Abstract
Recently, increasing evidence has shown that CDGSH iron sulfur domain 2 (CISD2) is involved in the initiation and metastasis of several cancers. However, the evidence of its potential role in pancreatic cancer is still lacking. In our present study, CISD2 was found to be increased in pancreatic cancer samples and multiple cell lines. Moreover, statistical analysis revealed that a high level of CISD2 was related to advanced clinical stage, advanced T-stage, positive vascular invasion, positive distant metastasis, and larger tumor size. In addition, multivariate analysis suggests that CISD2 was an independent prognostic factor in pancreatic cancer. Importantly, downregulation of CISD2 was capable of inhibiting the survival and growth of pancreatic cancer cells. Mechanistic study showed that inactivation of the Wnt/β-catenin pathway contributed to the CISD2 deficit-induced death of pancreatic cancer cells. Furthermore, we showed that CISD2 silencing significantly inhibited EMT via the Wnt/β-catenin pathway. Finally, in nude mice, the CISD2 deficit suppressed the tumorigenesis of pancreatic cancer cells. Collectively, our study demonstrated that CISD2 could be an independent prognostic factor for pancreatic cancer and suggested that the CISD2/Wnt/β-catenin pathway contributes to the proliferation of pancreatic cancer cells and EMT, hinting at a novel promising molecular target in the therapeutic strategy for pancreatic cancer.
Key words: Pancreatic cancer (PC), CDGSH iron sulfur domain 2 (CISD2), Proliferation, Prognosis, Epithelial-to-mesenchymal transition (EMT), Wnt/β-catenin
INTRODUCTION
Pancreatic cancer (PC) is a highly lethal and aggressive tumor in the digestive system1. PC is usually at an advanced stage when it is diagnosed, and there are currently no early detectable biomarkers available2,3. The prognosis is poor, and the overall 5-year survival rate is less than 5%4. The therapeutic methods for PC mainly include surgical removal, adjuvant chemotherapy, and radiation5. However, most patients with advanced PC are not eligible for surgery. Besides, because of drug resistance, chemotherapy is not very effective, especially in advanced stage PC6. Thus, developing new strategies and identifying new molecular targets for treating PC are essential.
The CDGSH iron sulfur domain 2 (CISD2) is an evolutionarily conserved gene7. CISD2 is primarily located in the mitochondrial outer membrane and participates in regulation of mitochondrial integrity and life span in mammals8,9. Recent studies have revealed the involvement of CISD2 in tumorigenesis. CISD2 promotes tumor growth in human breast cancer10. In addition, in early stage cervical cancer, a high level of CISD2 is related to metastasis and poor prognosis7. Moreover, CISD2 promotes proliferation of gastric cancer cells through the AKT pathway and could be an independent prognostic factor in human gastric cancer11. Furthermore, CISD2 is demonstrated to be an oncogene in hepatocellular carcinoma and laryngeal squamous cell carcinoma12,13. However, the role of CISD2 in PC is still unknown, and the molecular mechanism underlying its carcinogenesis is far from clear.
Epithelial-to-mesenchymal transition (EMT) is characterized by losing epithelial phenotype and gaining mesenchymal characteristics in epithelial cells, as evidenced by the decrease in E-cadherin/γ-cadherin and increase in vimentin/N-cadherin14. In PC, EMT contributes to metastasis and chemotherapy resistance15–17. PC patients with a high EMT have a worse prognosis and more remote metastasis including liver and lung metastasis, compared with those with less EMT18,19. Wnt/β-catenin is one of the pathways involved in the regulation of EMT. The nuclear translocation of β-catenin mediates the expression of its target genes, and these downstream factors of the Wnt/β-catenin pathway including ZEB1, SNAIL1, SNAIL2, and TWIST are demonstrated to play important roles in regulating EMT20–23. However, whether the Wnt/β-catenin/EMT pathway is involved in the carcinogenesis of CISD2, especially in PC, has never been reported.
In the present study, we first tested the level of CISD2 in both PC samples and several cell lines with multiple methods. Moreover, we analyzed the relationship between CISD2 and the clinical characteristics as well as the prognosis of PC. Further, we explored the effects of CISD2 on survival and proliferation of PC cells and investigated the underlying molecular mechanism. In addition, we demonstrated that CISD2 regulated EMT through the Wnt/β-catenin pathway. Finally, we determined the effect of CISD2 silencing on the carcinogenic ability of PC cells in nude mice.
MATERIALS AND METHODS
Clinical Sample Collection
This study was conducted with written informed consents from all patients and was approved by the ethical review board of the China–Japan Union Hospital of Jilin University. Forty fresh samples of PC and their adjacent non-PC tissues were collected postsurgically from patients with PC between January 1, 2015, and January 1, 2016, at the China–Japan Union Hospital of Jilin University and used for Western blot, qRT-PCR, and immunohistochemistry staining to determine the level of CISD2. Moreover, 120 paraffin-embedded PC samples were collected in the China–Japan Union Hospital of Jilin University between March 1, 2008, and January 1, 2014. All 120 patients were followed for up to 25 months. These data were used to analyze the relationship between CISD2 level and clinical characters as well as the prognosis of PC.
Cell Culture
Immortalized normal pancreatic cell line hTERT-HPNE was used as the control, and human PC cell lines PANC-1, SW1990, Capan-1, Capan-2, and Hs766T were all purchased from the Chinese Academy of Sciences and cultured in DMEM (Thermo Fisher Scientific, Shanghai, P.R. China) supplemented with 10% FBS (Thermo Fisher Scientific). These cells were maintained in a humidified 37°C incubator containing 5% CO2.
Nude Mice
Seven-week-old BALB/c nude mice (n = 6, three in each group) were purchased from the Animal Center of the China–Japan Union Hospital of Jilin University. All the animals were housed in a pathogen-free condition. Procedures for animal experiments were reviewed and approved by the ethical review board of the China–Japan Union Hospital of Jilin University. All possible efforts were made to minimize the suffering of these mice.
Western Blot
Western blot was conducted as previously described24. Briefly, protein samples were prepared with RIPA lysis buffer (Sigma-Aldrich, Cambridge, MA, USA) with protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific). After separation by sodium dodecyl sulfate polyacrylamide gel, proteins (30 μg) were transferred to a PVDF membrane (Millipore, Boston, MA, USA). After blocking with 5% nonfat milk, the PVDF membrane was incubated with primary antibody in a cold room overnight. After three washes with Tris-buffered saline Tween (TBST) for 15 min, the PVDF membrane was incubated with peroxidase-conjugated secondary antibody. Then after three washes with TBST for 15 min, the protein was visualized with ECL detection solution (Millipore). The primary antibodies were listed as follows: anti-CISD2, anti-GAPDH, anti-GSK3β, anti-p-GSK3β, anti-vimentin, and anti-N-cadherin (purchased from Sigma-Aldrich, St. Louis, MO, USA); anti-β-catenin, anti-p-β-catenin, anti-c-Myc, anti-E-cadherin, and anti-γ-catenin (purchased from Abcam, Cambridge, UK). GAPDH was used as internal control. The intensity of the protein bands was quantified with ImageJ and normalized to that of GAPDH.
Immunohistochemistry Staining
In order to detect the CISD2 level of the collected clinical PC tissues, immunohistochemistry staining was performed. Briefly, after fixation with formalin and paraffin, the tissues were sectioned with thickness of 15 μm. Heating was used to retrieve the antigen. Antibody-identifying CISD2 (Sigma-Aldrich) was applied, followed by nuclear counterstaining with hematoxylin.
Real-Time Quantitative PCR (qRT-PCR)
TRIzol reagent (Thermo Fisher Scientific) was used to extract RNA from samples. Then a total of 250 ng of RNA was used to perform reverse transcription with QuantiMir RT kit (System Biosciences, Palo Alto, CA, USA). The primers used were as follows: CISD2, 5′-GCAAGGTAGCCAAGAAGTGC-3′ (forward) and 5′-CCCAGTCCCTGAAAGCATTA-3′ (reverse); GAPDH, 5′-GCGAGATCGCACTCATCATCT-3′ (forward) and 5′-TCAGTGGTGGACCTGACC-3′ (reverse).
CISD2 was amplified as follows: step 1: denaturation at 95°C for 10 min; step 2: 40 cycles of 95°C for 15 s and 60°C for 1 min. Results were calculated with 2−ΔΔCT and normalized to GAPDH.
MTT Assay
PANC-1 and SW1990 cells whose CISD2 was downregulated by lentivirus carrying shCISD2 were seeded in a 96-well plate. At the indicated time points (0, 24, 48, and 72 h), the viability of cells in each well was determined with the MTT assay. Briefly, after adding 10 μl of MTT labeling reagent, the 96-well plate was covered and placed in a 37°C incubator for 4 h. Finally, the absorbance at a wavelength of 570 nm was measured.
TUNEL Assay
In order to detect apoptosis, TUNEL assay was used as previously described25. Briefly, cells were fixed with 4% paraformaldehyde solution for 0.5 h at room temperature. Then the cells were exposed to a methanol solution containing 0.2% H2O2 for 0.5 h to block endogenous peroxidase activity. TUNEL reaction mixture (Sigma-Aldrich) was then added, and the cells were placed in an incubator at 37°C for 60 min. Finally, laser confocal microscopy (A1; Nikon, Japan) was used to capture the fluorescence. Apoptosis index was quantified as the ratio of (TUNEL+ cells)/(total cells) × 100%.
Flow Cytometry
Briefly, cells were washed with culture medium twice and collected with trypsin. After centrifugation at 400 × g for 5 min, the supernatant was then discarded. The cells were incubated with an FITC Annexin-V Apoptosis Detection Kit with PI (BioLegend, San Diego, CA, USA) for 30 min. Flow cytometry (Becton Dickinson, San Jose, CA, USA) was used to analyze the ratio of apoptotic cells.
CISD2 Overexpression and Downregulation
Lentivirus carrying scramble (Lv-scramble) or CISD2 overexpression construct (Lv-oeCISD2) or shCISD2 (Lv-shCISD2) was bought from Shanghai GenePharma Co. Ltd. (Shanghai, P.R. China). PANC-1 and SW1990 PC cells were pretreated with Lv-scramble or Lv-shCISD2 or Lv-oeCISD2 with a MOI of 15, respectively. At 24 h postinfection, the cells were used for subsequent experiments.
Nuclear and Cytoplasmic Extraction
NE-PERTM Nuclear and Cytoplasmic Extraction Reagent (78833; Thermo Fisher Scientific, Waltham, MA, USA) was applied to separate nuclear and cytoplasmic components, according to the instructions of the manufacturer.
Tumorigenesis Assay
PANC-1 cells (8 × 106) were preinfected with Lv-scramble or Lv-shCISD2 at the MOI of 15 (Shanghai GenePharma Co. Ltd.). After being collected and diluted in 100 μl of PBS, all the cells were injected subcutaneously into the right groin of BALB/c nude mice (n = 6, three in each group). On the 40th day postinoculation, the tumors were removed. The weights of tumors were scaled, and the volumes were calculated (length × width2 × π/6).
Statistical Analysis
Analysis between categorical variables was performed with the chi-square test. Multivariate analysis was based on the Cox proportional hazards regression model. Survival analysis was conducted with Kaplan–Meier plot and log-rank tests. Data among or between groups were analyzed by one-way ANOVA or Student’s t-test, respectively. A value of p < 0.05 was considered to be statistically significant. All the analysis was done using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). All experiments were repeated in triplicate, and the results were expressed as mean ± SD.
RESULTS
CISD2 Was Increased in Pancreatic Cancer Samples and Cell Lines
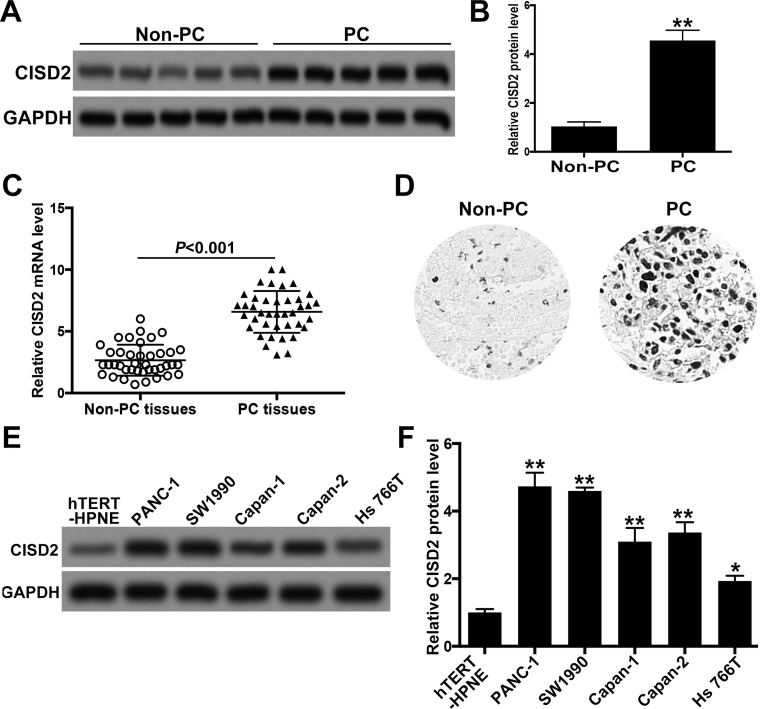
To determine the level of CISD2 in PC, we collected 40 fresh samples of PC and their adjacent non-PC tissues. Western blot showed that, compared with that of non-PC samples, CISD2 was significantly increased in PC samples (Fig. 1A and B). In addition, the elevated expression of CISD2 in PC tissues was confirmed by qRT-PCR and immunohistochemistry staining (Fig. 1C and D). We then explored the expression of CISD2 in several pancreatic cell lines with Western blot. Compared with that in hTERT-HPNE cells (an immortalized normal pancreatic cell line), CISD2 was obviously upregulated in PC cell lines PANC-1, SW1990, Capan-1, Capan-2, and Hs766T (Fig. 1E and F). Among these pancreatic cell lines, the increase in CISD2 in PANC-1 and SW1990 was the most significant; thus, we decided to use these two cell lines for subsequent experiments.
Figure 1.
CISD2 was increased in pancreatic cancer samples and cell lines. (A) We collected 40 fresh samples of pancreatic cancer (PC) and their adjacent non-pancreatic cancer (non-PC) tissues, and Western blot was used to determine the level of CISD2. (B) Relative level of CISD2 in (A) was measured using ImageJ and normalized to GAPDH. (C) qRT-PCR was applied to test the level of CISD2 mRNA in the collected PC and non-PC tissues. (D) Immunohistochemistry staining was used to confirm the increase in CISD2 level of PC tissues compared with non-PC tissues. (E) Western blot was applied to determine the level of CISD2 in several pancreatic cancer cell lines (PANC-1, SW1990, Capan-1, Capan-2, and Hs766T). hTERT-HPNE is an immortalized normal pancreatic cell line and acts as a control. (F) Relative level of CISD2 in (E) was measured with ImageJ and normalized to GAPDH. Data were presented as mean ± SD from at least three independent experiments. *p < 0.05 and **p < 0.01 compared with control group.
High Level of CISD2 Was Related With Advanced Clinicopathological Characteristics and Poor Prognosis in Patients With Pancreatic Cancer
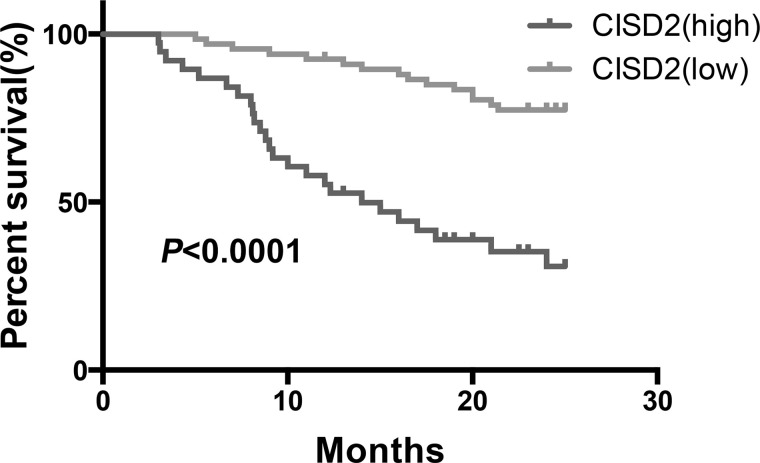
We collected 120 paraffin-embedded PC samples from patients who were histopathologically and clinically diagnosed, and these patients were followed for up to 25 months. High CISD2 expression was significantly associated with advanced clinical stage (p < 0.05), advanced T-stage (p < 0.05), positive vascular invasion (p < 0.05), positive distant metastasis (p < 0.05), and larger tumor size (p < 0.05). Moreover, multivariate analysis suggested that high CISD2 expression was closely correlated with the overall survival time of patients with PC and is an independent prognostic factor (p = 0.02). Also, our results demonstrated that distant metastasis (p < 0.001), clinical stage (p = 0.019), and vascular invasion (p = 0.021), but not nodal metastasis (p = 0.092), were independent prognostic factors for PC. Finally, Kaplan–Meier survival curves indicated that patients with a high CISD2 level have significantly shorter overall survival duration when compared with patients with low CISD2 expression (Fig. 2). Taken together, the above results demonstrated that a high level of CISD2 was closely related with advanced clinicopathological parameters of PC and is a promising independent prognostic factor.
Figure 2.
Kaplan–Meier survival curves indicated that patients with a high CISD2 level have significantly shorter overall survival duration when compared to patients with low CISD2 expression (p < 0.0001).
CISD2 Silencing Inhibited the Survival and Growth of Pancreatic Cancer Cells
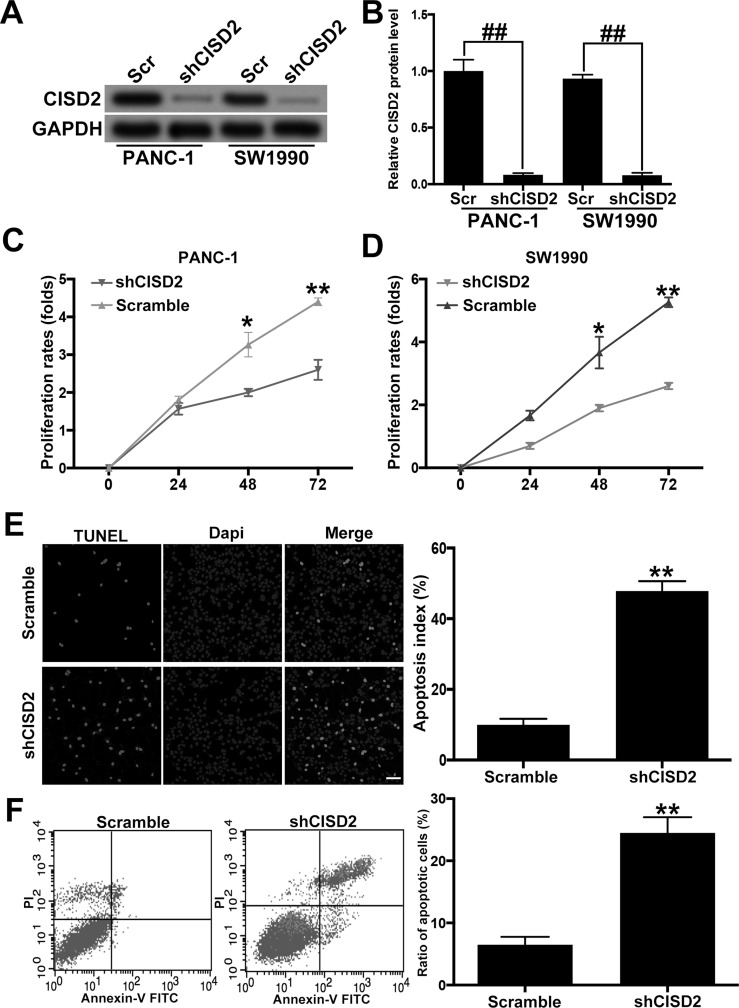
In order to downregulate CISD2 in PANC-1 and SW1990 PC cell lines, we applied lentivirus carrying shRNA specifically targeting CISD2 (shCISD2). Western blot confirmed that shCISD2 succeeded in significantly knocking down CISD2 in both cell lines (Fig. 3A and B). The MTT assay revealed that, compared with the scramble groups, CISD2 silencing significantly inhibited the growth rate of PANC-1 and SW1990 cell lines (Fig. 3C and D). The TUNEL assay indicated that shCISD2 increased the percentage of apoptotic cells in PANC-1 cells (Fig. 3E). Finally, flow cytometry confirmed the increase in number of necrotic and apoptotic cells by shCISD2 in SW1990 cells (Fig. 3F). Altogether, these data suggest that CISD2 silencing inhibited the survival and growth of PC cells.
Figure 3.
CISD2 silencing inhibited the survival and growth of pancreatic cancer cells. (A) We applied lentivirus carrying shRNA specifically targeting CISD2 (shCISD2) to downregulate the level of CISD2 in PANC-1 and SW1990 cells. The efficiency of knocking down CISD2 was determined by Western blot. Scr, scramble. (B) Relative level of CISD2 in (A) was measured using ImageJ and normalized to GAPDH. (C, D) The effect of CISD2 defect on the growth rate of PANC-1 and SW1990 cells was tested with the MTT assay. (E) The TUNEL assay indicated that the CISD2 defect increased the percentage of apoptotic cells in PANC-1 cells. (F) Flow cytometry confirmed the increase in number of necrotic and apoptotic cells by shCISD2 in SW1990 cells. Lower left (LL) quadrants: the live cells. Lower right (LR) quadrants: the early apoptotic cells. Upper right (UR) quadrants: terminal apoptotic cells. Upper left (UL) quadrants: the necrotic cells. Data were presented as mean ± SD from at least three independent experiments. *p < 0.05 and **p < 0.01 compared with control group and ##p < 0.01 compared with the indicated groups.
Inactivation of Wnt/β-Catenin Pathway Contributed to CISD2 Silencing-Induced Inhibition of Proliferation in Pancreatic Cancer Cells
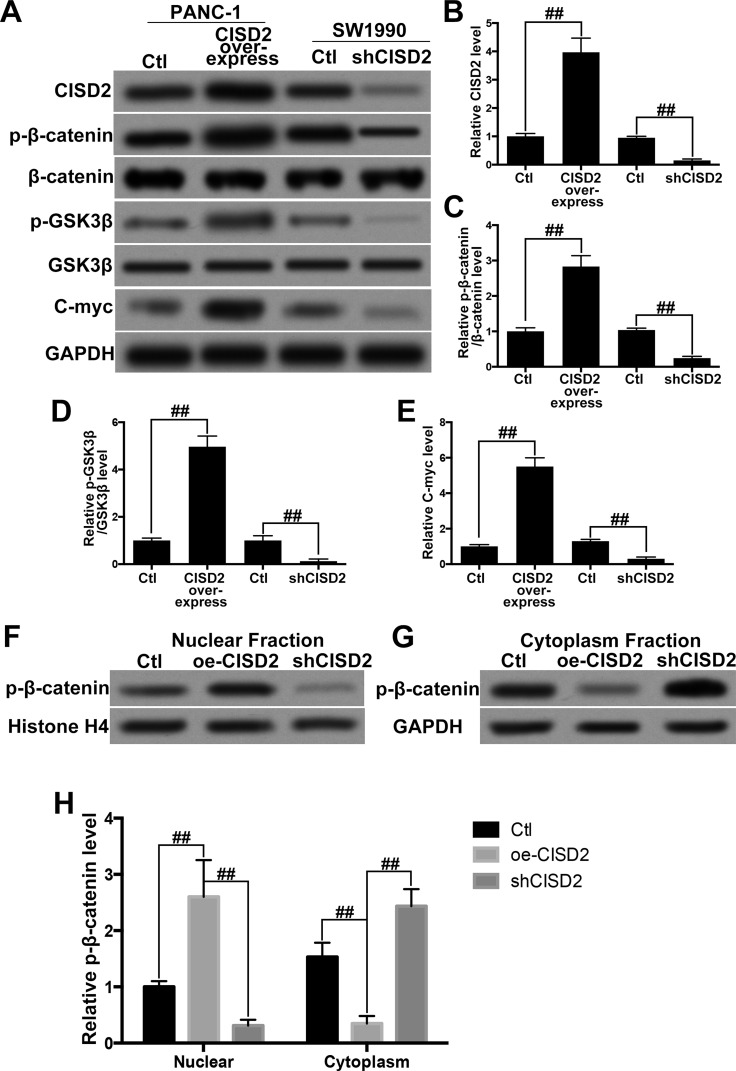
The Wnt/β-catenin pathway has been well established to participate in the regulation of proliferation and survival of PC cells26,27. Consequently, we explored the potential effect of CISD2 on the Wnt/β-catenin pathway. We overexpressed CISD2 in PANC-1 cells and downregulated CISD2 in SW1990 cells. The efficiency of manipulating CISD2 in these cells was confirmed by Western blot (Fig. 4A and B). In PANC-1 cells, the Western blot showed that CISD overexpression increased the ratio of p-β-catenin/β-catenin (Fig. 4A and C) and p-GSK3β/GSK3β (Fig. 4A and D), and enhanced the expression of the Wnt/β-catenin pathway targeting c-Myc (Fig. 4A and E). By contrast, in SW1990 cells, knocking down CISD2 resulted in a decreased ratio of p-β-catenin/β-catenin (Fig. 4A and C) and p-GSK3β/GSK3β (Fig. 4A and D), and reduced the c-Myc level (Fig. 4A and E). Furthermore, we manipulated CISD2 in PANC-1 cells and separated the nuclear fraction from the cytoplasm fraction to determine the location of p-β-catenin. The Western blot showed that, compared with the control group, CISD2 overexpression groups showed significantly more p-β-catenin in the nuclear parts and less in the cytoplasm parts (Fig. 4F and H). However, the CISD2 deficit significantly decreased CISD2 overexpression groups in the nuclear components and increased overexpression in the cytoplasm components (Fig. 4G and H). Taken together, these results suggested that inactivation of the Wnt/β-catenin pathway contributed to CISD2 silencing-induced inhibition of proliferation in PC cells.
Figure 4.
Inactivation of the Wnt/β-catenin pathway contributed to CISD2 silencing-induced inhibition of proliferation in pancreatic cancer cells. (A) We overexpressed CISD2 in PANC-1 cells and silenced CISD2 in SW1990 cells. Western blot was used to determine the level of CISD2, phosphorylated-β-catenin (p-β-catenin), β-catenin, phosphorylated-GSK3β (p-GSK3β), GSK3β, and c-Myc. Ctl, control. (B–E) Relative levels of CISD2 (B), ratio of p-β-catenin/β-catenin (C), ratio of p-GSK3β/GSK3β (D), and c-Myc (E) were measured by ImageJ and normalized to GAPDH. (F, G) Furthermore, we manipulated CISD2 in PANC-1 cells and separated the nuclear fraction from the cytoplasm fraction. Western blot was used to determine the level of p-β-catenin in the nuclear fraction (F) and the cytoplasm fraction (G). oe-CISD2, overexpression of CISD2. (H) Relative level of p-β-catenin in (F) and (G) was measured using ImageJ and normalized to histone H4 or GAPDH, respectively. Data were presented as mean ± SD from at least three independent experiments. ##p < 0.01 compared with the indicated groups.
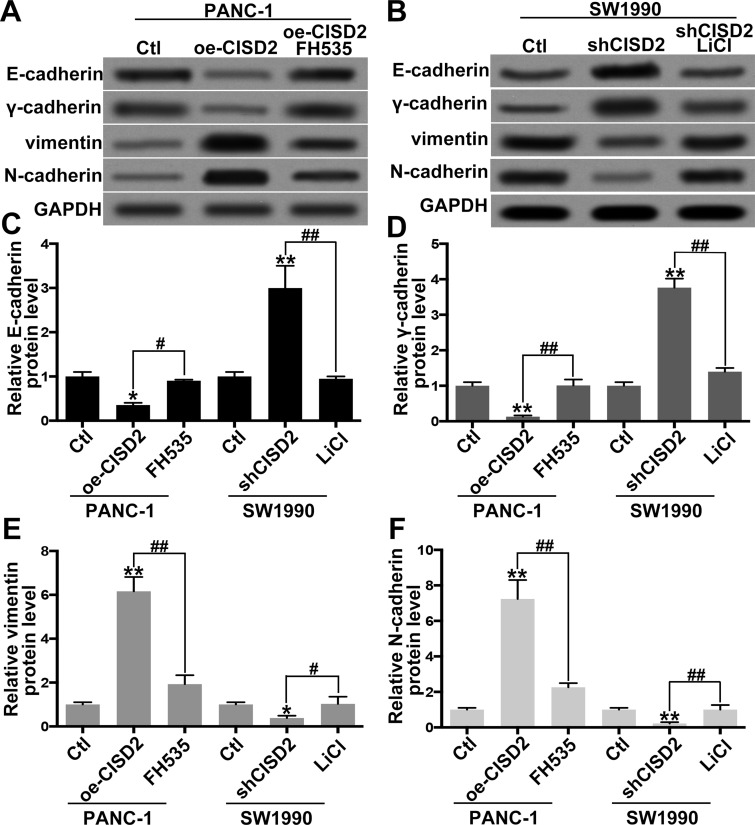
CISD2 Deficit Inhibited EMT Through Inactivating the Wnt/β-Catenin Pathway in Pancreatic Cancer Cells
Recently, increasing evidence supports the role of the Wnt/β-catenin pathway in promoting EMT in PC28,29. Thus, we further tested the effect of the CISD2/Wnt/β-catenin pathway on EMT in PC cells. In order to modulate activity of the Wnt/β-catenin pathway, FH535 (Wnt/β-catenin inhibitor) and LiCl (Wnt/β-catenin activator) were used27,30. The Western blot showed that, in PANC-1 cells, CISD2 upregulation markedly promoted EMT as evidenced by weakened expression of epithelial markers E-cadherin (Fig. 5A and C) and γ-catenin (Fig. 5A and D) and enhanced expression of the mesenchymal markers vimentin (Fig. 5A and E) and N-cadherin (Fig. 5A and F). However, the Wnt inhibitor FH535 significantly abolished CISD2-induced EMT (Fig. 5A and C–F). By contrast, in SW1990 cells, CISD2 knockdown significantly inhibited EMT as evidenced by an increase in E-cadherin (Fig. 5B and C) and γ-catenin (Fig. 5B and D) and a decrease in vimentin (Fig. 5B and E) and N-cadherin (Fig. 5B and F). Conversely, the Wnt activator LiCl reversed the suppression of shCISD2 on EMT (Fig. 5B and C–F). Thus, the above results demonstrated that a CISD2 deficit inhibited EMT through inactivating the Wnt/β-catenin pathway in PC cells.
Figure 5.
CISD2 deficit inhibited EMT through inactivating the Wnt/β-catenin pathway in pancreatic cells. (A) In PANC-1 cells, CISD2 was upregulated, and FH535 was used as the Wnt/β-catenin inhibitor. The epithelial markers E-cadherin and γ-catenin as well as the mesenchymal markers vimentin and N-cadherin were tested by Western blot. Ctl, control; oe-CISD2, overexpression of CISD2. (B) In SW1990 cells, CISD2 was downregulated, and LiCl was applied as Wnt/β-catenin activator. The epithelial markers E-cadherin and γ-catenin as well as the mesenchymal markers vimentin and N-cadherin were determined by Western blot. (C–F) Relative level of E-cadherin (C), γ-catenin (D), vimentin (E), and N-cadherin (F) in (A) and (B) was measured using ImageJ and normalized to GAPDH. Data were presented as mean ± SD from at least three independent experiments. *p < 0.05 and **p < 0.01 compared with control group and #p < 0.05 and ##p < 0.01 compared with the indicated groups.
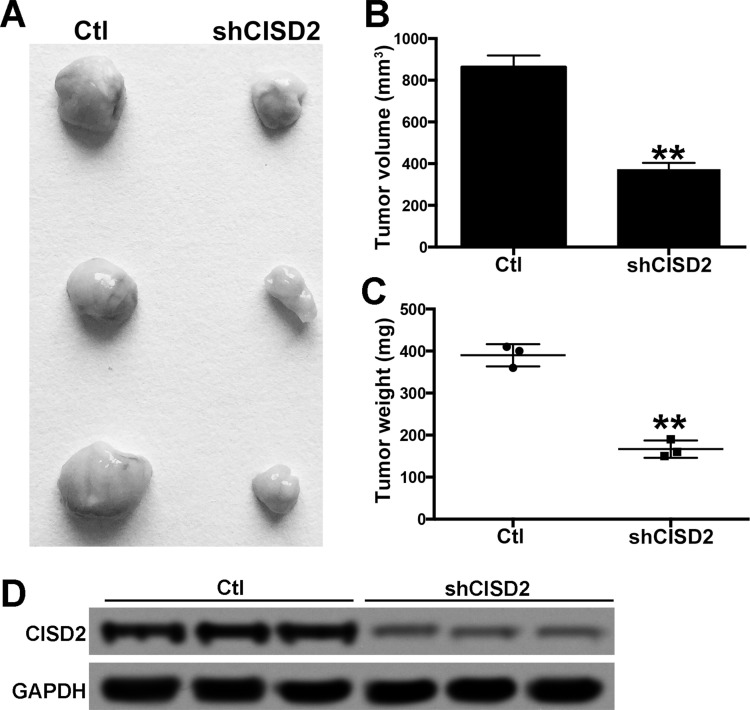
Knockdown of CISD2 Inhibited Tumorigenesis of Pancreatic Cancer Cells
Finally, the role of CISD2 in tumorigenecity of PC cells was determined in BALB/c nude mice. Scramble and shCISD2 PANC-1 cells were subcutaneously inoculated into nude mice. The tumors were carefully dissected on the 40th day postinoculation (Fig. 6A). Compared with the control groups, the CISD2 deficit significantly decreased the average volume and weight of tumors (Fig. 6B and C). Furthermore, the Western blot confirmed the intensive expression of CISD2 in the formed tumors of control groups; however, the CISD2 level was markedly reduced in the tumors of the shCISD2 groups (Fig. 6D). The above data suggested that CISD2 knockdown significantly inhibited tumorigenesis of PC cells in vivo.
Figure 6.
Knocking down of CISD2 inhibited tumorigenesis of pancreatic cancer cells. (A) Scramble and shCISD2 PANC-1 cells were subcutaneously inoculated in BALB/c nude mice, and 40 days later the tumors were carefully dissected. (B, C) The average volume and weight of tumors were measured in both groups. (D) Western blot was used to confirm the level CISD2 in the formed tumors of both groups. Data were presented as mean ± SD from at least three independent experiments. **p < 0.01 compared with control group.
DISCUSSION
In the present study, CISD2 was found to be increased in PC tissues and multiple cell lines. Moreover, a high level of CISD2 is correlated with advanced clinical stage, advanced T-stage, positive vascular invasion, positive distant metastasis, and larger tumor size. Multivariate analysis suggested that high CISD2 expression was closely correlated with the overall survival time of PC patients. Kaplan–Meier survival curves indicated that patients with a high CISD2 level have significantly shorter overall survival duration. Thus, CISD2 could be an independent prognostic factor in patients with PC. Importantly, CISD2 silencing was capable of inhibiting the survival and growth of PC cells. A mechanistic study showed that inactivation of the Wnt/β-catenin pathway contributed to the CISD2 deficit-induced death of PC cells. Furthermore, we showed that CISD2 silencing significantly inhibited EMT via the Wnt/β-catenin pathway. Finally, in nude mice, a CISD2 deficit suppressed the tumorigenesis of PC cells.
To our knowledge, this is the first study to describe the carcinogenic role of CISD2 in PC. Primarily, CISD2 is located in the mitochondrial outer membrane, and its deficit can damage mitochondrial integrity and lead to mitochondrial dysfunction and cell autophagy31,32. Recently, the involvement of CISD2 in tumorigenesis has attracted much interest. CISD2 promotes tumor proliferation by maintaining mitochondria homeostasis in human breast cancer10,33. In addition, in early stage cervical cancer, a high level of CISD2 is related with metastasis and poor prognosis7. Moreover, CISD2 promotes proliferation of gastric cancer cells through the AKT pathway and could be an independent prognostic factor in human gastric cancer11. Furthermore, CISD2 indicates a negative prognosis in hepatocellular carcinoma patients and laryngeal squamous cell carcinoma12,13. However, the role of CISD2 in PC is still unclear. Our study showed that CISD2 was significantly upregulated in PC samples and multiple cell lines. Importantly, statistical analysis revealed that a high level of CISD2 correlated with advanced clinicopathological characteristics and poor prognosis. Importantly, reducing CISD2 significantly inhibited proliferation of PC cells and suppressed the carcinogenic capability of PC cells in nude mice. These data support the suggestion that CISD2 could be a promising independent prognostic factor for patients with PC and favors an oncogenic role for CISD2 in PC.
The Wnt/β-catenin pathway is demonstrated to participate in the regulation of proliferation and survival of many cancer cells. The Wnt/β-catenin signal inhibitor HC-1 enhanced 5-fluorouracil-induced toxicity in oral squamous cell carcinoma cells34. Rab11a increases cancer metastasis in PC through Wnt/β-catenin signaling27. KLF8 modulates the Wnt/β-catenin pathway to sustain stem cell characteristics of hepatocellular carcinoma cells35. HMGA1 facilitates tumor progression through regulating the Wnt/β-catenin pathway in endometrial cancer36. Consistent with these early studies, our results demonstrated that inactivation of the Wnt/β-catenin pathway contributed to CISD2 silencing-induced inhibition of proliferation in PC cells. The mechanism underlying how CISD2 silencing inactivates the Wnt/β-catenin pathway needs further investigation.
Increasing evidence supports the suggestion that Wnt/β-catenin promotes EMT in many cancers, including PC. Loss of zfp36 expression enhances EMT in colorectal cancer via activating Wnt/β-catenin22. Oridonin inhibits PC cell migration and EMT by suppressing the Wnt/β-catenin signaling28. LncRNA UCA1 promotes the EMT of breast cancer cells via enhancing the Wnt/β-catenin pathway37. DKK3 inhibits β-catenin/EMT and sensitizes Bxpc-3 cells to gemcitabine29. Our study enhanced the evidence for the role of Wnt/β-catenin in promoting EMT in PC. Since EMT is closely related with the metastasis of cancers, the relationship between CISD2 and metastasis of PC warrants future studies.
In conclusion, our study demonstrated that CISD2 could be an independent prognostic factor for PC and suggested that the CISD2/Wnt/β-catenin pathway contributed to the proliferation of PC and EMT, hinting at a new promising molecular target for PC therapy.
ACKNOWLEDGMENTS
The authors thank Prof. Alex Baton from Robert Johnson University for the critical editing of our manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004;363:1049–57. [DOI] [PubMed] [Google Scholar]
- 3. Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, Goel R, Mathivanan S, Marimuthu A, Kashyap M, Vizza RF, Mayer RJ, Decaprio JA, Srivastava S, Hanash SM, Hruban RH, Pandey A. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang J, Ouyang J, Ouyang L, Ouyang L, Chen Y. Inhibition of cell proliferation and increase of chemosensitivity by simultaneous knockdown of XIAP and survivin in pancreatic carcinoma cells. Oncol Res. 2013;21:43–50. [DOI] [PubMed] [Google Scholar]
- 7. Liu L, Xia M, Wang J, Zhang W, Zhang Y, He M. CISD2 expression is a novel marker correlating with pelvic lymph node metastasis and prognosis in patients with early-stage cervical cancer. Med Oncol. 2014;31:183. [DOI] [PubMed] [Google Scholar]
- 8. Chen YF, Kao CH, Kirby R, Tsai TF. CISD2 mediates mitochondrial integrity and life span in mammals. Autophagy 2009;5:1043–5. [DOI] [PubMed] [Google Scholar]
- 9. Chen YF, Wu CY, Kirby R, Kao CH, Tsai TF. A role for the CISD2 gene in lifespan control and human disease. Ann NY Acad Sci. 2010;1201:58–64. [DOI] [PubMed] [Google Scholar]
- 10. Sohn YS, Tamir S, Song L, Michaeli D, Matouk I, Conlan AR, Harir Y, Holt SH, Shulaev V, Paddock ML, Hochberg A, Cabanchick IZ, Onuchic JN, Jennings PA, Nechushtai R, Mittler R. NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci USA 2013;110:14676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Ouyang F, Liu X, Wu S, Wu HM, Xu Y, Wang B, Zhu J, Xu X, Zhang L. Overexpressed CISD2 has prognostic value in human gastric cancer and promotes gastric cancer cell proliferation and tumorigenesis via AKT signaling pathway. Oncotarget 2016;7:3791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen B, Shen S, Wu J, Hua Y, Kuang M, Li S, Peng B. CISD2 associated with proliferation indicates negative prognosis in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:13725–38. [PMC free article] [PubMed] [Google Scholar]
- 13. Yang L, Hong S, Wang Y, He Z, Liang S, Chen H, He S, Wu S, Song L, Chen Y. A novel prognostic score model incorporating CDGSH iron sulfur domain 2 (CISD2) predicts risk of disease progression in laryngeal squamous cell carcinoma. Oncotarget 2016;7:22720–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, Zhang J, Wu X, Shan B. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol Res. 2013;21:165–71. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Wang D, Li Y, Liu Y, Xie X, Wu Y, Zhou Y, Ren J, Zhang J, Zhu H, Su Z. CCL21/CCR7 axis contributed to CD133+ pancreatic cancer stem-like cell metastasis via EMT and Erk/NF-kappaB pathway. PLoS One 2016;11:e0158529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Wang S, Su J, Chu G, You H, Chen Z, Sun H, Chen B, Zhou M. Interleukin-32alpha inactivates JAK2/STAT3 signaling and reverses interleukin-6-induced epithelial-mesenchymal transition, invasion, and metastasis in pancreatic cancer cells. Onco Targets Ther. 2016;9:4225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasegawa S, Nagano H, Konno M, Eguchi H, Tomokuni A, Tomimaru Y, Asaoka T, Wada H, Hama N, Kawamoto K, Marubashi S, Nishida N, Koseki J, Mori M, Doki Y, Ishii H. A crucial epithelial to mesenchymal transition regulator, Sox4/Ezh2 axis is closely related to the clinical outcome in pancreatic cancer patients. Int J Oncol. 2016;48:145–52. [DOI] [PubMed] [Google Scholar]
- 19. Subramani R, Gonzalez E, Arumugam A, Nandy S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan M, Dwivedi A, Lakshmanaswamy R. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci Rep. 2016;6:19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Douchi D, Ohtsuka H, Ariake K, Masuda K, Kawasaki S, Kawaguchi K, Fukase K, Oikawa M, Motoi F, Naitoh T, Katayose Y, Egawa S, Unno M. Silencing of LRRFIP1 reverses the epithelial-mesenchymal transition via inhibition of the Wnt/beta-catenin signaling pathway. Cancer Lett. 2015;365:132–40. [DOI] [PubMed] [Google Scholar]
- 21. Liu MH, Shi SM, Li K, Chen EQ. Knockdown of PFTK1 expression by RNAi inhibits the proliferation and invasion of human non-small lung adenocarcinoma cells. Oncol Res. 2016;24:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montorsi L, Guizzetti F, Alecci C, Caporali A, Martello A, Giacinto Atene C, Parenti S, Pizzini S, Zanovello P, Bortoluzzi S, Ferrari S, Grande A, Zanocco-Marani T. Loss of zfp36 expression in colorectal cancer correlates to wnt/β-catenin activity and enhances epithelial-to-mesenchymal transition through upregulation of zeb1, sox9 and macc1. Oncotarget 2016;7(37):59144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Li L, Huang Q, Xu W, Cai X, Zhang J, Yan W, Song D, Liu T, Zhou W, Li Z, Yang C, Dang Y, Xiao J. Wnt signaling through Snail1 and Zeb1 regulates bone metastasis in lung cancer. Am J Cancer Res. 2015;5:748–55. [PMC free article] [PubMed] [Google Scholar]
- 24. Wang B, Cai Z, Lu F, Li C, Zhu X, Su L, Gao G, Yang Q. Destabilization of survival factor MEF2D mRNA by neurotoxin in models of Parkinson’s disease. J Neurochem. 2014;130:720–8. [DOI] [PubMed] [Google Scholar]
- 25. Wang B, Cai Z, Tao K, Zeng W, Lu F, Yang R, Feng D, Gao G, Yang Q. Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy 2016;12:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang JX, Sun CY, Tian S, Yu C, Chen MY, Zhang H. Tumor suppressor Fbxw7 antagonizes WNT signaling by targeting beta-catenin for degradation in pancreatic cancer. Tumour Biol. 2016;37(10):13892–902. [DOI] [PubMed] [Google Scholar]
- 27. Yu L, Li X, Li H, Chen H, Liu H. Rab11a sustains GSK3beta/Wnt/beta-catenin signaling to enhance cancer progression in pancreatic cancer. Tumour Biol. 2016;37(10):13821–9. [DOI] [PubMed] [Google Scholar]
- 28. Liu QQ, Chen K, Ye Q, Jiang XH, Sun YW. Oridonin inhibits pancreatic cancer cell migration and epithelial-mesenchymal transition by suppressing Wnt/beta-catenin signaling pathway. Cancer Cell Int. 2016;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo Q, Qin W. DKK3 blocked translocation of beta-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J Cell Mol Med. 2015;19:2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T, Zhang H, Qiu H, Li B, Wang J, Du G, Ren C, Wan X. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/beta-catenin signaling in endometrial carcinoma. Oncotarget 2016;7:25712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen YF, Kao CH, Chen YT, Wang CH, Wu CY, Tsai CY, Liu FC, Yang CW, Wei YH, Hsu MT, Tsai SF, Tsai TF. CISD2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang NC, Nguyen M, Shore GC. BCL2-CISD2: An ER complex at the nexus of autophagy and calcium homeostasis? Autophagy 2012;8:856–7. [DOI] [PubMed] [Google Scholar]
- 33. Holt SH, Darash-Yahana M, Sohn YS, Song L, Karmi O, Tamir S, Michaeli D, Luo Y, Paddock ML, Jennings PA, Onuchic JN, Azad RK, Pikarsky E, Cabantchik IZ, Nechushtai R, Mittler R. Activation of apoptosis in NAF-1-deficient human epithelial breast cancer cells. J Cell Sci. 2016;129:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yokogi S, Tsubota T, Kanki K, Azumi J, Itaba N, Oka H, Morimoto M, Ryoke K, Shiota G. Wnt/Beta-catenin signal inhibitor HC-1 sensitizes oral squamous cell carcinoma cells to 5-fluorouracil through reduction of CD44-positive population. Yonago Acta Med. 2016;59:93–9. [PMC free article] [PubMed] [Google Scholar]
- 35. Shen YN, He HG, Shi Y, Cao J, Yuan JY, Wang ZC, Shi CF, Zhu N, Wei YP, Liu F, Huang JL, Yang GS, Lu JH. Kruppel-like factor 8 promotes cancer stem cell-like traits in hepatocellular carcinoma through Wnt/beta-catenin signaling. Mol Carcinog. 2017;56(2):751–60. [DOI] [PubMed] [Google Scholar]
- 36. Han X, Cao Y, Wang K, Zhu G. HMGA1 facilitates tumor progression through regulating Wnt/beta-catenin pathway in endometrial cancer. Biomed Pharmacother. 2016;82:312–8. [DOI] [PubMed] [Google Scholar]
- 37. Xiao C, Wu CH, Hu HZ. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20:2819–24. [PubMed] [Google Scholar]