Abstract
Chemotherapy is typically used to treat choriocarcinoma. However, a small proportion of this malignancy develops resistance to common chemotherapeutic drugs such as methotrexate (MTX) and floxuridine (FUDR). This study aimed to investigate the role and potential mechanisms of chloride intracellular channel protein 1 (CLIC1) in the development of chemoresistance in choriocarcinoma JeG3 cells. Two chemoresistant sublines were induced from their parental cell line JeG3 through intermittent exposure to MTX (named JeG3/MTX) or FUDR (named JeG3/FUDR). It was found that expression of CLIC1 was significantly higher in the chemoresistant sublines JeG3/MTX and JeG3/FUDR than in their parental cell line JeG3. Knockdown of CLIC1 by specific siRNA significantly increased cell sensitivity to MTX and FUDR in vitro and in vivo. Moreover, the high expression of CLIC1 in chemoresistant sublines was associated with upregulation of multidrug resistance-associated protein 1 (MRP1). Knockdown of CLIC1 decreased the expression of MRP1 accordingly. While reexpression of CLIC1 in the parental cell JeG3 increased its resistance to MTX and FUDR, depletion of MRP1 significantly blunted CLIC1 reexpression-mediated acquirement of chemoresistance in JeG3 cells. In conclusion, our results suggest that CLIC1 may serve as a critical mediator of chemoresistance in human choriocarcinoma JeG3 cells. The CLIC1-mediated chemoresistance is achieved through positive regulation of MRP1. Depletion of either CLIC1 or its downstream MRP1 may be a promising therapeutic strategy concerning reversing the chemoresistance in human choriocarcinoma JeG3 cells.
Key words: Chloride intracellular channel protein 1 (CLIC1), Multidrug resistance-associated protein 1 (MRP1), Choriocarcinoma, Chemoresistance, JeG3 cells
INTRODUCTION
Gestational trophoblastic neoplasia (GTN), including invasive moles, choriocarcinoma, epithelioid trophoblastic tumors, and, rarely, placental site trophoblastic tumors, is a term used to describe malignant lesions that originate from the chorionic villi and the extravillous trophoblast1–3. Of these GTNs, choriocarcinoma is a malignant lesion often arising after molar pregnancies. However, it can also occur after any form of gestation, such as miscarriages and term pregnancies. The incidence of choriocarcinoma differs among reports from different regions. Basically, higher occurrences were reported in Asia and Latin America compared to Europe and North American countries4.
Choriocarcinoma represents a large proportion of GTN cases worldwide. Previous opinions hold that despite its malignant property, choriocarcinoma is highly responsive to chemotherapy such as methotrexate (MTX) and floxuridine (FUDR) with an expected cure rate of greater than 90%, making it one of the most exciting successes in the treatment of human solid tumors by chemotherapy5. Current evidence has updated these data and shown that a few tumors acquire resistance to chemotherapy. It is estimated that in low-risk patients, approximately 10%–20% of nonmetastatic and 30%–50% of metastatic patients develop single-agent chemoresistance and thus require combined chemotherapy to achieve complete remission. In high-risk patients, about 20%–30% have an incomplete response to first-line multiagent chemotherapy6–8. The first-line chemotherapy MTX, for example, has witnessed approximately 30% drug resistance6. Chemoresistance has also been reported in other drugs such as FUDR, etoposide (VP), and actinomycin-D (KSM), among others. Therefore, chemoresistance is the primary cause for treatment failure in human choriocarcinoma.
Recently, multidrug resistance-associated proteins (MRPs) have been identified. Among these MRPs, MRP1 was first cloned and highly expressed in a doxorubicin-selected multidrug-resistant human lung carcinoma cell line H69AR9. The mechanism of MRP1 function is mainly the reduction of intracellular drug accumulation, in which ATP-binding cassette (ABC) transporters expressed in the plasma membrane are notorious mediators10. Because of this property, MRP1 confers resistance to not only doxorubicin but also many other common antineoplastic drugs such as MTX, VP, daunorubicin, and vincristine11. Hence, MRP1 has emerged as a key contributor to chemoresistance.
Chloride intracellular channel protein 1 (CLIC1) is a novel biomarker that has aroused researchers’ interests because of its extensive role in the development and progression of cancer. Primarily, CLIC1 has been reported to promote tumor metastasis in a wide range of cancer types, including gastric12,13, ovarian14, hepatocellular15,16, and colon17, among others. CLIC1 was also proposed as one oncogene in pancreatic cancer18. Of specific interest, a recent two-dimensional gel electrophoresis-based proteomic approach revealed that CLIC1 was a candidate prognostic biomarker for hydatidiform moles malignantly transforming into choriocarcinoma19. The high expression of CLIC1 was, for the first time, observed in choriocarcinoma. This report suggested that CLIC1 might also play a critical role in choriocarcinoma19.
The present study aimed to investigate whether CLIC1 had any role in the development of chemoresistance in human choriocarcinoma cell line JeG3. To this end, two sublines, JeG3/MTX and JeG3/FUDR, were induced from their parental cell line JeG3 through intermittent exposure to MTX or FUDR. Expression of CLIC1 was initially examined in the drug-resistant sublines JeG3/MTX and JeG3/FUDR. Furthermore, the role of CLIC1 in chemoresistance to MTX and FUDR was explored in vitro and in vivo. Finally, the potential mechanism underlying CLIC1-mediated chemoresistance was investigated with a specific focus on whether it had any regulative role in MRP1 expression.
MATERIALS AND METHODS
Cells and Reagents
The chemosensitive parental cell line JeG3 was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were grown in high-glucose RPMI-1640 (Invitrogen, Shanghai, P.R. China) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. Two chemoresistance sublines, JeG3/MTX and JeG3/FUDR, were induced from their parental JeG3 cells through intermittent exposure to MTX (namely, JeG3/MTX) or FUDR (namely, JeG3/FUDR) based on published protocols20,21. The sublines were maintained using the same method as their parental JeG3 cells.
For knockdown of CLIC1, a specific shRNA targeting CLIC1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Meanwhile, for the confirmation of CLIC1 knockdown, we also synthesized another shRNA against CLIC1 with the sequences 5′-GATGATGAGGAGATCGAGCTC-3′, which was confirmed to be effective22. For knockdown of MRP1, a specific siRNA was used based on published sequences23. These sequences were confirmed to be specific against MRP1. A scramble siRNA (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAATT-3′) was also synthesized. All sequences were synthesized by GenePharma Co., Ltd. (Shanghai, P.R. China). The expression plasmid of CLIC1 was purchased from Addgene (Cambridge, MA, USA). Primary antibodies as well as corresponding secondary antibodies were purchased from Santa Cruz Biotechnology.
Quantitative Real-Time PCR (qRT-PCR)
Total RNAs from cell culture were extracted using the RNeasy Plus Mini Kit (Cat. No. 74134) (Qiagen, Shanghai, P.R. China). RNAs were immediately converted into cDNAs using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. qRT-PCR was performed using the SYBR Premix Ex Taq Perfect Real Time (TaKaRa, Shiga, Japan). The primers for CLIC1 were as follows: forward, 5′-AGAGGCTGAATGAGCTGGAG-3′; reverse, 5′-TGGCAATTTTGGTCCATTTT-3′.
All values were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data were obtained from three independent assays with each assay in triplicate.
Western Blot Assays
Cells were seeded in six-well plates and allowed to grow up to 80% confluence. After the indicated treatments, total cell proteins were extracted and quantified. An equal amount of 40 μg of protein extract was loaded to each lane on a 12% SDS-PAGE gel. The proteins were then transferred to a nitrocellulose membrane (GE Healthcare, San Francisco, CA, USA). The membranes were later blocked in Tris-buffered saline (TBS) with 5% skim milk for 1 h and then incubated in the corresponding primary antibodies overnight. The membranes were then incubated with secondary antibodies at room temperature for 1 h. Immunoreactivity was detected using an ECL chemiluminescent substrate (Amersham Pharmacia Biotech, Piscataway, NJ, USA). GAPDH protein was synchronously detected as a loading control.
Cell Viability Determination
To assess cell viability under each condition, a cell counting kit-8 (CCK-8; Dojindo, Japan) assay was conducted. Briefly, cells with distinct treatments were trypsinized and seeded at an initial density of 3,000 cells/well in 96-well plates. After incubation for 24 h, cells were treated with MTX or FUDR as indicated. For each monitored day, a 10-μl CCK-8 solution was added to each well for 4 h at 37°C. Absorbance was then determined using a Multiskan plate reader at 490 nm. This assay was consecutively monitored for 5 days.
Xenograft Model of Choriocarcinoma
To assess the effects of CLIC1 knockdown on cell sensitivity to drugs (MTX or FUDR) in vivo, a xenograft model of human choriocarcinoma was established using the induced chemoresistant JeG3/MTX cells and JeG3/FUDR cells. JeG3/MTX cells and JeG3/FUDR cells that were stably depleted of CLIC1 were initially selected and collected. A total of 40 5-week-old athymic nude mice (BALB/cnu/nu) were used for the experiment. These mice were randomly assigned into four groups, with the former two groups treated with MTX and the latter two groups with FUDR. For the former two groups, an equal amount of 1 × 106 JeG3/MTX cells with CLIC1 depletion (group 2) or none (group 1) was injected subcutaneously into the right flank. After the exhibition of neoplasia, MTX (4.0 μg/20 μl per gram of mouse weight) was injected intraperitoneally into each tumor-bearing mouse each day. Tumor dimensions were then measured once every 3 days. For the latter two groups, an equal amount of 9.0 × 105 JeG3/FUDR cells with CLIC1 depletion (group 4) or none (group 3) was injected subcutaneously into the right flank. Each tumor-bearing mouse was injected with FUDR (10 μg/20 μl per gram of mouse weight) intraperitoneally each day. Tumor dimensions were also measured. The tumor volume was calculated as: TV = L × W 2/2, where L represents the longer dimension, and W represents the shorter dimension. Thirty-two days after inoculation, all mice were sacrificed, and tumors were dissected. The protocols of animal experiments were approved by the ethical committee of Cangzhou Central Hospital.
Statistical Analysis
Data were presented as mean ± standard deviation (SD). The Student’s t-test was used to evaluate the difference between two groups. A value of p < 0.05 was considered statistically significant.
RESULTS
CLIC1 Is Highly Expressed in Chemoresistant Sublines in Choriocarcinoma
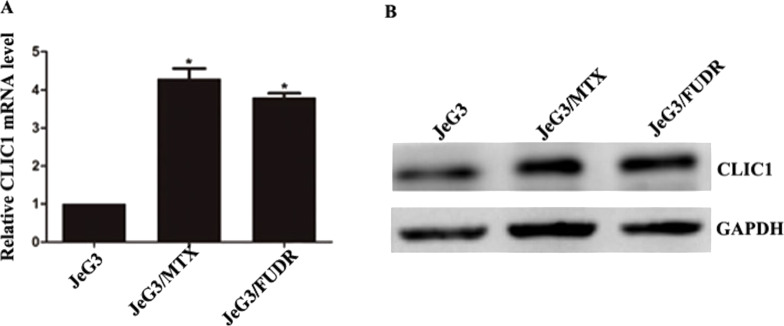
Initially, two chemoresistant sublines, JeG3/MTX and JeG3/FUDR, were induced from their parental JeG3 cells based on published protocols20,21. After a 10-month induction, the resistance index (RI) values were 26.81 for JeG3/MTX cells and 30.14 for JeG3/FUDR cells. By culturing the chemoresistant sublines and JeG3 cells, the expression of CLIC1 was examined by Western blot and qRT-PCR. It was found that the mRNA level of CLIC1 was significantly higher in the induced sublines than their parental cells (Fig. 1A). Likewise, the protein level of CLIC1 was also remarkably elevated in JeG3/MTX cells and JeG3/FUDR cells compared with JeG3 cells (Fig. 1B). These data suggest that CLIC1 is overexpressed in chemoresistant sublines.
Figure 1.
CLIC1 is highly expressed in chemoresistant sublines in choriocarcinoma. (A) qRT-PCR analysis of the relative mRNA level of CLIC1 in JeG3 cells and two sublines (JeG3/MTX and JeG3/FUDR). (B) Western blot analysis of the protein level of CLIC1 in the above three cell lines. *p < 0.05 versus JeG3 group.
Knockdown of CLIC1 in Chemoresistant Sublines Increases Cell Sensitivity to Drugs
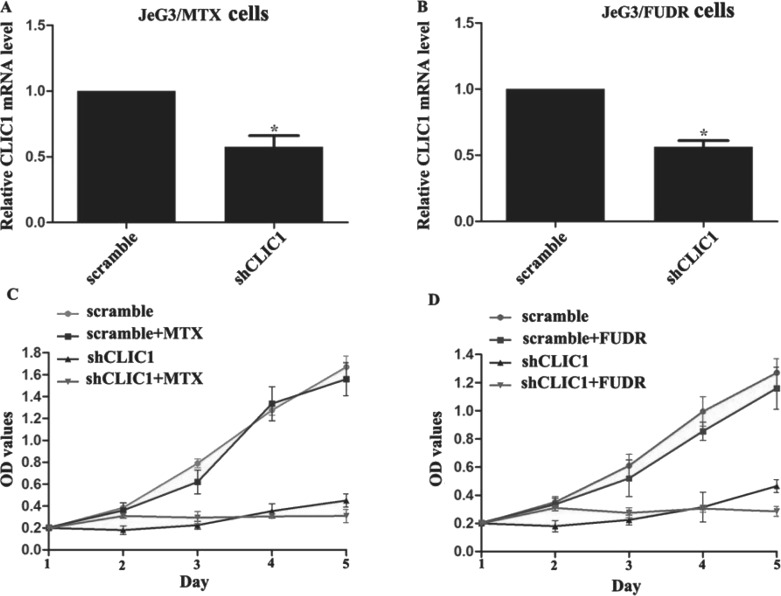
Next we adopted specific shRNAs against CLIC1 (shCLIC1) to deplete the expression of CLIC1 in the two chemoresistant sublines. Initially, a shCLIC1 confirmed by a previous report (shCLIC1-1) and another shCLIC1 commercially purchased (shCLIC1-2) were used. When both shCLIC1s were transfected, the relative CLIC1 mRNA was similar but significantly lower than that in the control groups (negative control and scramble shRNA groups) (see supplemental Fig. S1B, available at http://pan.baidu.com/share/link?shareid=2256097262&uk=1765598789). Immunoblotting also showed that knockdown of CLIC1 by either shRNA caused the remarkable decreases in CLIC1 protein level without affecting the protein level of GAPDH. The scramble shRNA caused no changes to either the CLIC1 or the GAPDH expression in JeG3 cells (see supplemental Fig. S1B, available at http://pan.baidu.com/share/link?shareid=2256097262&uk=1765598789). These preliminary data suggested that the synthesized shRNAs worked effectively to knock down the expression of CLIC1 without remarkable off-target effects. Since either shRNA was equally effective, shCLIC1-1 was randomly chosen for subsequent analysis. Thereafter, with this shRNA, qRT-PCR verified that the mRNA level of CLIC1 was also successfully depleted in sublines JeG3/MTX cells and JeG3/FUDR cells (Fig. 2A and B). Effects of CLIC1 knockdown on cell response to drugs were then assessed by CCK-8 proliferation assays. In JeG3/MTX cells, when CLIC1 was not depleted, cell viability was similar between cells treated with or without MTX. When CLIC1 was depleted, cell proliferation was significantly slowed down. This was particularly significant in MTX-treated CLIC1-depleted JeG3/MTX cells where cell viability barely differed among the monitored periods (Fig. 2C). Similarly, cell viability was not significantly different when CLIC1 was not depleted in JeG3/FUDR cells at the presence or absence of FUDR treatment. After knockdown of CLIC1, JeG3/FUDR cells showed full sensitivity to FUDR and failed to proliferate in 5 consecutive days (Fig. 2D). These observations suggest that knockdown of CLIC1 increases cell sensitivity to drugs in JeG3/MTX cells and JeG3/FUDR cells.
Figure 2.
Knockdown of CLIC1 in chemoresistant sublines increases cell sensitivity to drugs. (A, B) The specific shRNA against CLIC1 (shCLIC1-1) was transfected into JeG3/MTX cells and JeG3/FUDR cells, respectively. It was shown that the mRNA level of CLIC1 was significantly depleted by shCLIC1 in both sublines. (C) Cell proliferations of JeG3/MTX cells with or without CLIC1 knockdown were assessed at the presence or absence of MTX treatment. (D) Cell proliferations of JeG3/FUDR cells with or without CLIC1 knockdown were assessed at the presence or absence of FUDR treatment. All experiments were repeated three times. *p < 0.05 versus scramble group.
Knockdown of CLIC1 Increases Cell Sensitivity to Drugs in a Mouse Model of Choriocarcinoma
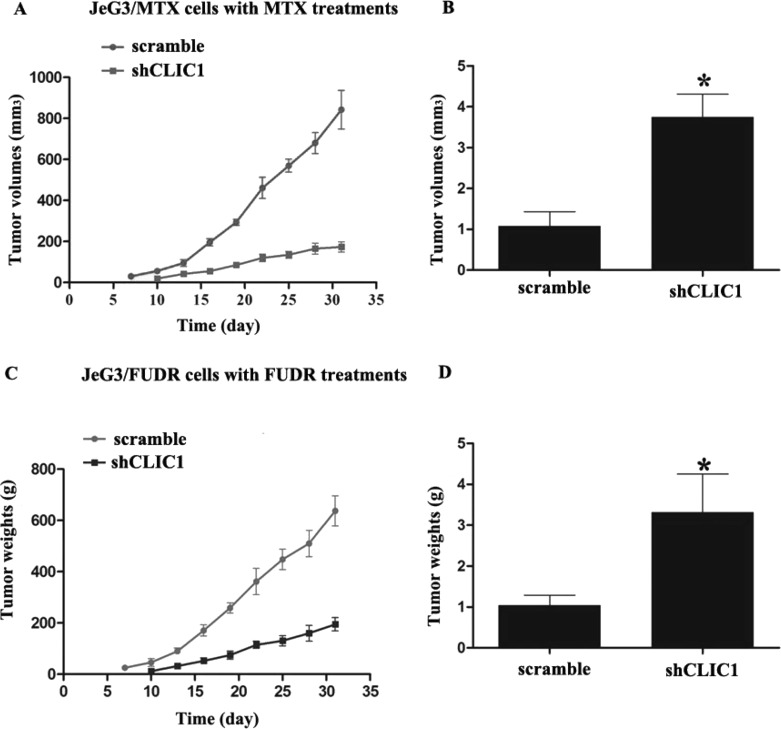
Furthermore, the effects of CLIC1 knockdown on tumor growth were assessed in vivo. JeG3/MTX cells with or without CLIC1 stable knockdown were injected subcutaneously into nude mice. It was observed that all mice exhibited neoplasia 7 days after injection in the CLIC1 undepleted group. In the CLIC1-depleted group, neoplasia was observed on the 10th day. All mice then received chemotherapy (MTX). After a total of 32 days of monitoring, the tumor volume in the CLIC1-depleted group was significantly smaller than that in its paired group, indicating that MTX treatment limited tumor growth after CLIC1 knockdown (Fig. 3A). Tumors also weighed significantly less, by up to 75% in the CLIC1-depleted mice (Fig. 3B). Comparable results were also observed in the mouse model bearing JeG3/FUDR-xenografted tumors. Tumor volume was changed approximately threefold by day 32 between the two groups with and without CLIC1 knockdown (Fig. 3C). The tumor weights were decreased 2.5-fold in the CLIC1-depleted mice as well (Fig. 3D). The shrinkage in tumor volume and weights suggests that knockdown of CLIC1 increases JeG3/MTX and JeG3/FUDR cell sensitivity to drugs in the mouse model.
Figure 3.
Knockdown of CLIC1 increases cell sensitivity to drugs in a mouse model of choriocarcinoma. In a mouse model bearing chemoresistant cells, JeG3/MTX cells with or without CLIC1 stable knockdown were injected into mouse (n = 10 each). After the exhibition of neoplasia, MTX was intraperitoneally injected, and tumor dimensions were measured once every 3 days (A). On day 32, all tumors were dissected, and tumor mass was weighed (B). In another mouse model bearing chemoresistant JeG3/FUDR cells, FUDR was intraperitoneally injected into each mouse, and tumor volume (C) and weights (D) were measured as stated above. *p < 0.05 versus scramble group.
CLIC1 Positively Regulates the Expression of MRP1 in Choriocarcinoma JeG3 Cells
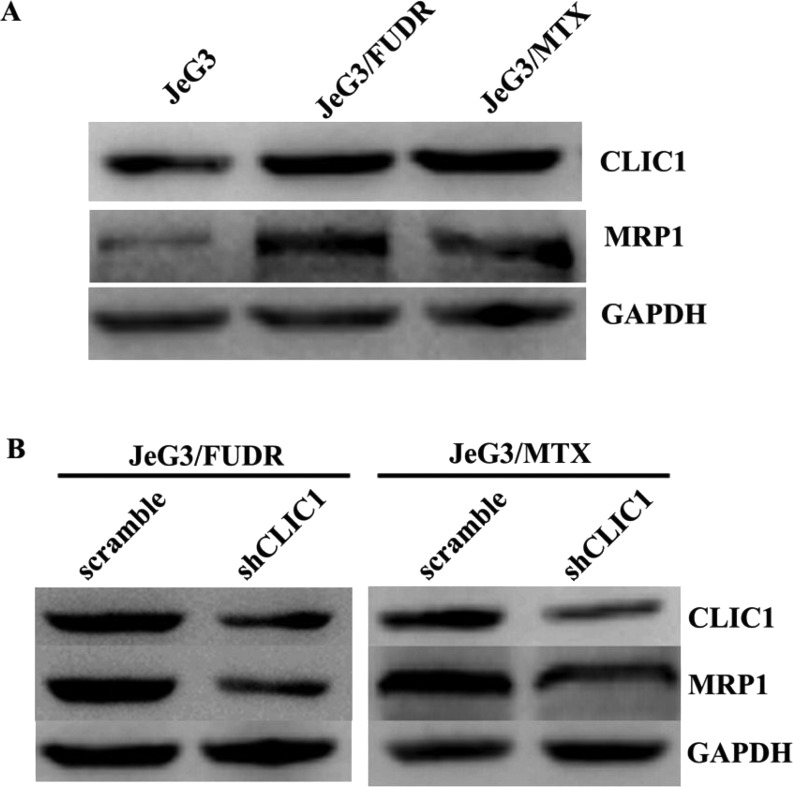
To evaluate the possible mechanism underlying CLIC1-mediated chemoresistance, we assessed the expression patterns of CLIC1 and the widely detected drug resistance-associated protein 1 (MRP1). In JeG3/MTX cells and JeG3/FUDR cells, while CLIC1 was elevated, the protein level of MRP1 was accordingly increased (Fig. 4A). Moreover, in JeG3/MTX cells, when CLIC1 was depleted, MRP1 was also downregulated. This was also true in JeG3/FUDR cells (Fig. 4B). These data suggest that CLIC1 positively regulates the expression of MRP1 in chemoresistant choriocarcinoma sublines.
Figure 4.
MRP1 is a target of CLIC1. (A) Expression patterns for CLIC1 and MRP1 in the parental cell line JeG3 and its two sublines JeG3/MTX and JeG3/FUDR. (B) In JeG3/MTX and JeG3/FUDR cells, knockdown of CLIC1 decreased the expression of MRP1 accordingly.
Knockdown of MRP1 Blunts CLIC1-Mediated Acquirement of Chemoresistance in JeG3 Cells
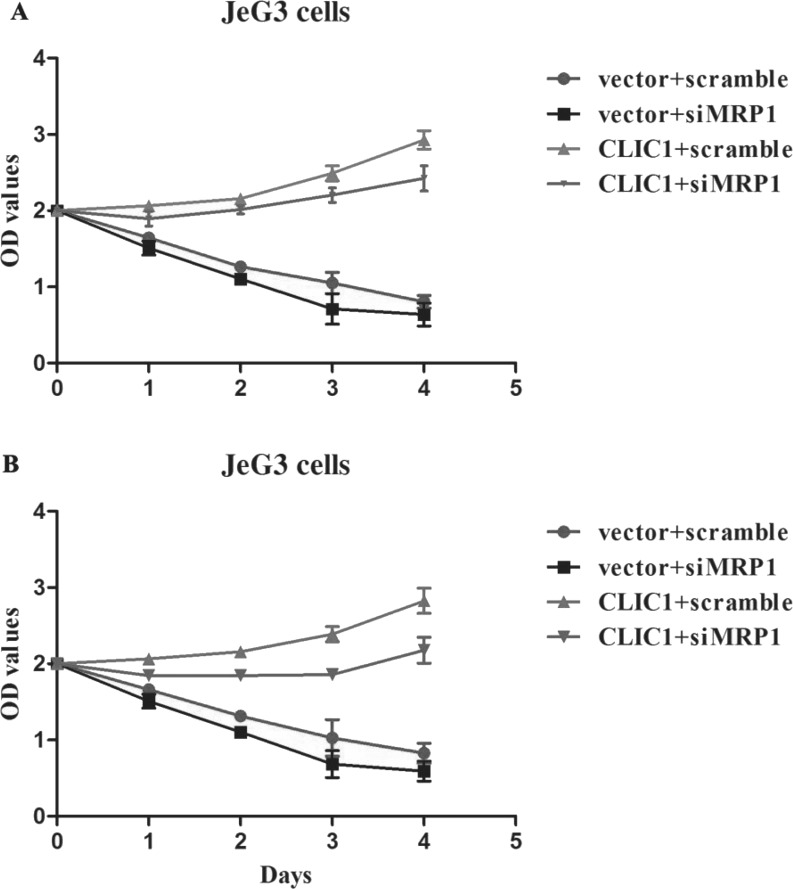
Considering that MRP1 is a downstream target of CLIC1, effects of MRP1 knockdown on CLIC1-mediated chemoresistance were then investigated. Parental JeG3 cells were sensitive to MTX and FUDR treatments, and knockdown of MRP1 resulted in further sensitivity to these drugs (Fig. 5). However, overexpression of CLIC1 in parental JeG3 cells caused less sensitivity of cells to MTX treatment, indicating that reexpression of CLIC1 could induce chemoresistance in nonresistant parental cell lines. Moreover, when MRP1 was depleted, JeG3 cells with CLIC1 reexpression showed high sensitivity to MTX treatment (Fig. 5A), indicating that knockdown of MRP1 abolished CLIC1-mediated development of chemoresistance in JeG3 cells. Similarly, while CLIC1 induced the development of chemoresistance to FUDR in JeG3 cells, knockdown of MRP1 abolished the above effects (Fig. 5B). All these data conclude that MRP1 is a key downstream target that mediates CLIC1-induced development of chemoresistance in JeG3 cells.
Figure 5.
Knockdown of MRP1 blunts CLIC1-mediated acquirement of chemoresistance in JeG3 cells. CLIC1-overexpressed JeG3 cells with or without MRP1 knockdown were treated with MTX (A) and FUDR (B). Cell viability was then monitored under each condition. While CLIC1 overexpression promoted cell proliferation even under MTX or FUDR treatment, knockdown of MRP1 blunted the CLIC1-mediated abovementioned effects. The “vector + scramble” group indicates JeG3 cells were without CLIC1 plasmid and siMRP1 transfection. The “vector + siMRP1” group indicates JeG3 cells were without CLIC1 plasmid transfection but were transfected with specific MRP1 siRNA.
DISCUSSION
Choriocarcinoma represents a large proportion of GTN. Prognosis of this malignancy is traditionally desirable because of the development of chemotherapy. However, a few tumor types acquire chemoresistance. It is estimated that approximately 10%–30% of patients have an incomplete response to first-line multiagent chemotherapy6–8. Therefore, drug resistance remains a major obstacle to the successful treatment of this malignancy. Hence, new therapeutic targets are desperately needed.
Recently, the CLIC1 protein has gained much research interest24. The CLIC gene family comprises at least six members, namely, CLIC1 to CLIC625. The functions of CLIC1 range from ion homeostasis to cell volume regulation, transepithelial transport, and regulation of electrical excitability21. Recently, CLIC1 was proposed as a novel prognostic biomarker and therapeutic agent in various types of solid tumors12,14–17. Of particular interest, a recent bioinformatic analysis revealed that CLIC1 was critically involved in the malignant transformation of hydatidiform moles to choriocarcinoma19. However, no study has been conducted as to whether CLIC1 confers any drug resistance in choriocarcinoma treatment.
The present study initially established two sublines from their parental JeG3 cells. RI values for the two sublines (JeG3/MTX and JeG3/FUDR) were 26.81 and 30.14, respectively, suggesting high drug resistance26 and the successful establishment of chemoresistant sublines from parental cells. By using the induced chemoresistant sublines, it was observed that the expression of CLIC1 was significantly higher. Knockdown of CLIC1 by shRNAs significantly increased cell sensitivity to MTX in JeG3/MTX cells or to FUDR in JeG3/FUDR cells in vitro and in vivo. Cell viability in response to drug treatment was decreased by up to 60%–75% after CLIC1 knockdown. Our observations collectively conclude that CLIC1 is a key mediator of chemoresistance in choriocarcinoma cells.
Of particular interest, CLIC1 functions by positive regulation of MRP1, an MRP. MRP1 was first cloned and highly expressed in a doxorubicin-selected multidrug-resistant human lung carcinoma cell line, H69AR9. MRP1 functions to reduce the intracellular drug accumulation, in which ABC transporters are notorious mediators10. Because of this property, MRP1 confers resistance to not only doxorubicin but also many other common antineoplastic drugs such as MTX, VP, daunorubicin, and vincristine11. Hence, MRP1 has emerged as a key contributor to chemoresistance. However, the molecular mechanism for the induction of MRP1 has not been fully clarified. Our study found that MRP1 was a target protein by CLIC1. Knockdown of MRP1 significantly abolished CLIC1-mediated acquirement of chemoresistance in JeG3 cells. These data suggest that CLIC1 exerts its chemoresistant property by promoting the expression of MRP1 in choriocarcinoma. Hence, this is the first report to our knowledge that identifies the role and one of the potential mechanisms of CLIC1 in choriocarcinoma chemoresistance.
The identification of CLIC1 as a key mediator of chemoresistance is of biological significance. On the one hand, a small proportion of choriocarcinoma patients have received an unfavorable prognosis due to chemoresistance. To date, no biomarker has been proposed as an effective indicator that can be used to differentiate chemosensitive and chemoresistant patients. Our report suggests that CLIC1 may serve as a candidate to indicate whether a patient is sensitive to common drugs. On the other hand, despite much knowledge of how MRP1 induces drug resistance in multiple solid tumors, the molecular mechanism that controls MRP1 expression remains largely unknown. Our data may suggest that MRP1 is regulated by CLIC1, leading to CLIC1-mediated development of chemoresistance in choriocarcinoma cells.
In all, this is the first report to identify the critical role of CLIC1 in the development of chemoresistance in JeG3 cells. Our data suggest that knockdown of CLIC1 or its target protein MRP1 may be promising therapeutic strategies for alternative treatment of chemoresistant choriocarcinoma patients.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Lurain JR. Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11–8. [DOI] [PubMed] [Google Scholar]
- 2. Lurain JR. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203:531–9. [DOI] [PubMed] [Google Scholar]
- 3. Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C, Group EGW. Gestational trophoblastic disease: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi39–50. [DOI] [PubMed] [Google Scholar]
- 4. Biscaro A, Braga A, Berkowitz RS. Diagnosis, classification and treatment of gestational trophoblastic neoplasia. Rev Bras Ginecol Obstet. 2015;37:42–51. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematol Oncol Clin North Am. 2012;26:111–31. [DOI] [PubMed] [Google Scholar]
- 6. Abrao RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: Comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecol Oncol. 2008;108:149–53. [DOI] [PubMed] [Google Scholar]
- 7. Lurain JR, Nejad B. Secondary chemotherapy for high-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2005;97:618–23. [DOI] [PubMed] [Google Scholar]
- 8. Lurain JR, Singh DK, Schink JC. Primary treatment of metastatic high-risk gestational trophoblastic neoplasia with EMA-CO chemotherapy. J Reprod Med. 2006;51:767–72. [PubMed] [Google Scholar]
- 9. Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992;258:1650–4. [DOI] [PubMed] [Google Scholar]
- 10. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. [DOI] [PubMed] [Google Scholar]
- 11. Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: The multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–302. [DOI] [PubMed] [Google Scholar]
- 12. Zhao W, Lu M, Zhang Q. Chloride intracellular channel 1 regulates migration and invasion in gastric cancer by triggering the ROS-mediated p38 MAPK signaling pathway. Mol Med Rep. 2015;12:8041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma PF, Chen JQ, Wang Z, Liu JL, Li BP. Function of chloride intracellular channel 1 in gastric cancer cells. World J Gastroenterol. 2012;18:3070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Y, Yin M, Huang B, Wang Y, Li X, Lou G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015;36:4175–9. [DOI] [PubMed] [Google Scholar]
- 15. Wei X, Li J, Xie H, Wang H, Wang J, Zhang X, Zhuang R, Lu D, Ling Q, Zhou L, Xu X, Zheng S. Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J Gastroenterol Hepatol. 2015;30:208–16. [DOI] [PubMed] [Google Scholar]
- 16. Li RK, Zhang J, Zhang YH, Li ML, Wang M, Tang JW. Chloride intracellular channel 1 is an important factor in the lymphatic metastasis of hepatocarcinoma. Biomed Pharmacother. 2012;66:167–72. [DOI] [PubMed] [Google Scholar]
- 17. Wang P, Zeng Y, Liu T, Zhang C, Yu PW, Hao YX, Luo HX, Liu G. Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. 2014;20:2071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang F, Song X, Gao G, Mu J, Wang Z, Ma F, Gu J. Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol. 2015;32:616. [DOI] [PubMed] [Google Scholar]
- 19. Shi ZH, Zhao C, Wu H, Wang W, Liu XM. CLIC1 protein A candidate prognostic biomarker for malignant-transformed hydatidiform moles. Int J Gynecol Cancer 2011;21:153–60. [DOI] [PubMed] [Google Scholar]
- 20. Han B, Xiang Y, Sha GH, Zhang H, Liu X. [Establishment of floxuridine-resistant JeG-3 subline and the role of thymidylate synthetase mRNA expression in chem-resistant-prediction.]. Zhonghua Fu Chan Ke Za Zhi 2009;44:851–5. [PubMed] [Google Scholar]
- 21. Zhao J, Xiang Y, Xiao C, Guo P, Wang D, Liu Y, Shen Y. AKR1C3 overexpression mediates methotrexate resistance in choriocarcinoma cells. Int J Med Sci. 2014;11:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Setti M, Savalli N, Osti D, Richichi C, Angelini M, Brescia P, Fornasari L, Carro MS, Mazzanti M, Pelicci G. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst. 2013;105:1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tivnan A, Zakaria Z, O’Leary C, Kogel D, Pokorny JL, Sarkaria JN, Prehn JH. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front Neurosci. 2015;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suh KS, Mutoh M, Gerdes M, Crutchley JM, Mutoh T, Edwards LE, Dumont RA, Sodha P, Cheng C, Glick A, Yuspa SH. Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor {alpha}-induced apoptosis, and inhibits tumor growth. Cancer Res. 2005;65:562–71. [PubMed] [Google Scholar]
- 25. Friedli M, Guipponi M, Bertrand S, Bertrand D, Neerman-Arbez M, Scott HS, Antonarakis SE, Reymond A. Identification of a novel member of the CLIC family, CLIC6, mapping to 21q22.12. Gene 2003;320:31–40. [DOI] [PubMed] [Google Scholar]
- 26. Snow K, Judd W. Characterisation of adriamycin- and amsacrine-resistant human leukaemic T cell lines. Br J Cancer 1991;63:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]