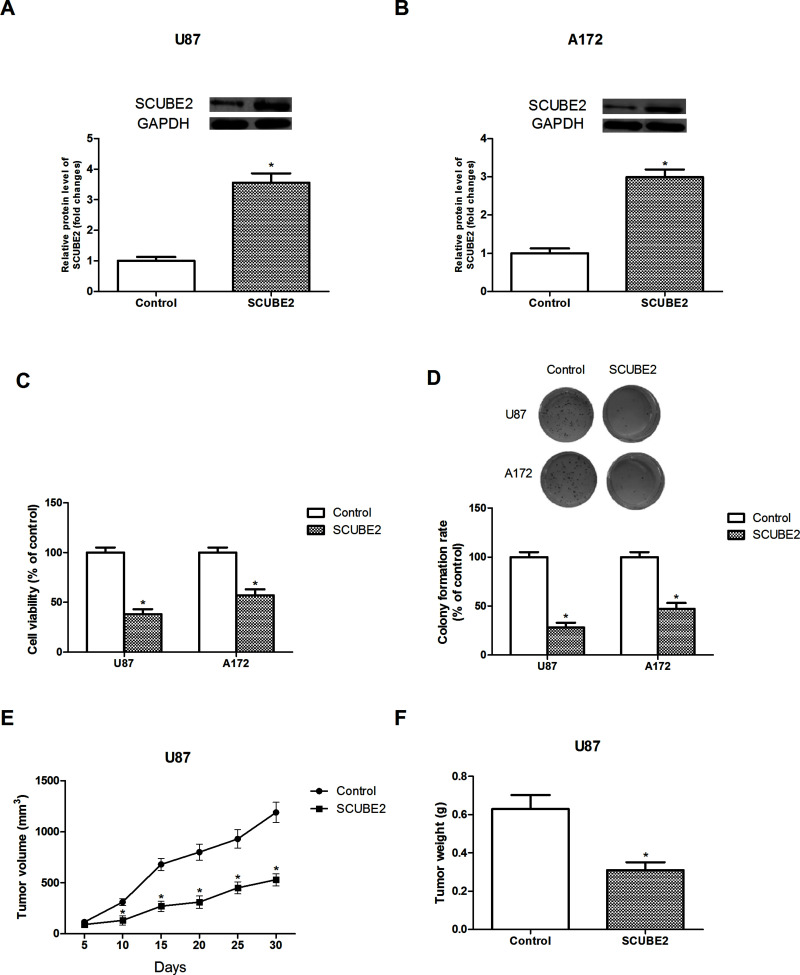
To investigate the biological function of SCUBE2 in glioma, we established a SCUBE2 overexpression model via transfection of pcDNA3.1-SCUBE2 into U87 and A172 cells. The Western blot assay showed a successful transfection (Fig. 2A and B).
Figure 2.
Overexpression of SCUBE2 inhibited glioma cell proliferation in vitro and in vivo. (A, B) Relative protein expression levels of SCUBE2 in U87 and A172 cells after transfection of pcDNA3.1-SCUBE2. (C, D) The effect of SCUBE2 overexpression on U87 and A172 cell proliferation was determined by MTT and colony formation assays. (E) The volume of tumors was measured every 5 days after injection of SCUBE2-transfected U87 cells into nude mice. (F) The weight of tumors was measured 30 days after injection of SCUBE2-transfected U87 cells into nude mice. *p < 0.05.
The effect of SCUBE2 overexpression on glioma cell proliferation in vitro was detected via MTT and colony formation assays. The MTT assay indicated that SCUBE2 overexpression remarkably inhibited cell viability in comparison with the control group (Fig. 2C). Similarly, the colony formation assay showed that SCUBE2 overexpression significantly decreased the number of colonies in comparison with the control group (Fig. 2D).
To verify the in vitro results, we performed in vivo experiments. After subcutaneous injection of SCUBE2-transfected U87 cells into nude mice, we measured tumor growth. The volume and weight of tumors were obviously reduced in the SCUBE2-transfected group in comparison with the control group (Fig. 2E and F).
Overexpression of SCUBE2 Inhibited Glioma Cell Migration and Invasion In Vitro
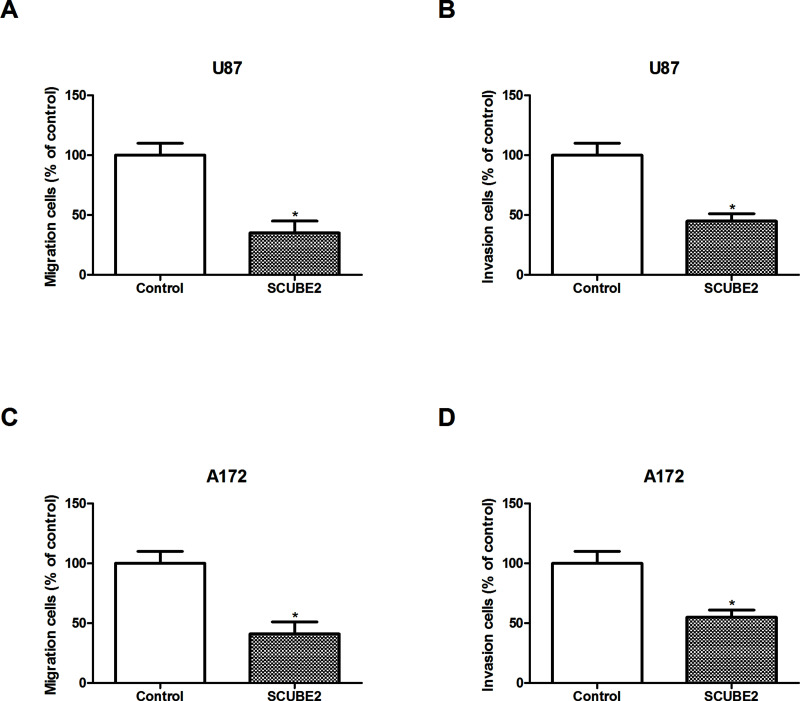
We also explored the effect of SCUBE2 overexpression on glioma cell migration and invasion. The Transwell assay showed that SCUBE2 overexpression markedly attenuated the migratory and invasive abilities of U87 cells (Fig. 3A and B). We obtained similar results for A172 cells (Fig. 3C and D).
Figure 3.
Overexpression of SCUBE2 inhibited glioma cell migration and invasion in vitro. (A, B) Transwell assay was performed to evaluate the effect of SCUBE2 overexpression on cell migration and invasion of U87 cells. (C, D) Transwell assay was performed to evaluate the effect of SCUBE2 overexpression on cell migration and invasion of A172 cells. *p < 0.05.
Overexpression of SCUBE2 Inhibited the Activity of the Shh Signaling Pathway
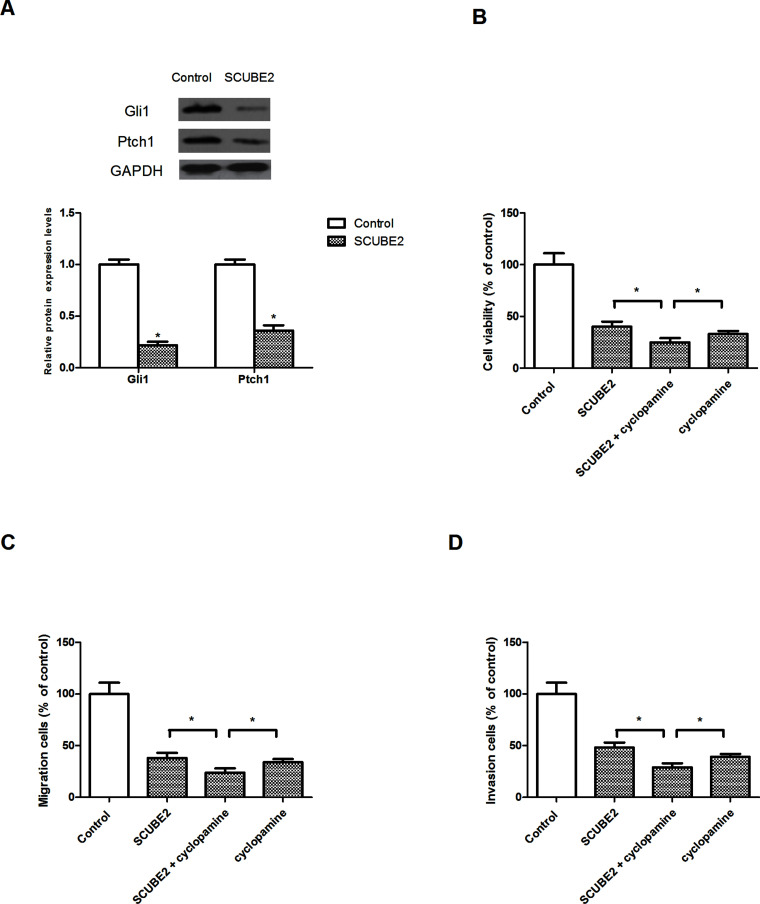
Many studies have demonstrated the important role of SCUBE2 in the regulation of the Shh signaling pathway20,21. Therefore, we determined whether SCUBE2 exerted its inhibitory effect on glioma cells via the Shh signaling pathway. SCUBE2 overexpression remarkably decreased the protein expression levels of Gli1 and Ptch1 in U87 cells in comparison with the control group (Fig. 4A). We also investigated the effect of cyclopamine (an inhibitor of the Shh signaling pathway) on glioma cell proliferation, migration, and invasion mediated by SCUBE2 overexpression. The results showed that cyclopamine obviously potentiated SCUBE2 overexpression-inhibited U87 cell proliferation (Fig. 4B), migration (Fig. 4C), and invasion (Fig. 4D).
Figure 4.
Overexpression of SCUBE2 inhibited activation of the Shh signaling pathway. (A) Western blot assay showed that SCUBE2 overexpression decreased the protein expression of Gli1 and Ptch1 in U87 cells. (B) U87 cells were transfected with pcDNA3.1-SCUBE2 or the empty vector in the presence or absence of cyclopamine (100 nM) for 48 h. MTT assay was performed to measure U87 cell proliferation. (C, D) Transwell assay was performed to detect U87 cell migration and invasion. *p < 0.05.
DISCUSSION
As a great threat to public health, glioma is a problem that urgently needs to be solved. To provide more effective therapeutic strategies, great effort has been put into the exploration of new mechanisms underlying glioma progression.
SCUBE2, a member of the SCUBE family of proteins, was recently found to have an inhibitory effect during cancer development16–18. Despite the findings on the functional significance of SCUBE2 in cancer, little is known regarding its role in glioma. In this study, we investigated the biological function of SCUBE2 in glioma. The study results showed that SCUBE2 was lowly expressed in glioma tissue and cell lines. In addition, SCUBE2 overexpression inhibited glioma cell proliferation, migration, and invasion in vitro. These results were consistent with a previous study where SCUBE2 had a decreased expression in colorectal cancer, and its upregulation exerted an inhibitory effect on colorectal cancer development18. In addition, SCUBE2 was found to serve as a tumor suppressor in breast cancer16,17. In our study, we also conducted in vivo experiments to confirm our in vitro results. In line with our expectation, SCUBE2 overexpression greatly reduced the volume and weight of tumors formed by SCUBE2-transfected U87 cells in nude mice in comparison with the control group. Based on these observations, we suggested the tumor-inhibiting function of SCUBE2 in glioma.
The hedgehog signaling pathway, a highly conserved system, plays a key role in tissue patterns as well as in cell proliferation and differentiation during embryonic development22,23. As a mammalian counterpart of the hedgehog pathway, the Shh signaling pathway shares similar functions24. It is activated by binding to the Ptch–Smo membrane–receptor complex25. There have been findings that the Shh signaling pathway is abnormally activated in various cancers, and many studies have suggested the contribution of its abnormal activation to carcinogenesis26–31. Recently, several studies reported the correlation between the Shh signaling pathway and glioma32,33. Increasing evidence has also demonstrated the importance of the Shh signaling pathway for self-renewal, proliferation, and tumorigenesis of glioma34,35. More importantly, SCUBE2 has been found to play a significant role in regulating the Shh signaling pathway20,21. From the above studies, we inferred that SCUBE2 had a fair chance of inhibiting glioma cell proliferation, migration, and invasion via the Shh signaling pathway. To verify our assumption, we examined the effect of SCUBE2 overexpression on Gli1 and Ptch1, which are important components of the Shh signaling pathway. The results showed that overexpressed SCUBE2 remarkably decreased the protein expression levels of Gli1 and Ptch1 in glioma cells. In addition, we investigated the effect of cyclopamine (an inhibitor of the Shh signaling pathway) on glioma cell proliferation, migration, and invasion mediated by SCUBE2 overexpression. The assay results indicated that cyclopamine greatly inhibited glioma cell proliferation, migration, and invasion, supporting the involvement of the Shh signaling pathway. Besides, cyclopamine markedly enhanced the suppressive effect of SCUBE2 overexpression on glioma cells, suggesting that SCUBE2 inhibited glioma cell proliferation, migration, and invasion partly through suppressing the Shh signaling pathway. Further studies are needed to reveal the definite mechanism behind this effect of SCUBE2.
Taken together, we demonstrated that SCUBE2 was lowly expressed in glioma tissue and cell lines. SCUBE2 overexpression inhibited glioma cell proliferation in vitro and in vivo as well as suppressed glioma cell migration and invasion in vitro. Furthermore, we found that the Shh signaling pathway was involved in the inhibitory effect of SCUBE2 overexpression on glioma cells. In light of the results obtained from our study, SCUBE2 may be regarded as a potential therapeutic target for glioma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C. Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol. 2009;3:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Barnholtz-Sloan JS. Current state of our knowledge on brain tumor epidemiology. Curr Neurol Neurosci Rep. 2011;11:329–35. [DOI] [PubMed] [Google Scholar]
- 3. Ma CC, Xiong Z, Zhu GN, Wang C, Zong G, Wang HL, Bian EB, Zhao B. Long noncoding RNA ATB promotes glioma malignancy by negatively regulating miR-200a. J Exp Clin Cancer Res. 2016;35:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torre LA, Freddie B, Siegel RL, Jacques F, Joannie LT, Ahmedin J. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 5. Goodenberger MKL, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205:613–21. [DOI] [PubMed] [Google Scholar]
- 6. Wang G, Li Z, Tian N, Han L, Fu Y, Guo Z, Tian Y. miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett. 2016;12:879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 8. Grimmond S, Larder R, Hateren NV, Siggers P, Hulsebos TJM, Arkell R, Greenfield A. Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1). Genomics 2000;70:74–81. [DOI] [PubMed] [Google Scholar]
- 9. Grimmond S, Larder R, Hateren NV, Siggers P, Morse S, Hacker T, Arkell R, Greenfield A. Expression of a novel mammalian epidermal growth factor-related gene during mouse neural development. Mech Dev. 2001;102:209–11. [DOI] [PubMed] [Google Scholar]
- 10. Haworth K, Smith F, Zoupa M, Seppala M, Sharpe PT, Cobourne MT. Expression of the SCUBE3 epidermal growth factor-related gene during early embryonic development in the mouse. Gene Expr Patt. 2007;7:630–4. [DOI] [PubMed] [Google Scholar]
- 11. Xavier GM, Panousopoulos L, Cobourne MT. SCUBE3 is expressed in multiple tissues during development but is dispensable for embryonic survival in the mouse. PLoS One 2013;8:e55274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson JLFA, Hall TE, Dyson JM, Sonntag C, Ayers K, Berger S, Gautier P, Mitchell C, Hollway GE, Currie PD. SCUBE activity is necessary for hedgehog signal transduction in vivo. Dev Biol. 2012;368:193–202. [DOI] [PubMed] [Google Scholar]
- 13. Woods IG, Talbot WS. The you gene encodes an EGF-CUB protein essential for hedgehog signaling in zebrafish. PLoS Biol. 3(3):e66; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu BT, Su YH, Tsai MT, Wasserman SM, Topper JN, Yang RB. A novel secreted, cell-surface glycoprotein containing multiple epidermal growth factor-like repeats and one CUB domain is highly expressed in primary osteoblasts and bones. J Biol Chem. 2004;279:37485–90. [DOI] [PubMed] [Google Scholar]
- 15. Mentese A, Fidan E, Sumer AU, Karahan SC, Sonmez M, Altay DU, Kavgaci H, Alver A. Is SCUBE1 a new biomarker for gastric cancer? Cancer Biomark. 2012;11:191–5. [DOI] [PubMed] [Google Scholar]
- 16. Cheng CJ, Lin YC, Tsai MT, Chen CS, Hsieh MC, Chen CL, Yang RB. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 2009;69:3634–41. [DOI] [PubMed] [Google Scholar]
- 17. Lin YC, Lee YC, Li LH, Cheng CJ, Yang RB. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition. J Cell Sci. 2014;127:85–100. [DOI] [PubMed] [Google Scholar]
- 18. Song Q, Li C, Feng X, Yu A, Tang H, Peng Z, Wang X. Decreased expression of SCUBE2 is associated with progression and prognosis in colorectal cancer. Oncol Rep. 2015;33:1956–64. [DOI] [PubMed] [Google Scholar]
- 19. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20. Jakobs P, Schulz P, Ortmann C, Schürmann S, Exner S, Rebollidorios R, Dreier R, Seidler DG, Grobe K. Bridging the gap: Heparan sulfate and SCUBE2 assemble Sonic hedgehog release complexes at the surface of producing cells. Sci Rep. 2016;6:26435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakobs P, Exner S, Schürmann S, Pickhinke U, Bandari S, Ortmann C, Kupich S, Schulz P, Hansen U, Seidler DG. SCUBE2 enhances proteolytic Shh processing from the surface of Shh-producing cells. J Cell Sci. 2014;127:1726–37. [DOI] [PubMed] [Google Scholar]
- 22. Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 2003;3:903–11. [DOI] [PubMed] [Google Scholar]
- 23. Kameda C, Tanaka H, Yamasaki A, Nakamura M, Koga K, Sato N, Kubo M, Kuroki S, Tanaka M, Katano M. The hedgehog pathway is a possible therapeutic target for patients with estrogen receptor-negative breast cancer. Anticancer Res. 2009;29:871–9. [PubMed] [Google Scholar]
- 24. Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993;75:1417–30. [DOI] [PubMed] [Google Scholar]
- 25. Zhai E, Chen J, Chen C, Qin C, Chen S, He Y, Wu H, Cai S. Autocrine Sonic hedgehog signaling promotes gastric cancer proliferation through induction of phospholipase Cγ1 and the ERK1/2 pathway. J Exp Clin Cancer Res. 2016;35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taipale J, Beachy PA. The hedgehog and Wnt signalling pathways in cancer. Nature 2001;411:349–54. [DOI] [PubMed] [Google Scholar]
- 27. Teglund S, Toftgård R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010;1805:181–208. [DOI] [PubMed] [Google Scholar]
- 28. Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 2004;145:3961–70. [DOI] [PubMed] [Google Scholar]
- 29. Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. J Urol. 2005;173:707–12. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez P, Hernández AM, Stecca B, Kahler AJ, Degueme AM, Barrett A, Beyna M, Datta MW, Datta S, Altaba ARI. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA 2004;101:12561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merchant JL, Saqui-Salces M, El-Zaatari M. Hedgehog signaling in gastric physiology and cancer. Prog Mol Biol Transl Sci. 2010;96:133–156. [DOI] [PubMed] [Google Scholar]
- 32. Rush SZ, Abel TW, Valadez JG, Pearson M, Cooper MK. Activation of the hedgehog pathway in pilocytic astrocytomas. Neuro Oncol. 2010;12:790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dahmane N, Sánchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Altaba ARI. The Sonic hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development 2001;128:5201–12. [DOI] [PubMed] [Google Scholar]
- 34. Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu JI, Kondo T. Essential role of the hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]