Abstract
The tripartite motif-containing protein 11 (TRIM11), a member of the TRIM protein family, has attracted much attention because of its involvement in the development of the central nervous system. It has gained renewed focus because of its newly found function in promoting tumors. However, little is known about its role in hepatocellular carcinoma (HCC). In the present study, we found TRIM11 to be overexpressed in HCC tissues and cell lines. Downregulation of TRIM11 inhibited HCC cell proliferation and invasion in vitro and in vivo as well as suppressed the epithelial–mesenchymal transition (EMT) process. In addition, downregulation of TRIM11 decreased the protein expression levels of p-PI3K and p-Akt in HCC cells and thus inhibited activation of the PI3K/Akt signaling pathway. Based on these results, we suggest the importance of TRIM11 in HCC progression and the potential of TRIM11 as a therapeutic target for HCC.
Key words: Tripartite motif-containing protein 11 (TRIM11), Proliferation, Invasion, Epithelial–mesenchymal transition (EMT), Hepatocellular carcinoma (HCC)
INTRODUCTION
Hepatocellular carcinoma (HCC), accounting for 80% to 90% of liver cancers, ranks third in the world among the leading causes of cancer-related deaths1–3. HCC has occurred more and more frequently in the past two decades because of various factors such as infection by hepatitis B and C viruses, alcoholism, and aflatoxin-contaminated food4,5. In spite of great improvement in the main therapies including hepatic resection and transplantation, HCC patients still suffer from a poor treatment outcome owing to a high recurrence rate, which impedes prolonged patient survival6,7. Therefore, we desperately need to gain a better understanding of the molecular mechanisms involved in HCC development for the purpose of making HCC treatment more effective.
The family of tripartite motif (TRIM)-containing proteins is composed of more than 70 members, which are further divided into 12 subgroups according to C-terminal domains8,9. This protein family is involved in a wide variety of cellular processes such as cell growth, development, differentiation, apoptosis, immunity, and inflammation10–15. In the past decade, much attention was paid to the investigation of the role the members of the TRIM family played in innate immunity and viral infection16. Currently, most researchers have focused on its function in promoting or suppressing tumors because many of the family members have been found to be implicated in the development of different kinds of cancers8. For example, TRIM11 has been reported to be upregulated in high-grade gliomas and have a promoting effect on the proliferation, migration, and invasion of gliomas17. In another report, TRIM11 was found to function as an oncogene in lung cancer via promoting cell proliferation and invasion18. However, there have been no reports or studies so far on the role of TRIM11 in HCC.
In the present study, we observed TRIM11 to be overexpressed in HCC tissues and cell lines. Downregulation of TRIM11 inhibited HCC cell proliferation and invasion in vitro and in vivo as well as suppressed the epithelial–mesenchymal transition (EMT) process. In addition, downregulation of TRIM11 decreased the protein expression levels of p-PI3K and p-Akt in HCC cells and thus inhibited activation of the PI3K/Akt signaling pathway. Based on all these results, we suggest the importance of TRIM11 in HCC progression and the potential of TRIM11 as a therapeutic target for HCC.
MATERIALS AND METHODS
Tissue Samples
Twenty pairs of HCC tissues and matched noncancerous tissues were obtained from patients who received surgery at the Zhejiang Cancer Hospital (P.R. China). Every patient participating in the study provided written consent. All tissue samples were collected and then frozen in liquid nitrogen for future experiments. The study was carried out with the approval of the ethics committee of the Zhejiang Cancer Hospital.
Cell Culture
MHCC97L, Huh1, and Hep3B are the human HCC cell lines, and THLE-2 is the normal liver cell line. All cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; Sigma-Aldrich) and kept at 37°C in a humidified atmosphere with 5% CO2.
RNA Extraction and Quantitative RT-PCR Analysis
Total RNA was isolated from tissues or cells using an RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s manual. Complementary DNA was synthesized with a TaqMan reverse transcription kit (Bio-Rad, Hercules, CA, USA). The following primers were used for gene amplification: TRIM11, 5′-CACCTAAGCTGCACAGTTCC-3′ (forward) and 5′-GGCTGCCTCCTAATTCTTCC-3′ (reverse); GAPDH, 5′-CACCCACTCCTCCACCTTTG-3′ (forward) and 5′-CCACCACCCTGTTGCTGTAG-3′ (reverse). The expression levels of genes were normalized with the GAPDH gene and measured using the comparative CT method (2−ΔΔCT)19.
Western Blot Analysis
Protein was extracted from tissues or cells using RIPA lysis buffer (Sigma-Aldrich). Protein concentration was determined using a BCA kit (Thermo Fisher Scientific, Waltham, MA, USA). Lysates were subjected to 10% SDS-PAGE and transferred onto nitrocellulose membranes. Subsequent to blocking for 30 min in 5% nonfat milk, the membranes were incubated overnight at 4°C with primary antibodies against TRIM11, E-cadherin, α-catenin, N-cadherin, vimentin, p-PI3K, PI3K, p-Akt, Akt, or GAPDH, followed by incubation for 2 h at room temperature with HRP-conjugated secondary antibody. All antibodies used for these experiments were purchased from Invitrogen (Carlsbad, CA, USA). Blots were visualized using an ECL detection kit (Bio-Rad) and analyzed using Scion Image software (Scion Corporation, Frederick, MD, USA).
Small Interfering RNA (siRNA) and Transfection
The siRNA targeting TRIM11 (siTRIM11; 5′-CAGAAGUUGUGCCUAUGGA-3′) and a nonspecific control siRNA (siNC) were synthesized and purified by GenePharma (Shanghai, P.R. China). Huh1 and Hep3B cells were transfected with siTRIM11 or siNC using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Twenty-four hours later, Western blot analysis was performed to evaluate the transfection efficiency.
Cell Proliferation Assay In Vitro
Cells were seeded in 96-well culture plates at a density of 5 × 104 cells/well and cultured for 24, 48, 72, or 96 h. At a different time point, 0.5 mg/ml MTT (Sigma-Aldrich) was added to each well, and cells were incubated for another 4 h at 37°C. Subsequently, the culture medium was removed, and 150 μl dimethyl sulfoxide (Sigma-Aldrich) was added. The absorbance was measured at 570 nm using a microplate reader (Bio-Rad).
Cell Invasion Assay In Vitro
Transwell chambers were used to perform the cell invasion assay. Cells were resuspended in serum-free medium and then placed in the Matrigel-coated upper chamber (5 × 104 cells/well). The lower chamber was filled with DMEM containing 20% FBS. After incubation for 24 h at 37°C, cells remaining in the upper chamber were removed with a cotton swab, and invading cells were fixed with methanol and stained with 0.1% crystal violet. The number of invading cells from four random areas was counted under a microscope (400×).
Tumorigenicity Assays in Nude Mice
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Zhejiang Cancer Hospital. All animals for experiments were handled according to the institutional guidelines. Male BALB/c nude mice (5 to 6 weeks old) were purchased from the Center for Experimental Animals of the Zhejiang Cancer Hospital. For the tumor growth assay, cells (6 × 106) transfected with siTRIM11 or siNC were suspended in 200 μl of normal saline and subcutaneously injected into the left flank of nude mice (n = 6/group). Every week, tumor volume was measured with a vernier caliper and calculated by the following formula: tumor volume = (largest diameter × perpendicular height2)/2. After 5 weeks, all mice were euthanized to weigh tumors. For the tumor metastasis assay, 200 μl of suspension of transfected cells (6 × 106) was intravenously inoculated into the tail of nude mice (n = 6/group). Five weeks later, all mice were euthanized to check the main organs for macroscopic metastasis. Tumor metastasis mainly appeared in the lungs.
Statistical Analysis
The results from three independent experiments were presented as mean ± standard deviation (SD). All data analysis was performed using SigmaPlot 2001 software (Systat Software, Chicago, IL, USA). Student’s t-tests were applied to compare the differences between two groups. A value of p < 0.05 was considered statistically significant.
RESULTS
TRIM11 Is Upregulated in HCC Tissues and Cell Lines
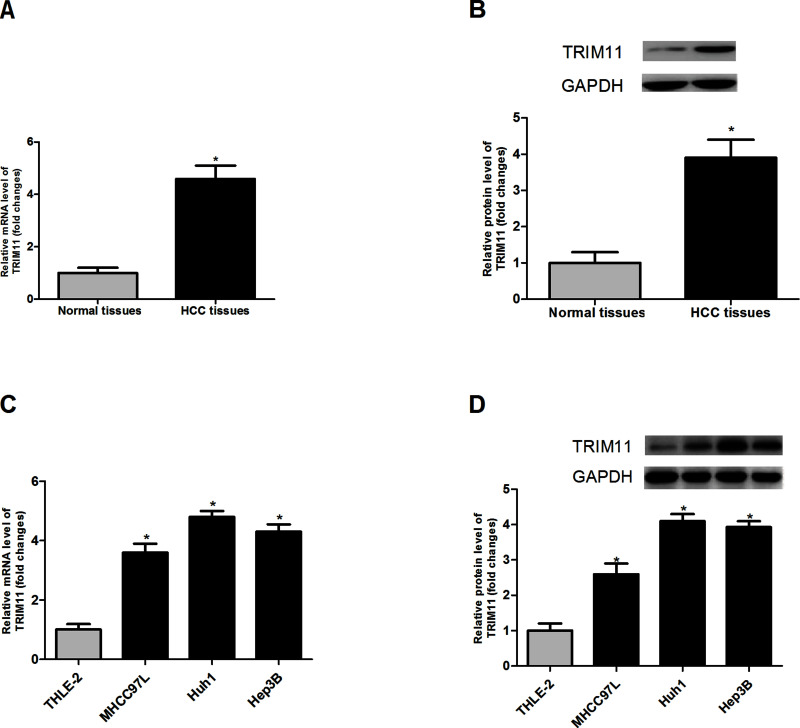
To make clear the relation between TRIM11 and HCC development, we detected the expression of TRIM11 in 20 pairs of HCC tissues and matched noncancerous tissues via RT-PCR and Western blot assays. The assay results indicated that the expression of TRIM11 in HCC tissues was markedly higher than that in normal tissues at both mRNA (Fig. 1A) and protein (Fig. 1B) levels. Additionally, we confirmed the expression of TRIM11 in three HCC cell lines (Huh1, Hep3B, and MHCC97L) as well as one normal liver cell line THLE-2. As expected, we found that the mRNA (Fig. 1C) and protein (Fig. 1D) expression levels of TRIM11 in the three HCC cell lines were much higher than those in THLE-2. Our results showed that TRIM11 was upregulated in HCC tissues and cell lines.
Figure 1.
TRIM11 is upregulated in HCC tissues and cell lines. (A, B) Expression of TRIM11 in HCC tissues and matched normal tissues. (C, D) Expression of TRIM11 in three HCC cell lines (MHCC97L, Huh1, and Hep3B) as well as one normal liver cell line THLE-2. *p < 0.05.
Downregulation of TRIM11 Inhibits HCC Cell Proliferation In Vitro and Cell Growth In Vivo
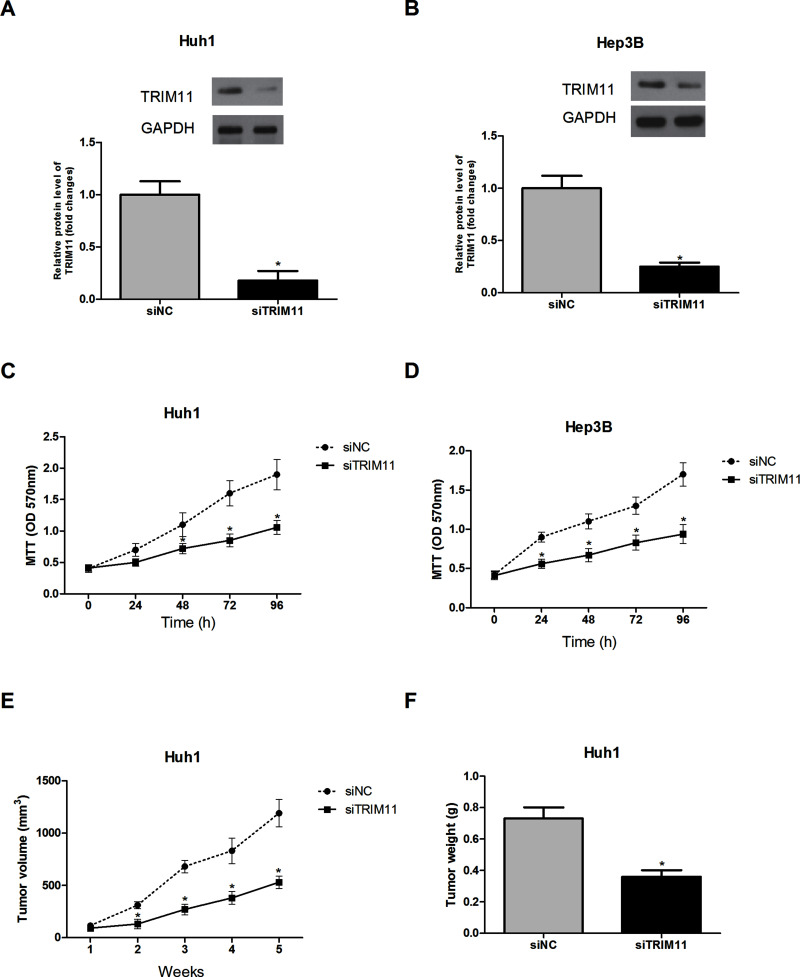
To determine the role of TRIM11 in HCC cells, TRIM11 was downregulated in Huh1 and Hep3B cells by transfection of siTRIM11. The protein expression levels of TRIM11 in Huh1 and Hep3B cells were remarkably decreased by siTRIM11 transfection (Fig. 2A and B).
Figure 2.
Downregulation of TRIM11 inhibits HCC cell proliferation in vitro and cell growth in vivo. (A, B) Expression of TRIM11 in Huh1 and Hep3B cells was downregulated by siRNA transfection. (C, D) The MTT assay showed that downregulated TRIM11 greatly inhibited the proliferation of Huh1 and Hep3B cells in comparison with the control cells. (E, F) Both tumor volume and tumor weight were significantly reduced in siTRIM11-Huh1 cell-injected mice, compared to the control group. *p < 0.05.
The MTT assay was performed to examine the effect of TRIM11 downregulation on HCC cell proliferation. The results showed that downregulation of TRIM11 markedly suppressed the proliferation of Huh1 (Fig. 2C) and Hep3B (Fig. 2D) cells, suggesting the involvement of TRIM11 in the regulation of HCC cell proliferation in vitro.
To test the in vivo effect of TRIM11 downregulation on tumorigenesis of HCC, siTRIM11-transfected Huh1 cells were subcutaneously injected into nude mice, and tumor growth was measured. Downregulation of TRIM11 markedly decreased the volume and weight of tumors formed by Huh1 cells in comparison with tumors formed by corresponding control cells (Fig. 2E and F). These in vivo results proved the positive correlation of TRIM11 to tumorigenesis of HCC.
Downregulation of TRIM11 Inhibits HCC Cell Invasion In Vitro and Metastasis In Vivo
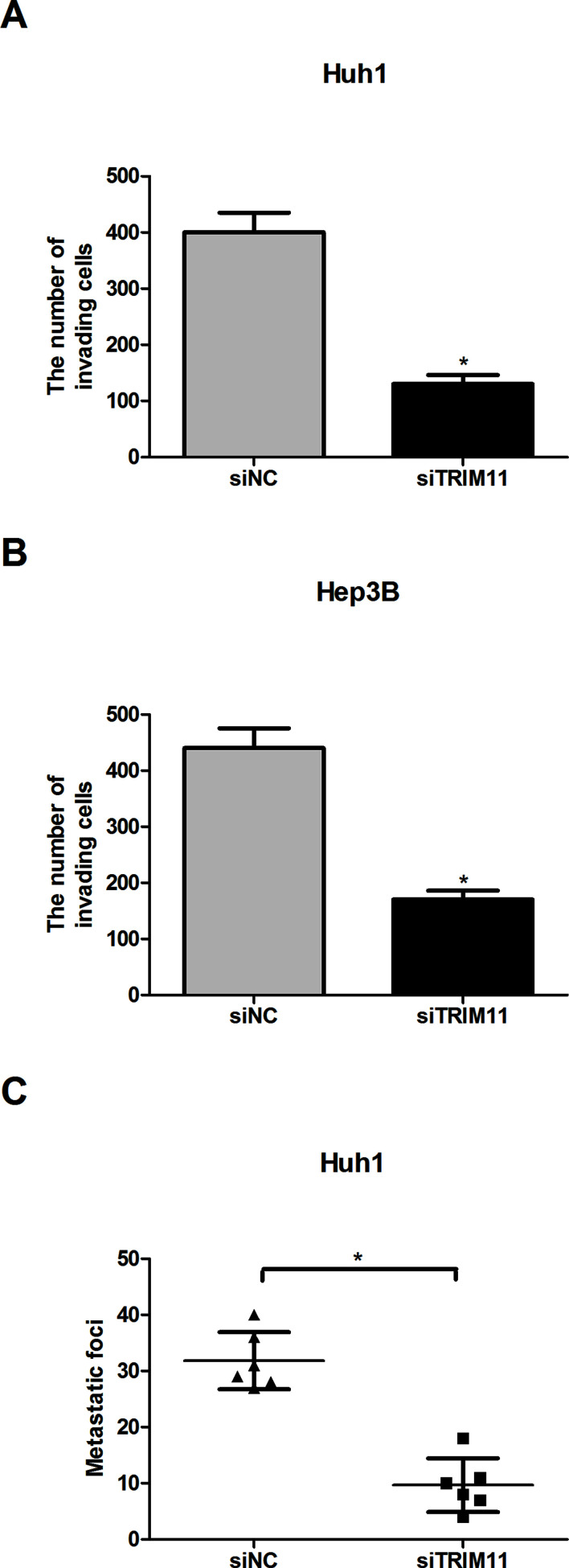
The effect of TRIM11 downregulation on HCC cells was further examined by the cell invasion assay in vitro. As measured by the assay, downregulation of TRIM11 dramatically reduced the invasive capability of both Huh1 (Fig. 3A) and Hep3B (Fig. 3B) cells, compared to corresponding control cells.
Figure 3.
Downregulation of TRIM11 inhibits HCC cell invasion in vitro and metastasis in vivo. (A, B) The representative results of the in vitro invasion assay for Huh1 and Hep3B cells. (C) Metastatic foci in the lungs 5 weeks after intravenous injection of siTRIM11-Huh1 or siNC-Huh1 cells. *p < 0.05.
To investigate whether TRIM11 downregulation inhibited HCC metastasis in vivo, we intravenously inoculated siTRIM11-transfected Huh1 cells into the tails of nude mice. Macroscopic metastasis was checked in the main organs of nude mice 5 weeks after injection. We found that metastatic foci in the lungs were significantly decreased by downregulated TRIM11 (Fig. 3C).
Downregulation of TRIM11 Inhibits the EMT Process in HCC Cells
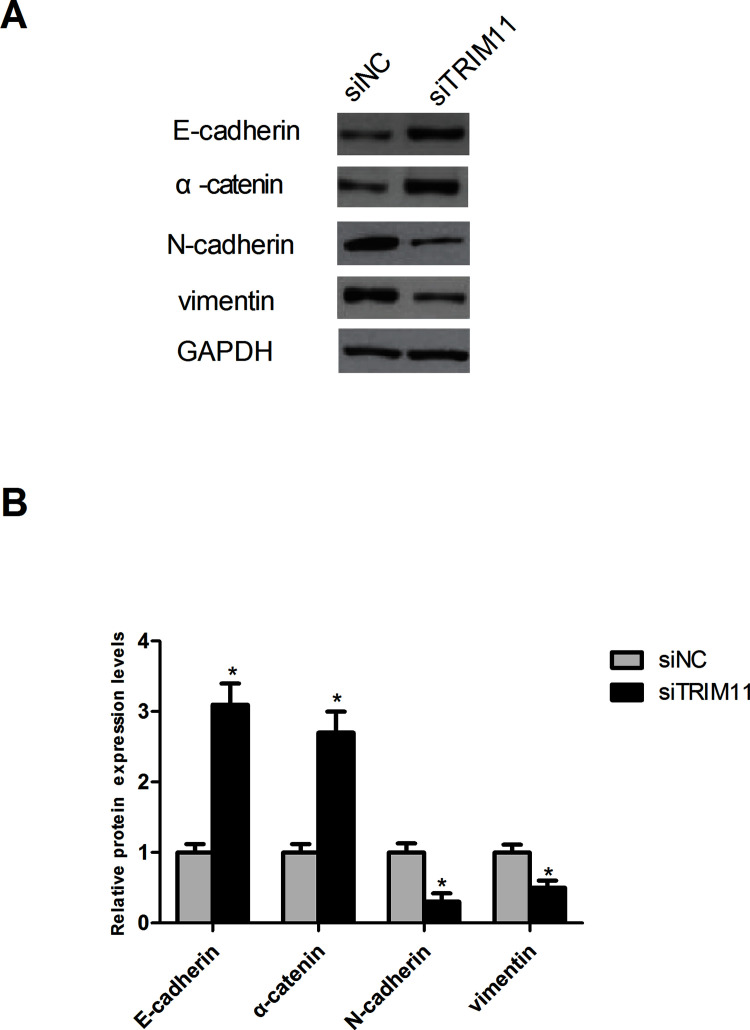
To further confirm the results of the cell invasion and metastasis assays, we investigated the effect of TRIM11 downregulation on the EMT process, which plays a significant role in driving invasion and metastasis of tumors20. For this purpose, we observed the change in the protein expression of epithelial markers (E-cadherin and α-catenin) and mesenchymal markers (N-cadherin and vimentin) in Huh1 cells. Consistent with the cell invasion and metastasis assays, the Western blot analysis showed that downregulated TRIM11 obviously increased the protein expression of E-cadherin and α-catenin but decreased the protein expression of N-cadherin and vimentin in Huh1 cells compared to the control cells (Fig. 4).
Figure 4.
Downregulation of TRIM11 inhibits the EMT process in HCC cells. (A) Detection of the protein expression of EMT-related markers in Huh1 cells by Western blot. (B) Quantification of the protein expression levels of EMT-related markers in Huh1 cells by Scion Image software. *p < 0.05.
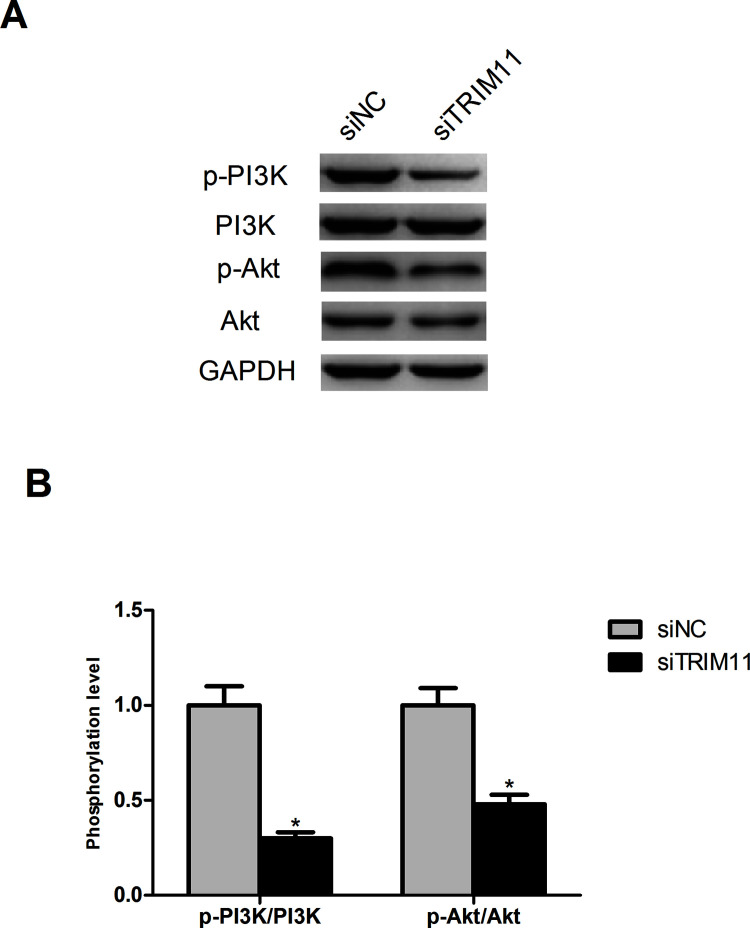
Downregulation of TRIM11 Inhibits Activation of the PI3K/Akt Signaling Pathway
It has been reported that TRIM11 downregulation suppresses the PI3K/Akt signaling pathway in lung cancer cells18. Therefore, we determined whether this pathway was inhibited by TRIM11 downregulation in HCC cells. The results showed that downregulated TRIM11 significantly decreased the protein expression levels of p-PI3K and p-Akt in Huh1 cells without changing the total protein levels of PI3K and Akt (Fig. 5).
Figure 5.
Downregulation of TRIM11 inhibits activation of the PI3K/Akt signaling pathway. (A) Detection of the protein expression levels of p-PI3K, PI3K, p-Akt, Akt, and GAPDH in Huh1 cells by Western blot. (B). The phosphorylation level of PI3K and Akt in Huh1 cells. *p < 0.05.
DISCUSSION
The TRIM-containing protein family attracted much attention in the past, mainly because of its role in innate immunity and viral infection16. Recently, this protein family is gaining focus because most of the family members have been found to be involved in the development of different kinds of cancers8. For instance, TRIM25 was suggested as an oncogene in gastric cancer owing to its promoting effect on cell migration and invasion21. Similarly, TRIM29 was found to function as an oncogene in gastric cancer via potentiating cell proliferation and attenuating apoptosis22. In the aforementioned findings, the TRIM family members had a tumor-promoting effect during cancer development. However, in the studies conducted by Li et al., TRIM16 was found to inhibit HCC cell migration and invasion and thus was defined as an inhibitor of EMT and metastasis in HCC23. Collectively, we infer that TRIM family members play different roles in various cancers. Therefore, a comprehensive understanding of the significance and mechanism of the TRIM family in cancer progression will be valuable for cancer treatment.
In our study, we made an investigation of the expression pattern and biological functions of TRIM11 in HCC. Our study results showed that TRIM11 was overexpressed in HCC tissues and cell lines. Moreover, downregulation of TRIM11 inhibited HCC cell proliferation and invasion in vitro. The in vitro results were confirmed by our in vivo experiments, which indicated a consistent inhibition of downregulated TRIM11 on HCC cell growth and metastasis. Di et al. obtained similar results from their studies—that TRIM11 downregulation suppressed proliferation, migration, and invasion of gliomas17. In another study, TRIM11 was inhibited, which resulted in reduced cell growth, motility, and invasion of lung cancer cells18. In our study, we also examined the effect of TRIM11 downregulation on the EMT process in HCC cells because the EMT process is considered essential for invasion and metastasis of tumors24. The results indicated that downregulated TRIM11 inhibited the EMT process in HCC cells via increasing the protein expression levels of epithelial markers (E-cadherin and α-catenin) and decreasing that of mesenchymal markers (N-cadherin and vimentin). These results were in line with the results of our cell invasion and metastasis assays. Taken together, we proved the tumor-promoting function of TRIM11 in HCC and thus further confirmed the oncogenic role of TRIM11 in cancer development.
Additionally, we investigated the molecular mechanism via which TRIM11 exerted its promoting effect on HCC cells. The PI3K/Akt signaling pathway is capable of promoting cell growth and metastasis25,26. Besides, the pathway is often found to be activated in various cancers27. A study of lung cancer cells showed a suppressive effect of TRIM11 downregulation on the PI3K/Akt signaling pathway18. In our study, we found that TRIM11 downregulation in HCC cells caused a similar inhibition of the PI3K/Akt signaling pathway by decreasing the protein expression levels of p-PI3K and p-Akt. However, in another study of glioblastoma multiforme cells, TRIM11 downregulation had exerted no effect on the activity of the PI3K/Akt signaling pathway17. The discrepancy between the results of our study and those of the previous study may be due to the different types of cells used in studies. Therefore, we need to further investigate the roles of TRIM11 in promoting HCC cells via the PI3K/Akt signaling pathway.
In summary, we demonstrated that TRIM11 was overexpressed in HCC tissues and cell lines. Downregulation of TRIM11 inhibited HCC cell proliferation and invasion in vitro and in vivo. In addition, the EMT process was suppressed in HCC cells after downregulation of TRIM11. We also found that downregulated TRIM11 decreased the protein expression levels of p-PI3K and p-Akt in HCC cells and thus inhibited activation of the PI3K/Akt signaling pathway. Finally, we demonstrated the tumor-promoting effect of TRIM11 on HCC cells, providing evidence in support of the oncogenic role of TRIM11 in the development of HCC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kudo M. Hepatocellular carcinoma in 2011 and beyond: From the pathogenesis to molecular targeted therapy. Oncology 2011;81(Suppl 1):1–10. [DOI] [PubMed] [Google Scholar]
- 2. Meguro M, Mizuguchi T, Kawamoto M, Hirata K. The molecular pathogenesis and clinical implications of hepatocellular carcinoma. Int J Hepatol. 2010;2011:818672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153–6. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, Ferlay JJ, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 5. Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 6. Tang ZY. Hepatocellular carcinoma surgery—Review of the past and prospects for the 21st century. J Surg Oncol. 2005;91:95–6. [DOI] [PubMed] [Google Scholar]
- 7. Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004;127:S5–S16. [DOI] [PubMed] [Google Scholar]
- 8. Zhan W, Han T, Zhang C, Xie C, Gan M, Deng K, Fu M, Wang JB. TRIM59 promotes the proliferation and migration of non-small cell lung cancer cells by upregulating cell cycle related proteins. PLos One 2015;10:e0142596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu C, Guo Y, Yang H, Shi G, Xu G, Shi J, Yin N, Chen D. TRIM66 overexpression contributes to osteosarcoma carcinogenesis and indicates poor survival outcome. Oncotarget 2015;6:23708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Chen DT, Kurtyka C, Rawal B, Fulp WJ, Haura EB, Cress WD. Tripartite motif containing 28 (Trim28) can regulate cell proliferation by bridging HDAC1/E2F interactions. J Biol Chem. 2012;287:40106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tocchini C, Keusch JJ, Miller SB, Finger S, Gut H, Stadler MB, Ciosk R. The TRIM-NHL protein LIN-41 controls the onset of developmental plasticity in Caenorhabditis elegans. PLoS Genet. 2014;10:761–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato T, Okumura F, Ariga T, Hatakeyama S. TRIM6 interacts with Myc and maintains the pluripotency of mouse embryonic stem cells. J Cell Sci. 2012;125:1544–55. [DOI] [PubMed] [Google Scholar]
- 13. Zaman MM, Nomura T, Takagi T, Okamura T, Jin W, Shinagawa T, Tanaka Y, Ishii S. Ubiquitination-deubiquitination by the TRIM27-USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2013;33:4971–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Versteeg GA, Benke S, García-Sastre A, Rajsbaum R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25:563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eames HL, Saliba DG, Krausgruber T, Lanfrancotti A, Ryzhakov G, Udalova IA. KAP1/TRIM28: An inhibitor of IRF5 function in inflammatory macrophages. Immunobiology 2012;217:1315–24. [DOI] [PubMed] [Google Scholar]
- 16. Manocha GD, Mishra R, Sharma N, Kumawat KL, Basu A, Singh SK. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflamm. 2014;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di K, Linskey ME, Bota DA. TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth. Oncogene 2013;32:5038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Shi W, Shi H, Lu S, Wang K, Sun C, He J, Jin W, Lv X, Zou H. TRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cells. J Exp Clinical Cancer Res. 2016;35:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20. De CB, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- 21. Zhu Z, Wang Y, Zhang C, Yu S, Zhu Q, Hou K, Yan B. TRIM25 blockade by RNA interference inhibited migration and invasion of gastric cancer cells through TGF-β signaling. Sci Rep. 2016;6:19070. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Qiu F, Xiong JP, Deng J, Xiang XJ. TRIM29 functions as an oncogene in gastric cancer and is regulated by miR-185. Int J Clin Exp Pathol. 2015;8:5053–61. [PMC free article] [PubMed] [Google Scholar]
- 23. Li L, Dong L, Qu X, Jin S, Lv X, Tan G. Tripartite motif 16 inhibits hepatocellular carcinoma cell migration and invasion. Int J Oncol. 2016;48:1639–49. [DOI] [PubMed] [Google Scholar]
- 24. Lu WD, Zuo Y, Xu Z, Zhang M. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. Int J Clin Exp Pathol. 2014;7:7286–96. [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298:1911–2. [DOI] [PubMed] [Google Scholar]
- 26. Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell 2007;129:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489–501. [DOI] [PubMed] [Google Scholar]