Abstract
Progestin and adipoQ receptor family member III (PAQR3), a member of the PAQR family, is frequently downregulated in different types of human cancer. However, its expression and functions in esophageal cancer are still unknown. This study aimed to explore the expression of PAQR3 in esophageal cancer cell lines and to investigate the role of PAQR3 in the development of esophageal cancer. Our data showed that PAQR3 is expressed in low amounts in human esophageal cancer cell lines. Overexpression of PAQR3 significantly suppressed the proliferation, migration, and invasion of esophageal cancer cells. In addition, overexpression of PAQR3 downregulated the protein expression levels of RAF1, p-MEK1, and p-ERK1/2 in esophageal cancer cells. Furthermore, overexpression of PAQR3 attenuated the tumor growth in a tumor xenograft model. In conclusion, we demonstrated that overexpression of PAQR3 suppresses cell proliferation, migration, and invasion in esophageal cancer in vitro and in vivo. Therefore, PAQR3 may act as a therapeutic target for human esophageal cancer.
Key words: Progestin and adipoQ receptor family member III (PAQR3), Esophageal cancer, Proliferation, Invasion
INTRODUCTION
Esophageal cancer is one of the most lethal malignancies of the gastrointestinal tract1. It is more common in developing countries, especially P.R. China2. During the last decade, the development of multiple therapeutic strategies for esophageal cancer including radical surgery, chemotherapy, and radiotherapy has improved the prognosis of patients with this malignancy3–5; however, the 5-year survival rate of esophageal cancer patients is less than 30%6. Thus, it is important to explore the molecular mechanisms underlying esophageal cancer tumorigenesis for the treatment of esophageal cancer.
Progestin and adipoQ receptor family member III (PAQR3), also called RKTG (Raf kinase trapping to Golgi), is a member of the PAQR family and has been demonstrated to function as a spatial regulator that negatively modulates the Ras/Raf/MEK/ERK signaling cascade and the GPCR Gβγ subunit signaling pathway7,8. PAQR3 is a Golgi-anchored membrane protein containing seven-transmembrane helices9 and plays an important role in modulating obesity, energy homeostasis, and leptin signaling10,11. In addition, previous studies have shown that PAQR3 is frequently downregulated in different types of human cancer, including prostate, hepatocellular, breast, and gastric, among others. Reduced expression of PAQR3 is involved in the initiation and progression of cancer12–15. Very recently, Wu et al. reported that the expression of PAQR3 was downregulated in human laryngeal squamous cell carcinoma (LSCC) tissues, and overexpression of PAQR3 greatly inhibited the proliferation and invasion of LSCC cells16. However, its expression and functions in esophageal cancer are still unknown. This study aimed to explore the expression of PAQR3 in esophageal cancer cell lines and to investigate the role of PAQR3 in the development of esophageal cancer.
MATERIALS AND METHODS
Cell Culture
Three human esophageal cancer cell lines (EC9706, TE13, and ECA109) and human esophageal epithelial cells (HEEC) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA) and 1% (v/v) penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA). All cells were incubated in a 5% CO2 atmosphere at 37°C.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from esophageal cancer cells using the TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. About 5 μg of the total RNA for each sample was reverse transcribed into the first-strand cDNA for qRT-PCR analysis. PCR amplification was performed on the ABI PRISM 7900 thermocycler (Applied Biosciences, Benicia, CA, USA) using SYBR Premix Taq. The following primer pairs were used for PCR amplification: PAQR3, 5′-TTTGGTCCCCTTCAACC-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′ (reverse); GADPH, 5′-ATCCCATCACCATCTTCCAG-3′ (forward) and 5′-CCATCACGCCACAGTTTCC-3′ (reverse). GAPDH was used as a control for normalizing the gene expression. The relative expression level was calculated using the 2−ΔΔCt cycle threshold method.
Western Blot
Esophageal cancer cells were harvested and washed three times with PBS and then lysed in RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) with protease and phosphatase inhibitors on ice for 10 min. Protein concentrations were determined with a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Equal amounts of protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). Nonspecific binding was then blocked by incubating with 5% skim milk in Tris-buffered saline with Tween (TBST; 10 Mm Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20) at room temperature for 1 h. Subsequently, the membranes were incubated at 4°C overnight with primary antibodies against PAQR3, E-cadherin, N-cadherin, vimentin, RAF1, MEK1, p-MEK1, ERK1/2, p-ERK1/2, and GAPDH (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3,000; Santa Cruz Biotechnology). Finally, the membranes were exposed to and visualized using enhanced chemiluminescence (ECL) reagent.
PAQR3 Expression Vector and Cell Transfection
For PAQR3 overexpression, PAQR3 cDNA were purified and cloned into a pGL3 vector (Promega, Madison, WI, USA) to generate pGL3-PAQR3 recombinant plasmids. An empty vector served as the scramble group. EC9706 cells (1 × 105 cells/well) were cultured in 24-well microplates 1 day prior to transfections. The cultures were 60%–80% confluent when the transfection assay was performed according to the manufacturer’s instructions. Briefly, EC9706 cells were transfected with PAQR3 or vector using Lipofectamine 2000 (Invitrogen).
Cell Proliferation Assay
Cell proliferation was evaluated using the Cell Counting Kit-8 (CCK-8; Beyotime Technology, Jiangsu, P.R. China) according to the manufacturer’s instructions. Briefly, infected EC9706 cells were seeded into 96-well plates (1 × 104 cells/well) and incubated with CCK-8 reagents at different time points (24, 48, 72, and 96 h) at 37°C for 3 h. The absorbance at 450 nm was determined by a microplate reader (Bio-Rad). Optical density was determined at a wavelength of 450 nm using a microplate reader (Bio-Rad).
Cell Migration and Invasion Assays
Cell migration was determined by Transwell assay. Briefly, infected EC9706 cells were plated at a density of 1 × 104 cells per insert in the upper chamber. DMEM with 10% FBS was added to the lower chamber as a chemoattractant. After incubation for 24 h, nonmigrating cells on the upper side of the filter were wiped off. The cells that migrated through the membrane were fixed in absolute methanol for 10 min, stained with 0.5% crystal violet for 30 min, and quantified by counting six random 100× fields per well under the microscope (Olympus, Tokyo, Japan). Cell invasion assay was performed by the same procedure, except that the membrane was precoated with Matrigel (1:3; BD Biosciences, San Jose, CA, USA).
In Vivo Xenograft Tumor Assay
Five-week-old BALB/c female nude mice were purchased from the Experimental Animal Centre of Huaihe Hospital of Henan University (P.R. China). All experiments in this study were performed according to the Institutional Animal Care and Use Committee of Huaihe Hospital of Henan University. For the in vivo study, infected EC9706 cells at 1 × 106 cells/0.1 ml were injected subcutaneously into the flank of nude mice (five animals per group). Tumor size was measured every 5 days and calculated according to the formula (a × b 2) × 0.5 (a is the long axis and b is the short axis of the tumor) using digital calipers. Twenty days after injection, the animals were sacrificed, and tumors were harvested and weighed.
Statistical Analysis
Statistical analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). The data are presented as means ± standard deviation (SD). A one-way ANOVA followed by a Student’s t-test was used to determine if the results were statistically significant. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
PAQR3 Is Lowly Expressed in Human Esophageal Cancer Cell Lines
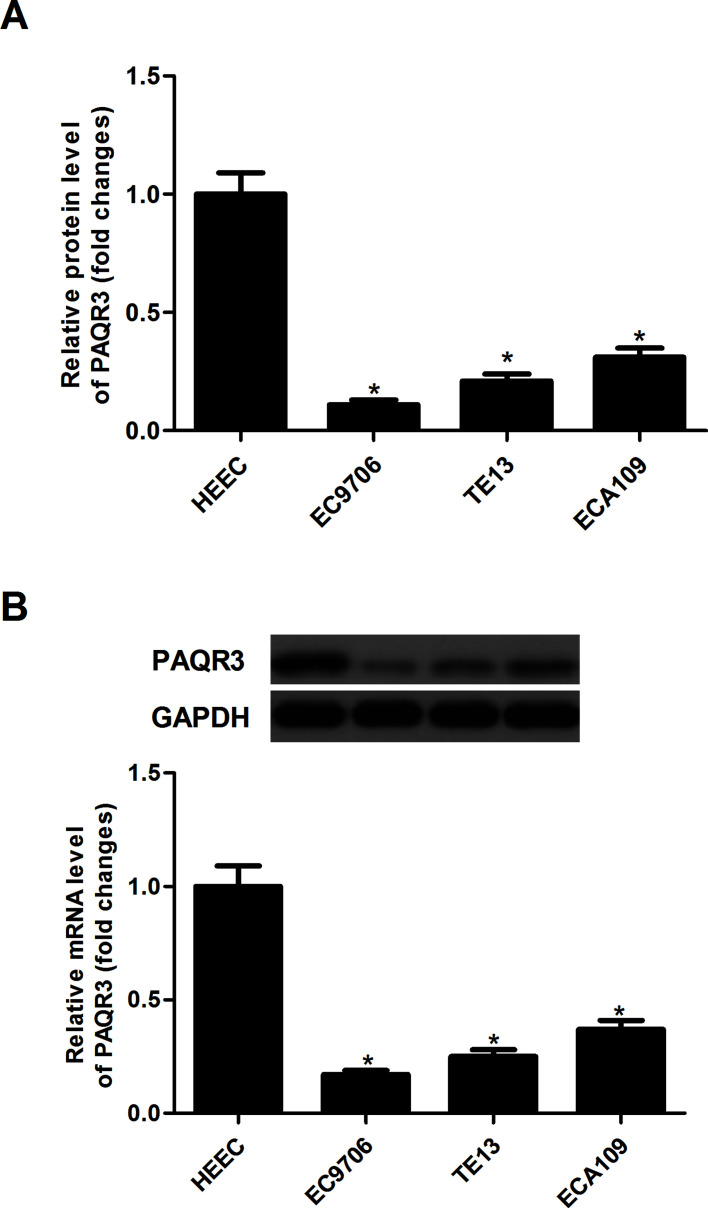
The mRNA expression of PAQR3 was detected in three esophageal cancer cell lines and a normal human esophageal epithelial cell line (HEEC) by qRT-PCR. The results demonstrated that the mRNA expression levels of PAQR3 were lower in esophageal cancer cell lines than in HEEC cells (Fig. 1A). Similarly, the Western blot analysis indicated that the protein expression levels of PAQR3 were significantly decreased in esophageal cancer cell lines when compared with the HEEC cells (Fig. 1B).
Figure 1.
PAQR3 is lowly expressed in human esophageal cancer cell lines. (A) The mRNA expression of PAQR3 in esophageal cancer cell lines was analyzed by qRT-PCR. (B) The mRNA expression of PAQR3 was detected in esophageal cancer cell lines by Western blot analysis and quantified using Image-Pro Plus 6.0 software. Data are expressed as mean ± SD. n = 3. *p < 0.05 versus HEEC group.
Overexpression of PAQR3 Inhibits Esophageal Cancer Cell Proliferation
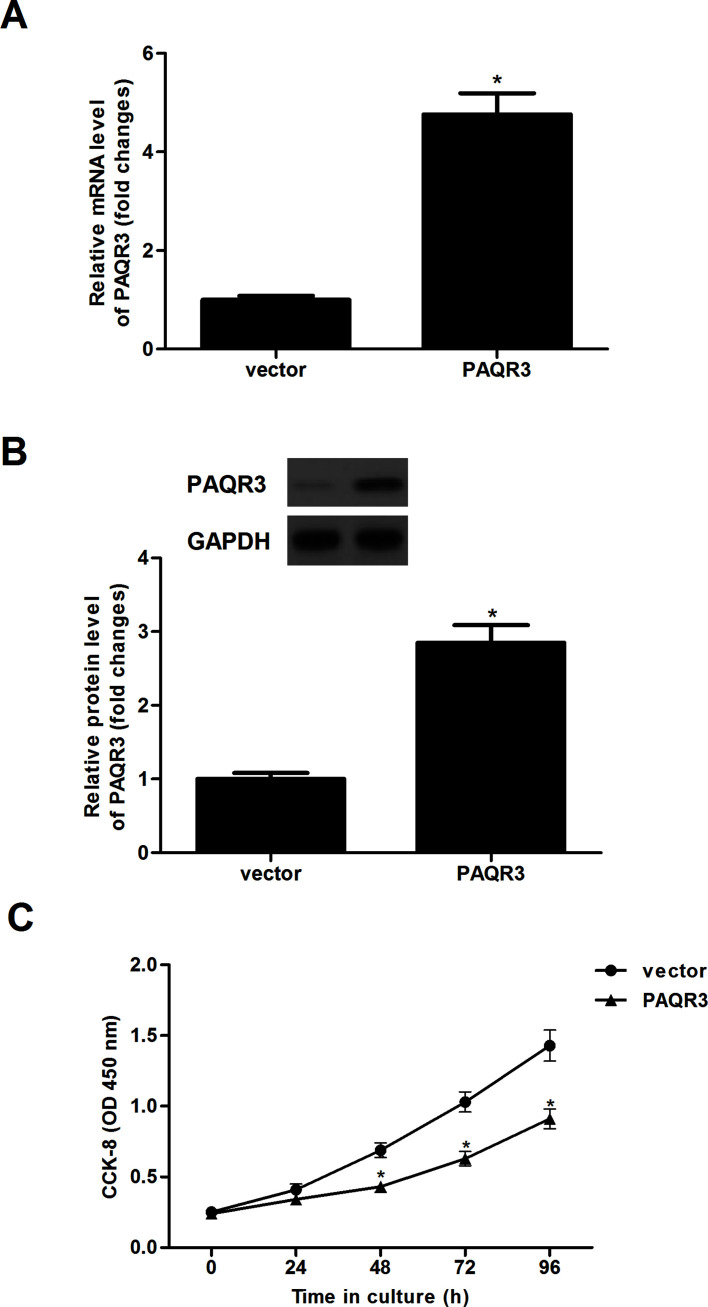
To further validate the role of PAQR3 in regulating esophageal cancer, we generated EC9706 cells that stably express PAQR3 or vector. The results of the qRT-PCR analysis demonstrated that the mRNA expression of PAQR3 in EC9706 cells with PAQR3 was significantly upregulated (Fig. 2A). Similarly, the protein expression of PAQR3 was also obviously upregulated in EC9706 cells (Fig. 2B). Furthermore, CCK-8 assay was performed to investigate the effects of PAQR3 on the proliferation of esophageal cancer cells. Results demonstrated that overexpression of PAQR3 dramatically inhibited the proliferation in EC9706 cells, compared with the vector group (Fig. 2C).
Figure 2.
Overexpression of PAQR3 inhibits esophageal cancer cell proliferation. EC9706 cells were infected with PAQR3 or vector for 24 h. (A) The mRNA expression of PAQR3 was detected by qRT-PCR. (B) The protein expression of PAQR3 was evaluated using Western blotting. (C) Cell proliferation was determined by the CCK-8 assay. Data are expressed as mean ± SD. n = 3. *p < 0.05 versus vector group.
Overexpression of PAQR3 Inhibits Esophageal Cancer Cell Migration and Invasion
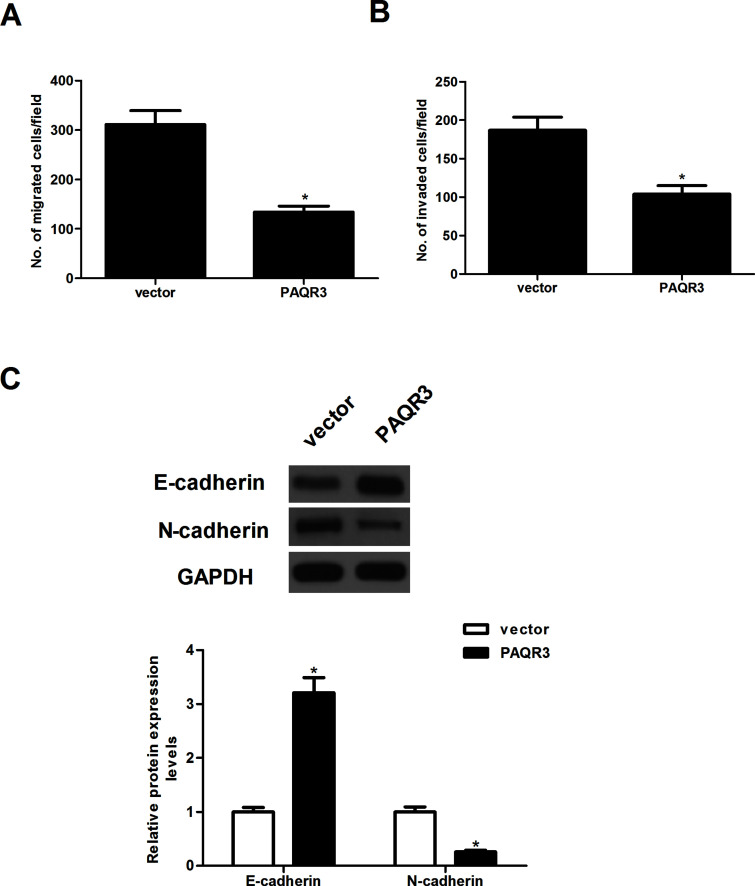
We further investigated the effects of PAQR3 on the migration and invasion of esophageal cancer cells. The Transwell migration assay demonstrated that the number of cells that migrated was significantly lower in EC9706 cells transfected with PAQR3 than that of control parental cells. EC9706 cells transfected with PAQR3 showed significantly less invasion compared with the vector group (Fig. 3B). Furthermore, we evaluated the expression of EMT-related markers in EC9706 cells transfected with PAQR3 using Western blotting. The results showed that overexpression of PAQR3 in EC9706 cells led to an increase in E-cadherin, and a decrease in N-cadherin and vimentin (Fig. 3C).
Figure 3.
Overexpression of PAQR3 inhibits esophageal cancer cell migration and invasion. EC9706 cells were infected with PAQR3 or vector for 24 h. (A) The migration of EC9706 cells was determined using in vitro Transwell migration assay. (B) The invasion of EC9706 cells was determined using in vitro Transwell insert invasion assay. (C) The protein levels of E-cadherin, N-cadherin, and vimentin were determined by Western blot. The relative protein levels were quantified using Image-Pro Plus 6.0 software. Data are expressed as mean ± SD. n = 3. *p < 0.05 versus vector group.
Overexpression of PAQR3 Inhibits the Activation of RAF/MEK/ERK Pathway in Esophageal Cancer Cells
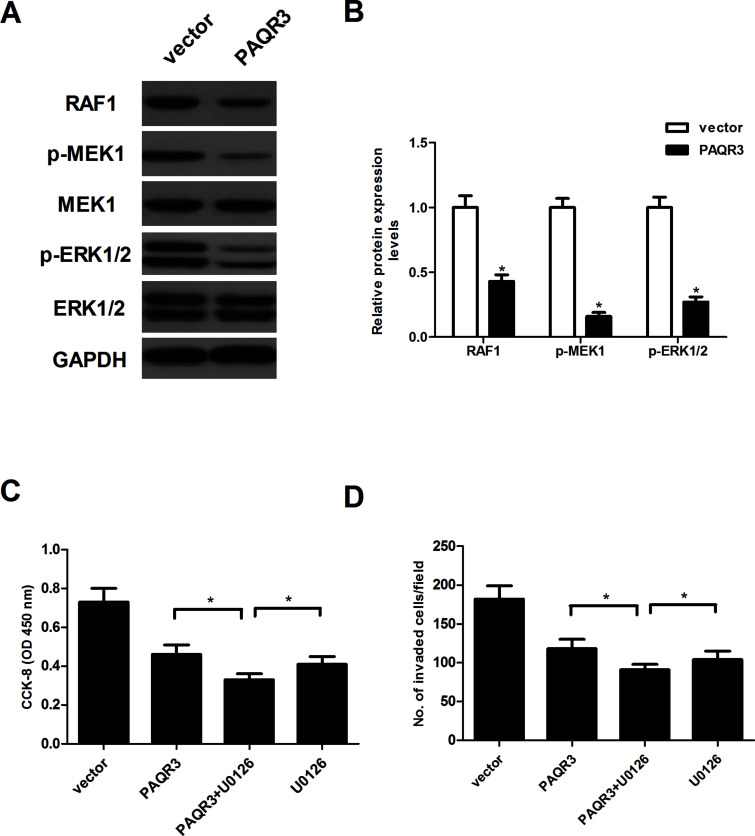
To further elucidate the molecular mechanism by which PAQR3 inhibits the progression of esophageal cancer, we examined the effects of PAQR3 on the protein levels of RAF1, MEK1, p-MEK1, ERK1/2, and p-ERK1/2 in EC9706 cells. The results of the Western blot analysis indicated that overexpression of PAQR3 dramatically downregulated the protein expression levels of RAF1, p-MEK1, and p-ERK1/2 in esophageal cancer cells, compared with the vector group (Fig. 4A). Next, we tested the effect of MEK-specific inhibitor U0126 on PAQR3-inhibited EC9706 cell proliferation and invasion. The results revealed that U0126 treatment greatly enhanced the inhibitory effects of PAQR3 on EC9706 cell proliferation (Fig. 4C) and invasion (Fig. 4D).
Figure 4.
Overexpression of PAQR3 inhibits the activation of the RAF/MEK/ERK pathway in esophageal cancer cells. EC9706 cells were infected with PAQR3 or vector for 24 h. (A) Protein levels of RAF1, MEK1, p-MEK1, ERK1/2, and p-ERK1/2 were determined by Western blot. (B) The relative protein levels were quantified using Image-Pro Plus 6.0 software. EC9706 cells were infected with PAQR3 or vector in the presence or absence of the MEK-specific inhibitor U0126 (1 μM) for 24 h. (C) Cell proliferation was detected by the CCK-8 assay. (D) Cell invasion was evaluated by the in vitro Transwell insert invasion assay. Data are expressed as mean ± SD. n = 3. *p < 0.05.
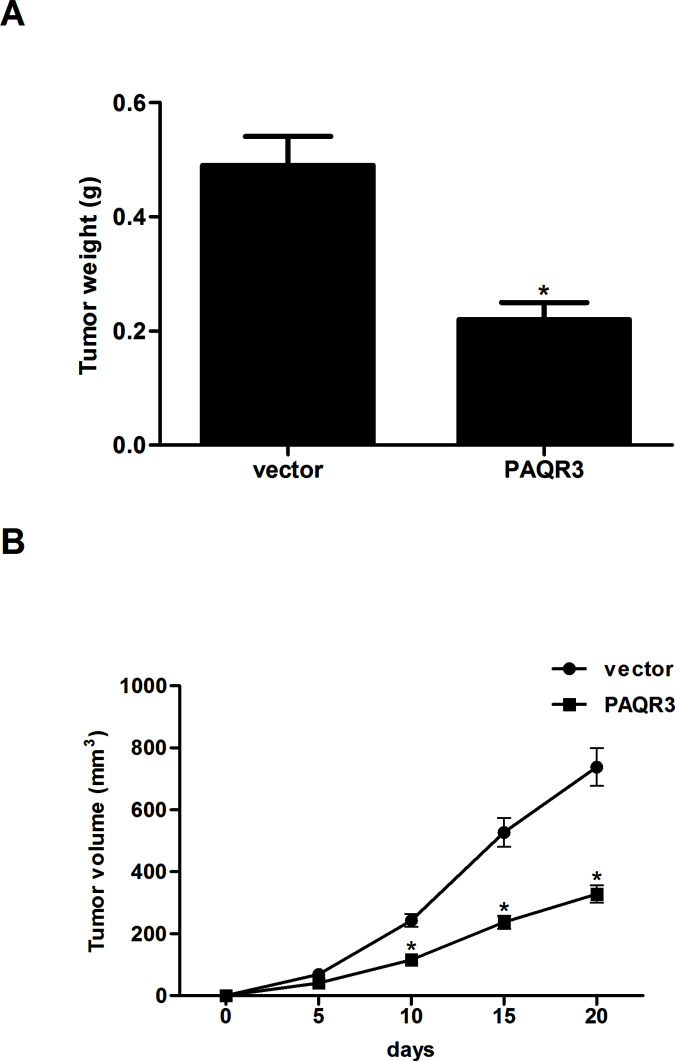
Overexpression of PAQR3 Attenuates the Growth of Xenograft Tumor
To examine the in vivo antitumor growth effects of PAQR3 in esophageal cancer, EC9706 cells infected with PAQR3 or vector were subcutaneously injected into the flank of nude mice. Compared with the vector group, we observed a significant decrease in tumor weight in EC9706 cells transfected with PAQR3 (Fig. 5A). In addition, we found that overexpression of PAQR3 significantly reduced the volume of the tumors (Fig. 5B).
Figure 5.
Overexpression of PAQR3 attenuates the growth of xenograft tumor. Infected EC9706 cells at 1 × 106 cells/0.1 ml were injected subcutaneously into the flank of nude mice. (A) Tumor weights were measured at day 20. (B) Tumor volumes were calculated in each group every 5 days from day 0 to day 20. Data are expressed as mean ± SD. n = 5. *p < 0.05 versus vector group.
DISCUSSION
In this study, a lower expression of PAQR3 was found in human esophageal cancer cell lines. Overexpression of PAQR3 significantly suppressed the proliferation, migration, and invasion of esophageal cancer cells. In addition, overexpression of PAQR3 downregulated the protein expression levels of RAF1, p-MEK1, and p-ERK1/2 in esophageal cancer cells. Furthermore, overexpression of PAQR3 attenuated the tumor growth in a tumor xenograft model.
Previous studies have reported that PAQR3 plays an important role in the progression and metastasis of various human cancers. For example, Ling et al. confirmed that PAQR3 was downregulated in gastric cancer and was closely associated with the progression and metastasis of gastric cancers15. Another study reported that the expression level of PAQR3 was significantly decreased in colorectal cancer samples in comparison with adjacent normal tissues. In addition, the expression level of PAQR3 was inversely associated with tumor grade in colorectal cancer samples17. Consistent with the results of previous studies, our study showed that PAQR3 was downregulated in human esophageal cancer cell lines. Moreover, cellular studies and experimental animal models showed that overexpression of PAQR3 significantly inhibited esophageal cancer cell proliferation and attenuated tumor growth in vivo. Collectively, the results obtained from both in vivo and in vitro experiments strongly suggest that PAQR3 may act as a tumor suppressor in the progression and development of esophageal cancer.
EMT is one of the major molecular mechanisms through which invasion and metastasis are promoted during the oncogenic process18. During EMT, the actin cytoskeleton is dramatically reorganized, and cells acquire increased cell–matrix contacts, leading to dissociation from surrounding cells and enhanced migratory and invasive abilities19. It was reported that PAQR3 overexpression suppressed EMT and metastasis of gastric cancer cells20. Similarly, we observed that overexpression of PAQR3 substantially suppressed migration and invasion, as well as inhibited the EMT process in esophageal cancer cells. These data suggest that PAQR3 might be an important contributor to EMT progression, thus facilitating the migration and invasion of esophageal cancer cells.
The Raf/MEK/ERK signaling pathway plays a critical role in the development and progression of tumors21–23. ERK is a downstream component of an evolutionarily conserved signaling module that is activated by the Raf serine/threonine kinases. ERK phosphorylation may control transcription by targeting several different regulators, which were involved in the regulation of normal cell proliferation, survival, and differentiation24,25. PAQR3 is a regulator that negatively modulates the Raf/MEK/ERK signaling pathway. Most recently, one study reported that overexpression of PAQR3 inhibited ERK phosphorylation in LSCC cells16. In line with the results of the prior study, we found that overexpression of PAQR3 dramatically downregulated the protein expression levels of RAF1, p-MEK1, and p-ERK1/2 in esophageal cancer cells. These data suggest that PAQR3 inhibited invasion and tumorigenesis in esophageal cancer cells through suppressing the RAF/MEK/ERK signaling pathway.
In conclusion, we demonstrated that overexpression of PAQR3 suppresses the proliferation, migration, and invasion of esophageal cancer in vitro and in vivo. Therefore, PAQR3 may act as a therapeutic target for human esophageal cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:134. [DOI] [PubMed] [Google Scholar]
- 3. Allum WH, Luigi B, Cassivi SD, Cuesta MA, Ming DZ, Nilton FV, Edgar F, Gatenby PAC, Leonie H, Ibraev MA. Surgical treatments for esophageal cancers. Ann NY Acad Sci. 2014;1325:242–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buzurovic IM, Hansen JL, Bhagwat MS, O’Farrell DA, Friesen S, Harris TC, Damato AL, Cormack RA, Martin NE, Devlin PM. Clinical implementation of a novel applicator in high-dose-rate brachytherapy treatment of esophageal cancer. J Contemp Brachytherapy 2016;8:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Nistelrooij AM, van Steenbergen LN, Spaander MC, Tilanus HW, van Lanschot JJ, Lemmens VE, Wijnhoven BP. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Oncol. 2014;109:561–6. [DOI] [PubMed] [Google Scholar]
- 6. Baquet CR, Commiskey P, Mishra SI. Esophageal cancer epidemiology: Racial and gender disparities in incidence, mortality and survival. Arthritis Res Ther. 2015;17:1–15.25566937 [Google Scholar]
- 7. Yu X, Li Z, Chan MT, Wu WK. PAQR3: A novel tumor suppressor gene. Am J Cancer Res. 2015;5:2562–8. [PMC free article] [PubMed] [Google Scholar]
- 8. Peng W, Qiang L, Zheng J, Hu Z. Characterization of Golgi scaffold proteins and their roles in compartmentalizing cell signaling. J Mol Histol. 2014;45:1–11. [DOI] [PubMed] [Google Scholar]
- 9. Feng L, Xie X, Ding Q, Luo X, He J, Fan F, Liu W, Wang Z, Chen Y. Spatial regulation of RAF kinase signaling by RKTG. Proc Natl Acad Sci USA 2007;104:14348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L, Wang X, Li Z, Xia T, Zhu L, Liu B, Zhang Y, Xiao F, Pan Y, Liu Y. PAQR3 has modulatory roles in obesity, energy metabolism, and leptin signaling. Endocrinology 2013;154:4525–35. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Wang L, Zhu L, Pan Y, Xiao F, Liu W, Wang Z, Guo F, Liu Y, Thomas WG. PAQR3 modulates insulin signaling by shunting phosphoinositide 3-kinase p110 to the Golgi apparatus. Diabetes 2013;62:444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang W, Guo W, You X, Pan Y, Dong Z, Jia G, Yang C, Chen Y. PAQR3 suppresses the proliferation, migration and tumorigenicity of human prostate cancer cells. Oncotarget 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu HG, Zhang WJ, Ding Q, Peng G, Zou ZW, Liu T, Cao RB, Fei SJ, Li PC, Yang KY. Identification of PAQR3 as a new candidate tumor suppressor in hepatocellular carcinoma. Oncol Rep. 2014;32:2687–95. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Wang F, Xu J, He Z, Lu Y, Wang Z. The role of PAQR3 gene promoter hypermethylation in breast cancer and prognosis. Oncol Rep. 2016;36:1612–8. [DOI] [PubMed] [Google Scholar]
- 15. Ling ZQ, Guo W, Lu XX, Zhu X, Hong LL, Wang Z, Chen Y. A Golgi-specific protein PAQR3 is closely associated with the progression, metastasis and prognosis of human gastric cancers. Ann Oncol. 2014;25:1363–72. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Zhuang K, Li H. PAQR3 plays a suppressive role in laryngeal squamous cell carcinoma. Tumor Biol. 2015;37:1–5. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Li X, Fan F, Jiao S, Wang L, Zhu L, Pan Y, Wu G, Ling ZQ, Fang J. PAQR3 plays a suppressive role in the tumorigenesis of colorectal cancers. Carcinogenesis 2012;33:2228–35. [DOI] [PubMed] [Google Scholar]
- 18. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. J Oral Maxil Pathol. 2009;139:871–90. [DOI] [PubMed] [Google Scholar]
- 19. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo W, Xue Y, Xu D, Zhang Y, Zheng W, Man K, Wang Z, Yan C. PAQR3 enhances TWIST1 degradation to suppress epithelial–mesenchymal transition and metastasis of gastric cancer cells. Carcinogenesis 2016;37:397–407. [DOI] [PubMed] [Google Scholar]
- 21. Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–34. [DOI] [PubMed] [Google Scholar]
- 22. Li L, Xing H, Liu E, Xie WD. CKBP002, an inhibitor of Raf/MEK/ERK signal pathway and potential cancer therapeutic agent. AAACR (Abstract 5644>) 2007;67(9, Suppl):564467. [Google Scholar]
- 23. Peng CL, Guo W, Ji T, Ren T, Yang Y, Li DS, Qu HY, Li X, Tang S, Yan TQ, Tang XD. Sorafenib induces growth inhibition and apoptosis in human synovial sarcoma cells via inhibiting the RAF/MEK/ERK signaling pathway. Cancer Biol Ther. 2009;8:1729–36. [DOI] [PubMed] [Google Scholar]
- 24. Roberts PJ, Der CJ. Targeting the RAF-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291–310. [DOI] [PubMed] [Google Scholar]
- 25. Mccubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. [DOI] [PubMed] [Google Scholar]