Abstract
Adherence has become an important issue in modern oncology treatment. Most studies have included heterogeneous target tumor types, regimens, and therapy settings. Our study focused on capecitabine during capecitabine plus oxaliplatin (XELOX) treatment as an adjuvant therapy for colorectal cancer. The main aims of this study were to evaluate real-life adherence to capecitabine and to investigate candidate factors that might decrease adherence. We studied 338 consecutive patients who received XELOX treatment between December 1, 2011, and April 30, 2015, at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. Our study assessed adherence to capecitabine through patient-reported treatment diaries and interviewed nonadherents to determine the reasons for not taking capecitabine at a pharmaceutical outpatient clinic. We calculated the adherence rate in a cycle as: number of times the patient took capecitabine/28. Relative dose intensities and factors associated with deteriorating adherence to capecitabine were retrospectively surveyed from electronic patient records. Uni- and multivariate logistic regression analyses were used to investigate factors associated with optimal adherence. The study covered 282 patients who received 2,055 cycles of XELOX. Median adherence rate was 94.0% in the first cycle, and median relative dose intensity of capecitabine was 77.8%. The most common reasons for nonadherence were nausea/vomiting and diarrhea. The presence of the following factors was not significantly associated with adherence: ECOG performance status ≥1 (p = 0.715), clinical stage (p = 0.408), primary tumor site (p = 0.576), age ≥70 years at study entry (p = 0.757), female gender (p = 0.504), and not living alone (p = 0.579). The adherence rate from this study was significantly higher than the adherence from metastatic settings. Adherence-enhancing interventions for capecitabine in XELOX treatment as adjuvant therapy comprised management of nausea/vomiting and diarrhea.
Key words: Capecitabine, Adherence, XELOX treatment, Pharmaceutical outpatient clinic, Oral anticancer drugs
INTRODUCTION
The use of oral anticancer therapy is increasing every year. In particular, cytotoxic agents and, more recently, targeted therapies are often administered orally. Pharmacoeconomic studies have shown that oral medications are superior to in-hospital intravenous treatment with respect to cost1. Most patients prefer to take medications orally2; therefore, the use of oral anticancer agents is likely to continue to increase. Oral anticancer agents are expected to improve the patient’s quality of life by decreasing treatment interference with work and social activities, eliminating travel time to clinics for infusion, and reducing the discomfort and potential complications associated with placing an intravenous line for each administration.
Until recently, the problem of assessing adherence was not a subject of major interest in cancer care. As recent developments in oral therapies for oncology could mark a turning point in the perceived need for assessing adherence among cancer patients, adherence has become an important issue in modern oncology. Ruddy et al. reported 16%–100% adherence and persistence rates with oral anticancer agents3. Several studies have reported patient adherence rates for capecitabine within the range of 58%–100%, mainly depending on the measurement method4. The superiority of capecitabine plus oxaliplatin (XELOX) compared with 5-flurouracil/leucovorin was maintained at 4 and 5 years, indicating that the benefits of XELOX are durable5. In particular, improving treatment adherence is critical to achieving optimal therapeutic efficacy in adjuvant therapy.
Adherence to treatment with oral anticancer agents (dasatinib, erlotinib, everolimus, etc.) and factors influencing adherence have been assessed6. Most such studies have included heterogeneous target tumor types, regimens, and therapy settings7. Our study focused on capecitabine during XELOX treatment as an adjuvant therapy for colorectal cancer. The main aim of this study was to evaluate real-life adherence to capecitabine on XELOX treatment and investigate candidate factors that might decrease adherence.
MATERIALS AND METHODS
Study Design and Treatment
We obtained data from 338 consecutive patients who received XELOX treatment as an adjuvant therapy for colorectal cancer between December 1, 2011, and April 30, 2015, at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. Our study assessed adherence with capecitabine using patient-reported treatment diaries and interviewed nonadherents regarding their reasons for not taking capecitabine at a pharmaceutical outpatient clinic every XELOX treatment cycle.
Adherence rate was defined as the number of times that a patient took capecitabine in a 14-day cycle divided by the prescribed 28 doses. Relative dose intensities8 and factors associated with deteriorating adherence to capecitabine were retrospectively surveyed from electronic patient records.
Patients were assigned to adjuvant treatment with the XELOX regimen. The XELOX regimen comprised a 2-h intravenous infusion of oxaliplatin 130 mg/m2 on day 1, and oral capecitabine at 1,000 mg/m2 twice daily was given for 14 days on a 3-week cycle, for a total of eight cycles (24 weeks). The first dose of capecitabine was given on the evening of day 1, and the last dose on the morning of day 15 for each cycle9.
Pharmaceutical Outpatient Clinic
In our hospital, proactive intervention by pharmacists to check adherence and side effects (which represent one cause of nonadherence) is considered necessary, and we hold a pharmaceutical outpatient clinic for patients receiving oral chemotherapy treatment10. The main tasks of XELOX performed in the pharmaceutical outpatient clinic are as follows: 1) use of the capecitabine treatment diary to check for capecitabine adherence and leftover medication; 2) provision of prescription support for XELOX treatment; 3) assessment of side effects; and 4) suggestion of prescriptions for medication as supportive therapy. In practice, prescription support for XELOX treatment consists of a pharmacist recording the capecitabine and oxaliplatin doses and administration period required for the next cycle in the electronic medical record. This is then checked and authorized by a physician to enable the prescription to be issued. If capecitabine is left over from the previous cycle, the amount to be prescribed is adjusted accordingly.
Study Population
Patients in this study were receiving XELOX treatment as an adjuvant therapy for colorectal cancer. Patients were excluded on the basis of previous cytotoxic chemotherapy or immunotherapy for colorectal cancer. All participants in this study visited a pharmaceutical outpatient clinic and completed XELOX treatment at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. The study protocol was reviewed and approved by the Clinical Research Ethics Review Committee at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (Approval No. 2012-1035).
Statistical Analysis
This analysis was based on 282 patients evaluated for adherence to capecitabine. The outcome variable was adherence, dichotomized according to success defined as adherence rate ≥95% in the first cycle. All independent variables were first tested in univariate analyses. Independent variables with a two-tailed p < 0.05 value in univariate analyses were included in multivariate logistic regression analysis, where forward selection was used to build the final model. Dichotomous independent variables included in the analyses were Eastern Cooperative Oncology Group (ECOG) performance status, age, gender, and living status (alone vs. with others). All other independent variables (clinical stage and primary tumor site) were included as continuous variables. Odds ratios and p values were computed for the variables in logistic regression models. All analyses were performed using SPSS version 11.5 (SPSS, Chicago, IL, USA).
RESULTS
Patients and Characteristics
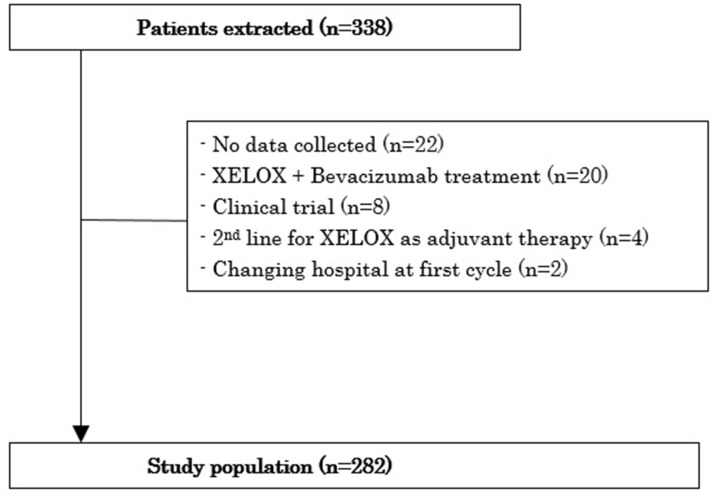
A total of 338 patients were extracted from outpatient pharmacy information systems in the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. A flowchart shows patients and the reason for exclusion (Fig. 1). Reasons for nonparticipation included no data collected (n = 22), XELOX + bevacizumab treatment (n = 20), participation in another clinical trial (n = 8), prior adjuvant treatment at another hospital (n = 4), and change in hospital after the first cycle (n = 2). Clinical and sociodemographic characteristics data for the 282 patients analyzed are reported in Table 1. The study included 135 male and 147 female patients, and median age was 60 years (range: 26–81 years). In terms of living status, 254 patients (90.1%) were living with family, and 28 (9.9%) were living alone. Mean duration of XELOX treatment was 7.28 cycles (range: 1–8 cycles), and total number of XELOX treatments was 2,055 cycles.
Figure 1.
Flowchart of patients and reasons for exclusion.
Table 1.
Clinical Characteristics and Sociodemographic of the Study Population
| Characteristic | No. of Patients (n = 282) |
|---|---|
| Gender | |
| Male | 135 (47.9%) |
| Female | 147 (52.1%) |
| Age at study entry (years) | |
| Median | 60 |
| Range | 26–81 |
| ECOG performance status | |
| 0 | 273 (96.8%) |
| 1 | 9 (3.2%) |
| Primary tumor site | |
| Colonic | 147 (52.1%) |
| Rectal | 109 (38.7%) |
| Others | 26 (9.2%) |
| Clinical stage | |
| Stage II | 31 (11.0%) |
| Stage IIIa | 141 (50.0%) |
| Stage IIIb | 81 (28.7%) |
| Stage IV | 29 (10.3%) |
| Living status | |
| Living with family | 254 (90.1%) |
| Living alone | 28 (9.9%) |
| Duration of use (cycle) | |
| Mean (SD) | 7.28 (1.84) |
| Range | 1–8 |
| Total treatment number | 2,055 |
ECOG, Eastern Cooperative Oncology Group.
Adherence Rate
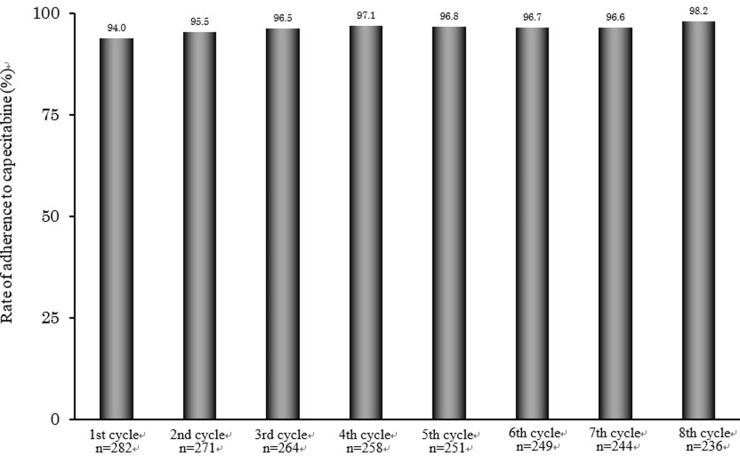
The capecitabine adherence rate was determined by pharmacists from patient-reported treatment diaries during XELOX treatment as adjuvant therapy (Fig. 2). The ratio of patients who completed capecitabine treatment on XELOX treatment was 83.6%. Median adherence rate was 94.0% (n = 282) in the first cycle of XELOX treatment, 95.5% (n = 271) in the second cycle, and rising to 98.2% (n = 236) in the eighth cycle. The median relative dose intensity of capecitabine was 77.8%.
Figure 2.
Capecitabine adherence rate on XELOX treatment as adjuvant therapy for colorectal cancer. Adherence rate with capecitabine during cycles 1–8. Adherence to capecitabine was checked by pharmacists via the self-reported treatment diaries at a pharmaceutical outpatient clinic.
Factors Reducing Capecitabine Adherence
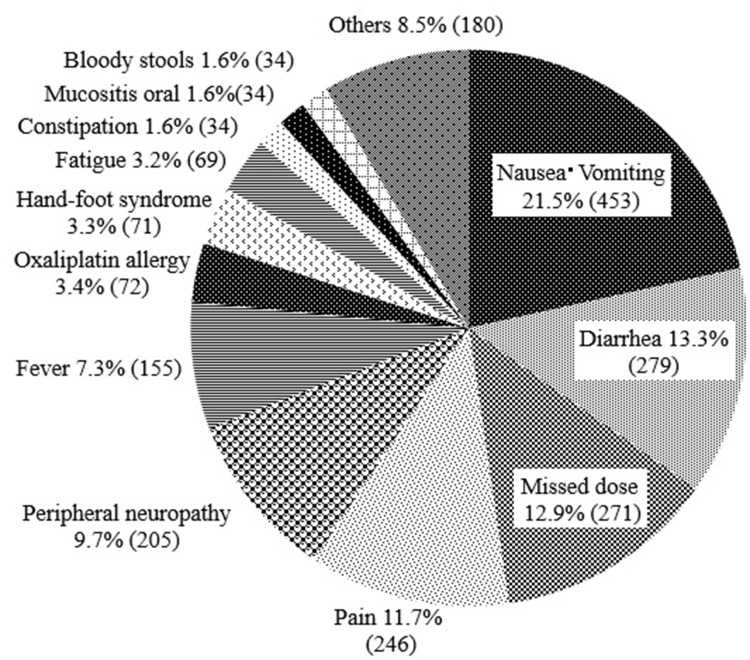
In total, nonadherence was seen in 2,103 instances. The most common reasons for nonadherence were nausea/vomiting (21.5%, 435 instances), diarrhea (13.3%, 279 instances), missed dose (13.0%, 271 instances), and pain (11.7%, 246 instances) (Fig. 3).
Figure 3.
Factors reducing adherence to capecitabine on XELOX treatment as adjuvant therapy (n = 2,103). Factors reducing adherence to capecitabine during cycles 1–8.
Multivariate Model of Factors Associated With Adherence
Results of multivariate analysis are shown in Table 2. Clinical and sociodemographic factors were not significantly associated with capecitabine adherence on XELOX in the first cycle. The presence of the following factors was not significantly associated with adherence: ECOG performance status ≥1 (p = 0.715), clinical stage (p = 0.408), primary tumor site (p = 0.576), age ≥70 years at study entry (p = 0.757), female sex (p = 0.504), and not living alone (p = 0.579).
Table 2.
Logistic Regression Analysis of Optimal Adherence Behavior in Relation to Sociodemographic and Clinical Factors
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sociodemographic factors | ||||
| Age at study entry (ref. <70 years) | 1.16 (0.45–2.99) | 0.757 | NA | NA |
| Gender (ref. male) | 1.28 (0.61–2.67) | 0.504 | NA | NA |
| Living status (ref. living alone) | 0.89 (0.25–3.14) | 0.579 | NA | NA |
| Clinical factors | ||||
| ECOG performance status (ref. 0) | 0.94 (0.11–7.77) | 0.715 | NA | NA |
| Clinical stage | 0.82 (0.52–1.30) | 0.408 | NA | NA |
| Primary tumor site | 0.85 (0.49–1.47) | 0.576 | NA | NA |
OR, odds ratio; CI, confidence interval; NA, not applicable.
DISCUSSION
The median capecitabine adherence rate on XELOX treatment in the first cycle as an adjuvant therapy for colorectal cancer as measured by patient self-report was 94.0%. The median relative dose intensity of capecitabine was comparable to a previous randomized phase III study of XELOX as adjuvant therapy for colorectal cancer (77.8% vs. 78.0%)11. Although the adherence rate was high, a considerable number of patients (16.4%) showed nonadherence. In analysis of the total number of instances of nonadherence (2,103 instances), the main reasons for nonadherence were nausea or vomiting (21.5%, 435 instances), diarrhea (13.3%, 279 instances), and missed dose (13.0%, 271 instances). Clinical and sociodemographic factors were not significantly associated with capecitabine adherence on XELOX treatment in the first cycle.
Our study focused on adjuvant therapy for colorectal cancer and on XELOX treatment in clinical practice. Previous studies have reported on several oral anticancer agents, tumor types, and therapy settings6,12,13. Our data focused on a specific agent, tumor type, country, and treatment setting will be highly valuable in clinical practice.
Studies investigating patient adherence to capecitabine have found that median adherence across all cycles and studies was 78%14, and median adherence rate was 93.5% in the first cycle of XELOX treatment for unresectable metastatic disease15. An interesting finding is that adjuvant chemotherapy was associated with a higher probability of adherence among breast cancer patients on oral endocrine therapy. This is consistent with a previous cohort study, which also used persistence as an outcome variable in conjunction with a 4- to 5-year follow-up16,17. Adherence rates in our study were higher than those reported previously14–16. The high adherence results in this study might be explained by the fact that patient state (e.g., performance status, kidney function, hepatic function, motivation for treatment) was better under an adjuvant therapy setting than under an unresectable metastatic setting. The incentive for patients to maintain adherence to oral chemotherapy would thus seem higher in the adjuvant setting where there is true intent compared to patients with advanced, metastatic cancer, in which case treatment is being administered in the palliative setting. Nonadherent instances were seen throughout the first to eighth cycles. However, the ratio of patients who completed capecitabine treatment on XELOX treatment was 83.6%. As a result, the median capecitabine adherence rate was 94.0% in the first cycle.
The high adherence rate in this study might have been associated with Japanese characteristics such as increased commitment to treatment, punctuality, earnestness, and listening more carefully to their physicians. Side effects of medications are known to represent major predictors of poor adherence18, and treatment-related side effects are the most frequently reported factor cited in previous studies of patients with nonadherence to oral anticancer drug therapy12,19. Diarrhea and hand–foot syndrome are consistent with adverse effects reported in previous studies in patients with nonadherence to capecitabine13. Our study indicated that nonadherence correlated with a higher number of adverse effects. We prescribed supportive therapy for all cases in this study beforehand, but appropriate antiemetic medication is necessary for patients with risk factors for chemotherapy-induced nausea and vomiting during highly emetogenic chemotherapy. We recommend prophylactic treatment with aprepitant for patients with high-risk factors of age <55 years, female sex, and nonhabitual alcohol intake20.
Factors influencing adherence among patients taking oral anticancer agents have recently been reviewed21. Low or very high age seems to be associated with lower adherence17,22–24, and women seem to be less adherent than men25. Cancer stage seems to have no influence on adherence17, but performance status is related to adherence24,26, as is the living status of patients6,27. The frequency of adverse events during adjuvant XELOX therapy in older patients was similar to that in younger patients, suggesting that age should not be a barrier to intensive adjuvant therapy5. Unfortunately, our study showed no significant predictors of adherence, although a large population was investigated.
For that reason, some limitations of this study should be taken into consideration. First, we might have missed relevant patient-related factors, such as education6 or out-of-pocket expenses7,23, which might be related to adherence. In terms of the pharmacoeconomic aspects of adherence to oral chemotherapy, one of the challenges with oral chemotherapy in the US relates to the financial burden on the patient, as such treatment may not be covered by insurance to the same extent as intravenous chemotherapy. In Japan, where patients are covered by the national health insurance system, this is not as much of an issue. Whether out-of-pocket expenses affect adherence to oral chemotherapy in Japan thus merits investigation. Second, we relied on patient self-reports (via diary) in assessing the key primary outcome of adherence. The World Health Organization recommends using one objective measure with a self-reported measure for the assessment of adherence28. Further research should consider using more objective methods such as pill count. Third, we used self-reported adherence based on patient-completed medication diaries, rather than a medication event monitoring system (MEMS) or the Morisky Medication Adherence Scale (MMAS). A MEMS is unsuitable for patients using oral cytotoxic agents, which require close monitoring of side effects and regular patient visits. Although various methods for measuring adherence are available, self-reporting is the most widely used29. The MMAS has been validated with outstanding reliability in patients with other chronic diseases30,31. In oncological settings, the MMAS allows for rapid assessment of adherence to oral adjuvant endocrine therapy32,33. The MMAS eight-item questionnaire contains “Did you take your medication yesterday?”—which is unsuitable for measuring adherence to anticancer drugs within the rest period. Previous studies of oral cytotoxic chemotherapeutic drugs have mainly used self-reported questionnaires17, which tend to overestimate adherence because patients are inclined to overreport to please their doctors.
In conclusion, the median capecitabine adherence rate on XELOX treatment as adjuvant therapy was 94.0% in the first cycle. The main reasons for nonadherence were nausea and vomiting. We recommend prophylactic treatment with an aprepitant for patients with high-risk factors such as age <55 years, female sex, or nonhabitual alcohol intake. ECOG performance status ≥1, clinical stage, primary tumor site, age ≥70 years, female sex, and living alone seem to be negatively associated with adherence. These findings may contribute to achieving higher adherence to capecitabine during XELOX treatment as adjuvant therapy for colorectal cancer.
REFERENCES
- 1. Cassidy J, Douillard JY, Twelves C, McKendrick JJ, Scheithauer W, Bustová I, Johnston PG, Lesniewski-Kmak K, Jelic S, Fountzilas G, Coxon F, Díaz-Rubio E, Maughan TS, Malzyner A, Bertetto O, Beham A, Figer A, Dufour P, Patel KK, Cowell W, Garrison LP. Pharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: The X-ACT trial. Br J Cancer 2006;9:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fallowfield L, Atkins L, Catt S, Cox A, Coxon C, Langridge C, Morris R, Price M. Patients’ preference for administration of endocrine treatments by injection or tablets: Results from a study of women with breast cancer. Ann Oncol. 2006;17:205–10. [DOI] [PubMed] [Google Scholar]
- 3. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. [DOI] [PubMed] [Google Scholar]
- 4. Bourmaud A, Henin E, Tinquaut F, Regnier V, Hamant C, Colomban O, You B, Ranchon F, Guitton J, Girard P, Freyer G, Tod M, Rioufol C, Trillet-Lenoir V, Chauvin F. Adherence to oral anticancer chemotherapy: What influences patients’ over or non-adherence? Analysis of the OCTO study through quantitative–qualitative methods. BMC Res Notes 2015;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, Price T, Lim R, Van Cutsem E, Park YS, McKendrick J, Topham C, Soler-Gonzalez G, de Braud F, Hill M, Sirzén F, Haller DG. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J Clin Oncol. 2007;25:102–9. [DOI] [PubMed] [Google Scholar]
- 6. Timmers L, Boons CC, Kropff F, van de Ven PM, Swart EL, Smit EF, Zweegman S, Kroep JR, Timmer-Bonte JN, Boven E, Hugtenburg JG. Adherence and patients’ experiences with the use of oral anticancer agents. Acta Oncol. 2014;53:259–67. [DOI] [PubMed] [Google Scholar]
- 7. Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treat Rev. 2013;39:610–21. [DOI] [PubMed] [Google Scholar]
- 8. Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–7. [DOI] [PubMed] [Google Scholar]
- 9. Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33:3733–40. [DOI] [PubMed] [Google Scholar]
- 10. Sugita K, Kawakami K, Yokokawa T, Sugisaki T, Takiguchi T, Aoyama T, Suzuki K, Suenaga M, Yamaguchi K, Inoue A, Machida Y, Yamaguchi T, Hama T. Self-reported adherence to trifluridine and tipiracil hydrochloride for metastatic colorectal cancer: A retrospective cohort study. Oncology 2016;91:224–30. [DOI] [PubMed] [Google Scholar]
- 11. Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll H. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71. [DOI] [PubMed] [Google Scholar]
- 12. Winterhalder R, Hoesli P, Delmore G, Pederiva S, Bressoud A, Hermann F, von Moos R, SAEDA Investigators Group (Swiss prospective cohort group). Self-reported compliance with capecitabine: Findings from a prospective cohort analysis. Oncology 2011;80:29–33. [DOI] [PubMed] [Google Scholar]
- 13. Simons S, Ringsdorf S, Braun M, Mey UJ, Schwindt PF, Ko YD, Schmidt-Wolf I, Kuhn W, Jaehde U. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer 2011;19:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG, Cohen HJ, Becker HP, Kartcheske PA, Wheeler JD, Perez EA, Wolff AC, Gralow JR, Burstein HJ, Mahmood AA, Magrinat G, Parker BA, Hart RD, Grenier D, Norton L, Hudis CA, Winer EP, CALGB Investigators. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawakami K, Nakamoto E, Yokokawa T, Sugita K, Mae Y, Hagino A, Suenaga M, Mizunuma N, Oniyama S, Machida Y, Yamaguchi T, Hama T. Patients’ self-reported adherence to capecitabine on XELOX treatment in metastatic colorectal cancer: Findings from a retrospective cohort analysis. Patient Prefer Adherence 2015;9:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Font R, Espinas JA, Gil-Gil M, Barnadas A, Ojeda B, Tusquets I, Segui MA, Margelí M, Arcusa A, Prat A, Garcia M, Borras JM. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: A retrospective cohort study in Catalonia, Spain. Br J Cancer 2012;107:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 19. Regnier DV, Poirson J, Nourissat A, Jacquin JP, Guastalla JP, Chauvin F. Adherence with oral chemotherapy: Results from a qualitative study of the behaviour and representations of patients and oncologists. Eur J Cancer Care 2011;20:520–7. [DOI] [PubMed] [Google Scholar]
- 20. Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy-induced nausea and vomiting: Index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence influencing factors in patients taking oral anticancer agents: A systematic review. Cancer Epidemiol. 2014;38:214–6. [DOI] [PubMed] [Google Scholar]
- 22. Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. [DOI] [PubMed] [Google Scholar]
- 23. Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 2006;42:2271–6. [DOI] [PubMed] [Google Scholar]
- 24. Noens L, van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, Martiat P, Mineur P, Van Eygen K, MacDonald K, De Geest S, Albrecht T, Abraham I. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood 2009;113:5401–11. [DOI] [PubMed] [Google Scholar]
- 25. Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, Hatfield A, Cortes J. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukemia. Pharmacoeconomics 2007;25:481–96. [DOI] [PubMed] [Google Scholar]
- 26. Zeeneldin AA, Ramadan M, Gaber AA, Taha FM. Clinico-pathological features of breast carcinoma in elderly Egyptian patients: A comparison with the non-elderly using population-based data. J Egypt Natl Cancer Inst. 2013;25:5–11. [DOI] [PubMed] [Google Scholar]
- 27. Efficace F, Baccarani M, Rosti G, Taha FM. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: An observational patient-centered outcome study. Br J Cancer 2012;107:904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Adherence to long-term therapies: Evidence for action. Geneva, Switzerland; 2003. [Google Scholar]
- 29. McCue DA, Lohr LK, Pick AM. Improving adherence to oral cancer therapy in clinical practice. Pharmacotherapy 2014;34:481–94. [DOI] [PubMed] [Google Scholar]
- 30. Castellucci LA, Shaw J, van der Salm K, Erkens P, Le Gal G, Petrcich W, Carrier M. Self-reported adherence to anticoagulation and its determinants using the Morisky Medication Adherence Scale. Thromb Res. 2015;136:727–31. [DOI] [PubMed] [Google Scholar]
- 31. Saeki H, Imafuku S, Abe M, Shintani Y, Onozuka D, Hagihara A, Katoh N, Murota H, Takeuchi S, Sugaya M, Tanioka M, Kaneko S, Masuda K, Hiragun T, Inomata N, Kitami Y, Tsunemi Y, Abe S, Kobayashi M, Morisky DE, Furue M. Poor adherence to oral and topical medication in 3069 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kesmodel SB, Goloubeva OG, Rosenblatt PY, Heiss B, Bellavance EC, Chumsri S, Bao T, Thompson J, Nightingale G, Tait NS, Nichols EM, Feigenberg SJ, Tkaczuk KH. Patient-reported adherence to adjuvant aromatase inhibitor therapy using the Morisky Medication Adherence Scale: An evaluation of predictors. Am J Clin Oncol. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33. Brier MJ, Chambless D, Gross R, Su HI, DeMichele A, Mao JJ. Association between self-report adherence measures and oestrogen suppression among breast cancer survivors on aromatase inhibitors. Eur J Cancer 2015;51:1890–6. [DOI] [PubMed] [Google Scholar]