Abstract
Emerging evidence has suggested that aberrantly expressed microRNAs (miRNAs) are associated with glioma development and progression. The aberrant expression of miR-409-3p has been reported in several human cancers. However, little is known about the function of miR-409-3p in gliomas. The aim of this study was to investigate the specific role and molecular mechanism of miR-409-3p in gliomas. In the present study, we found that miR-409-3p was downregulated in glioma tissue and cell lines. Overexpression of miR-409-3p inhibited glioma cell invasion and proliferation, whereas suppression of miR-409-3p promoted glioma cell invasion and proliferation. High-mobility group nucleosome-binding domain 5 (HMGN5), a well-known oncogene in gliomas, was identified as a functional target of miR-409-3p using bioinformatics, dual-luciferase reporter assay, real-time quantitative polymerase chain reaction, and Western blot analysis. Furthermore, miR-409-3p was found to regulate the expression of matrix metalloproteinase 2 and cyclin D1. Restoration of HMGN5 expression significantly reversed the inhibitory effects of miR-409-3p overexpression on glioma cell invasion and proliferation. Taken together, our results suggest that miR-409-3p inhibits glioma cell invasion and proliferation by targeting HMGN5, representing a potential therapeutic target for glioma.
Key words: Glioma, High-mobility group nucleosome-binding domain 5 (HMGN5), miR-409-3p, Invasion, Proliferation
INTRODUCTION
Glioma is one of the most common brain tumors and the leading cause of cancer-related death associated with the central nervous system1,2. Despite advances in cancer therapy over the past decades, glioma-related mortality remains high3. Improving the survival rate of glioma patients remains a challenge for clinical medicine. Efforts have been made to explore novel therapeutic targets in order to improve the diagnosis, prognosis, and treatment of glioma4. However, the molecular pathogenesis of glioma remains largely unknown.
MicroRNAs (miRNAs) are small, noncoding RNAs with a length of ∼22 nucleotides, which are emerging as novel regulators of gene expression5,6. miRNAs inhibit translation by binding to the 3′-untranslated region (3′-UTR) of mRNAs5,6. Thus, miRNAs are involved in many cellular biological processes, including cell proliferation, apoptosis, differentiation, migration, and invasion7. A growing body of evidence has shown that aberrantly expressed miRNAs are associated with cancer development and progression8,9; consequently, miRNAs have been investigated as potential therapeutic targets for the development of cancer therapies10,11. Studies have also shown that miRNAs play an essential role in glioma development and progression12,13. Target-specific miRNAs represent a promising approach for inhibiting glioma cell progression14–16. Therefore, a better understanding of the role of miRNAs in glioma progression will provide novel biomarkers for diagnosis and prognosis, as well as for identifying novel targets for treatment.
High-mobility group nucleosome-binding domain 5 (HMGN5), also known as nucleosome-binding protein 1, has been identified as an oncogene in many human cancers17. HMGN5 is a ubiquitous protein that plays a key role in DNA repair, replication, recombination, and transcription18–20. However, dysregulation of HMGN5 is associated with tumorigenesis17. A high expression of HMGN5 has been reported in numerous cancer types, including renal cell carcinoma21, prostate cancer22, and bladder cancer23. HMGN5 participates in tumorigenesis by regulating a series of tumor-associated genes, including those that encode matrix metalloproteinase (MMP) 2/9, cyclin B1, cyclin D1, and Bcl-221. Studies have also shown that HMGN5 is highly expressed in glioma tissue and is associated with glioma cell proliferation24. Therefore, HMGN5 may be a promising target for the treatment of glioma.
Aberrant expression of miR-409-3p has been reported in many human cancers25,26. A recent study suggested that miR-409-3p may be involved in glioma progression27. However, the clinical significance and function of miR-409-3p in glioma are not entirely clear. Thus, this study focused on investigating the association between miR-409-3p and glioma. We found that miR-409-3p was downregulated in glioma tissue and cell lines. Overexpression of miR-409-3p inhibited glioma cell invasion and proliferation. HMGN5 was identified as a functional target of miR-409-3p in glioma cells. Furthermore, miR-409-3p was found to regulate the expression of MMP2 and cyclin D1, which are associated with cancer metastasis and malignancy. Taken together, our results suggest that miR-409-3p inhibits glioma cell invasion and proliferation by targeting HMGN5. These findings provide novel insight into our understanding of the molecular pathogenesis of glioma and suggest a potential therapeutic target.
MATERIALS AND METHODS
Clinical Samples and Cell Lines
Glioma tissue samples were obtained from 20 glioma patients who underwent surgery in Tangdu Hospital. Normal brain tissue samples were obtained from patients undergoing internal decompression surgery, which served as the control samples. All samples were collected after receiving the patients’ informed consent and following approval from the Institutional Human Experiment and Ethics Committee of Tangdu Hospital. Experiments were carried out in accordance with the Helsinki Declaration. Glioma cell lines (A172, SHG44, U251, and U87) and normal human astrocytes (NHAs) were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China). Cells were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from clinical samples and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) or mirVana™ miRNA isolation kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturers’ instructions. To detect mRNA expression, cDNA was obtained using M-MLV reverse transcriptase (BioTeke, Beijing, P.R. China). To detect miRNA expression, cDNA was obtained using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). RT-qPCR was performed with SYBR Green PCR Master Mix (Applied Biosystems) with specific primers. GAPDH served as the internal control for the normalization of mRNA expression, and U6 served as the internal control for normalization of miRNA expression. Data were analyzed using the 2−ΔΔCt method. The primer sequences were as follows: HMGN5, 5′-GGTTGTCTGCTATGCTTGTG-3′ (forward) and 5′-ACTGCTTCTTGCTTGGTTTC-3′ (reverse); cyclin D1, 5′-GCTGCGAAGTGGAAACCATC-3′ (forward) and 5′-CCTCCTTCTGCACACATTTGAA-3′ (reverse); MMP2, 5′-AGGCCAAGTGGTCCGTGTGA-3′ (forward) and 5′-TAGGTGGTGGAGCACCAGAG-3′ (reverse); GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse); miR-409-3p, 5′-GAATGTTGCTCGGTGAACCCCT-3′ (forward) and 5′-TGGTGTCGTGGAGTCG-3′ (reverse); U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse).
Cell Transfection
The miR-409-3p mimics, inhibitor, and negative control (NC) were purchased from GenePharma (Shanghai, P.R. China). Cells were grown to 80% confluence prior to transfection. Transfection of the miR-409-3p mimics or inhibitor into glioma cells was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. HMGN5 was cloned into pcDNA vector (Invitrogen), and then pcDNA/HMGN5 vector was transfected into cells using Lipofectamine 2000 (Invitrogen). An empty vector served as the control. At 48 h posttransfection, transfection efficacy was measured by RT-qPCR or Western blot.
Cell Invasion Assay
The Transwell inserts were coated with 1 mg/ml Matrigel matrix (BD Biosciences, San Jose, CA, USA). After 48 h of transfection, cells (1 × 104) that had been suspended in 0.5 ml of medium without FBS were added to the inserts, and 0.5 ml of medium containing 10% FBS was added to the lower chamber. Cells were allowed to grow for 24 h at 37°C. The cells that remained on the upper surface of the membrane were discarded, and cells that had invaded the lower surface of the inserts were fixed and stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA). Cells were counted under a light microscope.
Water-Soluble Tetrazolium Salt (WST) Assay
Cells were seeded onto a 96-well plate (1 × 104 cells/well) and cultured overnight. After transfection of the miR-409-3p mimics or inhibitor for 48 h, cells were treated with 10 μl of cell counting kit solution (Sigma-Aldrich) and incubated for 4 h. The absorbance at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Colony Formation Assay
The transfected cells were plated into six-well plates at a density of 100 cells/well and grown in 0.4% agar medium for 14 days at 37°C. The colonies were then visualized with 0.1% crystal violet (Sigma-Aldrich) and counted under a microscope.
Cell Cycle Assay
Cells were serum starved for 24 h to synchronize the cell cycle before transfection. After 48 h of transfection, cells were harvested with trypsin, washed with phosphate-buffered saline, and fixed with 70% ethanol. Afterward, cells were treated with 100 μg/ml of propidium iodide (Sigma-Aldrich) in the presence of 10 μg/ml of RNase A and incubated for 30 min in the dark. Cell distribution was then detected using a FACSCalibur flow cytometer (BD Biosciences) and analyzed with CellQuest software.
Dual-Luciferase Reporter Assays
The HMGN5 3′-UTR, containing the seed-matched or mutated sequences of miR-409-3p, was cloned into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA). Cells were plated into 24-well plates and transfected with the miR-409-3p mimics or inhibitor, in addition to the luciferase reporter. Cells were harvested after 48 h of transfection and then detected using the Dual-GLO Luciferase Assay Kit (Promega).
Western Blot Analysis
Equivalent quantities (30 μg) of protein were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred to a polyvinylidene fluoride membrane (Millipore, Boston, MA, USA). The membrane was blocked with 5% nonfat milk, followed by incubation with primary antibodies (anti-HMGN5 and anti-GAPDH; Abcam, Cambridge, UK) at appropriate concentrations overnight at 4°C. The membrane was then incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:2,000 dilution for 1 h. Protein bands were visualized using a Pierce ECL Western Blotting Kit (Pierce, Rockford, IL, USA). The protein band intensity was determined using the Image-Pro Plus 6.0 software (Media Cybernetics Inc., Rockville, MD, USA).
Data Analysis
Results are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) with Student’s t-test or one-way analysis of variance, followed by Bonferroni’s post hoc test. A value of p < 0.05 was considered statistically significant.
RESULTS
Decreased Expression of miR-409-3p in Glioma Tissues and Cell Lines
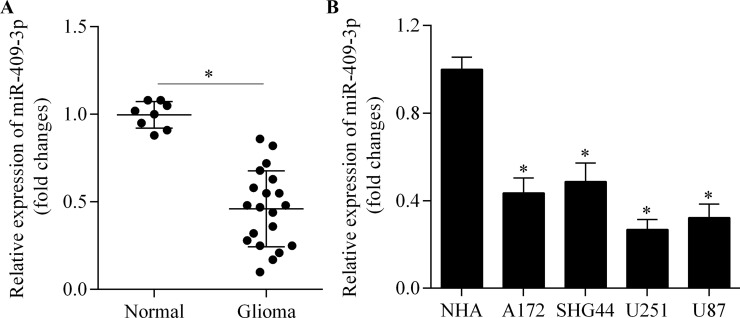
To investigate miR-409-3p expression in glioma, we first detected the expression pattern of miR-409-3p in glioma tissue by RT-qPCR. miR-409-3p showed a lower expression in glioma tissue compared with normal brain tissue (Fig. 1A). We further investigated miR-409-3p expression in a series of human glioma cell lines. Consistent with the results in brain tissue, miR-409-3p was significantly downregulated in the glioma cell lines compared with normal human astrocytes (Fig. 1B). Our data suggest that miR-409-3p may function as a tumor suppressor in glioma.
Figure 1.
Expression of miR-409-3p in glioma tissue and cell lines. (A) miR-409-3p expression in glioma tissue (n = 20) and normal brain tissue (n = 8) samples, as determined by real-time quantitative polymerase chain reaction (RT-qPCR). *p < 0.05. (B) miR-409-3p expression in A172, SHG44, U251, and U87 cells and normal human astrocytes (NHAs), as determined by RT-qPCR. *p < 0.05 versus NHA.
miR-409-3p Suppresses Glioma Cell Invasion
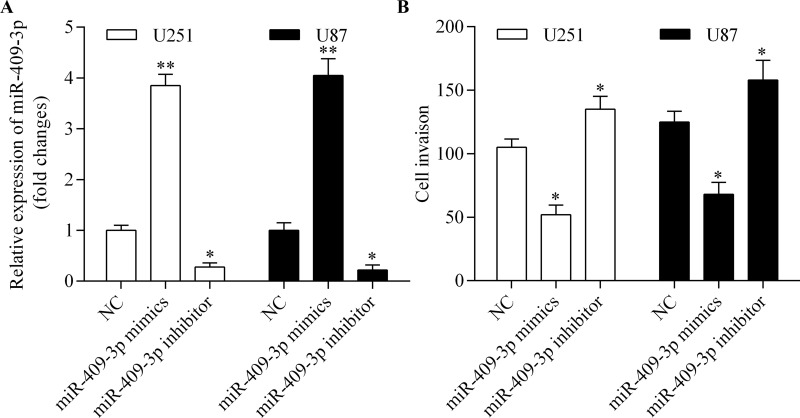
To investigate the antitumor activity of miR-409-3p in glioma, we performed gain-of-function and loss-of-function experiments using miR-409-3p mimics and inhibitor, respectively, in U251 and U87 cells (Fig. 2A). We then detected the role of miR-409-3p in the regulation of glioma cell invasion using a Transwell invasion assay. The results showed that overexpression of miR-409-3p significantly inhibited glioma cell invasion, while suppression of miR-409-3p promoted glioma cell invasion (Fig. 2B). These results suggest that miR-409-3p inhibits glioma cell invasion.
Figure 2.
miR-409-3p suppresses glioma cell invasion. U251 and U87 cells were transfected with miR-409-3p mimics or inhibitor for 48 h. (A) Expression of miR-409-3p measured by RT-qPCR. (B) Cell invasion measured using a Transwell invasion assay. *p < 0.05 and **p < 0.01 versus negative control (NC).
miR-409-3p Inhibits Glioma Cell Proliferation
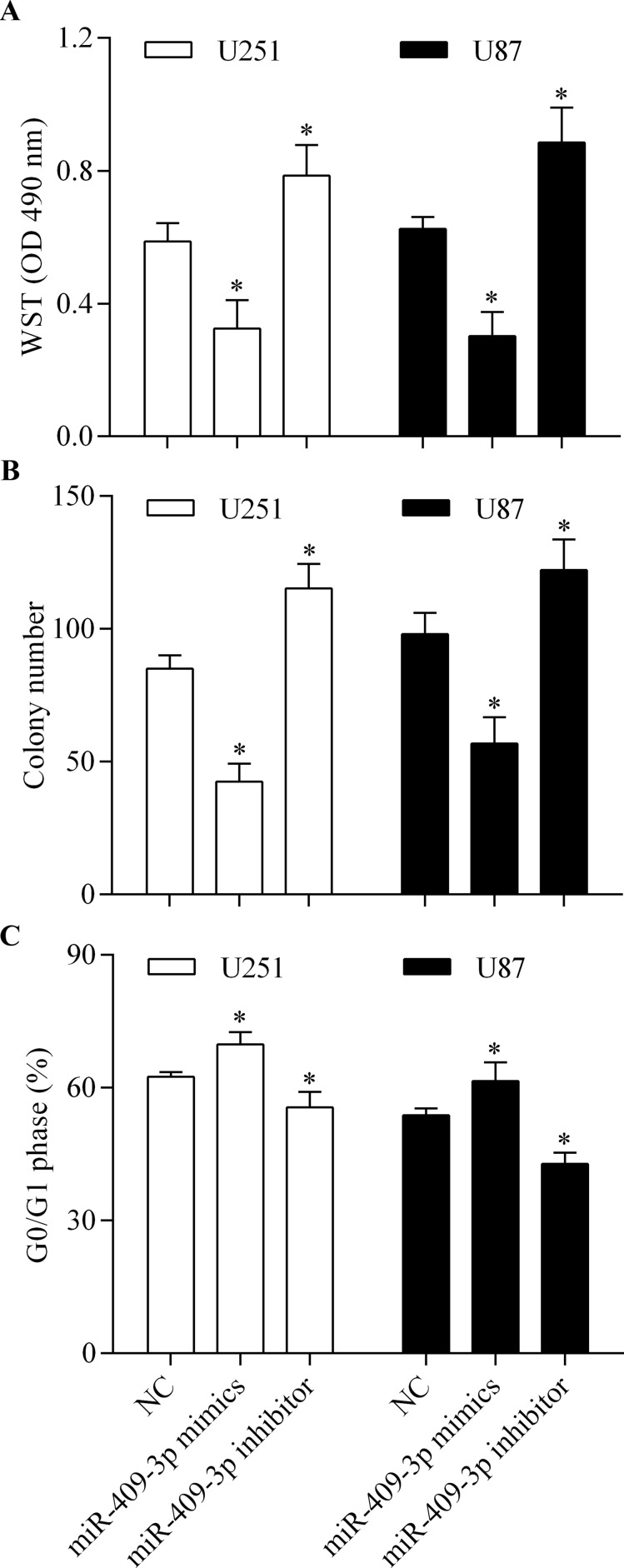
To further investigate the role of miR-409-3p in glioma, we examined the role of miR-409-3p in the regulation of glioma cell proliferation. The results showed that overexpression of miR-409-3p markedly inhibited cell proliferation (Fig. 3A) and colony formation (Fig. 3B) of glioma cells and induced cell cycle arrest (Fig. 3C) in the G0/G1 phase. In contrast, suppression of miR-409-3p showed the opposite effects. Collectively, these data suggest that miR-409-3p suppresses glioma cell proliferation.
Figure 3.
miR-409-3p suppresses glioma cell proliferation. U251 and U87 cells were transfected with miR-409-3p mimics or inhibitor for 48 h and then used for detection. (A) Cell proliferation detected by water-soluble tetrazolium salt (WST) assay. (B) Colony-forming capacity of glioma cells assessed using a colony formation assay. (C) Number of cells in the G0/G1 phase, detected by flow cytometry. *p < 0.05 versus NC.
HMGN5 Is a Target Gene of miR-409-3p in Glioma Cells
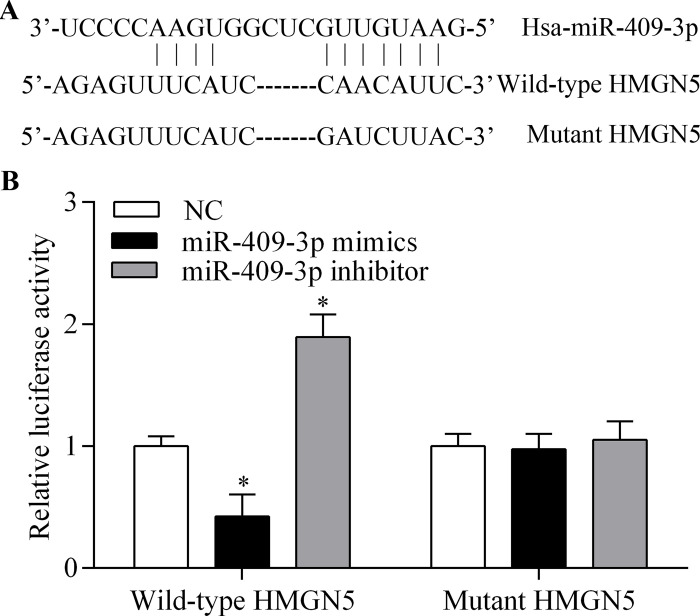
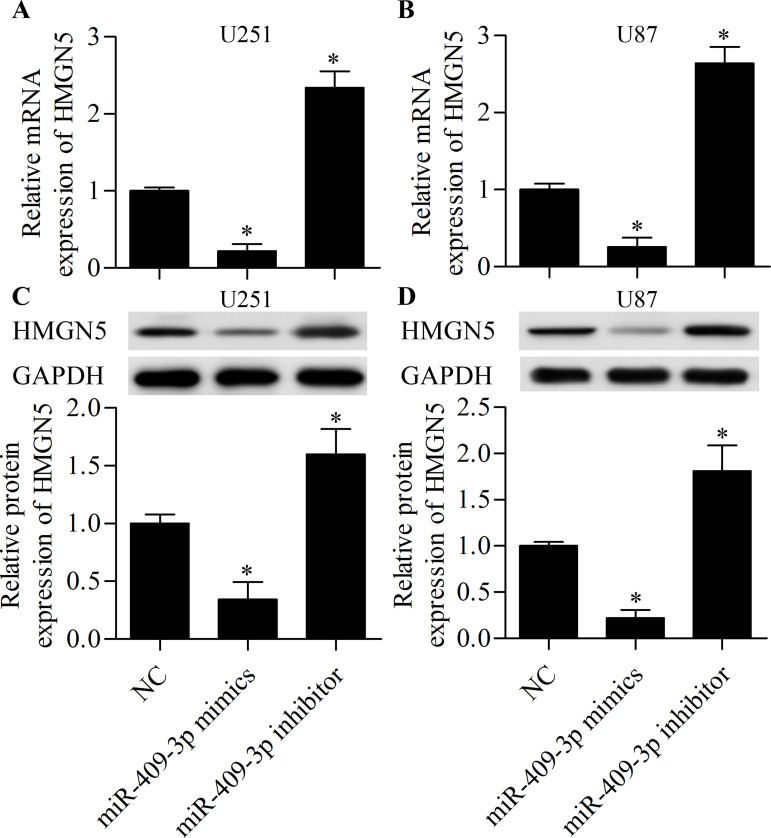
To investigate the underlying mechanism by which miR-409-3p inhibits glioma cell invasion and proliferation, we performed bioinformatics analysis to identify potential targets of miR-409-3p. We found that HMGN5 was a putative target gene of miR-409-3p. The predicted seed-matched sequences are presented in Figure 4A. To confirm binding of miR-409-3p with the HMGN5 3′-UTR, we carried out dual-luciferase assays. The results showed that the luciferase activity of the reporter vector containing the wild-type HMGN5 3′-UTR was markedly decreased as a result of miR-409-3p overexpression (Fig. 4B). Furthermore, suppression of miR-409-3p enhanced the luciferase activity of the reporter vector containing the wild-type HMGN5 3′-UTR (Fig. 4B). However, these effects were abolished by mutation of the binding sites (Fig. 4B), indicating that miR-409-3p directly targets the 3′-UTR of HMGN5. To further confirm that HMGN5 is the target gene of miR-409-3p, we examined the regulatory effect of miR-409-3p on HMGN5. We found that overexpression of miR-409-3p significantly inhibited the mRNA (Fig. 5A and B) and protein (Fig. 5C and D) expression of HMGN5 in glioma cells, whereas suppression of miR-409-3p promoted HMGN5 expression. Overall, these results indicate that HMGN5 is a direct target gene of miR-409-3p.
Figure 4.
miR-409-3p targets the 3′-untranslated region (3′-UTR) of high-mobility group nucleosome binding domain 5 (HMGN5). (A) Schematic of the miR-409-3p-binding sites in the HMGN5 3′-UTR. (B) Dual-luciferase assays of miR-409-3p and HMGN5 3′-UTR. Wild-type or mutated 3′-UTR of HMGN5 was cloned into luciferase reporter vectors. U251 cells were transfected with miR-409-3p mimics or inhibitor in addition to the luciferase reporter vector and incubated for 48 h. *p < 0.05 versus NC.
Figure 5.
miR-409-3p suppresses HMGN5 expression. U251 and U87 cells were transfected with miR-409-3p mimics or inhibitor for 48 h prior to detection. The mRNA expression of HMGN5 in U251 (A) and U87 (B) cells as detected by RT-qPCR. The protein expression of HMGN5 in U251 (C) and U87 (D) cells as detected by Western blot. *p < 0.05 versus NC.
miR-409-3p Inhibits the Expression of MMP2 and Cyclin D1
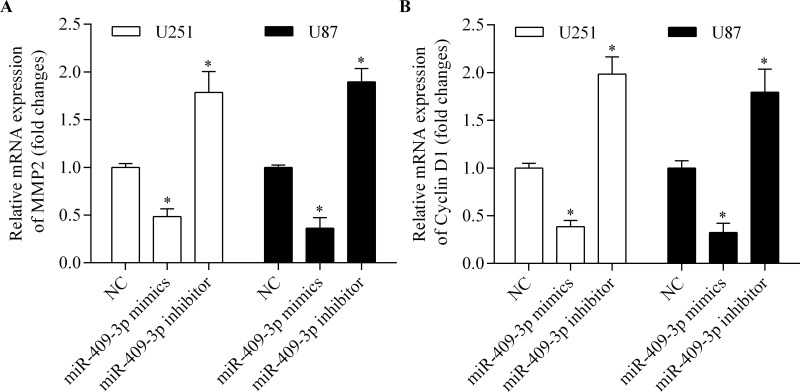
To further investigate the molecular basis of miR-409-3p in the regulation of glioma cell invasion and proliferation, we measured the expression of MMP2 and cyclin D1, both of which are associated with cancer cell metastasis and proliferation. The results showed that overexpression of miR-409-3p significantly suppressed the mRNA expression of MMP2 (Fig. 6A) and cyclin D1 (Fig. 6B) in glioma cells. In contrast, suppression of miR-409-3p markedly promoted the expression of MMP2 (Fig. 6A) and cyclin D1 (Fig. 6B). These results indicate that miR-409-3p inhibits glioma cell invasion and proliferation, which are associated with suppression of cyclin D1 and MMP2.
Figure 6.
miR-409-3p inhibits the expression of matrix metalloproteinase 2 (MMP2) and cyclin D1. U251 and U87 cells were transfected with miR-409-3p mimics or inhibitor for 48 h prior to detection. The mRNA expression of MMP2 (A) and cyclin D1 (B) in cells as detected by RT-qPCR. *p < 0.05 versus NC.
Restoration of HMGN5 Expression Abrogates Antitumor Effects of miR-409-3p
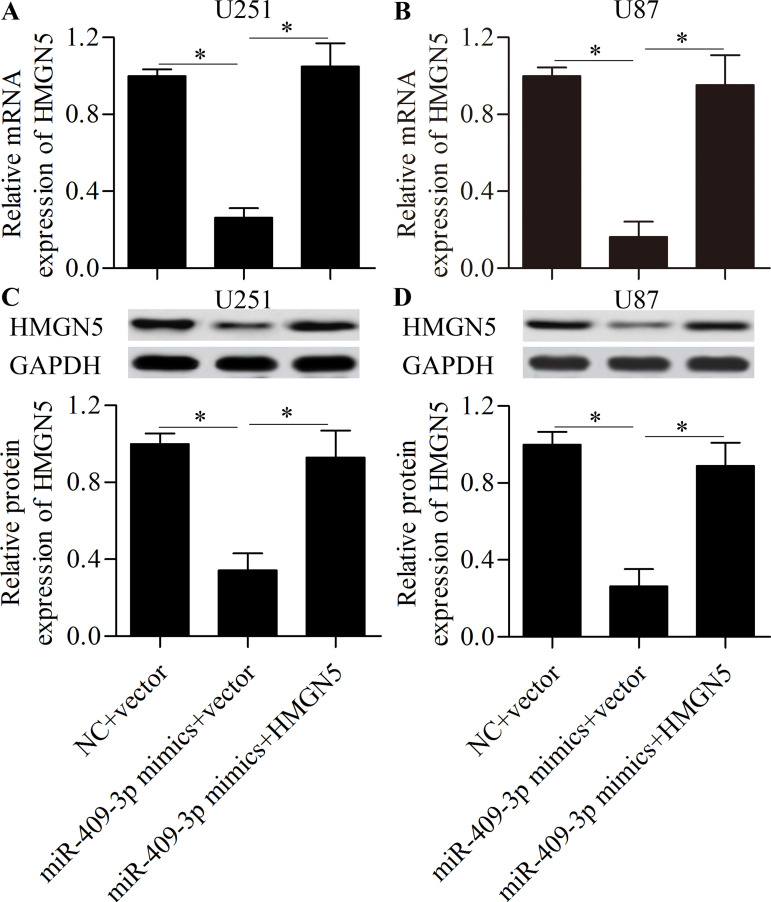
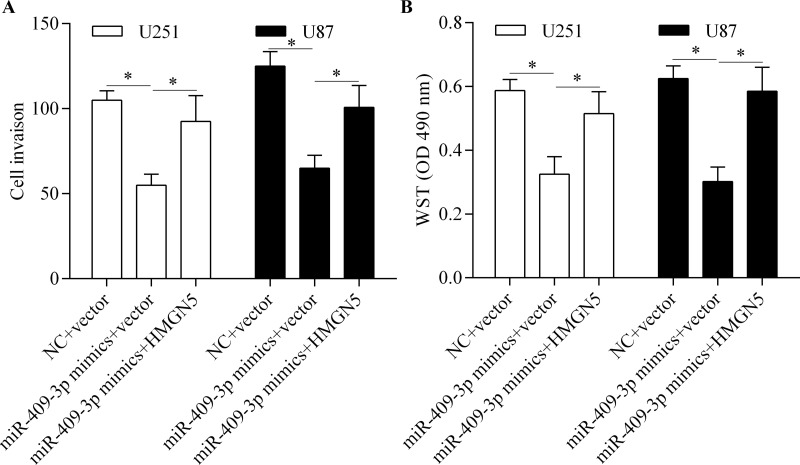
To investigate whether miR-409-3p inhibits glioma cell invasion and proliferation by targeting HMGN5, we performed rescue experiments by overexpressing HMGN5. Glioma cells were transfected with miR-409-3p mimics along with the pcDNA/HMGN5 vector without the 3′-UTR. The results showed that transfection of the pcDNA/HMGN5 vector significantly restored HMGN5 expression (Fig. 7A–D). We then detected the effect of restoring HMGN5 expression on miR-409-3p-mediated cell invasion and proliferation. The results showed that restoration of HMGN5 expression significantly reversed the inhibitory effect of miR-409-3p overexpression on glioma cell invasion (Fig. 8A) and proliferation (Fig. 8B). Taken together, these results suggest that miR-409-3p inhibits glioma cell invasion and proliferation by regulating HMGN5.
Figure 7.
Restoration of HMGN5 expression in miR-409-3p-transfected glioma cells. U251 and U87 cells were transfected with miR-409-3p mimics along with the pcDNA/HMGN5 vector without the 3′-UTR and incubated for 48 h. The mRNA expression of HMGN5 in U251 (A) and U87 (B) cells as detected by RT-qPCR. The protein expression of HMGN5 in U251 (C) and U87 (D) cells as detected by Western blot. *p < 0.05.
Figure 8.
Restoration of HMGN5 expression abrogates antitumor effects of miR-409-3p. U251 and U87 cells were transfected with miR-409-3p mimics along with the pcDNA/HMGN5 vector without the 3′-UTR and incubated for 48 h. (A) Cell invasion detected by the Transwell assay. (B) Cell proliferation assessed by the WST assay. *p < 0.05.
DISCUSSION
In recent years, accumulating evidence has confirmed the critical role of miRNAs in glioma28,29. Although the miRNA signatures in glioma have been well characterized, the role of dysregulated miRNAs on glioma progression and development remains largely unknown. A recent study reported that miR-409-3p expression may predict the expression of O 6-methylguanine-DNA methyltransferase in glioma patients undergoing alkylating chemotherapy27. However, the clinical significance and function of miR-409-3p in glioma were not entirely clear. In the present study, we identified miR-409-3p as a novel dysregulated miRNA in glioma. We found that miR-409-3p was decreased in glioma tissue and cell lines, and overexpression of miR-409-3p inhibited glioma cell invasion and proliferation, indicating a tumor suppressor role of miR-409-3p in glioma.
Previous studies have shown that miR-409-3p is dysregulated in many types of human cancers, where it participates in tumor development and progression25,26. Low expression of miR-409-3p has been reported in breast cancer specimens, associated with an advanced TNM stage, lymph node metastasis, and poorer pathological differentiation30. Venkatadri et al. reported that miR-409-3p functions as an apoptosis-related miRNA in resveratrol-induced breast cancer cell death31. Other studies have shown that miR-409-3p inhibits breast cancer cell growth and metastasis by targeting zinc-finger E-box-binding homeobox 126 and Akt132. The expression of miR-409-3p was found to be downregulated in colorectal cancer tissue, and overexpression of miR-409-3p suppresses cell proliferation, migration, and invasion of colorectal cancer cells by targeting GRB2-associated-binding protein 125 and nemo-like kinase33. Moreover, miR-409-3p sensitizes colon cancer cells to oxaliplatin-induced cell death by suppressing Beclin-1-mediated autophagy34. It has also been reported that miR-409-3p inhibits gastric cancer cell growth and metastasis by targeting PHD-finger protein 1035 and radixin36 and that overexpression of miR-409-3p suppresses osteosarcoma cell migration and invasion by inhibiting catenin-δ137. Furthermore, miR-409-3p inhibits the progression and development of fibrosarcoma38, lung adenocarcinoma39, and bladder cancer40 by targeting different oncogenes. These reports suggest that miR-409-3p functions as a tumor suppressor; however, an oncogenic role of miR-409-3p has also been reported. Josson et al. reported a high expression of miR-409-3p in human prostate cancer tissue and bone metastatic prostate cancer cell lines41, and that overexpression of miR-409-3p promotes tumorigenesis and metastasis of prostate cancer41. Thus, the precise role of miR-409-3p needs to be further investigated. In the current study, we observed a low expression pattern of miR-409-3p in glioma tissue and cell lines. Overexpression of miR-409-3p suppressed glioma cell invasion and proliferation, supporting a tumor-suppressor role for miR-409-3p.
In this study, we identified HMGN5 as a potential target gene of miR-409-3p in glioma cells. HMGN5 is a well-known oncogene, recognized in many types of cancers17. The silencing of HMGN5 in prostate cancer cells has been shown to suppress cell proliferation and promote cell apoptosis22,42. Furthermore, a high expression of HMGN5 has been reported in clear cell renal cell carcinoma tissue, and knockdown of HMGN5 was found to induce cell cycle arrest and apoptosis, also inhibiting cell invasion21. HMGN5 is highly expressed in breast cancer and promotes breast cancer cell proliferation and invasion43. A high expression of HMGN5 has also been reported in bladder cancer23,44, osteosarcoma45, and lung cancer38, also promoting tumorigenesis through cell proliferation and metastasis. The molecular basis of HMGN5 in tumorigenesis is associated with the regulation of various genes, including cyclin B1, cyclin D1, Bcl-2, MMP2/9, and vascular endothelial growth factor, which regulates cell cycle, proliferation, apoptosis, metastasis, and angiogenesis21–23,42,44,45. Moreover, HMGN5 promotes chemotherapy and radiotherapy resistance of prostate cancer46,47 and osteosarcoma48 cells. A high expression of HMGN5 has been reported in glioma tissue24, and silencing of HMGN5 was found to induce cell cycle arrest in the G0/G1 phase, in addition to delaying cell proliferation and promoting apoptosis of glioma cells24, indicating an oncogenic role for HMGN5 in glioma. In this study, we identified HMGN5 as a direct target gene of miR-409-3p in glioma cells. Suppression of HMGN5 by overexpression of miR-409-3p markedly repressed the invasion and proliferation of glioma cells, associated with the inhibition of cyclin D1 and MMP2 expression. Our results suggest that miR-409-3p/HMGN5 plays an important role in glioma tumorigenesis.
HMGN5 has been found to be regulated by several miRNAs, including miR-32649, miR-34050, and miR-18651, and suppression of HMGN5 by these miRNAs significantly represses the proliferation and metastasis of cancer cells49–51. These studies suggest that HMGN5-related tumor progression is epigenetically regulated by miRNAs. However, it was still unclear whether HMGN5 undergoes epigenetic regulation by miRNAs in glioma. Our study revealed that miR-409-3p specifically targeted and modulated HMGN5 in glioma cells, suggesting that this miRNA is a novel inhibitor of HMGN5. Therefore, targeting HMGN5 by miR-409-3p may show promise for the treatment of glioma.
Overall, our study reported a tumor-suppressor role for miR-409-3p in glioma. We observed decreased miR-409-3p expression in glioma, and overexpression of miR-409-3p repressed glioma cell invasion and proliferation by targeting HMGN5, an oncogene associated with glioma24. These findings add to our understanding of the molecular pathogenesis of glioma and suggest a potential therapeutic approach for the treatment of glioma by inhibiting HMGN5 with miR-409-3p.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Westphal M, Lamszus K. The neurobiology of gliomas: From cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495–508. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 4. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 6. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. [DOI] [PubMed] [Google Scholar]
- 7. Manikandan J, Aarthi JJ, Kumar SD, Pushparaj PN. Oncomirs: The potential role of non-coding microRNAs in understanding cancer. Bioinformation 2008;2:330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nohata N, Goto Y, Gutkind JS. Onco-GPCR signaling and dysregulated expression of microRNAs in human cancer. J Hum Genet. 2017;62:87–96. [DOI] [PubMed] [Google Scholar]
- 9. Liu HT, Gao P. The roles of microRNAs related with progression and metastasis in human cancers. Tumour Biol. 2016;37:15383–97. [DOI] [PubMed] [Google Scholar]
- 10. Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC, Jaggi M. miRNA nanotherapeutics for cancer. Drug Discov Today 2017;22:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer—An emerging concept. EBioMedicine 2016;12:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Dutta A, Abounader R. The role of microRNAs in glioma initiation and progression. Front Biosci. (Landmark Ed) 2012;17:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Besse A, Sana J, Fadrus P, Slaby O. MicroRNAs involved in chemo- and radioresistance of high-grade gliomas. Tumour Biol. 2013;34:1969–78. [DOI] [PubMed] [Google Scholar]
- 14. Nawaz Z, Patil V, Paul Y, Hegde AS, Arivazhagan A, Santosh V, Somasundaram K. PI3 kinase pathway regulated miRNome in glioblastoma: Identification of miR-326 as a tumour suppressor miRNA. Mol Cancer 2016;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Yuan J, Yuan X, Zhao J, Zhang Z, Weng L, Liu J. MicroRNA-200b inhibits the growth and metastasis of glioma cells via targeting ZEB2. Int J Oncol. 2016;48:541–50. [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Wang H, Zhu Z, Ye Y, Mao H, Zhang S. MicroRNA-105 targets SOX9 and inhibits human glioma cell progression. FEBS Lett. 2016;590:4329–42. [DOI] [PubMed] [Google Scholar]
- 17. Shi Z, Tang R, Wu D, Sun X. Research advances in HMGN5 and cancer. Tumour Biol. 2016;37:1531–9. [DOI] [PubMed] [Google Scholar]
- 18. Shirakawa H, Landsman D, Postnikov YV, Bustin M. NBP-45, a novel nucleosomal binding protein with a tissue-specific and developmentally regulated expression. J Biol Chem. 2000;275:6368–74. [DOI] [PubMed] [Google Scholar]
- 19. King LM, Francomano CA. Characterization of a human gene encoding nucleosomal binding protein NSBP1. Genomics 2001;71:163–73. [DOI] [PubMed] [Google Scholar]
- 20. Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji SQ, Yao L, Zhang XY, Li XS, Zhou LQ. Knockdown of the nucleosome binding protein 1 inhibits the growth and invasion of clear cell renal cell carcinoma cells in vitro and in vivo. J Exp Clin Cancer Res. 2012;31:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang N, Zhou LQ, Zhang XY. Downregulation of the nucleosome-binding protein 1 (NSBP1) gene can inhibit the in vitro and in vivo proliferation of prostate cancer cells. Asian J Androl. 2010;12:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wahafu W, He ZS, Zhang XY, Zhang CJ, Yao K, Hao H, Song G, He Q, Li XS, Zhou LQ. The nucleosome binding protein NSBP1 is highly expressed in human bladder cancer and promotes the proliferation and invasion of bladder cancer cells. Tumour Biol. 2011;32:931–9. [DOI] [PubMed] [Google Scholar]
- 24. Qu J, Yan R, Chen J, Xu T, Zhou J, Wang M, Chen C, Yan Y, Lu Y. HMGN5: A potential oncogene in gliomas. J Neurooncol. 2011;104:729–36. [DOI] [PubMed] [Google Scholar]
- 25. Bai R, Weng C, Dong H, Li S, Chen G, Xu Z. MicroRNA-409-3p suppresses colorectal cancer invasion and metastasis partly by targeting GAB1 expression. Int J Cancer 2015;137:2310–22. [DOI] [PubMed] [Google Scholar]
- 26. Ma Z, Li Y, Xu J, Ren Q, Yao J, Tian X. MicroRNA-409-3p regulates cell invasion and metastasis by targeting ZEB1 in breast cancer. IUBMB Life 2016;68:394–402. [DOI] [PubMed] [Google Scholar]
- 27. Khalil S, Fabbri E, Santangelo A, Bezzerri V, Cantu C, Di Gennaro G, Finotti A, Ghimenton C, Eccher A, Dechecchi M, Scarpa A, Hirshman B, Chen C, Ferracin M, Negrini M, Gambari R, Cabrini G. miRNA array screening reveals cooperative MGMT-regulation between miR-181d-5p and miR-409-3p in glioblastoma. Oncotarget 2016;7:28195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hummel R, Maurer J, Haier J. MicroRNAs in brain tumors: A new diagnostic and therapeutic perspective? Mol Neurobiol. 2011;44:223–34. [DOI] [PubMed] [Google Scholar]
- 29. Palumbo S, Miracco C, Pirtoli L, Comincini S. Emerging roles of microRNA in modulating cell-death processes in malignant glioma. J Cell Physiol. 2014;229:277–86. [DOI] [PubMed] [Google Scholar]
- 30. Cao GH, Sun XL, Wu F, Chen WF, Li JQ, Hu WC. Low expression of miR-409-3p is a prognostic marker for breast cancer. Eur Rev Med Pharmacol Sci. 2016;20:3825–9. [PubMed] [Google Scholar]
- 31. Venkatadri R, Muni T, Iyer AK, Yakisich JS, Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016;7:e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang G, Liu Z, Xu H, Yang Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem Biophys Res Commun. 2016;469:189–95. [DOI] [PubMed] [Google Scholar]
- 33. Liu M, Xu A, Yuan X, Zhang Q, Fang T, Wang W, Li C. Downregulation of microRNA-409-3p promotes aggressiveness and metastasis in colorectal cancer: An indication for personalized medicine. J Transl Med. 2015;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan S, Shi H, Ba M, Lin S, Tang H, Zeng X, Zhang X. miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Mol Med. 2016;37:1030–8. [DOI] [PubMed] [Google Scholar]
- 35. Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei M, Ju J, Yu Y, Yan M, Gu Q, Zhu Z, Liu B. MicroRNA-409-3p regulates cell proliferation and apoptosis by targeting PHF10 in gastric cancer. Cancer Lett. 2012;320:189–97. [DOI] [PubMed] [Google Scholar]
- 36. Zheng B, Liang L, Huang S, Zha R, Liu L, Jia D, Tian Q, Wang Q, Wang C, Long Z, Zhou Y, Cao X, Du C, Shi Y, He X. MicroRNA-409 suppresses tumour cell invasion and metastasis by directly targeting radixin in gastric cancers. Oncogene 2012;31:4509–16. [DOI] [PubMed] [Google Scholar]
- 37. Wu S, Du X, Wu M, Du H, Shi X, Zhang T. MicroRNA-409-3p inhibits osteosarcoma cell migration and invasion by targeting catenin-delta1. Gene 2016;584:83–9. [DOI] [PubMed] [Google Scholar]
- 38. Weng C, Dong H, Chen G, Zhai Y, Bai R, Hu H, Lu L, Xu Z. miR-409-3p inhibits HT1080 cell proliferation, vascularization and metastasis by targeting angiogenin. Cancer Lett. 2012;323:171–9. [DOI] [PubMed] [Google Scholar]
- 39. Wan L, Zhu L, Xu J, Lu B, Yang Y, Liu F, Wang Z. MicroRNA-409-3p functions as a tumor suppressor in human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem. 2014;34:1273–90. [DOI] [PubMed] [Google Scholar]
- 40. Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Zhu Y, Li S, Zheng X, Xie L. MicroRNA-409-3p inhibits migration and invasion of bladder cancer cells via targeting c-Met. Mol Cells 2013;36:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Josson S, Gururajan M, Hu P, Shao C, Chu GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, Lichterman J, Nandana S, Li Q, Rogatko A, Berel D, Posadas EM, Fazli L, Sareen D, Chung LW. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res. 2014;20:4636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang XY, Guo ZQ, Ji SQ, Zhang M, Jiang N, Li XS, Zhou LQ. Small interfering RNA targeting HMGN5 induces apoptosis via modulation of a mitochondrial pathway and Bcl-2 family proteins in prostate cancer cells. Asian J Androl. 2012;14:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weng M, Song F, Chen J, Wu J, Qin J, Jin T, Xu J. The high-mobility group nucleosome-binding domain 5 is highly expressed in breast cancer and promotes the proliferation and invasion of breast cancer cells. Tumour Biol. 2015;36:959–66. [DOI] [PubMed] [Google Scholar]
- 44. Gan Y, Tan J, Yang J, Zhou Y, Dai Y, He L, Yao K, Tang Y. Knockdown of HMGN5 suppresses the viability and invasion of human urothelial bladder cancer 5637 cells in vitro and in vivo. Med Oncol. 2015;32:136. [DOI] [PubMed] [Google Scholar]
- 45. Zhou X, Yuan B, Yuan W, Wang C, Gao R, Wang J. The expression and clinical significance of high mobility group nucleosome binding domain 5 in human osteosarcoma. Tumour Biol. 2014;35:6539–47. [DOI] [PubMed] [Google Scholar]
- 46. Guo Z, Zhang X, Li X, Xie F, Su B, Zhang M, Zhou L. Expression of oncogenic HMGN5 increases the sensitivity of prostate cancer cells to gemcitabine. Oncol Rep. 2015;33:1519–25. [DOI] [PubMed] [Google Scholar]
- 47. Su B, Shi B, Tang Y, Guo Z, Yu X, He X, Li X, Gao X, Zhou L. HMGN5 knockdown sensitizes prostate cancer cells to ionizing radiation. Prostate 2015;75:33–44. [DOI] [PubMed] [Google Scholar]
- 48. Yang C, Gao R, Wang J, Yuan W, Wang C, Zhou X. High-mobility group nucleosome-binding domain 5 increases drug resistance in osteosarcoma through upregulating autophagy. Tumour Biol. 2014;35:6357–63. [DOI] [PubMed] [Google Scholar]
- 49. Zhou S, Shen J, Lin S, Liu X, Xu M, Shi L, Wang X, Cai X. Downregulated expression of DIXDC1 in hepatocellular carcinoma and its correlation with prognosis. Tumour Biol. 2016;37:13607–16. [DOI] [PubMed] [Google Scholar]
- 50. Wei P, Qiao B, Li Q, Han X, Zhang H, Huo Q, Sun J. microRNA-340 suppresses tumorigenic potential of prostate cancer cells by targeting high-mobility group nucleosome-binding domain 5. DNA Cell Biol. 2016;35:33–43. [DOI] [PubMed] [Google Scholar]
- 51. Yao K, He L, Gan Y, Zeng Q, Dai Y, Tan J. MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn Pathol. 2015;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]