Abstract
The aim of this scoping review was to determine health-related impacts of poor oral health among community-dwelling seniors. Using MeSH terms and keywords such as elderly, general health, geriatrics, 3 electronic databases—Medline, CINAHL, and Age Line were searched. Title and abstracts were independently screened by 3 reviewers, followed by full-texts review. A total of 131 articles met our inclusion criteria, the majority of these studies were prospective cohort (77%, n = 103), and conducted in Japan (42 %, n = 55). These studies were categorized into 16 general health outcomes, with mortality (24%, n = 34), and mental health disorders (21%, n = 30) being the most common outcomes linked with poor oral health. 90% (n = 120) of the included studies reported that poor oral health in seniors can subsequently lead to a higher risk of poor general health outcomes among this population. Improving access to oral healthcare services for elderly can help not only reduce the burden of oral diseases in this population group but also address the morbidity and mortality associated with other general health diseases and conditions caused due to poor oral health. Findings from this study can help identify shortcomings in existing oral healthcare programs for elderly and develop future programs and services to improve access and utilization of oral care services by elderly.
Keywords: Aging, elderly, quality of life, general health, community-dwelling, chronic diseases, mortality, oral health
Background
A demographic transition is happening globally. The aging population is growing rapidly and older adults, aged 65 years or over, are expected to represent approximately 16% of the global population by 2050.1-3 According to the United Nations, those aged 80 years and older will represent 426 million in 2050, which is nearly triple their number in 2019.3 This is mainly attributable to increased life expectancy and decreased fertility.1 Canada is not an exception, where the latest data (for 2018) from the World Health Organization (WHO) showed that Canadian men and women are expected to live 81 and 85 years, respectively.4-6 In addition, adults aged 65 years or older are expected to constitute about one-quarter of the Canadian population by 2036.4 These demographic changes represent a significant challenge for health authorities and policymakers, particularly because senior adults are retaining more natural teeth for longer in life at a time and their oral health needs are not met.7,8 Prevalence of poor oral health conditions among older Canadians, puts them at a higher risk of developing serious general health conditions.4,9 Inadequate and inequitable access to oral care services, further negatively influences access to general healthcare, due to the use of emergency department resources and physician offices for treatment of oral conditions.10 This issue of resources allocation places a significant financial burden on the general health care services that could have been used more efficiently if used for diseases other than oral health problems, where the latter could have been treated more effectively in a dental care setting.10
The detrimental sequels of poor oral health extend far beyond the mouth as there is a growing body of evidence linking oral diseases with various systemic health conditions, especially amongst the elderly such as cardiovascular disorders, metabolic disorders, inflammatory disorders, mental health problems, and even increased mortality.4,11 Moreover, the quality of life of the elderly is compromised with respect to various domains including functional limitation, physical disability, psychological discomfort, pain, social disability, and handicap that might arise from oral health-related conditions.12,13
Elderly people live either independently in the community (ie, community-dwelling elderly) or in long-term care facilities including nursing homes, and chronic care and long-term care hospitals and residences (ie, institutionalized elderly).14 Institutionalized elderly are generally older, frailer, suffer from more co-morbidities, and have different challenges than community-dwelling elderly. It is important to understand the effects of poor oral health on general health for all elderly but as the community dwelling and institutionalized elderly are different in their health status and needs, reviewing literature separately for the 2 populations will be more helpful from the health policy and programmatic perspective. This review will be solely focusing on community-dwelling seniors and a separate review for institutionalized elderly would be conducted at a later date.
Methods
A scoping review was conducted from May 2019 to August 2019 following the 5-stage methodological framework proposed by Arskey and O’Malley (2005),15 and further modified by Levac and colleagues (2010).16 (Table 1).This review aimed at addressing the following research question: what are the health-related impacts of poor oral health among community-dwelling seniors? Our scope was to have a comprehensive understanding of health impacts of poor oral health among community-dwelling seniors both subjectively and objectively. Therefore, a broad research question was crucial to guide the search strategy. We conducted this type of review to identify the existing literature regarding the health-related effects of poor oral health among community-dwelling seniors and summarized the findings. Importantly, we recognized and noted the gaps in the existing evidence. No quality assessment of the included studies was performed in this review.
Table 1.
| Arksey and O’Malley Framework Stages | Description |
|---|---|
| 1. Identifying the research question | To identify the research question to be addressed as this guides the subsequent stages. A broad research question is crucial to guide the search strategy. |
| 2. Identifying relevant studies | To identify the relevant studies to answer the central research question. This involves a search strategy for research evidence as comprehensive as possible. |
| 3. Study selection | This involves the selection of post hoc inclusion and exclusion criteria |
| 4. Charting the data | To extract data from the included studies using a data-charting form. |
| 5. Collating, summarizing and reporting the results | This involves the presentation of an overview of all the materials reviewed but not the synthesis of the evidence. An analytical framework and thematic analysis are provided. |
Eligibility criteria
In this scoping review, studies were included based on the inclusion and exclusion criteria summarized in Table 2. No study design restrictions were applied; however, we limited the search to studies published only in English language. Cross-sectional studies, commentaries, and studies in countries other than those in the Organization for Economic Co-operation and Development (OECD) were excluded. We restricted the study population to community-dwelling seniors aged 60 years and above. Studies that included other age groups in addition to the elderly were included if data specific to those aged 60 years and above could be extracted. Publications, where the authors focused on the elderly population with an age range 50 years and above, were also eligible for inclusion. Our eligibility criteria were guided by the components of the PECO model (Population, Exposure, Comparison, and Outcome). Therefore, we selected the eligible studies based on the criteria outlined below:
Table 2.
Inclusion and exclusion criteria.
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Age limit | Seniors aged 60 years and above | Children and Adults younger than 60 years |
| Population | Community-dwelling seniors | Institutionalized seniors |
| Study design | All study designs | Cross-sectional studies and commentaries |
| Association of interest | Poor oral health (exposure) and a broad range of outcomes including general health, Quality of life, mortality, etc. | Studies measuring the association in a reverse direction, or studies that are not relevant to the study scope |
| Time limit | January 2000 up to May 2019 | Studies prior to 2000 |
| Language | English | Non-English |
| Study location | OECD* countries | Non-OECD countries |
Organization for Economic Co-operation and Development.
Population: Community-dwelling seniors
Exposure: Poor oral health
Comparison: No comparison group/Seniors with good oral health
Outcome: Any self-perceived or professionally assessed health/disease outcome, subjective measure of oral health-related quality of life, mortality, etc.
Search strategy
In collaboration with a library information specialist at Public Health Ontario, a literature search was performed including articles published from January 2000 up to May 2019 in order to include newer studies of recent years that are more relevant epidemiologically and from the health system services perspective. The information specialist at the library assisted the reviewers in identifying the key terms and the databases to be searched that are relevant to the research question scope. The following 3 electronic databases were searched: MEDLINE, CINAHL, and AgeLine using MeSH terms and keywords. A sample of the search strategy used in Medline Ovid is shown in Table 3. Additional articles were also retrieved from screening the reference lists of relevant studies; however, a grey literature search was not conducted in this review as the search strategy was restricted to good quality peer-reviewed evidence that had rigorous scientific evidence which is often lacking in gray literature.
Table 3.
Example search strategy.
| # | Searches | Results |
|---|---|---|
| 1 | *Oral Health/ or *Dentin Sensitivity/ or *Dental Caries/ or *Dental Deposits/ or *Dental Plaque/ or *Dental Pulp Diseases/ or exp *Mouth, Edentulous/ or *Periodontal Diseases/ or exp *Periodontitis/ or *Gingival Diseases/ or *Gingival Recession/ or *Gingivitis/ or *Pericoronitis/ or *Tooth Abrasion/ or *Tooth Attrition/ or *Tooth Avulsion/ or *Tooth Demineralization/ or *Tooth Discoloration/ or *Tooth Diseases/ or *Tooth Erosion/ or *Tooth Fractures/ or *Tooth Injuries/ or *Tooth Loss/ or *Tooth Wear/ or *Toothache/ or *Xerostomia/ or ((“Geriatric* Oral Health Assessment Index” or “oral health” or “periodontal disease*” or “tooth ache*” or (“dental pulp” adj3 (disease* or expos* or calcif* or necros*)) or ((dental or tooth or teeth) adj3 (deposits or plaque or calculus)) or ((dentin or dental or tooth or teeth) adj3 (sensitive* or hypersensitiv*)) or ((gingival or gum or gums) adj3 (recession or reced* or atroph* or disease* or inflam*)) or ((tooth or teeth) adj5 (abras* or attrit* or avuls* or cavity or cavities or crack* or decay* or demineralis* or demineraliz* or de-mineralis* or de-mineraliz* or discolo* or disease* or erod* or erosion or fractur* or injur* or lose or losing or loss or lost or wear or worn)) or (dry adj3 mouth) or “dental loss” or caries or edentul* or gingivitis or pericoronitis or periodontitis or pulpitis or toothache* or xerostomia).kf,kw,ti. and (“in data review” or in process or publisher or “pubmed not medline”).st.) | 128680 |
| 2 | “Aged, 80 and Over”/ or Aged/ or Aging/ or Frail Elderly/ or Geriatrics/ or Health Services for the Aged/ or Dental Care for Aged/ or Geriatric Dentistry/ or ((aged.ti. or (elderly or geriatric* or seniors or gerodontolog*).ti,ab,kf,kw. or ((old* or aging or aged) adj3 (adult* or people or person* or patient* or population* or male* or female* or resident* or man or men or woman or women)).ti,ab,kf,kw. or ((“6#” or “7#” or “8#” or “9#” or sixty* or seventy* or eighty* or ninety*) adj1 (age? or “years old” or “years of age?”)).ab,kf,kw,ti.) and (“in data review” or in process or publisher or “pubmed not medline”).st.) | 3252131 |
| 3 | Malnutrition/ or Nutrition Disorders/ or Nutritional Physiological Phenomena/ or Nutritional Status/ or Weight Loss/ or (malnourish* or malnutrition* or (weight adj3 (loss* or lost or lose* or losing)) or nutrition*).kf,kw,ti. or “Mini Nutritional Assessment”.ab,kf,kw,ti. | 207214 |
| 4 | Quality of Life/ or *Activities of Daily Living/ or *Independent Living/ or *Life Style/ or *Personal Satisfaction/ or (“daily life” or “daily living” or “life satisfaction” or “personal satisfaction” or “life style” or “quality of life” or “well being” or ((live or living) adj3 independen*) or lifestyle or QoL or wellbeing or wellness).kf,kw,ti. or (“Geriatric* Oral Health Assessment Index” or “Oral Health Impact Profile”).ab,kf,kw,ti. | 258840 |
| 5 | “Rejection (Psychology)”/ or Interpersonal Relations/ or Loneliness/ or Phobia, Social/ or Reinforcement, Social/ or Shyness/ or Social Adjustment/ or Social Behavior/ or Social Distance/ or Social Identification/ or Social Isolation/ or Social Participation/ or Social Perception/ or Social Skills/ or Social Stigma/ or Social Support/ or (socialis* or socializ* or ((social* or interpersonal*) adj3 (activities or activity or adjust* or behav* or distanc* or engag* or exclud* or exclusion or factor or factors or frail* or identif* or interact* or isolat* or network* or participat* or perceiv* or percep* or phobi* or reinforc* or reject* or relationship* or skills or support*)) or loneliness or lonely or shy* or stigma or stigmati*).kf,kw,ti. | 262381 |
| 6 | Alzheimer Disease/ or Cognition Disorders/ or Cognition/ or Cognitive Aging/ or Cognitive Dysfunction/ or Dementia/ or Executive Function/ or Mental Processes/ or Neurobehavioral Manifestations/ or Neurocognitive Disorders/ or (“executive function*” or ((mental* or cogni*) adj3 (age or aging or declin* or dysfunction* or function*)) or Alzheimer* or cognition or cognitive or dementia).kf,kw,ti. | 361311 |
| 7 | Affect/ or Affective Symptoms/ or Anxiety Disorders/ or Anxiety/ or Depression/ or Depressive Disorder/ or exp Psychiatric Status Rating Scales/ or Irritable Mood/ or Mental Disorders/ or Mental Fatigue/ or Mental Health/ or Mood Disorders/ or Stress, Psychological/ or (“affective disorder*” or “mental disorder*” or “mental health” or “psychological outcome*” or ((mental* or psych*) adj2 (disorder* or disease* or fatigue or ill* or health* or well* or unwell* or status or symptom*)) or anxiety or anxious* or depress* or mood or moods or stress*).kf,kw,ti. | 915234 |
| 8 | Body Image/ or Personal Autonomy/ or Self Care/ or Self Concept/ or Self Efficacy/ or Self-Neglect/ or (“body image” or “personal autonomy” or “self care” or “self concept” or “self confidence” or “self efficacy” or “self esteem” or “self image” or (self adj3 neglect*) or “self perceived” or “self perception” or “self worth” or “self-rated health” or “sense of self”).kf,kw,ti. | 137249 |
| 9 | Diabetes Mellitus/ or Diabetes Mellitus, Type 1/ or Diabetes Mellitus, Type 2/ or Glucose Intolerance/ or Hyperglycemia/ or Prediabetic State/ or (diabet* or hyperglycemi* or (glucose* adj3 intoler*) or prediabet*).kf,kw,ti. | 443836 |
| 10 | Adenoviridae Infections/ or Coronavirus Infections/ or Influenza, Human/ or Paramyxoviridae Infections/ or Pneumonia, Aspiration/ or Pneumonia, Viral/ or Pneumonia/ or Respiratory Syncytial Virus Infections/ or Respiratory Tract Infections/ or (adenovirus* or (respiratory adj3 (virus* or viral* or infect*)) or bocavirus* or coronavirus* or flu or influenza* or metapneumovirus* or parainfluenza or pneumonia or rhinovirus*).kf,kw,ti. | 245585 |
| 11 | Arthritis/ or Arthritis, Rheumatoid/ or Osteoporosis/ or Osteoporosis, Postmenopausal/ or (arthrit* or osteoporos*).kf,kw,ti. | 213784 |
| 12 | Emergency Service, Hospital/ or Hospitalization/ or Hospitals/ or Office Visits/ or Patient Admission/ or Patient Readmission/ or Physicians' Offices/ or (hospitaliz* or hospitalis* or hospital or hospitals or ((doctor* or physician* or clinician* or provider*) adj3 office*) or ((office* or doctor* or physician* or clinician* or provider* or ambulatory or “primary care” or medical or hospital) adj3 visit*) or (emergency adj3 (room* or department* or unit* or ward*)) or “primary care” or “ambulatory care”).kf,kw,ti. | 543117 |
| 13 | Cardiovascular Diseases/ or Cerebrovascular Disorders/ or Cholesterol/bl or Coronary Artery Bypass/ or C-Reactive Protein/ or Endothelium, Vascular/ or exp Coronary Disease/ or exp Endocarditis/ or exp Heart Arrest/ or exp Heart Failure/ or exp Myocardial Infarction/ or exp Myocardial Ischemia/ or exp Stroke/ or Heart Diseases/ or Models, Cardiovascular/ or Myocardial Revascularization/ or Percutaneous Coronary Intervention/ or Vascular Diseases/ or Vasodilation/ or (“acute coronary” or “artery bypass*” or “artery disease*” or “cardiovascular disease*” or “cardiovascular event*” or “cerebrovascular disease*” or “coronary disease” or “coronary intervention” or “coronary syndrome” or “c-reactive protein” or “heart attack*” or “heart disease*” or “heart failure” or “myocardial infarction*” or “myocardial ischemia” or “unstable angina” or (cardiovascular adj3 mortality) or (endothel* adj3 (function* or dysfunction*)) or cardiopulmonary or cholesterol or endocarditis or revascularis* or revasculariz* or stroke or strokes).kf,kw,ti. | 1320432 |
| 14 | Economics, Medical/ or Economics/ or exp “Costs and Cost Analysis”/ or exp Economics, Hospital/ or Health Services Accessibility/ or exp Models, Economic/ or Financing, Government/ or Health Services Accessibility/ or “Health Services Needs and Demand”/ or Healthcare Financing/ or Public Expenditures/ or Resource Allocation/ or Utilization Review/ or ec.fs. or (cost* or expenditure* or economic* or burden* or “health resources” or (resource* adj3 allocat*) or ((uptake or “use” or utilis* or utiliz* or access*) adj3 (care or healthcare or service*)) or funding or funded or financ* or fiscal*).kf,kw,ti. | 719350 |
| 15 | Disease/ or Chronic Disease/ or Geriatric Assessment/ or Health Status/ or Health/ or Holistic Health/ or Dentin Sensitivity/ae or Dental Caries/ae or Dental Deposits/ae or Dental Plaque/ae or Dental Pulp Diseases/ae or exp Mouth, Edentulous/ae or Periodontal Diseases/ae or exp Periodontitis/ae or Gingival Diseases/ae or Gingival Recession/ae or Gingivitis/ae or Pericoronitis/ae or Tooth Abrasion/ae or Tooth Attrition/ae or Tooth Avulsion/ae or Tooth Demineralization/ae or Tooth Discoloration/ae or Tooth Diseases/ae or Tooth Erosion/ae or Tooth Fractures/ae or Tooth Injuries/ae or Tooth Loss/ae or Tooth Wear/ae or Toothache/ae or Xerostomia/ae or (effect or effects).ti. or (“general health” or “overall health” or “physical outcome*” or “geriatric assessment” or (health adj3 (effect or effects)) or (health adj3 (outcome or outcomes)) or (health adj3 (impact or impacts)) or (health adj3 (risk or risks)) or (health adj3 (concern or concerns)) or (health adj3 (condition or conditions)) or (health adj3 (benefit or benefits)) or (health adj3 improv*) or (health adj3 (problem or problems)) or ((health or negative* or adverse* or positive*) adj3 (impact* or affect* or effect*)) or ((risk or morbidity) adj3 (increas* or decreas*)) or ((associat* or link* or relationship*) adj3 (risk* or disease* or illness*)) or “chronic disease*” or “health status”).kf,kw,ti. | 2238458 |
| 16 | 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 6564777 |
| 17 | 1 and 2 and 16 | 6062 |
| 18 | (exp Africa/ or exp Caribbean Region/ or exp Central America/ or exp Latin America/ or exp South America/ or Asia/ or exp Asia, Central/ or exp Asia, Northern/ or exp Asia, Southeastern/ or exp Asia, Western/ or Far East/ or exp China/ or exp Korea/ or Mongolia/ or Taiwan/ or Mexico/ or Developing Countries/ or exp USSR/ or exp Transcaucasia/ or exp Europe, Eastern/) not (North America/ or exp Canada/ or exp United States/ or exp Australia/ or New Zealand/ or Europe/ or exp United Kingdom/ or exp “Scandinavian and Nordic Countries”/ or Spain/ or Switzerland/ or Netherlands/ or Ireland/ or Italy/ or Germany/ or France/ or Belgium/ or Austria/ or exp Developed Countries/ or exp Japan/) | 1190290 |
| 19 | 17 not 18 | 5257 |
| 20 | exp Animals/ not (exp Animals/ and Humans/) | 4580550 |
| 21 | 19 not 20 | 5243 |
| 22 | (comment or editorial or letter or news or case reports).pt. | 3673940 |
| 23 | 21 not 22 | 5027 |
| 24 | limit 23 to yr = “2000 -Current” | 3707 |
| 25 | remove duplicates from 24 | 3707 |
Ovid MEDLINE(R) ALL <1946 to May 16, 2019>.
Study screening and selection
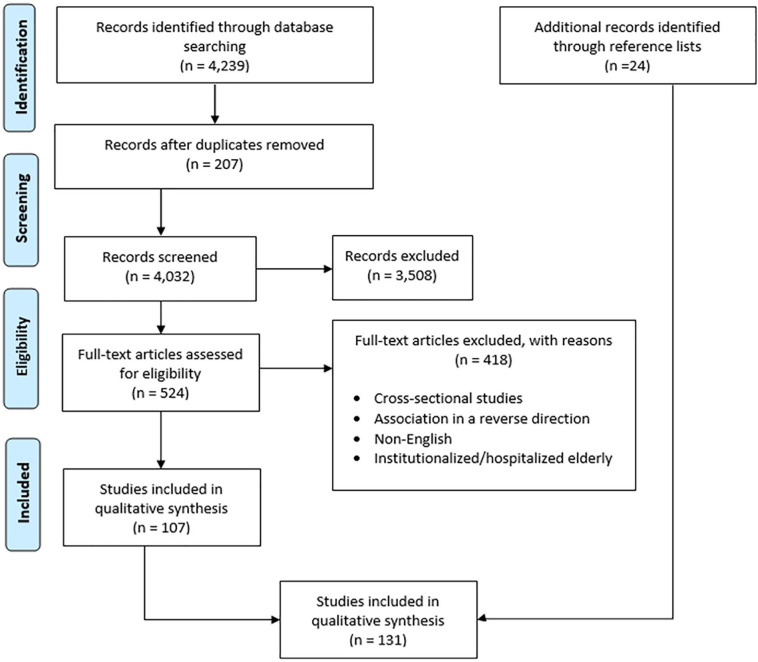
The Preferred Reported Items in Systematic Reviews and Meta-analysis (PRISMA) flowchart shown in Figure 1 describes the screening and selection procedure of studies. Three reviewers (HS, RB, and CQ) independently screened the titles and abstracts of the retrieved articles to select studies eligible for full-text screening. Inter rater reliability (IRR) for the 3 reviewers was 78.65%, which was assessed on 12% (n = 500) of the retrieved studies. Any discrepancies in study inclusion were resolved by consensus between the reviewers, before proceeding on to the next stage of screening. The references of the final studies included for the review were screened to include additional relevant articles.
Figure 1.
PRISMA flow chart for study screening and selection process.
Adapted with permission from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097. Figure 1, Flow of information through the different phases of a systematic review. Available from: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097.
Charting the data
After reviewing the full texts of studies, we extracted the data from each eligible study and developed a data-charting form to organize the study variables using the following headings: last name of the first author and year of publication, study location, study design, population characteristics (and sample size if applicable), objectives, oral health indicator, general health indicator, follow-up period, and author’s conclusion.
Collating, summarizing and reporting the results
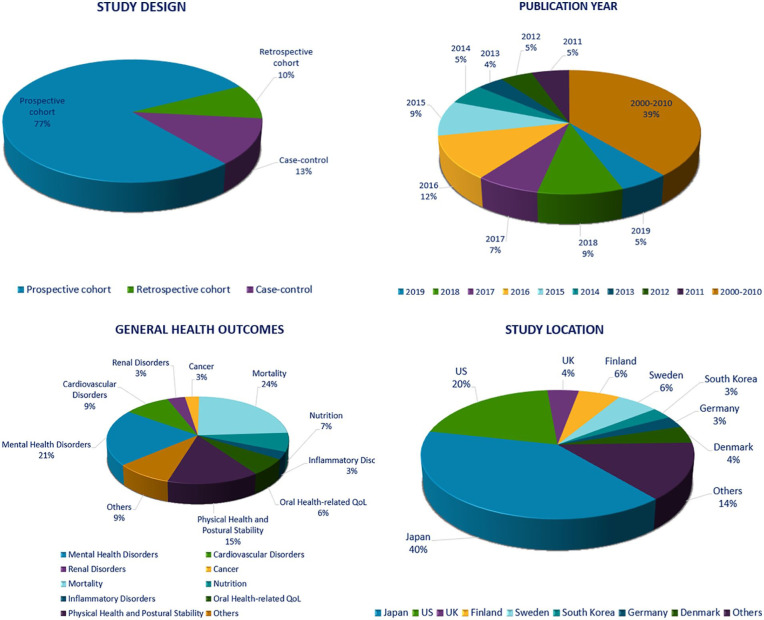
As recommended by Levac and colleagues,16 we started this stage by sorting all the data extracted from each study with respect to characteristics such as the study design, study location, population characteristics, year of publication, exposure and outcome variables, and conclusion, using an excel sheet. Two reviewers (HS, RB) analyzed this data after which a descriptive analysis was developed. Finally, similarities in the data between studies were summarized and a qualitative thematic analysis was conducted with findings represented in tables and charts. (See Figure 2 and Table 4)
Figure 2.
Characteristics of included studies.
Table 4.
Suggested links between oral health and general health of the elderly.
| Oral health indicators | General health indicators | Number of articles |
|---|---|---|
| General health outcome: mortality | (n = 34) | |
| Tooth loss | All-cause mortality | 23,27-31,49-76 |
| Swallowing dysfunction | Mortality due to chronic diseases | |
| Periodontitis | ||
| Maximum bite/occlusal force | ||
| Chewing/eating dysfunction | ||
| Missing, unrestored dentition | ||
| DMFT index | ||
| General health outcome: mental health disorders | (n = 30) | |
| Periodontitis/periodontal disease | Dementia | 24,36-38,77-102 |
| Number of teeth | Cognitive decline, cognitive impairment | |
| Tooth loss | Alzheimer’s disease | |
| Serological markers of periodontal infection | Mild memory impairment | |
| Eating disability | Depression | |
| Self-reported dental health variables: the number of teeth and/or use of dentures, ability to chew, presence/absence of a regular dentist, and taking care of dental health | ||
| General health outcome: physical health and postural stability | (n = 21) | |
| Number of teeth | Frailty, fatigue | 17,72,73,100-117 |
| Eating disability | Physical/mobility disability | |
| Tooth loss/ edentulousness | Gait instability | |
| Periodontitis | Handgrip muscle strength | |
| Masticatory dysfunction | Functional disability | |
| Dry mouth | Falls | |
| General health outcome: cardiovascular disorders | (n = 13) | |
| Tooth loss/ edentulism | Cardiac arrest | 23,26,33-35,45,46,48,118,122 |
| Dental caries | Hypertension | |
| Periodontitis | Non-fatal ischemic stroke | |
| Masticatory efficiency | Coronary heart disease (CHD) | |
| Number of functional occlusal units | Acute myocardial infarction (AMI) | |
| Chronic oral/periapical infections | Cerebrovascular disease | |
| Oral hygiene | Peripheral Artery Disease | |
| Carotid stenosis | ||
| General health outcome: nutrition | (n = 10) | |
| Edentulism/tooth loss Periodontal disease |
Malnutrition Weight loss |
47,123-131 |
| Number of teeth | Impaired diet quality | |
| General health outcome: nutrition | (n = 10) | |
| Toothache | Impaired diet intake | |
| Self-rated oral health | Swallowing dysfunction | |
| Xerostomia | ||
| Impaired dentition (<5 FTUs) | ||
| General health outcome: quality of life | (n = 9) | |
| Denture loss | Quality of life | 17,32,38,104,132-136 |
| Occlusal force | ||
| Xerostomia | ||
| Number of teeth | ||
| Tooth loss | ||
| Oral health problems | ||
| General health outcome: renal disorders | (n = 5) | |
| Periodontitis | Nephropathy and end-stage renal disease | 19,137-140 |
| Kidney function decline | ||
| General health outcome: cancers | (n = 4) | |
| Periodontitis | Non-Hodgkin lymphoma | 18,21,25,141 |
| Tooth loss | Prostate cancer | |
| Breast cancer | ||
| Orodigestive cancer | ||
| Squamous cell carcinoma of the head and neck (SCCHN) | ||
| General health outcome: inflammatory disorders | (n = 4) | |
| Four types of periodontal pathogens: Aggregatibacter actinomycetemcomitans (Aa), Eikenella corrodens (Ec), Porphyromonas gingivalis (Pg) and | Rheumatoid arthritis (RA) | 40-42,52 |
| Prevotella intermedia (Pi) | C-reactive protein levels | |
| Periodontitis | ||
| General health outcome: healthcare costs | (n = 3) | |
| OH related conditions | Hospital admissions | 142,144 |
| Periodontitis | Medical costs | |
| General health outcome: metabolic disorders | (n = 2) | |
| Periodontal disease | Diabetes mellitus (type 1 and type 2) | 39,145 |
| General health outcome: skeletal disorders | (n = 2) | |
| Periodontal disease | Bone mineral density (BMD) | 20,146 |
| Hip fractures | ||
| General health outcome: cerebrovascular disorders | (n = 2) | |
| Periodontitis | Lacunar infarct | 22,43 |
| Cerebrovascular diseases | ||
| General health outcome: general health | (n = 2) | |
| Non-intact teeth | General health | 136,147 |
| Chewing difficulty | ||
| Dry mouth | ||
| Self-rated oral health (SROH) | ||
| General health outcome: respiratory disorders | (n = 1) | |
| Periodontal infections | Decline in forced expiratory volume | 103 |
| Complete prostheses | ||
| General health outcome: hepatic disorders | (n = 1) | |
| Periodontitis | Liver disease progression | 44 |
Results
Our search yielded 4239 articles through the electronic database searching. After removing duplicates, 4032 articles were eligible for title and abstract screening. Of these, 524 articles remained eligible for full-text screening, after applying our inclusion and exclusion criteria (summarized in Table 2). After full-text analysis, 107 articles were found eligible for inclusion. Screening the reference list of these articles yielded an additional 24 articles, and thus a total of 131 articles were included for qualitative synthesis for this scoping review. The process of study selection and screening is described in the Preferred Reported Items in Systematic Reviews and Meta-analysis (PRISMA) flowchart shown in Figure 1.
Thematic analysis
Oral health indicators
Oral health status of older adults has been assessed using various oral health indicators, with periodontitis and tooth loss being the most commonly used measures. A summary of the different oral health indicators extracted from the studies and their possible links with the general health of the elderly is shown in Table 4.
General health indicators
Evidence on the causal links between oral health and general health of the elderly has been focused primarily on 16 general health indicators that have been outlined in this review. Below is a description of each indicator and whether or not it has been causally associated with poor oral health conditions among older adults as implied by the included 131 studies. General health indicators are ordered based on the number of articles found in each section. Data regarding the number of studies, study design, study location, follow-up period, oral health indicators, health outcomes, range of risk, and adjusted confounding variables is included for each of the indicators outlined below. With respect to mortality, there were studies investigating the impact of poor oral health on all-cause mortality and mortality due to chronic diseases such as cardiovascular diseases, cancer, and pneumonia. Studies, where mortality due to chronic diseases was the outcome of interest, were included in the mortality section discussed below, and not in the chronic disease section.
Descriptive analysis
Based on the nature of our research question, observational studies are considered the most appropriate study design to answer this question with poor oral health being the exposure variable. That is the reason why all the studies in our scoping review retrieved from the literature were observational. No randomized controlled trials were identified as this study design cannot be used to answer our question due to ethical reasons. Of the 131 articles included in this review, 103 were prospective cohort (77%), 16 case-control (13%), and 12 retrospective cohort (10%) studies. There were no systematic reviews that matched our inclusion criteria in entirety, and thus only relevant primary studies within them were extracted. The majority of the studies were from Japan and the US accounting for 42% and 21% of the included studies, respectively. The remainder 37%, were studies conducted in Finland, UK, Sweden, South Korea, Germany, Denmark, Australia, Austria, Netherlands, Spain, Switzerland, Ireland, France, Greece, Belgium, Europe, and New Zealand. Regarding the population characteristics, 6 studies focused exclusively on older males,17-22 whereas 3 studies focused only on older females.23-25 The remaining studies did not selectively focus on a particular sex. Thirteen studies included other age groups including those aged 60 years and above, but data were segregated for the elderly group.18,21,22,26-35 Nineteen studies focused on the elderly population with participants aged 50 years and above.17,23,25,36-51
List of outcomes
Health outcomes identified in the 131 included articles in our search were as follows: mortality, mental health disorders, physical health and postural stability, cardiovascular disorders, nutrition, oral health-related quality of life (OHRQoL), renal disorders, cancer, inflammatory disorders, healthcare costs, metabolic disorders, skeletal disorders, cerebrovascular disorders, general health, respiratory disorders, and hepatic disorders. Summary of the oral health indicators and the associated general health outcomes are represented in Table 4.
Mortality
Thirty-four of the included articles addressed whether older adults with indicators of poor oral health had a higher risk of mortality.23,27-31,49-76 The majority of studies investigated the risk of all-cause mortality among the elderly, with the exception of 6 studies reporting the risk of cardiovascular diseases (CVD) mortality,30,31,49,50,52,53 4 studies reporting the risk of pneumonia mortality,53,55,57,65 and 2 studies reporting the mortality risk due to cancer.53,54 The majority of studies were from Japan (n = 18), and the other studies were from Finland, the US, Denmark, Sweden, Europe, and Germany. 33 articles were prospective cohort with follow-up periods ranging from 1 to 23 years, and 1 article was a retrospective cohort study.64 The majority of the articles found a significant (P-values < .05) association between poor oral health status as measured by tooth loss, swallowing dysfunction, periodontitis, maximum bite/occlusal force, edentulism, and increased all-cause mortality risk after adjusting for confounding variables such as chronic diseases, self-rated general health, sex, and smoking status. Yoshida et al reported that, during 8 years follow-up period, the risk of all-cause mortality in Japanese elderly aged ⩾ 65 years with dental occlusion in at least the bilateral premolar regions was 22% (HR = 0.78, 95% CI: 0.60-0.99) lower than for those with no dental occlusion.69 Likewise, Paganini-Hill et al suggested edentulousness as a predictor of all-cause mortality, where the risk of mortality among edentulous Americans aged between 52 and105 years was 30% (HR = 1.33, 95% CI: 1.13-1.56) higher than for those having 20+ teeth.51 Moreover, the risk of all-cause mortality over a 5-year follow-up was 3.9 (HR = 3.94, 95% CI 1.77-8.78) times higher among Finnish elderly aged ⩾ 85 years in urgent need for dental treatments due to oral infections (eg, periodontal infection, oral abscess, and acute necrotising ulcerative gingivitis) than those in no such need, as reported by Hamalainen et al.70 However, not all the studies suggested an increased risk of mortality for older individuals with poor oral health. Saremi et al and Xu et al reported periodontitis as a strong predictor of all-cause mortality among younger subjects (<60 years), but this was not the case in older age groups (⩾60 years).31,49
Six studies assessed the relationship between poor oral health indicators and mortality due to cardiovascular diseases (CVD),30,31,49,50,52,53 2 of which reported dental status and eating ability as significant predictors of 4.28 and 10-year cardiovascular mortality in Japanese and German elderly respectively.50,53 Ajwani et al also found that Finnish elderly aged ⩾ 75 years with periodontitis had twice (RR = 1.97, 95% CI: 1.01-3.85) the risk for incident CVD mortality when compared to those with healthy periodontium.52 Saremi et al reported a significant (P-value < .05) association between severe periodontal disease and cardiorenal deaths (deaths due to ischemic heart disease (IHD) and diabetic nephropathy combined) among Americans aged ⩾35 years with type 2 diabetes, however the risk of deaths was higher in the younger age group 35 to 54 years (HR = 14.8, 95% CI: 3.5-63.2) than in the older age-group ⩾55 years (HR = 3.3, 95% CI: 0.8-14.0).49 Nevertheless, Xu et al reported a significant association between periodontal disease and risk of CVD mortality only among younger age groups (<65 years), whereas no association was found in those aged ⩾ 65 years.31 Two studies assessed the association between poor oral health indicators and mortality due to cancer, one of which reported tooth loss as a significant predictor of mortality due to orodigestive cancer among Japanese elderly aged ⩾ 80 years over a 12-year follow-up period.53,54 In this study, tooth loss significantly increased the risk of cancer by 6% (HR = 1.06, 95% CI: 1.01-1.13, P-value = .015) However, the second study by Aida et al reported no significant association between oral health status as measured by the number of remaining teeth and eating difficulty and cancer mortality among Japanese elderly aged ⩾ 65 years.53 Four studies assessed the relationship between poor oral health indicators and mortality due to respiratory disorders among the Japanese elderly.53,55,57,65 Awano et al reported that the risk of 4-year mortality from pneumonia in Japanese elderly aged >80 years with ⩾ 10 teeth with periodontal pockets was 3.9 (HR = 3.9, 95% CI: 1.1-13.9, P-value < .05) times higher than those without.65 These findings are in accordance with a 7-year study by Iwasaki et al where the risk of mortality from pneumonia in Japanese elderly with a mean age of 64 years having periodontal disease was 3.49 (adjusted sub hazard ratio = 3.49, 95% CI: 1.14-10.64, P-value < .05) times greater than those with a healthy periodontium.57 The aforementioned findings suggest the fact that, in the elderly, poor oral health indicators are associated with increased risk for mortality, however, a causal association between the 2 variables needs to be further investigated.
Mental health disorders
Thirty articles studied the relationship between oral health and mental health.24,36-38,77-102 The various mental health outcomes studied in these articles included depression, cognitive impairment, cognitive decline, memory impairment, dementia, and Alzheimer’s disease. The majority of these studies were prospective cohort studies conducted in Japan. There were differences in reporting the oral and dental health status of older adults between the studies. Multiple oral health indicators were used, including self-reported oral health, periodontitis, serologic markers of periodontitis (serum IgG antibodies), tooth loss, number of teeth, eating/chewing ability, and dentures use. For assessing the cognitive function of participants, most studies used the Mini-Mental State Examination (MMSE) test which is the most commonly used test globally to screen for orientation, concentration, immediate and delayed memory, short-term recall and language among the elderly. It has a maximum score of 30 with a score of 23 or lower indicating cognitive impairment. A variety of other tests were also used in some studies such as the Alzheimer’s Disease Assessment Scale (ADAS-cog), the Geriatric Depression Scale (GDS), Delayed Word Recall examination (DWR); Digit Symbol Substitution (DSS); and Word Fluency (WF). The majority of studies were prospective cohort (n = 23) with follow-up periods ranging between 6 months to 37 years, 4 studies were case-control and 3 were retrospective cohort studies. Twenty-eight studies reported a significant (P-values < .05) association between oral health measures and mental health disorders where older adults with poor oral health condition at baseline were found to be at higher risk of developing mental health disorders during the follow-up period compared to those with better oral health after adjusting for confounding variables such as age, sex, smoking status, drinking habits, physical activity, education level, hearing ability, body mass index, depression, hypertension, and diabetes. A recent study by Iwasaki et al reported that, over 5 years, the odds of suffering from mild cognitive impairment (MCI) among Japanese elderly aged ⩾ 75 years with severe periodontitis were 3.58 (adjusted OR = 3.58, 95% CI = 1.45-8.87) times greater than those without.95 These findings are in accordance with 6 years study by Nilsson et al in which the odds of incident cognitive decline among elderly Swedish aged 60 to 96 years suffering from periodontal bone loss were 2.2 (adjusted OR = 2.2, 95% CI 1.2-3.8) times greater than those without.92 In regards to the impact of tooth loss on mental health disorders, Okamoto et al suggested tooth loss as a possible risk factor for memory disorders where among edentulous Japanese elderly aged ⩾ 65 years, the odds of mild memory impairment (MMI) were 2.39 (OR = 2.39, 95% CI: 1.48-3.86) times greater than those having 25 to 32 teeth.84 However, only 1 study, Stewart et al did not report an association between lower teeth count among French elderly and the incidence of dementia during a 37-year follow-up period.24 Surprisingly, Arrive et al in his study found that having ⩾ 11 missing teeth is associated with a lower risk of dementia among French elderly aged 66 to 80 years with lower education level, however, this was not the case among those with higher education, where lower teeth count was associated with a higher risk of dementia.81 It can be hypothesized that this might be due to the fact that increasing the number of missing teeth among those with low education can improve the periodontal condition and therefore defeating the chronic inflammation induced by periodontal diseases. However, those with higher education most probably had better periodontal condition throughout their life compared to those with lower education levels, therefore, having high teeth count will not induce a chronic inflammatory response, unlike the lower education group. Findings from this study raise concerns regarding the oral health disparities among the elderly with respect to the education level. Based on findings from the majority of studies, indicators of poor oral health can be considered a possible risk factor for mental health disorders among the elderly population.
Physical health and postural stability
Physical health and postural stability have been linked to poor oral health conditions in 21 articles.17,72,73,100-117 The majority of these were prospective cohort studies conducted in Japan (n = 12). Avlund et al reported that Danish elderly aged ⩾ 70 years with a fewer number of teeth (1-9 teeth) had almost twice (adjusted OR = 1.91, 95% CI: 0.79-4.62) the odds of fatigue as those having ⩾ 20 teeth over 10 years after adjusting for confounding variables such as smoking status and socioeconomic factors.112 This is in accordance with findings reported by 2 prospective cohort studies linking the development of frailty in older age to the number of teeth, periodontal health status, and masticatory functions.105,108 In Finland, poor dental status assessed by the number of remaining teeth and periodontal infections has been associated with loss of handgrip muscle strength in 2 studies which place this vulnerable population at high risk of disability.103,116 Two studies by Mochida et al and Yamamoto et al reported that poor self-reported oral health conditions, number of teeth and chewing disability were significantly (P-values < .05) associated with incident falls in Japanese older adults where findings from 1 study reported that the odds of incident falls among Japanese elderly aged ⩾ 65 years having 19 or fewer teeth were 2.5 (OR = 2.50, 95% CI: 1.21-5.17) times greater than those having ⩾ 20 teeth over a 3-year follow-up period, after adjusting for confounding variables such as sex, functional disability during follow-up period, depression, self-rated health and education.106,111 Poor oral health status has also been associated with increased risk of functional and physical disability in eleven studies.17,72,73,100-102,104,107,109,113,114 A recent study by Ramsay et al reported that edentulous elderly aged between 71 and 92 years were associated with a 2-fold (OR = 1.90, 95%CI = 1.03-3.52) increase in the risk of incident frailty compared to those having teeth over 3 years after adjusting for confounding variables such as smoking status, social class, history of cardiovascular disease or diabetes mellitus, and medications related to dry mouth.107 Moreover, in the same study, the risk of incident frailty among elderly suffering from 3 or more oral health problems such as eating difficulty was 2.7 (OR = 2.72, 95% CI = 1.11-6.64) times greater than those without problems. Two additional studies reported tooth loss as having a negative impact on the postural stability of older adults, where findings from the study by Brand et al showed that edentulous Swedish elderly aged ⩾ 65 years experienced lower gait velocity compared to those who are dentate or having fixed restoration after adjusting for confounding variables.110,115
Cardiovascular disorders
Thirteen articles evaluated the risk of cardiovascular diseases due to poor oral health.23,26,33-35,45,46,48,118-122 The majority of the studies were prospective cohort, 5 studies were case-control,33,45,48,119,120 and only 1 study was a retrospective cohort.46 Outcomes for cardiovascular diseases included stroke, cardiac arrest, hypertension, coronary heart disease, non-fatal ischemic stroke, acute myocardial infarction, cerebrovascular disease, peripheral artery disease, carotid stenosis, pulmonary embolism, and heart failure. Oral health variables such as the presence of dental caries, periodontitis, periapical infections, tooth loss and the number of teeth significantly (P-values < .05) increased the risk of cardiovascular diseases among the elderly population in 7 studies after adjusting for confounding variables such as smoking status, obesity, diabetes, and sex.23,45,46,48,118,120,122 Schillinger et al suggested oral hygiene and dental status, particularly edentulousness, as significant predictors of progression of carotid stenosis, where edentulous Australian elderly aged between 62 and 76 years had twice (adjusted OR = 2.10, 95% CI: 1.06-4.16) the risk for disease progression when compared to those with teeth.118 With respect to periodontitis, Chen et al reported that Japanese elderly having periodontitis aged between 53 and 73 years with periodontitis had 5 (OR = 5.45, 95%CI: 1.57-18.89) times the risk for peripheral arterial disease (PAD) compared to those with healthy periodontium.120 However, no significant association was found among older individuals as opposed to their younger counterparts (<60 years) in 5 studies.26,33-35,119 Johansson et al over an 8-year follow up period also did not report periodontal disease as a determinant of coronary artery disease (CAD) among Swedish elderly aged between 60 and 79 years.121 Findings from the majority of studies suggest poor oral health indicators as predictors of CVD, however, more longitudinal and intervention studies need to be conducted to investigate such association among different age groups.
Nutrition
Ten prospective cohort studies evaluated the association between oral health indicators and nutritional status in older adults, the majority of which were conducted in Japan.47,123-131 Two articles suggested a relationship between self-reported and objective measures of oral health and the incidence of weight loss among the elderly.128,130 Weyant et al reported that the risk of incident weight loss among American elderly aged ⩾ 65 years with periodontal disease was 1.53 (OR = 1.53, 95% CI: 1.32-1.77) times greater than those without periodontal diseases over 2 years after adjusting for confounding variables such as education, race, smoking status, number of remaining teeth, diabetes mellitus, and body mass index.130 In Japan and US, impaired dentition assessed by having ⩽5 functional tooth units (FTUs),123 tooth loss,125,126 self-reported oral health and chewing disability,127,129 were significantly (P-values < .05) associated with impaired dietary intake and swallowing problems among older adults in 5 of the included articles.123,126,127,129,131 Over a 5-year follow-up period, Iwasaki et al reported that Japanese elderly aged ⩾ 75 years with impaired dentition suffered from a significant decline in the intake of multiple nutrients, dietary fibers, vegetables, and meat, compared to those without impaired dentition, after adjusting for confounding variables such as body mass index and race.123 Results from a study by Okamoto et al also showed that the risk of developing swallowing problems in Japanese elderly aged ⩾ 65 years having 0 to 12 teeth was 2.5 (OR = 2.49, 95% CI: 1.68-3.69) times greater than those having 27 to 32 teeth over a 5-year follow-up period.125 Three of the articles suggested poor oral health status as a determinant of incident malnutrition.47,124,131 Kiesswetter et al suggested that, among elderly aged between 55 and 80 years suffering from a toothache while chewing, the risk of 9-year incidence of malnutrition was 2.14 (OR = 2.14, 95% CI: 1.10-4.19) times higher than those without a toothache, after adjusting for confounders including sex and socioeconomic status.47 Overall, findings from the above studies suggest poor oral health indicators as possible determinants of malnutrition among the elderly, however, there is a need for more longitudinal studies with longer follow-up periods as most of the follow-ups in the ten included studies ranged from 1 to 2 years.
Oral Health-Related Quality of life
How poor oral health might impact the quality of life (QoL) of the elderly adults have been investigated in 9 of the included articles.17,32,38,104,132-136 This association is best measured by observing changes in oral health-related QoL (OHRQoL) over time. Of these 9 articles, 7 were prospective cohort studies, and 2 were retrospective cohort studies.17,132 The follow-up period in these studies ranged from 2 to 15 years. Various questionnaires have been developed to measure the OHRQoL of individuals, including the Rosenberg Self-Esteem Scale,136 Geriatric Oral Health Assessment Index (GOHAI), the Gero-oncology Health and Quality of Life Assessment,17 and Oral Health Impact Profile (OHIP-14).133 Findings from the 9 articles postulated a significant (P-values < .05) association between oral health problems as tooth loss, xerostomia, denture loss, occlusal force and the quality of life of the elderly in regard to their self-esteem, eating/chewing ability, social function and communication, homeboundness, the performance of daily activities and pain. Over a 7-year follow-up period, Enoki et al showed that Japanese elderly aged ⩾ 60 years who experienced tooth loss, as well as a decrease in the occlusal force, reported higher scores of GOHAI index after adjusting for age, sex and baseline GOHAI score, therefore indicating poorer OHRQoL.133 Sato et al reported that the risk for eating difficulties, speech problems, embarrassment upon smiling, laughing, or showing their teeth, emotional distress, and problems related to social interaction was 2.6 (RR = 2.65, 95% CI = 1.90-3.69), 4.3 (RR = 4.37, 95% CI = 2.46-7.76), 5.3 (RR = 5.32, 95% CI = 2.34-12.1), 2.3 (RR = 2.38, 95% CI = 1.41-4.03), and 6.9 (RR = 6.97, 95% CI = 1.75-27.7) times greater among Japanese elderly who lost their dentures, compared to those who haven’t lost their dentures, respectively.132
Renal disorders
Five articles investigated the effect of periodontal disease on the development of overt nephropathy, end-stage renal disease and kidney function decline among community-dwelling older adults with preserved renal function at baseline.19,137-140 These articles included 3 retrospective,19,137,140 and 2 prospective cohort studies.138,139 Of these 5, 3 were conducted in the US,19,137,138 and 2 were from Japan.139,140 Periodontal disease was associated with decreased kidney function and was reported as a possible predictor of renal diseases in all of the 5 articles after adjusting for confounding variables such as diabetes, hypertension, smoking status, and sociodemographic characteristics (race and education). Grubbs et al reported that, in a cohort of elderly American males, having severe periodontitis was associated with a 2-fold (incidence rate ratio (IRR) = 2.01 95% CI: 1.21-3.44) increase in the rate of incident chronic kidney disease (CKD) compared to those with non-severe periodontal disease, after a mean follow-up period of 4.9 years.19 Moreover, severe periodontitis was associated with a 4-fold (adjusted IRR = 4.18, 95%CI: 1.68-10.39) increased rate of incident CKD in a similar study.137 The above findings suggest a possible association between periodontal diseases and renal disorders among the elderly, however, more studies investigating the impact of other poor oral health indicators such as tooth loss and masticatory efficiency on renal disorders need to be considered.
Cancer
All of the 4 articles measuring the relationship between either periodontitis or tooth loss, and different types of cancer including prostate cancer, breast cancer, non-Hodgkin lymphoma, orodigestive cancer and squamous cell carcinoma of head and neck (SCCHN) reported an association, after adjusting for confounding variables such as sex, household income, insurance status, hypertension, diabetes mellitus, cerebral infarction, myocardial infarction, smoking status, and alcohol intake.18,21,25,141 Three studies were prospective cohort and one was a case-control study. Three studies were from the US,18,25,141 and 1 study was from South Korea..21 Findings from a 22-year follow-up study by Bertrand et al reported that the risk of non-Hodgkin lymphoma among American elderly aged > 65 years with periodontal disease was 33% (HR = 1.33, 95%CI: 1.09-1.62) higher than for those having healthy periodontium, however, these findings were not statistically significant (P-value = .47).18 Divaris et al reported that periodontal disease, as measured by self-reported tooth loss indicators, moderately increased the risk of squamous cell carcinoma of head and neck (SCCHN) by 33% (OR = 1.33, 95% CI: 1.07-1.65) among American elderly aged ⩾ 60 years after adjusting for age, sex, race, education, smoking status, and intensity, drinking status, cumulative ethanol consumption, and fruit and vegetable consumption.141 Conflicting evidence exists regarding the significant association between poor oral health and cancer, therefore there is a need for more longitudinal studies to elucidate the nature of such association.
Inflammatory disorders
Four articles addressed whether or not older adults with poor oral health had higher rates of inflammatory disorders.40-42,52 These articles included 3 case-control and a prospective cohort study. Kodovazenitis et al found periodontal disease to be a contributory factor to the elevated levels of C-reactive protein (CRP) in acute myocardial infarction (AMI) patients after controlling for confounders such as diabetes and smoking status.40 In this study, Greek elderly aged between 57 and 79 years having periodontal disease had significantly higher levels of CRP compared to those without periodontal disease. Another study found that mucosal lesions among the edentulous Finnish elderly aged ⩾ 75 years are strong predictors of elevated CRP levels, where those with mucosal lesions had twice (OR = 2.18, 95% CI: 1.03-4.61, P-value<.05) the risk of having elevated CRP over 10 years after adjusting for sex, history of smoking, alcohol consumption, blood pressure, and social class.52 The other 2 studies highlighted that patients with rheumatoid arthritis (RA) suffered the most from periodontal disease and infection by periodontal pathogens, in particular Prevotella intermedia (Pi) and Porphyromonas gingivalis (Pg).41,42 Although these studies reported an association between poor oral health indicators and inflammatory disorders, however, the exact mechanisms explaining this association are still unclear. Therefore, there is a need for robust longitudinal studies to further investigate the nature of this association among the elderly.
Healthcare costs
Our scoping review suggests that evidence on the association between poor oral health and health services utilization and costs among the elderly is limited. Three articles measured the association between oral health conditions, and medical expenditure and hospital admissions.142-144 Of these, 2 were prospective cohort,143,144 and the other was a retrospective cohort study.142 Kruger et al reported dental caries as being the most common oral-health related condition contributing to increased rates of hospitalizations among Australian elderly patients aged ⩾ 65 years over 10 years.142 The other 2 found periodontitis and tooth loss to be significantly associated with a future rise in medical expenditures among older Japanese adults aged ⩾ 80 years.143,144
Metabolic disorders
Two of the included articles assessed the relationship between dental status and the development of diabetes mellitus among community-dwelling seniors who were free of diabetes at baseline.39,145 The 2 articles were prospective cohort conducted in Ireland and Germany with periodontitis used as the oral health variable in both. Winning et al in a cohort of elderly males reported that the risk of incident Type 2 diabetes among Irish elderly aged between 58-72 years with moderate /severe periodontitis was 1.69 (HR = 1.69, 95% CI: 1.06-2.69, P-value = .03) times higher than those with no/ mild periodontitis over a median follow-up period of 7.8 years after adjusting for confounding variables such as the number of teeth, smoking status, tooth brushing frequency, body mass index, cholesterol, history of atherosclerotic cardiovascular disease/hypertension, education years, marital status and socio-economic status.39 The second study reported that diabetes-free older adults suffering from periodontal disease were at high risk of A1C or fasting plasma glucose progression during 5 years follow-up when compared to those with healthy periodontium.145 Findings from both studies suggest an association between periodontitis and the development of diabetes, however, the issue of causality needs to be further investigated through future studies as the magnitude of the impact of periodontitis over diabetes is expected to be higher than what was reported.39,145
Skeletal disorders
Two prospective cohort studies conducted in Sweden and the US assessed the relationship between periodontitis and skeletal disorders among older adults.20,146 Persson et al reported that over a 3-year follow-up period, Swedish elderly aged between 62 and 96 years with periodontitis had almost twice (OR = 1.8, 95% CI: 1.0-3.3, P-value < .05) the risk of hip/hand fractures as those without periodontitis after adjusting for age and sex, however, the magnitude of the impact was stronger when both periodontitis and osteoporosis were combined, where the risk of hip/hand fracture among those having periodontitis and osteoporosis was 12.2 (OR = 12.2, 95% CI: 3.5-42.3, P-value < .001) times greater than those without.146 A study by Phipps et.al, however, reported no possible association between periodontal disease and tooth loss with bone mineral density (BMD) in a large cohort of American older men aged ⩾ 65 years.20 More longitudinal studies need to be conducted to further elucidate the nature of the association between poor oral health and skeletal disorders among the elderly.
Cerebrovascular disorders
Our scoping review suggests that evidence on the association between poor oral health and cerebrovascular disorders among the elderly is limited.22,43 One prospective cohort and 1 case-control study assessed such association. Leira et al suggested chronic periodontitis as a determinant of Lacunar infarct (LI) among Spanish older adults aged between 58 and 71 years, where the risk of LI among those having chronic periodontitis was 4.2 (OR = 4.20, 95%CI: 1.81-10.20, P-value = .001) times higher than those with healthy periodontium after adjusting for age, hypertension and diabetes mellitus.43 However, in a cohort of American older men, Jimenez et al reported no association between periodontitis and cerebrovascular diseases among those aged ⩾ 60 years, as opposed to their younger counterparts (aged <60 years).22 Further longitudinal studies are needed in order to investigate the causal mechanisms that can explain this association.
General health
The general health of older adults has been studied as the outcome measure in 2 of the included articles.136,147 One of the articles measured the association between 3 oral health indicators including chewing difficulty, non-intact teeth, and dry mouth, and 4 dimensions assessing general health with respect to activities of daily living functioning, cognition, depression, and health instability.147 In this study, older adults aged ⩾ 65 years with poor oral health indicators, in particular those experiencing chewing difficulty (OR = 10.88, 95% CI: 7.35-16.29, P-value < .05), were found to be at higher risk of suffering from poor general health when compared to those without these indicators.147 Benyamini et al postulated an independent correlation between self-rated oral health (SROH) and future self-rated health (SRH) over a 5-year follow-up period after adjusting for socio-demographic factors and baseline SRH.136
Respiratory disorders
One prospective cohort study evaluated the possible association between oral health status and the incidence of pulmonary diseases.103 This article reported that Finnish older adults aged ⩾ 80 years suffering from periodontitis were the most likely to develop a decline in forced expiratory volume (FEV1) during the first second over a 5-year follow-up period after adjusting for confounding variables such as the existence of pulmonary diseases, smoking status, height, education, and handgrip strength. More longitudinal and interventional studies assessing the association between poor oral health and respiratory disorders among community-dwelling seniors need to be considered.
Hepatic disorders
The association between oral health indicators and the risk of developing hepatic diseases is scarcely investigated in the literature. One retrospective cohort study by Nagao et al found that Japanese elderly patients aged between 50 and 72 years with periodontal disease and hepatitis C virus (HCV) and/or hepatitis B virus (HBV) infection at baseline were at higher risk of liver disease progression when compared to those with slight or no periodontitis.44
Discussion
The aim of this scoping review was to determine the impacts of poor oral health among community-dwelling seniors and identify the gaps in the existing literature regarding this association which would be of benefit for future research. This review highlighted that a significant association exists between poor oral health and various general health indicators which underscores the fact that oral and general health are inter-related and that maintaining and improving oral health is an integral part of the general health and well-being of populations worldwide. It is interesting to see that the most commonly studied oral-systemic linkages are for mortality (all-cause mortality and mortality due to chronic diseases) and mental health disorders, representing 24% and 21% of the included articles, respectively.
In recent years, the association between poor oral health and increased cognitive decline has been of interest, and although several studies postulated a significant association, most of the evidence relies on cross-sectional data, which despite being useful, a temporal association cannot be inferred using this study design.148 Several biological mechanisms have been proposed to explain the association between poor oral health and cognitive impairment. It has been implied that it is mainly one of the 2 pathways, an inflammatory pathway or a nutritional pathway.148 Inflammatory oral conditions as periodontitis contribute to the overall systemic inflammatory burden where inflammatory mediators, cytokines, and bacterial pathogens enter the circulation crossing the blood-brain barrier causing neuroinflammation and possibly cognitive decline.149 In addition, tooth loss affects the masticatory efficiency and nutrients intake which has also been linked to cognitive impairment and mortality among the elderly.150,151 The inflammatory pathway has also been proposed as the most likely mechanism explaining the association between poor oral health and mortality owing to the increase in the levels of cytokines and C-reactive protein during periodontitis, oral infections as well as prior to death.152 However, the exact mechanisms supporting these associations remain unclear, and therefore, future studies assessing this association would be helpful.
The majority of studies in this review that reported no association between poor oral health and a general health outcome investigated the possible links with cardiovascular disorders (CVD). These studies included our relevant participants (elderly ⩾ 60 years) only as a sub-group with their results supporting a strong significant association only among younger age groups. It has been hypothesized that the risk of developing CVD decreases continuously with advancing age and that younger adults suffering from periodontitis are more susceptible to experiencing diseases.26 Perhaps this can also be explained by the fact that age is a risk factor for both CVD and periodontitis, and with older adults being highly exposed to other CVD risk factors, this might contribute to the decreased power of tooth loss to predict the incidence of CVD among this population.34 However, more longitudinal and intervention studies need to be conducted to elucidate such association amongst different populations.
There were differences in the assessment of oral health between the studies, with periodontitis and tooth loss being the most commonly used measures. In fact, this is expected owing to the high prevalence of periodontitis among older adults which eventually results in tooth loss. However, with dental caries being more common than periodontitis and tooth loss, the association of dental decay with general health outcomes has been scarcely reported. It is also worth mentioning that these 3 oral health conditions contribute to a significant portion of the burden of oral diseases globally.153
Another important finding was how healthy aging and improving the oral health of the elderly is a priority in the Japanese society, considering a majority of articles (42%, n = 55) found in our search included studies that were conducted in Japan. This can be attributable to the rapid increase in their aging population which is expected to reach 28.9% of the total population in 2025.1,154 Moreover, Japan is ranked the first amongst all OECD countries with respect to the public funds allocated to dental care services compared to Canada which is closer to the last.10 This supports the fact that the Japanese society values the welfare of their elderly population and seeks to implement strategies to enhance their quality of life and diminish inequity in dental care.
Our review did not find any studies linking poor oral health among community-dwelling seniors with aspiration pneumonia or other related respiratory disease outcomes. This is not surprising, as such association is more common among hospitalized or institutionalized, debilitated older adults and those living in nursing homes and long-term care facilities, whereas this review only focused on community-dwelling seniors.155 Various studies reported periodontal disease as a strong predictor of pneumonia among institutionalized and hospitalized elderly where bacterial periodontal pathogens have been identified in the lower respiratory tract.1,156,157 This can be elucidated by the fact that they aspirate oral secretions loaded with bacterial pathogens in larger amounts and more frequently and have worse oral hygiene than community-dwelling seniors.155 Consequently, they become at a higher risk of experiencing respiratory infections particularly pneumonia which is one of the main causes of mortality among the elderly.155
Given the rapid increase in the proportion of the aging population in Canada, it is surprising that in this review there was a lack of studies conducted that measure the impacts of poor oral health among older Canadians. This should be taken into consideration because addressing oral health needs of the elderly needs to become a priority to avoid the burden of diseases associated with poor oral health, which consequently inflates healthcare expenditures across the system. Globally, countries’ health care systems further need to integrate oral health care into general health care services of their populations through collaborations among administrators, legislators, regulators, and health care professionals. Oral healthcare for the elderly is not a priority in most countries despite their high burden of oral health diseases and inequities in accessing oral health care. With poor oral health being an issue in itself and also having impacts on both general health outcomes and health care systems, improving access to oral healthcare services through development of equitable interventions for the elderly, can help reduce their overall disease burden and also burden on healthcare systems. We can expect less hospitalizations, emergency room visits and physician visits for both oral health and general health conditions. Evidence suggests that providing dental treatment to individuals with systemic diseases can reduce the medical per-capita costs and emergency departments use158,159; studies have shown savings in medical costs up to $5681 per person, and reduction in hospitalization rates down to 39.4%.158,159 In 2010, reports from the ADA’s Health Policy Institute showed that the economic burden on the health care systems as a result of treating oral conditions in emergency departments can reach between $867 million and $2.1 billion.159,160 Studies from this review reported how poor oral health among elderly can negatively impact healthcare costs and hospitalization rates and these findings constitute formidable challenges and health authorities should be concerned about the massive burden hospital admissions and medical costs place on the healthcare systems internationally. Therefore, in order to reduce healthcare costs, it is essential to specifically target the increasing usage of hospital emergency departments while implementing strategies to improve oral healthcare such as including dental care services within the publicly funded healthcare systems and strengthening the linkage between oral and general health at both the structural and the practice levels. Furthermore, policy and decision makers need to focus on improving the quality of care when addressing the increased healthcare costs associated with oral diseases, through adapting the Donabedian’s classic paradigm which highlights how improving the infrastructure and the resources needed to provide care as well as improving how dental care services are delivered, administered, and financed can influence individuals’ oral health status and reduce health-related costs.161,162
Findings from this review can create a ground plan for health policymakers and stakeholders to invest in dental care programs addressing the current unmet oral health needs and the future general health needs of this population.
Strengths and limitations
Unlike systematic reviews, quality assessment of the included articles is not a mandatory step while conducting a scoping review. The main strength of this review is the exclusion of studies with a cross-sectional design and including observational studies such as cohort studies or case-control studies, which provide better evidence regarding a temporal and causal association between our variables of interest. However, the majority of the studies included in this review reported an association rather than causation between the exposure and outcome variables as a result of a number of limitations in the included studies such as the lack of adjustment for confounding variables, the magnitude of effects being not strong enough to report a causal association and short follow-up periods. As a result, a cause-effect relationship between poor oral health and the general health indicators included in this review cannot be inferred due to the limitations in the existing literature. Therefore, further robust longitudinal studies are needed in order to investigate the causal association between poor oral health and general health indicators among the elderly population.
This review has some limitations that are noteworthy. Publication bias existed, as the search was only limited to articles published in English and conducted only in OECD countries. Moreover, we did not retrieve grey literature articles, thus some relevant studies might have been missed. We also did not specifically search for special subgroups, such as Indigenous people, immigrants, etc. that may have a higher burden of oral health problems compared to the general elderly population. These groups are known to experience additional disadvantageous socioeconomic conditions and barriers that further complicate their oral and general health conditions.
Conclusion
This scoping review found that poor oral health can have significant implications on general health of the elderly. This highlights the importance of providing effective oral healthcare to this population to help reduce the burden of not just their oral diseases but also general health consequences. In countries such as Canada, it has important economic implications because the health care system is publicly funded; investing in oral health care can be a positive return on investment as it can reduce the health care costs potentially resulting in saving more dollars to the government. Further, existing literature on the impact of poor oral health on the elderly is based predominantly on an association rather than causation, therefore, more longitudinal research is needed to assess the causality link between oral health and general health conditions and to better understand the healthcare implications of providing dental services and improving the quality of care to guide evidence-informed policy decisions and implement strategies to improve oral and general health of older adults.
Acknowledgments
We would like to thank the library information specialists at Public Health Ontario and the research coordinator Sarah Muir for their valuable contributions. This project would not have been achievable without their help and support. We would also like to acknowledge the generous financial support of Green Shield Canada.
Footnotes
Authorship contributions: Below are the specific contributions made by each author.
Section 1
Conception and design of study: Carlos Quiñonez, Rana Badewy, Harkirat Singh;
Acquisition of data: Carlos Quiñonez, Rana Badewy, Harkirat Singh;
Analysis and/or interpretation of data: Rana Badewy, Harkirat Singh, Sonica Singhal;
Section 2
Drafting the manuscript: Rana Badewy, Harkirat Singh, Sonica Singhal;
Revising the manuscript critically for important intellectual content: Rana Badewy, Harkirat Singh, Sonica Singhal.
Section 3
Approval of the version of the manuscript to be: Sonica Singhal, Harkirat Singh, Rana Badewy, Carlos Quiñonez.
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Author(s) received financial support for the publication of this article through a philanthropic gift from Green Shield Canada.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Carlos Quiñonez receives remuneration from Green Shield Canada for consulting on dental care-related issues.
ORCID iD: Harkirat Singh  https://orcid.org/0000-0003-3031-3529
https://orcid.org/0000-0003-3031-3529
References
- 1. Kandelman D, Petersen PE, Ueda H. Oral health, general health, and quality of life in older people. Spec Care Dentist. 2008;28:224-236. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Active ageing: A policy framework. Geneva, Switzerland: World Health Organization; 2002. Accessed August 28, 2019 https://apps.who.int/iris/bitstream/handle/10665/67215/WHO_NMH_NPH_02.8.pdf;jsessionid=6F90900FC196430047265D465228D73A?sequence=1. [PubMed] [Google Scholar]
- 3. United Nations, Department of Economic and Social Affairs,Population Division. World population prospects 2019: Highlights . New York: United Nations; 2019. Accessed August 28, 2019 https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf. [Google Scholar]
- 4. Yao CS, MacEntee MI. Inequity in oral health care for elderly canadians: Part 1. oral health status. J Can Dent Assoc. 2013;79:d114. [PubMed] [Google Scholar]
- 5. National Population Projections team, Bohnert N, Chagnon J, Dion P. Population projections for canada (2013 to 2063), provinces and territories (2013 to 2038). Ottawa, ON: Statistics Canada; 2015. Accessed August 28, 2019 https://www150.statcan.gc.ca/n1/en/pub/91-520-x/91-520-x2014001-eng.pdf?st=3slj933u. [Google Scholar]
- 6. Life expectancy in canada. Available at: https://www.worldlifeexpectancy.com/canada-life-expectancy.
- 7. Yellowitz JA, Schneiderman MT. Elder's oral health crisis. J Evid Based Dent Pract. 2014;14 Suppl:191-200. [DOI] [PubMed] [Google Scholar]
- 8. White DA, Tsakos G, Pitts NB, et al. Adult dental health survey 2009: Common oral health conditions and their impact on the population. Br Dent J. 2012;213:567-72. [DOI] [PubMed] [Google Scholar]
- 9. Health Canada. Summary report on the findings of the oral health component of the canadian health measures survey 2007–2009. Ottawa, ON: Her Majesty the Queen in Right of Canada, represented by the Minister of Health, 2010; 2010 Accessed May 28, 2020 http://www.caphd.ca/sites/default/files/CHMS-E-summ.pdf. [Google Scholar]
- 10. Canadian Academy of Health Sciences. Improving Access to Oral Health Care for Vulnerable People Living in Canada. Canadian Academy of Health Sciences; 2014. Accessed August 28 2019 https://cahs-acss.ca/wp-content/uploads/2015/07/Access_to_Oral_Care_FINAL_REPORT_EN.pdf. [Google Scholar]
- 11. Galgut PN. Periodontal disease and poor health outcomes. BMJ. 2010;340:c2735. [DOI] [PubMed] [Google Scholar]
- 12. Puturidze S, Margvelashvili M, Bilder L, Kalandadze M, Margvelashvili V. Relationship between general health, oral health and healthy lifestyle in elderly population (review). Georgian Med News. 2018;(Issue):17-21. [PubMed] [Google Scholar]
- 13. Miyazaki H, Jones JA, Beltran-Aguilar ED. Surveillance and monitoring of oral health in elderly people. Int Dent J. 2017;67:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turcotte M, Sawaya C. Insights on Canadian Society- Senior Care: Differences by Type of Housing. Statistics Canada; 2015. Available from: https://www150.statcan.gc.ca/n1/en/pub/75-006-x/2015001/article/14142-eng.pdf?st=GtfQSlfi. Accessed 2020 May 27. [Google Scholar]
- 15. Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. [Google Scholar]
- 16. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69,5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingram SS, Seo PH, Sloane R, et al. The association between oral health and general health and quality of life in older male cancer patients. J Am Geriatr Soc. 2005;53:1504-1509. [DOI] [PubMed] [Google Scholar]
- 18. Bertrand KA, Shingala J, Evens A, Birmann BM, Giovannucci E, Michaud DS. Periodontal disease and risk of non-hodgkin lymphoma in the health professionals follow-up study. Int J Cancer. 2017;140:1020-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grubbs V, Vittinghoff E, Taylor G, et al. The association of periodontal disease with kidney function decline: A longitudinal retrospective analysis of the MrOS dental study. Nephrol Dial Transplant. 2016;31:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phipps KR, Chan BK, Madden TE, et al. Longitudinal study of bone density and periodontal disease in men. J Dent Res. 2007;86:1110-1114. [DOI] [PubMed] [Google Scholar]
- 21. Lee JH, Kweon HH, Choi JK, Kim YT, Choi SH. Association between periodontal disease and prostate cancer: Results of a 12-year longitudinal cohort study in south korea. J Cancer. 2017;8:2959-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. 2009;66:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LaMonte MJ, Genco RJ, Hovey KM, et al. History of periodontitis diagnosis and edentulism as predictors of cardiovascular disease, stroke, and mortality in postmenopausal women. J Am Heart Assoc. 2017;6:10.1161/JAHA.116.004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart R, Stenman U, Hakeberg M, Hagglin C, Gustafson D, Skoog I. Associations between oral health and risk of dementia in a 37-year follow-up study: The prospective population study of women in gothenburg. J Am Geriatr Soc. 2015;63:100-105. [DOI] [PubMed] [Google Scholar]
- 25. Freudenheim JL, Genco RJ, LaMonte MJ, et al. Periodontal disease and breast cancer: Prospective cohort study of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2016;25:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117:1668-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukai K, Takiguchi T, Ando Y, et al. Functional tooth number and 15-year mortality in a cohort of community-residing older people. Geriatr Gerontol Int. 2007;7:341-347. [Google Scholar]
- 28. Fukai K, Takiguchi T, Ando Y, et al. Mortality rates of community-residing adults with and without dentures. Geriatr Gerontol Int. 2008;8:152-159. [DOI] [PubMed] [Google Scholar]
- 29. Jansson L, Lavstedt S, Frithiof L. Relationship between oral health and mortality rate. J Clin Periodontol. 2002;29:1029-1034. [DOI] [PubMed] [Google Scholar]
- 30. Jansson L, Lavstedt S, Frithiof L, Theobald H. Relationship between oral health and mortality in cardiovascular diseases. J Clin Periodontol. 2001;28:762-768. [DOI] [PubMed] [Google Scholar]
- 31. Xu F, Lu B. Prospective association of periodontal disease with cardiovascular and all-cause mortality: NHANES III follow-up study. Atherosclerosis. 2011;218:536-542. [DOI] [PubMed] [Google Scholar]
- 32. Chavers LS, Gilbert GH, Shelton BJ. Chronic oral disadvantage, a measure of long-term decrements in oral health-related quality of life. Qual Life Res. 2004;13:111-123. [DOI] [PubMed] [Google Scholar]
- 33. Sim SJ, Kim HD, Moon JY, et al. Periodontitis and the risk for non-fatal stroke in korean adults. J Periodontol. 2008;79:1652-1658. [DOI] [PubMed] [Google Scholar]
- 34. Lee HJ, Choi EK, Park JB, Han KD, Oh S. Tooth loss predicts myocardial infarction, heart failure, stroke, and death. J Dent Res. 2019;98:164-170. [DOI] [PubMed] [Google Scholar]
- 35. Darnaud C, Thomas F, Pannier B, Danchin N, Bouchard P. Oral health and blood pressure: The IPC cohort. Am J Hypertens. 2015;28:1257-1261. [DOI] [PubMed] [Google Scholar]
- 36. Naorungroj S, Schoenbach VJ, Wruck L, et al. Tooth loss, periodontal disease, and cognitive decline in the atherosclerosis risk in communities (ARIC) study. Community Dent Oral Epidemiol. 2015;43:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rouxel P, Tsakos G, Chandola T, Watt RG. Oral health-A neglected aspect of subjective well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2018;73:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: A prospective cohort study. J Clin Periodontol. 2017;44:266-274. [DOI] [PubMed] [Google Scholar]
- 40. Kodovazenitis G, Pitsavos C, Papadimitriou L, et al. Periodontal disease is associated with higher levels of C-reactive protein in non-diabetic, non-smoking acute myocardial infarction patients. J Dent. 2011;39:849-854. [DOI] [PubMed] [Google Scholar]
- 41. Kimura Y, Yoshida S, Takeuchi T, et al. Periodontal pathogens participate in synovitis in patients with rheumatoid arthritis in clinical remission: A retrospective case-control study. Rheumatology (Oxford). 2015;54:2257-2263. [DOI] [PubMed] [Google Scholar]
- 42. Mikuls TR, Payne JB, Yu F, et al. Periodontitis and porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leira Y, Lopez-Dequidt I, Arias S, et al. Chronic periodontitis is associated with lacunar infarct: A case-control study. Eur J Neurol. 2016;23:1572-1579. [DOI] [PubMed] [Google Scholar]
- 44. Nagao Y, Kawahigashi Y, Sata M. Association of periodontal diseases and liver fibrosis in patients with HCV and/or HBV infection. Hepat Mon. 2014;14:e23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willershausen B, Kasaj A, Willershausen I, et al. Association between chronic dental infection and acute myocardial infarction. J Endod. 2009;35:626-630. [DOI] [PubMed] [Google Scholar]
- 46. Willershausen I, Weyer V, Peter M, et al. Association between chronic periodontal and apical inflammation and acute myocardial infarction. Odontology. 2014;102:297-302. [DOI] [PubMed] [Google Scholar]
- 47. Kiesswetter E, Hengeveld LM, Keijser BJ, Volkert D, Visser M. Oral health determinants of incident malnutrition in community-dwelling older adults. J Dent. 2019;85:73-80. [DOI] [PubMed] [Google Scholar]
- 48. Lafon A, Tala S, Ahossi V, Perrin D, Giroud M, Bejot Y. Association between periodontal disease and non-fatal ischemic stroke: A case-control study. Acta Odontol Scand. 2014;72:687-693. [DOI] [PubMed] [Google Scholar]
- 49. Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27-32. [DOI] [PubMed] [Google Scholar]
- 50. Schwahn C, Polzer I, Haring R, et al. Missing, unreplaced teeth and risk of all-cause and cardiovascular mortality. Int J Cardiol. 2013;167:1430-1437. [DOI] [PubMed] [Google Scholar]
- 51. Paganini-Hill A, White SC, Atchison KA. Dental health behaviors, dentition, and mortality in the elderly: The leisure world cohort study. J Aging Res. 2011;2011:156061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ajwani S, Mattila KJ, Narhi TO, Tilvis RS, Ainamo A. Oral health status, C-reactive protein and mortality–a 10 year follow-up study. Gerodontology. 2003;20:32-40. [DOI] [PubMed] [Google Scholar]
- 53. Aida J, Kondo K, Yamamoto T, et al. Oral health and cancer, cardiovascular, and respiratory mortality of japanese. J Dent Res. 2011;90:1129-35. [DOI] [PubMed] [Google Scholar]
- 54. Ansai T, Takata Y, Yoshida A, et al. Association between tooth loss and orodigestive cancer mortality in an 80-year-old community-dwelling japanese population: A 12-year prospective study. BMC Public Health. 2013;13:814,2458-13-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manabe K, Tanji F, Tomata Y, Zhang S, Tsuji I. Preventive effect of oral self-care on pneumonia death among the elderly with tooth loss: The ohsaki cohort 2006 study. Tohoku J Exp Med. 2019;247:251-257. [DOI] [PubMed] [Google Scholar]
- 56. Furuta M, Takeuchi K, Adachi M, et al. Tooth loss, swallowing dysfunction and mortality in japanese older adults receiving home care services. Geriatr Gerontol Int. 2018;18:873-880. [DOI] [PubMed] [Google Scholar]
- 57. Iwasaki M, Taylor GW, Awano S, et al. Periodontal disease and pneumonia mortality in haemodialysis patients: A 7-year cohort study. J Clin Periodontol. 2018;45:38-45. [DOI] [PubMed] [Google Scholar]
- 58. Iinuma T, Arai Y, Takayama M, et al. Association between maximum occlusal force and 3-year all-cause mortality in community-dwelling elderly people. BMC Oral Health. 2016;16:82,016-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iwasaki M, Yoshihara A, Sato N, et al. Maximum bite force at age 70 years predicts all-cause mortality during the following 13 years in japanese men. J Oral Rehabil. 2016;43:565-574. [DOI] [PubMed] [Google Scholar]
- 60. Suzuki R, Kikutani T, Yoshida M, Yamashita Y, Hirayama Y. Prognosis-related factors concerning oral and general conditions for homebound older adults in japan. Geriatr Gerontol Int. 2015;15:1001-1006. [DOI] [PubMed] [Google Scholar]
- 61. Hayasaka K, Tomata Y, Aida J, Watanabe T, Kakizaki M, Tsuji I. Tooth loss and mortality in elderly japanese adults: Effect of oral care. J Am Geriatr Soc. 2013;61:815-820. [DOI] [PubMed] [Google Scholar]
- 62. Avlund K, Schultz-Larsen K, Krustrup U, Christiansen N, Holm-Pedersen P. Effect of inflammation in the periodontium in early old age on mortality at 21-year follow-up. J Am Geriatr Soc. 2009;57:1206-1212. [DOI] [PubMed] [Google Scholar]
- 63. Ansai T, Takata Y, Soh I, et al. Relationship between tooth loss and mortality in 80-year-old japanese community-dwelling subjects. BMC Public Health. 2010;10:386,2458-10-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Onder G, Liperoti R, Soldato M, Cipriani MC, Bernabei R, Landi F. Chewing problems and mortality in older adults in home care: Results from the aged in home care study. J Am Geriatr Soc. 2007;55:1961-1966. [DOI] [PubMed] [Google Scholar]
- 65. Awano S, Ansai T, Takata Y, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87:334-349. [DOI] [PubMed] [Google Scholar]
- 66. Osterberg T, Carlsson GE, Sundh V, Steen B. Number of teeth–a predictor of mortality in the elderly? A population study in three nordic localities. Acta Odontol Scand. 2007;65:335-340. [DOI] [PubMed] [Google Scholar]
- 67. Ansai T, Takata Y, Soh I, et al. Relationship between chewing ability and 4-year mortality in a cohort of 80-year-old japanese people. Oral Dis. 2007;13:214-219. [DOI] [PubMed] [Google Scholar]
- 68. Morita I, Nakagaki H, Kato K, et al. Relationship between survival rates and numbers of natural teeth in an elderly japanese population. Gerodontology. 2006;23:214-218. [DOI] [PubMed] [Google Scholar]
- 69. Yoshida M, Morikawa H, Yoshikawa M, Tsuga K, Akagawa Y. Eight-year mortality associated with dental occlusion and denture use in community-dwelling elderly persons. Gerodontology. 2005;22:234-237. [PubMed] [Google Scholar]
- 70. Hamalainen P, Meurman JH, Kauppinen M, Keskinen M. Oral infections as predictors of mortality. Gerodontology. 2005;22:151-157. [DOI] [PubMed] [Google Scholar]
- 71. Soikkonen K, Wolf J, Salo T, Tilvis R. Radiographic periodontal attachment loss as an indicator of death risk in the elderly. J Clin Periodontol. 2000;27:87-92. [DOI] [PubMed] [Google Scholar]
- 72. Tanaka T, Takahashi K, Hirano H, et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73:1661-1667. [DOI] [PubMed] [Google Scholar]
- 73. Holm-Pedersen P, Schultz-Larsen K, Christiansen N, Avlund K. Tooth loss and subsequent disability and mortality in old age. J Am Geriatr Soc. 2008;56:429-435. [DOI] [PubMed] [Google Scholar]
- 74. Nakanishi N, Fukuda H, Takatorige T, Tatara K. Relationship between self-assessed masticatory disability and 9-year mortality in a cohort of community-residing elderly people. J Am Geriatr Soc. 2005;53:54-58. [DOI] [PubMed] [Google Scholar]
- 75. Ajwani S, Mattila KJ, Tilvis RS, Ainamo A. Periodontal disease and mortality in an aged population. Spec Care Dentist. 2003;23:125-130. [DOI] [PubMed] [Google Scholar]
- 76. Hamalainen P, Meurman JH, Keskinen M, Heikkinen E. Relationship between dental health and 10-year mortality in a cohort of community-dwelling elderly people. Eur J Oral Sci. 2003;111:291-296. [DOI] [PubMed] [Google Scholar]
- 77. Tran TD, Krausch-Hofmann S, Duyck J, et al. Association between oral health and general health indicators in older adults. Sci Rep. 2018;8:8871,018-26789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yoo JJ, Yoon JH, Kang MJ, Kim M, Oh N. The effect of missing teeth on dementia in older people: A nationwide population-based cohort study in south korea. BMC Oral Health. 2019;19:61,019-0750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gatz M, Mortimer JA, Fratiglioni L, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2:110-7. [DOI] [PubMed] [Google Scholar]
- 80. Starr JM, Hall RJ, Macintyre S, Deary IJ, Whalley LJ. Predictors and correlates of edentulism in the healthy old people in edinburgh (HOPE) study. Gerodontology. 2008;25:199-204. [DOI] [PubMed] [Google Scholar]
- 81. Arrive E, Letenneur L, Matharan F, et al. Oral health condition of french elderly and risk of dementia: A longitudinal cohort study. Community Dent Oral Epidemiol. 2012;40:230-238. [DOI] [PubMed] [Google Scholar]
- 82. Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between self-reported dental health status and onset of dementia: A 4-year prospective cohort study of older japanese adults from the aichi gerontological evaluation study (AGES) project. Psychosom Med. 2012;74:241-248. [DOI] [PubMed] [Google Scholar]
- 83. Stewart R, Weyant RJ, Garcia ME, et al. Adverse oral health and cognitive decline: The health, aging and body composition study. J Am Geriatr Soc. 2013;61:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Okamoto N, Morikawa M, Tomioka K, Yanagi M, Amano N, Kurumatani N. Association between tooth loss and the development of mild memory impairment in the elderly: The fujiwara-kyo study. J Alzheimers Dis. 2015;44:777-786. [DOI] [PubMed] [Google Scholar]
- 85. Noble JM, Scarmeas N, Celenti RS, et al. Serum IgG antibody levels to periodontal microbiota are associated with incident alzheimer disease. PLoS One. 2014;9:e114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Iwasaki M, Yoshihara A, Kimura Y, et al. Longitudinal relationship of severe periodontitis with cognitive decline in older japanese. J Periodontal Res. 2016;51:681-688. [DOI] [PubMed] [Google Scholar]
- 87. Ide M, Harris M, Stevens A, et al. Periodontitis and cognitive decline in alzheimer's disease. PLoS One. 2016;11:e0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Okamoto N, Morikawa M, Amano N, Yanagi M, Takasawa S, Kurumatani N. Effects of tooth loss and the apolipoprotein E varepsilon4 allele on mild memory impairment in the fujiwara-kyo study of japan: A nested case-control study. J Alzheimers Dis. 2017;55:575-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamamoto T, Aida J, Kondo K, et al. Oral health and incident depressive symptoms: JAGES project longitudinal study in older japanese. J Am Geriatr Soc. 2017;65:1079-1084. [DOI] [PubMed] [Google Scholar]
- 90. Takeuchi K, Ohara T, Furuta M, et al. Tooth loss and risk of dementia in the community: The hisayama study. J Am Geriatr Soc. 2017;65:e95-e100. [DOI] [PubMed] [Google Scholar]
- 91. Gil-Montoya JA, Barrios R, Santana S, et al. Association between periodontitis and amyloid beta peptide in elderly people with and without cognitive impairment. J Periodontol. 2017;88:1051-1058. [DOI] [PubMed] [Google Scholar]
- 92. Nilsson H, Sanmartin Berglund J, Renvert S. Longitudinal evaluation of periodontitis and development of cognitive decline among older adults. J Clin Periodontol. 2018;45:1142-1149. [DOI] [PubMed] [Google Scholar]
- 93. Saito S, Ohi T, Murakami T, et al. Association between tooth loss and cognitive impairment in community-dwelling older japanese adults: A 4-year prospective cohort study from the ohasama study. BMC Oral Health. 2018;18:142,018-0602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hatta K, Ikebe K, Gondo Y, et al. Influence of lack of posterior occlusal support on cognitive decline among 80-year-old japanese people in a 3-year prospective study. Geriatr Gerontol Int. 2018;18:1439-1446. [DOI] [PubMed] [Google Scholar]
- 95. Iwasaki M, Kimura Y, Ogawa H, et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J Periodontal Res. 2019;54:233-240. [DOI] [PubMed] [Google Scholar]
- 96. Kato H, Takahashi Y, Iseki C, et al. Tooth loss-associated cognitive impairment in the elderly: A community-based study in japan. Intern Med. 2019;58:1411-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Paganini-Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: The leisure world cohort study. J Am Geriatr Soc. 2012;60:1556-1563. [DOI] [PubMed] [Google Scholar]
- 98. Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB. Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res. 2010;89:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the nun study. J Am Dent Assoc. 2007;138:1314,22; quiz 1381-2. [DOI] [PubMed] [Google Scholar]
- 100. Sato Y, Aida J, Kondo K, et al. Tooth loss and decline in functional capacity: A prospective cohort study from the japan gerontological evaluation study. J Am Geriatr Soc. 2016;64:2336-2342. [DOI] [PubMed] [Google Scholar]
- 101. Tsakos G, Watt RG, Rouxel PL, de Oliveira C, Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc. 2015;63:91-9. [DOI] [PubMed] [Google Scholar]
- 102. Aida J, Kondo K, Hirai H, et al. Association between dental status and incident disability in an older japanese population. J Am Geriatr Soc. 2012;60:338-343. [DOI] [PubMed] [Google Scholar]
- 103. Hamalainen P, Suominen H, Keskinen M, Meurman JH. Oral health and reduction in respiratory capacity in a cohort of community-dwelling elderly people: A population-based 5-year follow-up study. Gerodontology. 2004;21:209-215. [DOI] [PubMed] [Google Scholar]
- 104. Koyama S, Aida J, Kondo K, et al. Does poor dental health predict becoming homebound among older japanese? BMC Oral Health. 2016;16:51,016-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Horibe Y, Ueda T, Watanabe Y, et al. A 2-year longitudinal study of the relationship between masticatory function and progression to frailty or pre-frailty among community-dwelling japanese aged 65 and older. J Oral Rehabil. 2018;45:864-870. [DOI] [PubMed] [Google Scholar]
- 106. Mochida Y, Yamamoto T, Fuchida S, Aida J, Kondo K. Does poor oral health status increase the risk of falls?: The JAGES project longitudinal study. PLoS One. 2018;13:e0192251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ramsay SE, Papachristou E, Watt RG, et al. Influence of poor oral health on physical frailty: A population-based cohort study of older british men. J Am Geriatr Soc. 2018;66:473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Iwasaki M, Yoshihara A, Sato N, et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. J Oral Rehabil. 2018;45:17-24. [DOI] [PubMed] [Google Scholar]
- 109. Komiyama T, Ohi T, Miyoshi Y, et al. Association between tooth loss, receipt of dental care, and functional disability in an elderly japanese population: The tsurugaya project. J Am Geriatr Soc. 2016;64:2495-2502. [DOI] [PubMed] [Google Scholar]
- 110. Brand C, Bridenbaugh SA, Perkovac M, et al. The effect of tooth loss on gait stability of community-dwelling older adults. Gerodontology. 2015;32:296-301. [DOI] [PubMed] [Google Scholar]
- 111. Yamamoto T, Kondo K, Misawa J, et al. Dental status and incident falls among older japanese: A prospective cohort study. BMJ Open. 2012;2:e001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Avlund K, Schultz-Larsen K, Christiansen N, Holm-Pedersen P. Number of teeth and fatigue in older adults. J Am Geriatr Soc. 2011;59:1459-1464. [DOI] [PubMed] [Google Scholar]
- 113. Okuyama N, Yamaga T, Yoshihara A, et al. Influence of dental occlusion on physical fitness decline in a healthy japanese elderly population. Arch Gerontol Geriatr. 2011;52:172-176. [DOI] [PubMed] [Google Scholar]
- 114. Fukai K, Takiguchi T, Ando Y, et al. Associations between functional tooth number and physical complaints of community-residing adults in a 15-year cohort study. Geriatr Gerontol Int. 2009;9:366-371. [DOI] [PubMed] [Google Scholar]
- 115. Yoshida M, Kikutani T, Okada G, Kawamura T, Kimura M, Akagawa Y. The effect of tooth loss on body balance control among community-dwelling elderly persons. Int J Prosthodont. 2009;22:136-139. [PubMed] [Google Scholar]
- 116. Hamalainen P, Rantanen T, Keskinen M, Meurman JH. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology. 2004;21:155-160. [DOI] [PubMed] [Google Scholar]
- 117. Avlund K, Holm-Pedersen P, Schroll M. Functional ability and oral health among older people: A longitudinal study from age 75 to 80. J Am Geriatr Soc. 2001;49:954-962. [DOI] [PubMed] [Google Scholar]
- 118. Schillinger T, Kluger W, Exner M, et al. Dental and periodontal status and risk for progression of carotid atherosclerosis: The inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke. 2006;37:2271-2276. [DOI] [PubMed] [Google Scholar]
- 119. Geismar K, Stoltze K, Sigurd B, Gyntelberg F, Holmstrup P. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547-1554. [DOI] [PubMed] [Google Scholar]
- 120. Chen YW, Umeda M, Nagasawa T, et al. Periodontitis may increase the risk of peripheral arterial disease. Eur J Vasc Endovasc Surg. 2008;35:153-158. [DOI] [PubMed] [Google Scholar]
- 121. Johansson CS, Ravald N, Pagonis C, Richter A. Periodontitis in patients with coronary artery disease: An 8-year follow-up. J Periodontol. 2014;85:417-425. [DOI] [PubMed] [Google Scholar]
- 122. Suematsu Y, Miura S, Zhang B, et al. Association between dental caries and out-of-hospital cardiac arrests of cardiac origin in japan. J Cardiol. 2016;67:384-391. [DOI] [PubMed] [Google Scholar]
- 123. Iwasaki M, Yoshihara A, Ogawa H, et al. Longitudinal association of dentition status with dietary intake in japanese adults aged 75 to 80 years. J Oral Rehabil. 2016;43:737-744. [DOI] [PubMed] [Google Scholar]
- 124. Okabe Y, Furuta M, Akifusa S, et al. Swallowing function and nutritional status in japanese elderly people receiving home-care services: A 1-year longitudinal study. J Nutr Health Aging. 2016;20:697-704. [DOI] [PubMed] [Google Scholar]
- 125. Okamoto N, Morikawa M, Yanagi M, et al. Association of tooth loss with development of swallowing problems in community-dwelling independent elderly population: The fujiwara-kyo study. J Gerontol A Biol Sci Med Sci. 2015;70:1548-1554. [DOI] [PubMed] [Google Scholar]
- 126. Yoshihara A, Watanabe R, Nishimuta M, Hanada N, Miyazaki H. The relationship between dietary intake and the number of teeth in elderly japanese subjects. Gerodontology. 2005;22:211-218. [DOI] [PubMed] [Google Scholar]
- 127. Kwon J, Suzuki T, Kumagai S, Shinkai S, Yukawa H. Risk factors for dietary variety decline among japanese elderly in a rural community: A 8-year follow-up study from TMIG-LISA. Eur J Clin Nutr. 2006;60:305-311. [DOI] [PubMed] [Google Scholar]
- 128. Ritchie CS, Joshipura K, Silliman RA, Miller B, Douglas CW. Oral health problems and significant weight loss among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2000;55:M366-M371. [DOI] [PubMed] [Google Scholar]
- 129. Bailey RL, Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Jensen GL. Persistent oral health problems associated with comorbidity and impaired diet quality in older adults. J Am Diet Assoc. 2004;104:1273-1276. [DOI] [PubMed] [Google Scholar]
- 130. Weyant RJ, Newman AB, Kritchevsky SB, et al. Periodontal disease and weight loss in older adults. J Am Geriatr Soc. 2004;52:547-553. [DOI] [PubMed] [Google Scholar]
- 131. Lee JS, Weyant RJ, Corby P, et al. Edentulism and nutritional status in a biracial sample of well-functioning, community-dwelling elderly: The health, aging, and body composition study. Am J Clin Nutr. 2004;79:295-302. [DOI] [PubMed] [Google Scholar]
- 132. Sato Y, Aida J, Takeuchi K, et al. Impact of loss of removable dentures on oral health after the great east japan earthquake: A retrospective cohort study. J Prosthodont. 2015;24:32-36. [DOI] [PubMed] [Google Scholar]
- 133. Enoki K, Matsuda KI, Ikebe K, et al. Influence of xerostomia on oral health-related quality of life in the elderly: A 5-year longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:716-721. [DOI] [PubMed] [Google Scholar]
- 134. Enoki K, Ikebe K, Matsuda KI, Yoshida M, Maeda Y, Thomson WM. Determinants of change in oral health-related quality of life over 7 years among older japanese. J Oral Rehabil. 2013;40:252-257. [DOI] [PubMed] [Google Scholar]
- 135. Warren JJ, Watkins CA, Cowen HJ, Hand JS, Levy SM, Kuthy RA. Tooth loss in the very old: 13-15-year incidence among elderly iowans. Community Dent Oral Epidemiol. 2002;30:29-37. [DOI] [PubMed] [Google Scholar]
- 136. Benyamini Y, Leventhal H, Leventhal EA. Self-rated oral health as an independent predictor of self-rated general health, self-esteem and life satisfaction. Soc Sci Med. 2004;59:1109-1116. [DOI] [PubMed] [Google Scholar]
- 137. Grubbs V, Vittinghoff E, Beck JD, et al. Association between periodontal disease and kidney function decline in african americans: The jackson heart study. J Periodontol. 2015;86:1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306-311. [DOI] [PubMed] [Google Scholar]
- 139. Chen YT, Shih CJ, Ou SM, et al. Periodontal disease and risks of kidney function decline and mortality in older people: A community-based cohort study. Am J Kidney Dis. 2015;66:223-230. [DOI] [PubMed] [Google Scholar]
- 140. Iwasaki M, Taylor GW, Nesse W, Vissink A, Yoshihara A, Miyazaki H. Periodontal disease and decreased kidney function in japanese elderly. Am J Kidney Dis. 2012;59:202-209. [DOI] [PubMed] [Google Scholar]
- 141. Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: The carolina head and neck cancer study. Cancer Causes Control. 2010;21:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kruger E, Tennant M. Hospital admissions of older people for oral health-related conditions: Implications for the future. Gerodontology. 2016;33:490-498. [DOI] [PubMed] [Google Scholar]
- 143. Sato M, Iwasaki M, Yoshihara A, Miyazaki H. Association between periodontitis and medical expenditure in older adults: A 33-month follow-up study. Geriatr Gerontol Int. 2016;16:856-864. [DOI] [PubMed] [Google Scholar]
- 144. Iwasaki M, Sato M, Yoshihara A, Ansai T, Miyazaki H. Association between tooth loss and medical costs related to stroke in healthy older adults aged over 75 years in japan. Geriatr Gerontol Int. 2017;17:202-210. [DOI] [PubMed] [Google Scholar]
- 145. Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: Longitudinal results from the study of health in pomerania (SHIP). Diabetes Care. 2010;33:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Persson GR, Berglund J, Persson RE, Renvert S. Prediction of hip and hand fractures in older persons with or without a diagnosis of periodontitis. Bone. 2011;48:552-556. [DOI] [PubMed] [Google Scholar]
- 147. de Almeida Mello J, Tran TD, Krausch-Hofmann S, et al. Cross-country validation of the association between oral health and general health in community-dwelling older adults. J Am Med Dir Assoc. 2019;20:1137-1142.e2. [DOI] [PubMed] [Google Scholar]
- 148. Nangle MR, Riches J, Grainger SA, Manchery N, Sachdev PS, Henry JD. Oral health and cognitive function in older adults: A systematic review. Gerontology. 2019:65:659-672. [DOI] [PubMed] [Google Scholar]
- 149. Teixeira FB, Saito MT, Matheus FC, et al. Periodontitis and alzheimer's disease: A possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front Aging Neurosci. 2017;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Adolph M, Darnaud C, Thomas F, et al. Oral health in relation to all-cause mortality: The IPC cohort study. Sci Rep. 2017;7:44604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cerutti-Kopplin D, Feine J, Padilha DM, et al. Tooth loss increases the risk of diminished cognitive function: A systematic review and meta-analysis. JDR Clin Trans Res. 2016;1:10-19. [DOI] [PubMed] [Google Scholar]
- 152. Harris TB, Ferrucci L, Tracy RP. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506-512. [DOI] [PubMed] [Google Scholar]
- 153. Marcenes W, Kassebaum NJ, Bernabe E, et al. Global burden of oral conditions in 1990-2010: A systematic analysis. J Dent Res. 2013;92:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. United Nations, Department of Economic and Social Affairs,Population Division. World Population Ageing. United Nations; 2015. Accessed August 28 2019 https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf. [Google Scholar]
- 155. Boehm TK, Scannapieco FA. The epidemiology, consequences and management of periodontal disease in older adults. J Am Dent Assoc. 2007;138 Suppl:26S-33S. [DOI] [PubMed] [Google Scholar]
- 156. Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465-1482. [DOI] [PubMed] [Google Scholar]
- 157. Mojon P, Budtz-Jorgensen E, Michel JP, Limeback H. Oral health and history of respiratory tract infection in frail institutionalised elders. Gerodontology. 1997;14:9-16. [DOI] [PubMed] [Google Scholar]
- 158. Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med. 2014;47:166-174 [DOI] [PubMed] [Google Scholar]
- 159. Snyder A. Oral health and the triple aim: evidence and strategies to improve care and reduce costs. The National Academy for State Health Policy. 2015. [Google Scholar]
- 160. Wall T, Nasseh K. Dental-related emergency department visits on the increase in the United States. Health Policy Resources Center Research Brief. American Dental Association. April 2013. http://www.ada.org/sections/professionalResources/pdfs/HPRCBrief_0413_4.pdf
- 161. Nuckols TK, Escarce JJ, Asch SM. The effects of quality of care on costs: a conceptual framework. Milbank Q. 2013;91:316-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Donabedian A, Wheeler JR, Wyszewianski L. Quality, cost, and health: an integrative model. Med Care. 1982;20:975-92. [DOI] [PubMed] [Google Scholar]