Abstract
Introduction
According to the taxonomy of the International Association for the Study of Pain (IASP 2011), neuropathic pain (NeuP) is defined as “pain caused by a lesion or disease of the somatosensory nervous system”. NeuP is currently well-defined clinically, despite a high degree of etiological variation, and it has become a significant public health problem. This work aimed to study the situation regarding NeuP in current practice in Mali, as well as to analyze the therapeutic environment of the patients.
Methodology
This was a retrospective and cross-sectional study, carried out in two phases: (1) compilation of the files of patients according to the ICD-11, over a period of 24 months (2) a second prospective phase regarding the Knowledge, Attitudes, and Practices (KAP) of general practitioners and neurologists in regard to NeuP. The focus of the first phase of the study was the files of the patients who had undergone a consultation at the Gabriel Touré UHC. The second phase of the study focused on the general practitioners (Community Health Centers (comHC) of Bamako) and neurologists (Malian or not).
Results
Over the period of the study, 7840 patients were seen in consultation in the Department of Neurology, of whom 903 for NeuP, thus amounting to a NeuP frequency of 11.5%. Women accounted for 58.9% (532/903), with a sex ratio of 1.4. Using a comparative normal law, the difference in frequency was statistically significant between males and females (p < 10−7) and between two age groups (p 〈10−3). The 49–58 years of age group was represented the most. Diabetic NeuP (21%), lumbar radiculopathies (14%), HIV/AIDS NeuP (13%), and post-stroke NeuP (11%) were the most represented. The survey among the carers revealed: a need for training, a low level of compliance with the therapeutic guidelines, and the use of traditional medicine by the patients.
Discussion/conclusion
This work confirms that NeuP is encountered frequently in current practice, and its optimal management will involve specific training of carers and improvement of access to the medications recommended in this indication. In light of this issue, we revisit the debate regarding the concept of essential medications and the relevance of taking into account effective medications for the treatment of NeuP.
Keywords: Chronic pain, Neuropathic pain, DN4 questionnaire, Pain management, Integrative medicine, Mali, Africa
Highlights
-
•
NeuP pain, irrespective of its type (diabetic NeuP, lumbar radiculopathies, pain linked to HIV, and pain linked to stroke), is encountered frequently in our current neurology practice in Mali.
-
•
Failed to comply with the NeuP treatment guidelines, thus highlighting the need for better training of health professionals involved in NeuP management in Mali.
-
•
Amitriptyline is the only validated drug that is readily available and affordable for most patients with NeuP.
-
•
Other effective drugs to treat NeuP should be on the list of essential drugs. Optimal management of NeuP in Africa also needs to take into account the local socio-cultural context and practices regarding pain.
1. Introduction
The incidence of neurological diseases is steadily increasing throughout the world [1]. In this context, neuropathic pain (NeuP), which is well-defined clinically despite a high degree of etiological variation, is presently a major public health problem [2,3]. It is estimated that 6% to 10% of adults in the world suffer from NeuP [4,5]. In Africa, this symptom is a common comorbidity of certain endemic pathologies on the continent such as diabetes, human immunodeficiency virus and acquired immune deficiency syndrome (HIV/SAIDS), infection by hepatitis C virus, leprosy, stroke, and traumatic lesions of the limbs and the spinal cord [[6], [7], [8], [9], [10]]. In Western Africa, its prevalence at Paraku in Benin has been reported to be 6.3% in the general population [11]. In Dakar (Senegal), it has been reported to be 7.1% in the geriatric population [12]. In Ouagadougou in Burkina Faso, it has been reported to be 49.5% in people with low back pain [13]. Furthermore, its prevalence is expected to increase with the aging of the population, the diabetes epidemic, and improvement in the survival of cancer and of the HIV/AIDS pandemic [12,14].
Moreover, NeuP is associated with a significant decrease in the quality of life and the socioeconomic well-being of patients, more so than non-neuropathic chronic pain [15,16]. Furthermore, its treatment in countries with low incomes such as Mali is subject to the problem of accessibility and availability of the recommended medications [[17], [18], [19], [20]], thereby making management of this pathology in the world in general, and in Africa in particular, a challenge [[21], [22], [23], [24]].
Despite the high prevalence of NeuP, this pathology has been particularly well-studied in Africa in subgroups of the population (diabetics, people with low back pain, leprosy, elderly individuals, and people with HIV/AIDS [6,9,10,12,13]. Despite its well-documented socioeconomic consequences, the extent of the phenomena induced by NeuP in the hospital environment remains unknown to date. There have been very few studies in Mali and in Africa regarding the care that is provided for NeuP. In light of this lack of information, we have hypothesized that a global analysis of the situation regarding NeuP constitutes an indispensable pre-condition for devising a strategy tailored to the health systems of poor countries. The aim of this preliminary work in regard to the summary of our data from consultations, in parallel with a KAP survey among sufferers, was to study the status of NeuP in current practice in Mali, as well as to analyze the therapeutic environment of patients through the opinions of the sufferers. In this work we discuss the determinants of the application in sub-Saharan Africa of a conceptual framework, tailored to the sociocultural realities, that will ensure effective treatment of patients suffering from NeuP. We also revisit the concept of essential medications, based on the model list of essential medications of the World Health Organization (WHO). Most countries in sub-Saharan Africa have adopted this model list. We have reflected on the relevance of a possible integration of effective essential medications in the treatment of NeuP.
2. Methodology
2.1. Operational definitions
2.1.1. DN4
a validated questionnaire for identification of neuropathic pain based on the score obtained for 10 questions to be answered per patient, each question being awarded one point. When the score is equal to or greater than 4/10, the test is positive (sensitivity of 82.9%; specificity of 89.9%) [26,27].
2.1.2. Probable NeuP
Patients exhibiting features suggestive of NeuP such as a type of paresthesia, tingling, numbness, electric shocks, etc., for whom the questioning and the clinical examination allow establishment of (1) prior incidents of neurological lesions or disease resulting in NeuP (diabetes, HIV, stroke, low back pain); (2) symptoms in keeping with an anatomical correlation compatible with nerve damage; and (3) testing (electrophysiological, imaging) suggesting neurological damage.
2.1.3. Certain NeuP
Patients exhibiting pain that is neuropathic in nature (paresthesia, tingling, numbness, electrical shocks, etc.) in a context of neurological damage in conjunction with a well-documented underlying pathology (imaging, electromyoneurography) and positivity of the DN4 questionnaire (a score greater than 4/10).
2.2. Type and timing of the study
This was a retro-prospective, descriptive, cross-sectional study carried out in two phases, A first retrospective phase regarding the status of NeuP in Mali in the Department of Neurology of the Gabriel Touré UHC of Bamako based on the compilation of patient files over a period of 24 months from January 1st, 2017 to December 31st, 2018. A second prospective phase comprising an evaluation of the knowledge, attitudes, and practices (KAP) of the general practitioners and neurologists in regard to the treatment of NeuP, which took place from January 2nd, 2019 to June 30th, 2019.
2.3. Site of the study
The Department of Neurology of the Gabriel Touré University Hospital of Bamako (GT UHC) constituted the main site of the study. It is a 3rd reference facility of the health pyramid of the country. This department is strategic in terms of training of general practitioners and specialists in neurology. Three-quarters of the neurologists of the department obtained formal training in regard to the diagnosis and management of pain from peripheral neuropathy during their syllabus in general neurology.
2.4. Population of the study
The focus of the first phase of the study was the files of the patients who had undergone a consultation at the Gabriel Touré UHC. The second phase of the study focused on the general practitioners (Community Health Centers (comHC) of Bamako) and neurologists (Malian or not).
2.5. Sampling
The sampling for the first phase included all of the files of the eligible patients. The sampling for the second phase of the study was carried out by random selection of general practitioners in each of the 57 comHC of Bamako. In this group, 45 general practitioners agreed to participate in the study. We undertook a simple random sampling of 60 neurologists who were members of the African Federation of Neurology (AFAN). We contacted these 60 neurologists individually by email and we sent them the KAP questionnaire. Given the number of respondents among the general practitioners, the research team decided to retain the first 45 participants who responded to our request out of the total of 53 neurologists who replied to the email. These comprised 20 West African neurologists from the following countries: Mali, Benin, Ivory Coast, Guinea, Senegal, Niger, Togo, and Nigeria; 13 neurologists from central Africa (Cameroon, Chad, Gabon, and Central Africa); nine (9) practitioners from the Maghreb (Morocco, Tunisia, and Mauritania); three neurologists from Eastern and Southern Africa (Djibouti and Kenya). The comparison between the two groups of practitioners involved the same number.
2.6. Criteria for inclusion
The first phase of the study included the files of the patients seen at the GT UHC who underwent an exhaustive clinical evaluation (questioning and physical examination revealing a pathology resulting in NeuP; presence in the file of paraclinical examination necessary for the diagnosis). In the second phase, we surveyed health professionals (neurologists throughout Africa and general practitioners at 57 community health centers in Bamako).
2.7. Criteria for non-inclusion
Incomplete files or those for which the neuropathic nature had not been proven by the clinical elements and the diagnostic tool (the DN4 questionnaire) were not included in the first phase of the study.
2.8. Tools and procedures for collection of the data
The data for the first phase of the study were collected from patient files containing sociodemographic, clinical (questioning, neurological and physical examination), the data for the additional and the therapeutic examinations, and the follow-up data. The files were compiled according to the guidelines of the International Association for the Study of Pain (IASP) and the WHO. Files of patients suffering from NeuP were retained: persistent or recent pain lasting more than three (3) months [25]. The files were categorized according to the 11th edition of the International Classification of Diseases (ICD-11) [26,27].
The first questionnaire for the collection of data in the consultation registries was in regard to the sociodemographic characteristics of the patients, the typologies, the clinical data, and the type of treatment received.
The 2nd questionnaire was in regard to the knowledge, attitudes, and practices of the carers (general practitioners versus neurologists). This questionnaire was designed specifically for the practitioners: their sociodemographic data and their knowledge, attitudes, and their practices regarding NeuP, the place of pain/NeuP and the most common types of NeuP in their daily practices, and their opinions regarding their patients' behaviors, especially in terms of their treatment of NeuP.
2.9. Statistical analysis of the data
The data were collected and analyzed with Prism GraphPad version 8.0 software. Frequency tables were generated, and calculation of the means were undertaken. The Chi2 and Fisher's exact test were used to compare the proportions, with a threshold of significance set at p = 0.05. Simple logistic regression was used for the measures of association, with presentation of the odds ratios and their 95% confidence intervals.
2.10. Ethical considerations
The data in the patient files were collected in a strictly anonymous manner and with approval from the relevant authorities of the UHC. The Medical Work Committee of the UHC, which oversees the ethics committee and has an advisory role regarding the management of clinical research activities provided approval for this study to be undertaken. For active involvement of the comHC, the national health board of Mali was informed in writing. The AFAN administration was informed of the study by email. The practitioners did not receive any payment for participation in the study. The practitioners were informed of the objective and the benefits of the study, and their free and informed consent was obtained by email prior to their inclusion.
3. Results
3.1. Epidemiological and clinical situation of patients with NeuP in our practice
3.1.1. Sociodemographic characteristics
During the two years of the study, 7840 patients were seen in outpatient consultation in the department, and 903 cases of the patients exhibiting NeuP were recorded, thus amounting to a frequency of 11.5%. Women were represented more, at 58.9% (532/903) of the patients, thus amounting to a sex-ratio of 1.4. The 49–58 years of age, the 39–48 years of age, and the 29–38 years age groups were represented the most at 25.7% (217/903); 24.1% (217/903); and 22.1% (200/903), respectively. Using a comparative normal law, the difference in frequency was statistically significant between men and women (p < 10−7) and between two age groups (p < 10−3) (Table 1).
Table 1.
Sociodemographic characteristics of the study population.
| Sociodemographic characteristics | Files surveyed |
95% CI | p | |
|---|---|---|---|---|
| N (%) | ||||
| Gender | Women | 532 (58.9) | [55.7–62.1] | 10–7* |
| Men | 371 (41.1) | [37.9–44.3] | ||
| Sex ratio | 1.4 in favor of female gender | |||
| Age bracket in years | 18–28 | 74 (8.2) | [6.5–10.1] | 10–3* |
| 29–38 | 200 (22.1) | [19.5–24.9] | ||
| 39–48 | 217 (24.1) | [21.3–26.9] | ||
| 49–58 | 232 (25.7) | [22.9–28.6] | ||
| 59–68 | 110 (12.2) | [10.2–14.4] | ||
| 69–78 | 40 (4.4) | [3.2–5.9] | ||
| > 79 | 30 (3.3) | [2.3–4.6] | ||
3.1.2. The main types of neuropathic pain
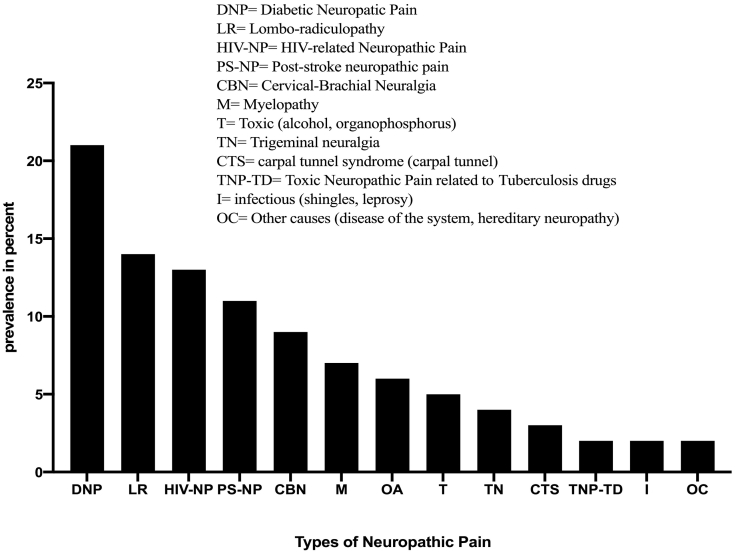
Diabetic neuropathic pain was the most represented type, at 21% (186/903), followed by lumbar radiculopathies, at 14% (129/903). Toxic neuropathic pain linked to antituberculosis drugs, pain of infectious origin (leprosy, Zona virus); the so-called “rare” causes in our study (pain linked to diseases of the nervous system, hereditary, fibromyalgia, Pudendal neuralgia, Arnold's neuralgia, and glossopharyngeal neuralgia) were less represented, at 2% for each group (Fig. 1).
Fig. 1.
Neuropathic pain typologies in our practice.
3.1.3. Clinical characteristics of the NeuP
Women were more represented in eight types of neuropathic pain. This predominance was much more the case for neuropathic pain linked to HIV, trigeminal neuralgia, entrapment syndrome, and diabetic neuropathy, at 73.1% (87/119); 72.9% (27/37); 70.9% (22/31), and 69.9% (130/186), respectively. There was a statistically significant difference between gender and the types of neuropathic pain (with Chi2 = 54.69; p < 0.0001). With trigeminal neuralgia as the reference, there were more men suffering from all types of neuropathic pain than women. Post-stroke neuropathic pain (2.98 times), toxic neuropathic pain linked to antituberculosis drugs (4.63 times), lumbar radiculopathy (2.74 times), myelopathy (4.26 times), and alcohol and organophosphorus-induced neuropathic pain (3.45 times) were more frequent in men compared to women (Table 2).
Table 2.
Distribution of the various types of neuropathic pain according to gender.
| Neuropathic pain typologies | Gender of the patients (%) |
Odds Ratio |
|||
|---|---|---|---|---|---|
| Files surveyed |
M |
F |
OR [95% CI] |
p-value |
|
| N | n (%) | n (%) | |||
| Post-stroke neuropathic pain | 103 | 54 (52.4) | 49 (47.6) | 2.98 [1.31;6.77] | 0.008 |
| Diabetic neuropathic pain | 186 | 56 (30.1) | 130 (69.9) | 1.16 [0.53;2.56] | 0.71 |
| HIV-linked neuropathic pain | 119 | 32 (26.9) | 87 (73.1) | 0.99 [0.43;2.28] | 0.99 |
| Toxic neuropathic pain linked to antituberculosis drugs | 19 | 12 (63.2) | 7 (36.8) | 4.63 [1.42;15.08] | 0.009 |
| Cervicobrachial neuralgia | 81 | 32 (39.5) | 49 (60.5) | 1.76 [0.75;4.13] | 0.19 |
| Lumbar radiculopathy | 129 | 65 (50.4) | 64 (49.6) | 2.74 [1.23;6.12] | 0.01 |
| Myelopathy | 67 | 41 (61.2) | 26 (38.8) | 4.26 [1.77;10.23] | 0.0008 |
| Toxic (alcohol. organophosphorus) | 41 | 23 (56.1) | 18 (43.9) | 3.45 [1.33;8.94] | 0.009 |
| Infectious (Zona, leprosy) | 17 | 9 (52.9) | 8 (47.1) | 3.04 [0.92;10.06] | 0.06 |
| Other radiculopathies (lumbar spinal stenosis, post-surgery of the spinal cord) | 57 | 23 (40.4) | 34 (59.6) | 1.83 [0.74;4.48] | 0.19 |
| Entrapment syndrome (carpal tunnel) | 31 | 9 (29.0) | 22 (71.0) | 1.10 [0.38;3.19] | 0.85 |
| Other causes (nervous system disease, hereditary neuropathy, fibromyalgia, Pudendal neuralgia, glossopharyngeal. Arnold's neuralgia) | 16 | 5 (31.2) | 11 (68.8) | 1.23 [0.34;4.42] | 0.75 |
| Trigeminal neuralgia | 37 | 10 (27) | 27 (73.0) | 1 (ref) | – |
| Total | 903 | 371 (41.1) | 532 (58.9) | ||
Bold is to focus on the P that is significant.
A high intensity of neuropathic pain was associated with certain etiologies: diabetic neuropathic pain 38.6% (46/119), toxic neuropathic pain linked to antituberculosis drugs 54.3% (44/81), cervicobrachial neuralgias 57.9% (11/19), lumbar-radiculopathies 39.5% (51/129), myelopathies 40.3% (27/67), neuropathies of toxic origin 51.2% (21/41), entrapment syndromes 38.7% (12/31), and systemic or hereditary causes 56.3% (9/16) (Fig. 2). They were mainly considered to be moderate for post-stroke neuropathic pain and neuropathic pain linked to HIV at 41.7% (43/103) and 59.1% (110/186), respectively (Fig. 2). The pain was deemed to be very intense for neuropathies of infectious origin, at 52.9% (9/17), and trigeminal neuralgias, at 56.8% (21/37) (Fig. 2). A statistically significant difference was observed between the intensity of the pain and the various etiologies of neuropathic pain (p < 0.0001).
Fig. 2.
Distribution of the intensity of the pain according to the neuropathic type.
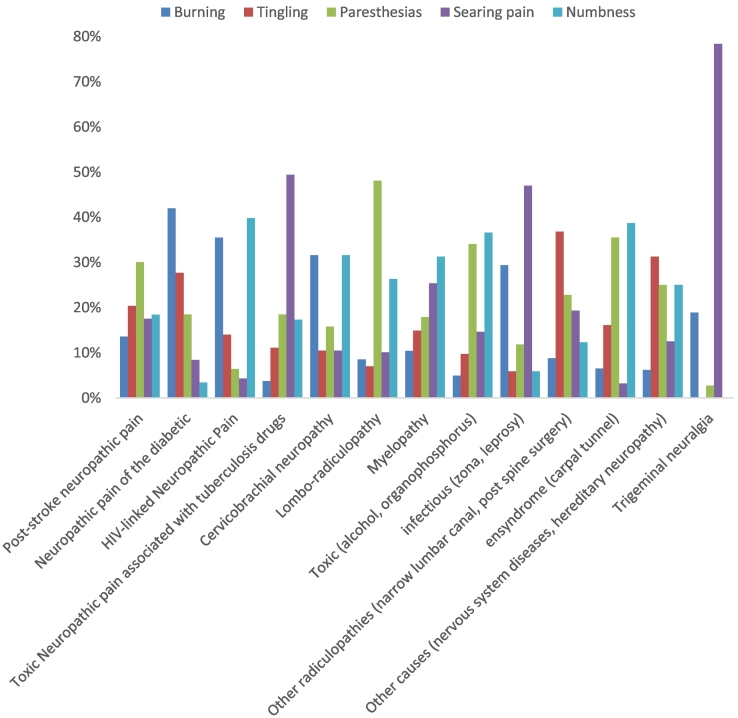
A burning sensation was the type of pain encountered most in diabetic neuropathies, at 42% (50/119) (Fig. 3). Paresthesia was the most frequently mentioned type of pain by patients suffering from post-stroke neuropathic pain, at 30.1% (31/103), and lumbar radiculopathies, at 48.1% (62/129) (Fig. 3). A burning sensation was encountered most with toxic neuropathic pain linked with antituberculosis drugs, at 49.4% (40/81); neuropathies of infectious origin, at 47% (8/17); and trigeminal neuralgias, at 78.4% (29/37) (Fig. 3). Numbness was mainly encountered with neuropathic pain linked to HIV, at 39.8% (74/186); myelopathies, at 31.3% (21/67); neuropathies of toxic origin, at 36.6% (15/41); and entrapment syndrome, at 38.7% (12/31) (Fig. 3). With cervicobrachial neuralgias, a distribution of burning and numbness was noted, with 31.6% (6/19) each, as well as burning and searing pain, each at 10.5% (2/19) (Fig. 3).
Fig. 3.
Distribution of the type of pain according to the neuropathic type.
3.1.4. Knowledge, attitudes, and practices (KAP) of the prescribers in regard to NeuP
The KAP survey involved 45 general practitioners and 45 neurologists representing participation rates of 79% and 75%, respectively.
3.1.5. Knowledge of the doctors regarding NeuP
The general practitioners and the neurologists mainly mentioned HIV and diabetes as the etiologies of neuropathic pain encountered in consultation. For the general practitioners, the frequencies were 31.1% and 26.7%, respectively versus 20% for each type for the neurologists (Table 3). Sleep disorders (28%) and anxiety (26.7%) were mentioned as the main consequences of the neuropathy by the neurologists, versus sleep disorders (24.4%) and a lower productivity (20%) for the general practitioners (Table 3).
Table 3.
Knowledge of the carers regarding NeuP.
| GPs |
Neurologists |
Odds ratio (95% CI) | p | ||
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| The main etiologies according to the carers | Lumbar radiculopathies | 5 (11.1) | 7 (15.5) | Ref | |
| Diabetes | 12 (26.7) | 9 (20) | 0.5 [0.1; 2.3] | 0.39449 | |
| HIV | 14 (31.1) | 9 (20) | 0.5 [0.1; 1.9] | 0.28293 | |
| Alcohol | 3 (6.7) | 4 (8.9) | 1 [0.1; 6.3] | 0.95957 | |
| Stroke | 4 (8.9) | 8 (17.8) | 1.4 [0.3; 7.5] | 0.67378 | |
| Toxic | 2 (4.4) | 2 (4.4) | 0.7 [0.1; 6.9] | 0.77154 | |
| Medications | 4 (8.8) | 3 (6.7) | 0.5 [0.1; 3.5] | 0.51663 | |
| Deficiencies | 1 (2.2) | 3 (6.7) | 2.1 [0.2; 27.1] | 0.55608 | |
| Presumed impact on the quality of life according to the carers | Sleep impairment | 11 (24.4) | 13 (28.9) | Ref | |
| Reduced productivity | 9 (20) | 5 (11.1) | 0.5 [0.1; 1.8] | 0.2754 | |
| Anxiety | 8 (17.8) | 12 (26.7) | 1.3 [0.4; 4.2] | 0.69748 | |
| Depression | 9 (20) | 11 (24.4) | 1 [0.3; 3.4] | 0.95592 | |
| Dependence/loss of autonomy | 4 (8.9) | 1 (2.2) | 0.2 [0; 2.2] | 0.19205 | |
| Relationship problems | 4 (8.9) | 3 (6.7) | 0.6 [0.1; 3.5] | 0.59981 | |
| Drugs that are effective against NeuP according to the carers | Amitriptyline | 7 (15.5) | 12 (26.7) | Ref | |
| Gabapentin | 5 (11.1) | 9 (20) | 1.1 [0.2; 4.4] | 0.94693 | |
| Pregabapentin | 4 (8.9) | 8 (17.8) | 1.2 [0.3; 5.3] | 0.84241 | |
| Antidepressant (IRS) | 1 (2.2) | 2 (4.4) | 1.2 [0.1; 15.3] | 0.9066 | |
| Carbamazepine | 7 (15.5) | 4 (8.9) | 0.3 [0.1; 1.6] | 0.16262 | |
| Opioids | 2 (4.4) | 1 (2.2) | 0.3 [0; 3.8] | 0.34834 | |
| Cannabinoids | 2 (4.4) | 2 (4.4) | 0.6 [0.1; 5.1] | 0.62643 | |
| Capsaicin patch | 1 (2.2) | 2 (4.4) | 1.2 [0.1; 15.3] | 0.9066 | |
| Botulinum toxin | 1 (2.2) | 1 (2.2) | 1.2 [0.1; 15.3] | 0.9066 | |
| Paracetamol | 4 (8.9) | 1 (2.2) | 0.1 [0; 1.6] | 0.11305 | |
| Tramadol | 6 (13.3) | 2 (4.4) | 0.1 [0; 1] | 0.04828 | |
| NSAIDs | 5 (11.1) | 1 (2.2) | 0.1 [0; 1.2] | 0.07202 | |
| Main criteria for choosing a drug in the treatment of NeuP | Cost | 17 (37.8) | 15 (33.3) | Ref | |
| Efficacy | 12 (26.7) | 11 (24.4) | 1 [0.4; 3] | 0.94444 | |
| Availability | 13 (28.9) | 12 (26.7) | 1 [0.4; 3] | 0.93273 | |
| Tolerance | 3 (6.7) | 7 (15.5) | 2.6 [0.6; 12.1] | 0.20996 | |
At the therapeutic level, the neurologists mainly prescribe amitriptyline (26.7%), gabapentin (20%), and pregabalin (17.8%) as the three most effective drugs to treat neuropathy, versus amitriptyline (15.5%), carbamazepine (15.5%), and tramadol (13.3%) for the general practitioners (Table 3). The cost of the drug and its availability constituted the main criteria for the choice, both for the general practitioners (37.8% and 28.9%, respectively) and the neurologists (33.3% and 26.7%) (Table 3). The survey among the neurologists provided the price of several drugs in African countries that are currently used for NeuP, relative to the minimum wage (IGMW) of the country (Table 4).
Table 4.
Cost of some of the drugs used in the medical treatment of neuropathic pain in 10 African countries relative to the minimum industrial wage of the countries (official sources).
| Country | Drugs; Market price for one month of treatment (US dollars) |
Minimum wage (US dollars) | ||||
|---|---|---|---|---|---|---|
| Neurontin® Gabapentin 300 mg | Lyrica 75 mg® Pregabalin 75 mg | Topalgic 100® Tramadol 100 mg | Laroxyl 25 mg® Amitriptyline 25 mg | Tegretol® Carbamazepine 400 mg | ||
| Mali | 48 | 62 | 68.70 | 7.17 | 9.29 | 80.69 |
| Benin | 39.49 | 83.05 | 19.85 | 5.46 | 9.46 | 80.28 |
| Cameroon | – | 51.03 | 4.19 | 5.31 | 9.45 | 72.53 |
| Ivory Coast | 68.50 | 52.99 | 8.32 | 5.34 | 11.14 | 119.99 |
| Djibouti | 61 | 38 | 5 | 7.50 | 9.14 | 85.89 |
| Morocco | 67.34 | 116.60 | 21.70 | 16.47 | 8.95 | 336.67 |
| Niger | 41.28 | 52.38 | 16.48 | 5.85 | 11.60 | 60.08 |
| Senegal | 87.36 | 65.65 | 16.64 | 5.50 | 8.70 | 95.39 |
| Burkina Faso | 45.80 | 44.27 | 6.10 | 4.58 | 10.68 | 64.42 |
| Togo | 53.78 | 48.12 | 4.26 | 6.30 | 10.20 | 69.99 |
Tolerability (safety) of a drug was raised by 15.5% of the neurologists as a criterion for the choice versus 6.7% of the general practitioners (Table 5).
Table 5.
Attitudes and practices of the carers in regard to NeuP.
| Evaluation of the last 3 weeks of consultation | GPs |
Neurologists |
Odds ratio (95% CI) | p | |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Proportion chronic pain | Less than 20% of the consultations | 4 (8.9) | 4 (8.9) | Ref | |
| Between 20 and 40% | 13 (28.9) | 9 (20) | 1.1 [0.2; 5.4] | 0.86681 | |
| Between 40 and 60% | 12 (26.7) | 18 (40) | 0.8 [0.3; 2.4] | 0.6798 | |
| More than 60% | 16 (35.5) | 14 (31.1) | 1.7 [0.6; 4.8] | 0.30211 | |
| Reasons for the consultation for pain | Headaches/migraine | 13 (28.9) | 12 (26.7) | Ref | |
| Low back pain and lumbar radiculopathy | 15 (33.3) | 11 (24.4) | 0.8 [0.3; 2.4] | 0.68315 | |
| Neurological pain | 7 (15.5) | 11 (24.4) | 1.7 [0.5; 5.8] | 0.3951 | |
| Osteo-articular pain | 4 (8.9) | 5 (11.1) | 1.4 [0.3; 6.3] | 0.6974 | |
| Oral facial pain | 2 (4.4) | 3 (6.7) | 1.6 [0.2; 11.5] | 0.6242 | |
| Post-traumatic pain | 3 (6.7) | 1 (2.2) | 0.4 [0.0; 0.4] | 0.3904 | |
| Psychogenic pain | 1 (2.2) | 2 (4.4) | 2.2 [0.2; 27.1] | 0.5412 | |
| Elements indicative of neuropathy | Context of the occurrence (notion of nerve injury) | 7 (15.5) | 16 (35.5) | Ref | |
| Description of the pain upon questioning (burning, heat, discharge) | 17 (37.8) | 12 (26.7) | 0.3 [0.1; 1] | 0.04624 | |
| Presence of discomfort/pain (numbness, tingling; paresthesias) | 19 (42.2) | 7 (15.5) | 0.4 [0.1; 1.2] | 0.09565 | |
| Location of the pain (vicinity of a nerve) | 2 (4.4) | 10 (22.2) | 0 [0; 0] | 0.99938 | |
| Tools for diagnosis known and used | No tool | 29 (64.4) | 3 (6.7) | Ref | |
| Yes (DN4) | 15 (33.3) | 35 (77.8) | 22.6 [5.9; 85.6] | 0.0001 | |
| Other diagnostic tools in addition to the DN4 | 1 (2.2) | 7 (15.5) | 67.7 [6.1; 752.6] | 0.001 | |
| Tools for evaluation of the intensity of the pain | Questioning | 18 (40) | 5 (11.1) | Ref | |
| Numerical scale (NS) | 11 (24.4) | 11 (24.4) | 3.6 [1; 13.2] | 0.05276 | |
| Analogue scale (AS) | 9 (20) | 16 (35.5) | 6.4 [1.8; 23.1] | 0.0046 | |
| Simple visual scale (SVS) | 7 (15.5) | 13 (28.9) | 6.7 [1.7; 25.8] | 0.00585 | |
Bold is to focus on the P that is significant.
3.1.6. Attitudes and practices in the treatment of NeuP
The majority of general practitioners (35.5%) stated that chronic pain was involved in more than 60% of their consultations, unlike the neurologists, of whom the majority (40%) asserted that this proportion ranged from 40 to 60% (Table 5). Headaches/migraines, low back pain and lumbar-radiculopathies, as well as neurological pain, were also mentioned as reasons for a consultation by 28.9%, 33.3%, and 15.5% of the general practitioners and 26.7%, 24.4%, and 24.4% of the neurologists, respectively (Table 5). There was a statistically significant difference between the neurologists and the general practitioners in the diagnostic approach (elements of orientation), with an odds ratio (OR) of 0.3 [0.1–1] and p = 0.04624 (Table 5). The majority of the general practitioners (64.4%) stated that they did not use any known diagnostic tool, unlike the neurologists, for whom the majority (77.8%) stated that they used the DN4 questionnaire as a diagnostic tool (Table 5). The neurologists were 22.6 [5.9–85.6] times more likely to use the DN4 questionnaire than the general practitioners. This probability was 67.7 [6.1–752.6] times for the use of other tools in addition to the DN4 questionnaire (p = 0.001). The intensity of the pain was evaluated during the questioning by the majority of the general practitioners (40%) and based on an analog scale (AS) by the majority of the neurologists (35.5%). The neurologists were 6.4 [1.8–23.1] and 6.7 [1.7–25.8] times more likely to use an analog scale and a simple visual scale (SVS), respectively, than the general practitioners (p = 0.0046 and p = 0.00585) (Table 5).
In terms of the views of the doctors regarding the therapeutic program of the patients, the majority of the general practitioners (84.4% or 38/45) and the neurologists (64.4% or 29/45) asserted that the patients used both conventional and traditional medicine. There was a larger number of general practitioners who stated cases of combinations of the two therapeutic methods, with an odds ratio of 0.4211 [0.2289–0.7472] and p-value of 0.003. The general practitioners, as well as the neurologists, for the most part, indicated that the beliefs of the populations were the main cause for the use of traditional practices, at 28.9% (13/45) and 33.3% (15/45), respectively. This attitude of the patients was tolerated by the majority of the carers who were questioned (general practitioners and neurologists). The most widely used traditional practice according to the general practitioners was phytotherapy, at 26.7% (13/45), while for the neurologists, phytotherapy and scarification were designated as being the most widely used, at 28.9% (13/45) each (Table 6).
Table 6.
Opinions of the doctors regarding the therapeutic environment of the patients in regard to NeuP.
| Opinions of the prescribers | GPs |
Neurologists |
Odds ratio (95% CI) | p | |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| According to your experience, what is the proportion of your patients who have recourse to: | Strict conventional medicine | 7 (15.6) | 16 (35.6) | Ref | |
| Traditional medicine associated with conventional medicine | 38 (84.4) | 29 (64.4) | 0.421 [0.2289; 0.7472] | 0.0013 | |
| According to your experience, what is the factor that explains recourse to traditional medicine by patients with chronic pain? | Beliefs | 13 (28.9) | 15 (33.3) | Ref | |
| High cost of medications in conventional medicine | 11 (24.4) | 12 (26.7) | 1.273 [0.5729; 2.885] | 0.34 | |
| High cost of consultations in conventional medicine | 7 (15.6) | 5 (11.1) | 2 [0.8149; 5.291] | 0.06 | |
| The efficacy of traditional medicine | 5 (11.1) | 4 (8.9) | 2.8 [1.038; 8.671] | 0.02 | |
| The accessibility of traditional therapists | 7 (15.6) | 4 (8.9) | 2 [0.8149; 5.291] | 0.06 | |
| The difficulties obtaining a consultation in conventional medicine | 2 (4.4) | 5 (11.1) | 7 [1.815: 45.48] | 0.001 | |
| How do you view the use of traditional medicine by the patients? | I appreciate this practice | 7 (15.6) | 8 (17.8) | Ref | |
| I tolerate this practice | 16 (35.5) | 17 (37.8) | 0.5 [0.2025; 1.157] | 0.05 | |
| I believe in the efficacy of this practice | 12 (26.7) | 7 (15.5) | 0.6667 [0.2595;1.639] | 0.19 | |
| I think that the association of this practice is better | 5 (11.1) | 4 (8.9) | 1.6 [0.5173; 5.376] | 0.2 | |
| I do not appreciate this practice | 3 (6.7) | 6 (13.3) | 2.667 [0.7298; 12.42] | 0.07 | |
| I recommend against this practice | 2 (4.4) | 3 (6.7) | 4 [0.9249; 27.57] | 0.03 | |
| According to your experience, what is the traditional practice most used by your patients with chronic pain/NeuP? | Phytotherapy | 12 (26.7) | 13 (28.9) | Ref | |
| Recourse to marabouts (gris-gris, reciting Koranic verses | 8 (17.8) | 11 (24.4) | 1.625 [0.6731; 4.125] | 0.14 | |
| Traditional manual methods (massage) | 9 (20) | 5 (11.1) | 1.444 [0.6141; 3.52] | 0.2 | |
| scarification | 5 (11.1) | 13 (28.9) | 2.6 [0.9505; 8.123] | 0.03 | |
| Prayers | 11 (24.4) | 3 (6.7) | 1.182 [0.523; 2.709] | 0.3 | |
Bold is to focus on the P that is significant.
4. Discussion
4.1. NeuP is a prominent issue in current practice
This work, the first of its kind in Western Africa, was purposefully carried out in two phases (retrospective and prospective) to undertake a situational analysis of NeuP in our department and to carry out a KAP study of carers in terms of the treatment of NeuP, respectively. NeuP was, therefore, assessed from different perspectives in our context. Our results combined with the existing literature yielded preliminary data on the burden of NeuP in Mali and laid the foundation for context-tailored therapeutic strategies.
The use of validated tools minimized the common selection bias seen in NeuP studies [26]. Based on the ICD-11 guidelines, NeuP was categorized as peripheral or central. We included solely patient files with a valid neurological exam and a completed NeuP DN4 questionnaire, (if needed). The diagnosis of NeuP was based on a rigorous and purely clinical approach along with the use of diagnostic tools. These tools are relevant, but the clinical assessment remains the cornerstone of NeuP studies [27,28,29].
NeuP was presented as moderate to severe burning, paresthesias, tingling, searing pain, or numbness depending on the underlying causes, in keeping with the literature [12,30,31,36,37]. NeuP represented 11.5% of all outpatient visits in our Department of Neurology. We noted a relatively higher prevalence of NeuP (11.5%) compared to 6.3% in Benin [11] and 7.1% in Senegal [12]. This difference could be due to several reasons: (1) the hospital setting of our study, which took place in a department of neurology; (2) the expertise of our team was essentially comprised of neurologists, of whom ¾ had received specific formal training in regard to pain; and (3) current and frequent use of the DN4 questionnaire in our department. We emphasize that this questionnaire is easy to use and had a sensitivity of 80% and a specificity of 92%. [30]. In practice, our results were in keeping with the data in the world literature, for which the estimates of the prevalence of NeuP are between 6.5% and 17.9% [31]. These findings support the fact that the DN4 questionnaire, which is a more reliable tool than the painDETECT questionnaire, has been translated and validated in many languages in the world [32,33].
4.2. Sociodemographic characteristics
This work revealed that NeuP primarily affects working-age adults (i.e., individuals less than 60 years of age) and particularly women, with predictable socio-economic consequences. In this regard, our results confirm the data of the literature in Africa and in the world in general [11,31,34]. In France, Bouhassira et al. reported NeuP in 8% of women versus 5.7% in men, and 8.9% in patients over 50 years old versus 5.6% in those <49 years of age [35].
4.3. Etiologies of NeuP in our context
Many central and/or peripheral nervous system disorders can cause NeuP [41]. In 78.7%, NeuP was linked to a peripheral nervous system pathology such as diabetic neuropathy, neuropathy linked with HIV/AIDS, toxic neuropathy (antituberculosis drugs, alcohol, organophosphorus compounds), infectious neuropathy (Varicella Zona virus, leprosy), lumbar-radiculopathy, cervicobrachial neuralgia, lumbar spinal stenosis radiculopathy, postsurgery spinal radiculopathy, progressive polyneuropathy, genetic neuropathy, fibromyalgia, Pudendal nerve entrapment, trigeminal neuralgia, and Chiari malformation. In 19.2%, NeuP was linked to a central nervous system disease such as post-stroke neuropathic pain and NeuP in relation to a myelopathy. NeuP can also be idiopathic [31].
Diabetic NeuP (21%), lumbar-radiculopathies (14%), HIV/AIDS NeuP (13%), and post-stroke NeuP (11%) were the four most common etiologies of NeuP. Similar trends have typically been reported in the literature [[35], [36], [37], [38], [39], [40]].
In 21%, NeuP was linked to diabetes due to its high prevalence in Mali and in Africa in general. In total, 7.1 million Africans currently suffer from diabetes, and this number is expected to increase to 18.6 million by 2030 [6]. Mali has a 7% prevalence of diabetes [42]. Diabetic polyneuropathy (DPN) with or without neuropathic pain potentially affects 50% of people with diabetes [[43], [44], [45]]. The prevalence of painful diabetic polyneuropathy was as high as 30.3% in a large multicentric study in South Africa [46].
In 14%, NeuP was linked to lumbar radiculopathy in our study. NeuP was found in 49.5% of the individuals with low back pain in Burkina Faso [13] and 40% in Germany [47]. In regard to NeuP, low back pain deserves more attention from the scientific community and the policy-makers as suggested in Lancet [48,49].
In 13%, NeuP was linked to HIV/AIDS in our study (Table 2) due to a 1.1% prevalence of HIV in Mali. Distal neuropathy has become very common with combined antiretroviral therapy [50]. It is not only severe but also often resistant to the currently available NeuP therapeutic arsenal [52,53]. In 2011, the prevalence of NeuP using the DN4 questionnaire in 600 patients with HIV was 20% [54,55]. In the Democratic Republic of Congo, 3.12% of the patients with HIV had neurological complications, and over 80% had NeuP [56]. NeuP can occur at any stage of the HIV/AIDS progression and it affects 55% of the patients taking ARV drugs for various reasons (the virus itself, HIV-related infections, ARV, and anti-TB drugs) [51,52]. It has been well-documented that ARTs result in NeuP, which negatively impacts the quality of life of patients. It is, therefore, important to facilitate patient access to drugs that are effective against NeuP through NeuP management programs. In addition to the effort to make ARVs widely available to patients with HIV, the potential to cause NeuP should be also considered [57].
In 11%, NeuP was linked to post-stroke outcomes in our study (Table 2). In a study in Nigeria, 50% of the patients exhibited post-stroke NeuP after a 3-month follow-up [62]. NeuP can affect up to 70% of patients after a stroke if properly diagnosed [[63], [64], [65], [66], [67], [68]]. An increased prevalence of NeuP after stroke has been reported in low-income countries [58,59]. In sub-Saharan Africa, stroke has been increasing in prevalence in line with demographic transitions, lifestyle changes, and subsequent health risk factors (hypertension, diabetes, obesity, etc.) [60]. For instance, stroke is by far the primary cause of hospitalization stays and mortality in our neurology department [61].
4.4. Prominent place of NeuP in daily medical and neurology practices
NeuP represented over 40% of the outpatient visits for general medical practitioners (55.4%) and neurologists (71%) (Table 5), which suggests a relatively high prevalence of NeuP in Africa [[69], [70], [71], [72]]. The current neurology curriculum at the medical school does not adequately prepare general medical practitioners to diagnose and refer NeuP patients for treatment. Postgraduate training is needed for this purpose. Similarly, young neurologists should be trained in the proper management of NeuP in clinical practice in “real life” [72].
4.5. Need for training in NeuP management
Most general medical practitioners in our study reported using the WHO list of essential drugs for the management of NeuP. However, they are often unaware that antidepressant and antiepileptic drugs could be used in NeuP in addition to their indication in depression and epilepsy, respectively [80,84]. Both the WHO and the International Association for the Study of Pain have emphasized the lack of qualified personnel as well as the paucity of diagnostic and therapeutic tools in Africa [2,22,24,73,74]. Patients with NeuP are cared for by poorly trained health professionals [75].
On the one hand, we found that the general medical practitioners (64.4%) and the neurologists (6.6%) were not aware of any diagnostic tool for NeuP. On the other hand, a patient satisfaction survey showed that less than one-third experienced over 30% pain relief [2]. The diagnostic and management of chronic pain/NeuP should be part of any effective continuing training for general medical practitioners and neurologists [72,76,77,78,79].
4.6. Limited compliance with current NeuP therapeutic guidelines in Mali
In our survey, amitriptyline, carbamazepine, and tramadol were the drugs most often prescribed by the general practitioners (Table 3), while for the neurologists these were amitriptyline; gabapentin, and pregabalin. Amitriptyline, which is the most available NeuP drug, is the only drug featured in the guidelines for general medical practitioners [2]. It is validated for treating NeuP and featured on the WHO model list of essential medications [80], despite its side effects [74,82]. For both the general medical practitioners and the neurologists in our study, amitriptyline was the most prescribed drug for patients with NeuP, which is in keeping with the literature [83]. General medical practitioners tend to prescribe the most accessible and the least expensive drugs for their patients [24]. No analgesic medications on this list of the WHO are recommended as first-line treatment of NeuP [2,24,81], which hinders NeuP treatment [24]. Consequently, current health policies do not favor optimal management of NeuP in Africa because, except for amitriptyline, no other effective drugs against NeuP are listed as essential medicines, which limits the range of available drugs for the treatment of NeuP. The WHO should revise its list of essential medicines to accommodate drug-based treatment of NeuP [24,[85], [86], [87]].
In practice, a sufficiently strong therapeutic need and sufficient evidence have been shown to warrant inclusion of the additional medications recommended for the treatment of NeuP in the next editions of the model lists of the WHO [24,88]. This will certainly allow the medications to be cheaper and more accessible to patients with NeuP.
4.7. Optimal management of NeuP in the Malian and African contexts
Traditional medicine was reported to be an integral part of the therapeutic program of patients with pain in our study. It remains the first option for most people in rural Africa, whether or not they are literate [89,90]. Both general medical practitioners and neurologists tolerated its use because it is strongly embedded in our culture and because conventional medicines are also less affordable due to their cost. Patients with pain often resort to phytotherapy, fumigation, incantations, gris-gris, scarification, marabouts, and prayers, which stems directly from the cultural representations of pain. The disease is thought to be caused by occult forces in addition to natural causes [93,94], which may justify the simultaneous use of traditional and conventional medicines.
In NeuP, burning, electric discharge, tingling, and numbness are what mislead many patients and general medical practitioners in Africa. In Parakou (Benin, Western Africa), patients described NeuP as “unusual, odd, or even bizarre”. This unusual symptomatology could in part explain the alternative choice of traditional and herbal therapists [11]. In Nigeria, a study of musculoskeletal pathologies in people with HIV/AIDS revealed nearly systematic patient recourse to a combination of traditional medicine and conventional medicine [91]. Even in the U.S., indigenous populations (American Indians) generally rely on traditional medicine. A greater complementarity between conventional and non-conventional medicines could become standard in facilities that provide health care to multiethnic and racial groups [92]. American Indians explain pain in a very vague way compared to Caucasians. Therefore, currently used standard pain scales and questionnaires may not be a good match for these populations [[94], [95], [96]].
The next step in the management of NeuP in Africa will require contextualization of NeuP in the African setting and the validation of NeuP diagnostic tools in local languages. In recent years, we have assisted with the translation of these tools (DN4, Neuropathic Pain Questionnaire, S-LANSS; painDETECT) into various languages (Arab, Chinese, Spanish, Mongolian, etc.) for diagnosing NeuP [[97], [98], [99], [100]]. African researchers hence ought to translate these tools into the main African languages (Kiswahili, Haoussa, Yorouba, Malinké, Lingala, Fulani, etc.). Furthermore, it is recognized that the cultural expression of suffering is based mainly on the words that are used to describe the pain [101]. Taking into account the wording is, therefore, useful in order to avoid erroneous interpretation of psychosocial distress as physical pain, which can lead to inappropriate treatments or to poor use of resources [102]. In this context, it has been shown that health interventions regarding suffering and distress that take into account the sociocultural context by integration of linguistic aspects yield better results [103].
In this context of conceptualization, the physiological and neurobiological specificities, particularly the ethnic, racial, and genetic aspects of African patients should be integrated into the process of managing NeuP in Africa. The role of ethnic and racial disparities has been examined in models of North American studies. These studies are based essentially on the cultural differences between whites, African Americans, and Hispanics. Certain studies have also shown differences in perceptions (frequency, response to a given stimulus, pain threshold, and tolerance to pain) and the strategies for coping (strategies for dealing with the pain) between the various racial groups in the USA [104]. In this context, a review of 28 articles was able to show a higher prevalence of pain in minorities versus the white population in the United States. The authors of this study recommended implementation of a conceptual framework to better understand and treat pain experienced by ethnic and racial minorities [104]. In terms of an explanation, this ethnic and racial disparity appears to involve biological, sociocultural, and environmental factors [105]. For all of these reasons, some authors recommend the development of tools to measure pain that are culturally appropriate [106,107].
In terms of neurobiological aspects, one of the key elements to take into account with NeuP is genetic diversity. The discovery of genetic variants of ion channels constitutes compelling evidence for an influence of human genetics in the field of pain [108]. This genetic diversity appears to be the result of a complex interaction between environmental and genetic factors that alter both the vulnerability and the resilience of the somatosensory nervous system [108,109]. A better understanding of the genetic architecture of pain is, therefore, indispensable for optimal management of the issue of chronic and particularly neuropathic pain [109]. We are convinced that the African continent, with its great geographic, ethnic, and racial diversity, could provide a valuable contribution to this important step in the study and the treatment of pain.
Other than these considerations, these neurobiological aspects and beliefs constitute important elements to be integrated in relation to pain to devise therapeutic strategies. In this regard, North American studies have shown the relevance of integrating beliefs. For example, a study of cancer-related pain in Ojibway women (the third-largest autonomous group of American Indians in the United States and Canada) has allowed reticence to be shown based on the culture of discussing pain [108]. The authors of this work identified a common response to pain in Ojibway women that they labeled “blockage”. Thus, Ojibway are convinced that it is up to the carer to perceive and sense the pain of the patient in order to treat it. Moreover, Ojibway women described their interactions with the carers as being poor, unlike the white patients who found this interaction to be satisfactory [108].
In our experience, these erroneous beliefs tend to overestimate the capacities of practitioners of conventional medicine, who are often on equal footing as the healers in Mali and in Western Africa in general [92]. Following is the type of statements that African patients make regarding the carers “if the doctor really has solid scientific know-how, they should be able to identify my illness/pain and cure it, as they are otherwise not in a position to be a carer. It is not up to me to tell them the nature of my illness”. Moreover, we think that the acceptance and the success of certain so-called complementary practices (Chinese medicine, religion-based medicine, traditional practices) could be explained by this sociocultural imprint [110,111].
Based on our experience, the results of this work, and data in the literature, we call for cross-sectional and integrated management of chronic/neuropathic pain in Africa. Sociocultural adaptation of the health programs regarding pain would allow patients and their families to accept the interventions. The adaptation and the conceptualization will require win/win alliances between patients, researchers, clinicians, religious leaders, traditional therapists, and decision-makers. In our opinion, the implementation of this conceptual framework constitutes a crucial stage for effective management of neurological pathologies in general and chronic and neuropathic pain in particular.
5. Limitations of the study
The retrospective nature of the first part of our study (compilation of the patient data phase), despite the rigorous methodology for selection of the files, could lead to some information being lost, as all of the incomplete files or those for which the neuropathic nature was not proven were excluded. We, therefore, believe that our results have underestimated the extent of the phenomenon of NeuP in our practice. A prospective approach would be needed to provide an update. Regarding the second part, the selection of the 45 first responders resulted in others not being taken into account. In both cases, there could, therefore, have been selection bias.
6. Conclusion
This work allowed us to confirm the extent of NeuP in our context of low-income countries. In this context, characterized by a pronounced lack of technical platforms, simple and low-cost tools (such as the DN4 questionnaire) associated with rigorous clinical examination suffice to diagnose NeuP. The study of KAP among carers allowed us to note that optimal management of this pathology will involve: (1) better training of carers, and (2) improvement of the accessibility and the availability of medications. In light of this work, we have restarted the debate in Mali and in Africa in general regarding the concept of essential medications that should be tailored to the new epidemiological realities of the pathologies. Lastly, we believe that optimal management of chronic and neuropathic pain in Africa requires a process in which researchers will be engaged to customize and to contextualize the treatment of NeuP.
References
- 1.GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnerup Nanna B., Attal Nadine, Haroutounian Simon, McNicol Ewan, Baron Ralf, Dworkin Robert H., Gilron Ian, Haanpaa Maija, Hansson Per, Jensen Troels S., Kamerman Peter R., Lund Karen, Moore Andrew, Raja Srinivasa N., Rice Andrew S.C., Rowbotham Michael, Sena Emily, Siddall Philip, Smith Blair H., Wallace Mark. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond M., Breivik H., Jensen T.S., Scholten W., Soyannwo O., Treede R.-D. Pain Associated with Neurological Disorders. World Health Organization; Switzerland: 2006. Neurological disorders: public health challenges; pp. 127–139. [Google Scholar]
- 4.Van Hecke O., Austin S.K., Ra Khan, Smith B.H., Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Holloway K.A., Henry D. WHO essential medicines policies and use in developing and transitional countries: an analysis of reported policy implementation and medicines use surveys. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroun O.M.O., Hietaharju A., Bizuneh E., Tesfaye F., Brandsma J.W., Haanpää M., Rice A.S.C., Lockwood D.N.J. Investigation of neuropathic pain in treated leprosy patients in Ethiopia: a cross-sectional study. Pain. 2012;153:1620–1624. doi: 10.1016/j.pain.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Hsu E., Cohen S.P. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. doi: 10.2147/JPR.S32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadosky A., McDermott A.M., Brandenburg N.A., Strauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8:45–56. doi: 10.1111/j.1533-2500.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 9.Maiga Y., Toloba Y., M’belesso P., Daniele R., Cissoko Y., Illiassou S., Maiga M.Y., Traore H.A. Neuropathic pain during tuberculosis treatment in Bamako (Mali) Med Sante Trop. 2012;22:312–316. doi: 10.1684/mst.2012.0090. [DOI] [PubMed] [Google Scholar]
- 10.Cherry C.L., Wadley A.L., Kamerman P.R. Painful HIV-associated sensory neuropathy. Pain Manag. 2012;2:543–552. doi: 10.2217/pmt.12.67. [DOI] [PubMed] [Google Scholar]
- 11.Adoukonou T., Gnonlonfoun D., Kpozehouen A., Adjien C., Tchaou B., Tognon-Tchegnonsi F., Adechina H., Covi R., Houinato D. Prevalence and characteristics of chronic pain with neuropathic component at Parakou in northern Benin in 2012. Rev Neurol. 2014;170:703–711. doi: 10.1016/j.neurol.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Lekpa F.K., Ndongo S., Ka O., Zeba D. Compaore' C, Pouye a, et al. sociodemographic and clinical profile of chronic pain with neuropathic characteristics in sub-Saharan African elderly. Eur J Pain. 2013;17(6):939–943. doi: 10.1002/j.1532-2149.2012.00243.x. [DOI] [PubMed] [Google Scholar]
- 13.Ouedraogo D.D., Nonguierma V., Napon C., Kabre A., Tie’no H, Guira O, et al. Prevalence of neuropathic pain among black African patients suffering from common low back pain. Rheumatol Int. 2012;32:2149–2153. doi: 10.1007/s00296-011-1945-4. [DOI] [PubMed] [Google Scholar]
- 14.van Hecke O., Austin S.K., Khan R.A., Smith B.H., Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Huang W., Calvo M., Pheby T., Bennett D.L., Rice A.S. A rodent model of HIV protease inhibitor indinavir-induced peripheral neuropathy. Pain. 2017;158:75–85. doi: 10.1097/j.pain.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 16.Poliakov I., Toth C. The impact of pain in patients with polyneuropathy. Eur J Pain. 2011;15:1015–1022. doi: 10.1016/j.ejpain.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Smith B.H., Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16:191–198. doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 18.Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Finnerup Nanna B., Haroutounian Simon, Baron Ralf, Dworkin Robert H., Gilron Ian, Haanpaa Maija, Jensen Troels S., Kamerman Peter R., McNicol Ewan, Moore Andrew, Raja Srinivasa N., Andersen Niels T., Sena Emily S., Smith Blair H., Rice Andrew S.C., Attal Nadine. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain. 2018;159:2339–2346. doi: 10.1097/j.pain.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attal N., Bouhassira D., Baron R., Dostrovsky J., Dworkin R.H., Finnerup N., Gourlay G., Haanpaa M., Raja S., Rice A.S.C., Simpson D., Treede R.-D. Assessing symptom profiles in neuropathic pain clinical trials: can it improve outcome? Eur J Pain. 2011;15:441–443. doi: 10.1016/j.ejpain.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett Stephen W., Ofosu-Amaah Samuel. Strengthening health services for MCH in Africa: the first four years of the ‘Bamako initiative’. Health Policy Plan. 1992;7:164–176. [Google Scholar]
- 22.Torrance N., Ferguson J.A., Afolabi E. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154:690–699. doi: 10.1016/j.pain.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shipton Elspeth E., Bate Frank, Garrick Raymond, Steketee Carole, Shipton Edward A., Visser Eric J. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7:139–161. doi: 10.1007/s40122-018-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamerman Peter R., Wadley Antonia L., Davis Karen, Hietaharju Aki, Jain Parmanand, Kopf Andreas, Meyer Ana-Claire, Raja Srinivasa N., Rice Andrew S.C., Smith Blair H., Treede Rolf-Detlef, Wiffen Phillip J. World Health Organization (WHO) essential medicines lists: where are the drugs to treat neuropathic pain? Pain. 2015;156(5):793–797. doi: 10.1097/01.j.pain.0000460356.94374.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treede R.-D., Rief W., Barke A., Aziz Q., Bennett M.I., Benoliel R., Cohen M., Evers S., Finnerup N.B., First M.B., Giamberardino M.A., Kaasa S., Kosek E., Lavand’homme P., Nicholas M., Perrot S., Scholz J., Schug S., Smith B.H., Svensson P., Vlaeyen J.W., Wang S.-J. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treede R.-D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., Hansson P., Hughes R., Nurmikko T., Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 27.Timmerman H. Investigating the validity of the DN4 in a consecutive population of patients with chronic pain. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0187961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The IASP classification of chronic pain for ICD-11: chronic neuropathic painPain. 2019;160:53–59. doi: 10.1097/j.pain.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg D.S., McGee S.J. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhassira Didier, Attal Nadine, Fermanian Jacques, Alchaar Haiel, Gautron Michèle, Masquelier Etienne, Rostaing Sylvie, Lanteri-Minet Michel, Collin Elisabeth, Grisart Jacques, Boureau François. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Colloca Luana, Ludman Taylor, Bouhassira Didier, Baron Ralf, Dickenson Anthony H., Yarnitsky David, Freeman Roy, Truini Andrea, Attal Nadine, Finnerup Nanna B., Eccleston Christopher, Kalso Eija, Bennett David L., Dworkin Robert H., Raja Srinivasa N. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attal Nadine, Bouhassira Didier, Baron Ralf. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018;17:456–466. doi: 10.1016/S1474-4422(18)30071-1. [DOI] [PubMed] [Google Scholar]
- 33.Themistocleous A.C. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amara Fahmy, Hafez Sherif, Orabi Abbas, Rahim Aly Ahmed Abdel, Zakaria Ebtissam, Koura Farouk, Talaat Farouk Mohamed, Gawish Hanan, Attia Ihab, Aziz Mohamed Fahmy Abdel, El Hefnawy Mohamed Hesham Mohamed Fahmy, Kamar Mohamed, Halawa Mohamed Reda, El-Sayed Mohamed Shawky, El Kafrawy Nabil Abdelfatah, AssaadKhalil Samir Helmy, Assaad Samir Naem. Review of diabetic polyneuropathy: pathogenesis, diagnosis and management according to the consensus of Egyptian experts. Current Diabetes Reviews. 2019;1:340–345. doi: 10.2174/1573399815666190226150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouhassira Didier, Lantéri-Minet Michel, Attal Nadine, Laurent Bernard, Touboul Chantal. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Freynhagen R. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22:529–537. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 37.Finnerup Nanna, Haroutounian Simon, Kamerman Peter, Baron Ralf, Bennett David, Bouhassira Didier, Cruccu Giorgio, Freeman Roy, Hansson Per, Nurmikko Turo, Raja Srinivasa, Rice Andrew, Serra Jordi, Smith Blair, Treede Rolf-Detlef, Jensen Troels. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J.A. Longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 39.Alleman C.J., Westerhout K.Y., Hensen M., Chambers C., Stoker M., Long S., van Nooten F.E. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109:215–225. doi: 10.1016/j.diabres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Klit H., Finnerup N.B., Andersen G., Jensen T.S. Central poststroke pain: a population-based study. Pain. 2011;152:818–824. doi: 10.1016/j.pain.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberger Daniela C., Blechschmidt Vivian, Timmerman Hans, Wolff André, Treede Rolf-Detlef. Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm. 2020;127:589–624. doi: 10.1007/s00702-020-02145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundufu Abu James, Bockarie Cecil N., Jacobsen Kathryn H. The prevalence of Type 2 diabetes in urban Bo, Sierra Leone, and in the 16 Countries of the West Africa Region. Diabetes Metab Res Rev. 2017;33(7) doi: 10.1002/dmrr.2904. [DOI] [PubMed] [Google Scholar]
- 43.Dyck P.J., Kratz K.M., Karnes J.L., Litchy W.J., Klein R., Pach J.M., Wilson D.M., O’Brien P.C., Melton L.J., 3rd, Service FJ The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 44.Van Acker K., Bouhassira D., De Bacquer D., Weiss S., Matthys K. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35:206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Tesfaye S., Boulton A.J., Dyck P.J., Freeman R., Horowitz M., Kempler P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacovides A., Bogoshi M., Distiller L.A., Mahgoub E.Y., Omar M.K., Tarek I.A., Wajsbrot D.B. An epidemiological study to assess the prevalence of diabetic peripheral neuropathic pain among adults with diabetes attending private and institutional outpatient clinics in South Africa. J Int Med Res. 2014;42:1018–1028. doi: 10.1177/0300060514525759. [DOI] [PubMed] [Google Scholar]
- 47.Freynhagen R. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22:529–537. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 48.Buchbinder R., van Tulder M., Öberg B. Low back pain: a call for action. Lancet. 2018;391:2384–2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 49.Hartvigsen J., Hancock M.J., Kongsted A. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 50.Keltner John R., Fennema-Notestine Christine, Vaida Florin, Wang Dongzhe, Franklin Donald R., Dworkin Robert H., Sanders Chelsea, McCutchan J. Allen, Archibald Sarah L., Miller David J., Kesidis George, Cushman Clint, Kim Sung Min, Abramson Ian, Taylor Michael J., Theilmann Rebecca J., Julaton Michelle D., Notestine Randy J., Corkran Stephanie, Cherner Mariana, Duarte Nichole A., Alexander Terry, Robinson-Papp Jessica, Gelman Benjamin B., Simpson David M., Collier Ann C., Marra Christina M., Morgello Susan, Brown Greg, Grant Igor, Atkinson Hampton, Jernigan Terry L., Ellis Ronald J., for the CHARTER Group HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol. 2014;20:209–218. doi: 10.1007/s13365-014-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuate C.T., Maiga Y. Seizures, epilepsy and HIV infection in Africa. Épilepsies. 2010;22(2):134–142. [Google Scholar]
- 52.Aouizerat B.E., Miaskowski C.A., Gay C., Portillo C.J., Coggins T., Davis H., Pullinger C.R., Lee K.A. Risk factors and symptoms associated with pain in HIV-infected adults. J Assoc Nurses AIDS Care. 2010;21:125–133. doi: 10.1016/j.jana.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard S. Smith. Treatment considerations in painful HIV-related neuropathy. Pain Physician. 2011;14:E505–E524. [PubMed] [Google Scholar]
- 54.Togo Josue, Maiga Almoustapha Issiaka, Sylla Mariam, Kone Bourahima, Dolo Oumar, Traore Fatoumata Tata, Sangare Samba Adama, Maiga Mamoudou, Diallo Souleymane, Murphy Robert, Calvez Vincent, Marcelin Anne-Genevieve. Evaluation of two HIV rapid diagnostic tests in a context of Strains’ genetic diversity in Mali. AIDS Res Hum Retroviruses. 2019;35:145–149. doi: 10.1089/aid.2017.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiga Y., Diakité S., Diallo F., Mbelesso P., Diallo Sh., My Maiga, Ha Traoré. Neuropathic pain in HIV / AIDS patients on antiretroviral therapy and followed as outpatients in Bamako, Mali. Rev Neurol. 2011;I67S:A3–A53. [Google Scholar]
- 56.Kabongo Joe Katabwa, Kaputu-Kalala-Malu Célestin, Luboya Oscar, Mutombo Valerien, Ntambwe Abel, Mapatano Mala Ali, Mukendi Kavulu Mayamba. Les neuropathies liées au VIH/SIDA: une étude clinique chez les patients infectés par le VIH au Centre d'Excellence VIH/SIDA de l'Université de Lubumbashi. Pan Afr Med J. 2015;20:392. doi: 10.11604/pamj.2015.20.392.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braitstein P., Brinkhof M.W., Dabis F., Schechter M., Boulle A., Miotti P. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 58.Murray C.J., Lopez A.D. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 59.Strong K., Mathers C., Bonita R. Preventing stroke: saving lives around world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 60.Sagui E. Stroke in sub-Saharan Africa. Med Trop. 2007;67:596–600. [PubMed] [Google Scholar]
- 61.Maiga Y., Albakaye M., Diango D., Kanikomo D., Hassane S., Minta I., Diakité S., Traoré Ha Et Guillon B. Modalities of stroke management in Mali (West Africa): a survey of practices. Mali Medical. 2013;28:30–35. [PubMed] [Google Scholar]
- 62.Bashir A.H., Abdullahi A., Abba M.A., Mukhtar N.B. Central Poststroke pain: its profile among stroke survivors in Kano, Nigeria. Behav Neurol. 2017;2017:9318597. doi: 10.1155/2017/9318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naess H., Lunde L., Brogger J. The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: the Bergen stroke study. Vasc Health Risk Manag. 2012;8:407–413. doi: 10.2147/VHRM.S32780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klit H., Finnerup N.B., Andersen G., Jensen T.S. Central poststroke pain: a population-based study. Pain. 2011;152:818–824. doi: 10.1016/j.pain.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 65.Langhorne P., Stott D.J., Robertson L., MacDonald J., Jones L., McAlpine C. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 66.Widar M., Samuelsson L., Karlsson-Tivenius S., Ahlstrom G. Long-term pain conditions after a stroke. J Rehabil Med. 2002;34:165–170. doi: 10.1080/16501970213237. [DOI] [PubMed] [Google Scholar]
- 67.Tang W.K., Liang H., Mok V., Ungvari G.S., Wong K.S. Is pain associated with suicidality in stroke? Arch Phys Med Rehabil. 2013;94:863–866. doi: 10.1016/j.apmr.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 68.Jonsson A.C., Lindgren I., Hallstrom B., Norrving B., Lindgren A. Prevalence and intensity of pain after stroke: a population-based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77:590–595. doi: 10.1136/jnnp.2005.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Upshur C.C., Luckmann R.S., Savageau J.A. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21:652–655. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mantyselka P., Kumpusalo E., Ahonen R., Kumpusalo A., Kauhanen J., Viinamaki H. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–180. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 71.Institute of Medicine, Committee on Advancing Pain Research C, Education . National Academies Press; Washington, DC: 2011. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. [PubMed] [Google Scholar]
- 72.Haanpaa M., Attal N., Backonja M. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 73.Cruccu Giorgio, Truini Andrea. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6(Suppl. 1):S35–S42. doi: 10.1007/s40122-017-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elspeth E., Bate Frank, Garrick Raymond, Steketee Carole, Shipton Edward A., Visser Eric J. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7:139–161. doi: 10.1007/s40122-018-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Lippe P.M., Brock C., David J., Crossno R., Gitlow S. The first national pain medicine summit—final summary report. Pain Med. 2010;11:1447–1468. doi: 10.1111/j.1526-4637.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 77.Shipton Elspeth Erica, Steketee Carole, Bate Frank, Visser Eric John. Exploring assessment of medical students' competencies in pain medicine: A review. Pain Rep. 2019;4 doi: 10.1097/PR9.0000000000000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holloway K.A., Henry D. WHO essential medicines policies and use in developing and transitional countries: an analysis of reported policy implementation and medicines use surveys. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laing R., Waning B., Gray A., Ford N. 25 years of the WHO essential medicines lists: progress and challenges. Lancet. 2003;361:1723–1729. doi: 10.1016/S0140-6736(03)13375-2. [DOI] [PubMed] [Google Scholar]
- 80.Daniel Bates B., Schultheis Carsten, Hanes Michael C., Jolly Suneil M., Chakravarthy Krishnan V., Deer Timothy R., Levy Robert M., Hunter Corey W. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019;20(Suppl. 1) doi: 10.1093/pm/pnz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall Gillian C., Carroll Dawn, McQuay Henry J. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002–2005. BMC Fam Pract. 2008;9:26. doi: 10.1186/1471-2296-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holloway K.A., Henry D. WHO essential medicines policies and use in developing and transitional countries: an analysis of reported policy implementation and medicines use surveys. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haque Mainul. Essential medicine utilization and situation in selected ten developing countries: a compendious audit. J Int Soc Prev Community Dent. 2017;7:147–160. doi: 10.4103/jispcd.JISPCD_224_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Godman Brian, Bucsics Anna, Bonanno Patricia Vella, Oortwijn Wija, Rothe Celia C., Ferrario Alessandra. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328. doi: 10.3389/fpubh.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The Lancet Commission on Essential Medicines Policies. http://www.bu.edu/lancet-commission-essential-medicines-policies
- 86.Wirtz Veronika J., Dr PhD, Hogerzeil Hans V., Gray Andrew L., Bigdeli Maryam, de Joncheere Cornelis P., Ewen Margaret A., Gyansa-Lutterodt Martha, Jing Sun, Luiza Vera L., Mbindyo Regina M., Möller Helene, Moucheraud Corrina, Pécoul Bernard, Rägo Lembit, Rashidian Arash, Ross-Degnan Dennis, Stephens Peter N., Teerawattananon Yot, Ellen F., Wagner Anita K., Yadav Prashant, Michael R. Essential medicines for universal health coverage. Lancet. 2017;389:403–476. doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oluwafemi O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J Inflamm Res. 2018;11:307–317. doi: 10.2147/JIR.S167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omodanisi E.I., Aboua G.Y., Oguntibeju O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of Moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules. 2017;22:439–445. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fink D., Oladele D., Etomi O., Wapmuk A., Musari-Martins T., Agahowa E., Ekama S., Okechukwu A., Mallen C., Ezechi O., Salako B. Musculoskeletal symptoms and non-prescribed treatments are common in an urban African population of people living with HIV. Rheumatol Int. 2019;39:285–291. doi: 10.1007/s00296-018-4188-9. [DOI] [PubMed] [Google Scholar]
- 90.Nebelkopf E., King J. A holistic system of care for native Americans in an urban environment. J Psychoactive Drugs. 2003;35:43–52. doi: 10.1080/02791072.2003.10399992. [DOI] [PubMed] [Google Scholar]
- 91.Sanou M., Jean A., Marjolet M., Pécaud D., Meas Y., Enguehard C., Moret L., Emane A. Conventional medical attitudes to using a traditional medicine vodou-based model of pain management: survey of French dentists and the proposal of a pain model to facilitate integration. Journal of Chiropractic Humanities. 2012;19:24–35. doi: 10.1016/j.echu.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nortje G., Oladeji B., Gureje O., Seedat S. Effectiveness of traditional healers in treating mental disorders: a systematic review. Lancet Psychiatry. 2016;3(2):154–170. doi: 10.1016/S2215-0366(15)00515-5. [DOI] [PubMed] [Google Scholar]
- 93.Kramer B.J., Harker J.O., Wong A.L. Arthritis beliefs and self-care in an urban American Indian population. Arthritis Rheum. 2002;47:588–594. doi: 10.1002/art.10795. [DOI] [PubMed] [Google Scholar]
- 94.Kramer B.J., Harker J.O., Wong A.L. Descriptions of joint pain by American Indians: comparison of inflammatory and non-inflammatory arthritis. Arthritis Rheum. 2002;47:149–154. doi: 10.1002/art.10325. [DOI] [PubMed] [Google Scholar]
- 95.Kim Ho-Joong, Park Joon-Hee, Bouhassira Didier, Shin Jae-Hoon, Chang Bong-Soon, Lee Choon-Ki, Baek Chang Hyun, Yeom Jin S. Validation of the Korean Version of the DN4 Diagnostic Questionnaire for Neuropathic Pain in Patients with Lumbar or Lumbar-Radicular Pain. Yonsei Med J. 2016;57:449–454. doi: 10.3349/ymj.2016.57.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harifi G., Ouilki I., El Bouchti I., Ouazar M.A., Belkhou A., Younsi R., Amine M., Tazi I., Abouqal R., Niamane R., El Hassani S. Validity and reliability of the Arabic adapted version of the DN4 questionnaire (Douleur Neuropathique 4 Questions) for differential diagnosis of pain syndromes with a neuropathic or somatic component. Pain Pract. 2011;11:139–147. doi: 10.1111/j.1533-2500.2010.00399.x. [DOI] [PubMed] [Google Scholar]
- 97.Li Jun, Feng Yi, Han Jisheng, Fan Bifa, Wu Dasheng, Zhang Daying, Dongping Du, Liu Hui, Lin Jian, Wang Jiashuang, Jin Yi, Zhijian Fu. Linguistic adaptation, validation and comparison of 3 routinely used neuropathic pain questionnaires. Pain Physician. 2012;15:179–186. [PubMed] [Google Scholar]
- 98.Perez Concepcion, Galvez Rafael, Huelbes Silvia, Insausti Joaquin, Bouhassira Didier, Diaz Silvia, Rejas Javier. Validity and reliability of the Spanish version of the DN4 (Douleur Neuropathique 4 questions) questionnaire for differential diagnosis of pain syndromes associated to a neuropathic or somatic component. Health Qual Life Outcomes. 2007;5:66. doi: 10.1186/1477-7525-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kohrt B.A., Rasmussen A., Kaiser B.N., Haroz E.E., Maharjan S.M., Mutamba B.B., de Jong J.T., Hinton D.E. Cultural concepts of distress and psychiatric disorders: literature review and research recommendations for global mental health epidemiology. Int J Epidemiol. 2014;43:365–406. doi: 10.1093/ije/dyt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keys H.M., Kaiser B.N., Kohrt B.A., Khoury N.M., Brewster A.R. Idioms of distress, ethnopsychology, and the clinical encounter in Haiti’s central plateau. Soc Sci Med. 2012;75:555–564. doi: 10.1016/j.socscimed.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 101.Das V. Language and body: Transactions in the construction of pain. In: Kleinman A., Das V., Lock M.M., editors. Editors of Social Suffering. University of California Press; Berkeley: 1997. pp. 67–92. [Google Scholar]
- 102.Buchwald D., Goldberg J., Noonan C., Beals J., Manson S. Relationship between post-traumatic stress disorder and pain in two American Indian tribes. Pain Med. 2005;6:72–79. doi: 10.1111/j.1526-4637.2005.05005.x. [DOI] [PubMed] [Google Scholar]
- 103.Jimenez N., Garroutte E., Kundu A., Morales L., Buchwald D. A review of the experience, epidemiology, and management of pain among American Indian, Alaska Native, and Aboriginal Canadian peoples. J Pain. 2011;12:511–522. doi: 10.1016/j.jpain.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campbell C.M., Edwards R.R., Fillingim R.B. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 105.Garroutte E.M., Westcott K.D. The Stories Are Very Powerful. A Native American Perspective on Health, Illness and Narrative. In: Crawford S., editor. Religion and Healing in Native America. Praeger Press; Westport: 2008. pp. 163–184. [Google Scholar]
- 106.Pascal M.M.V., Themistocleous A.C., Baron R., Binder A., Bouhassira D., Crombez G., Finnerup N.B., Gierthmühlen J., Granovsky Y., Groop L. DOLORisk: study protocol for a multi-centre observational study to understand the risk factors and determinants of neuropathic pain. Wellcome Open Res. 2019;3:63. doi: 10.12688/wellcomeopenres.14576.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Forstenpointner J., Otto J., Baron R. Individualized neuropathic pain therapy based on phenotyping: are we there yet? Pain. 2018;159:569–575. doi: 10.1097/j.pain.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 108.Barkwell D. Cancer pain: voices of the Ojibway people. J Pain Symptom Manage. 2005;30:454–464. doi: 10.1016/j.jpainsymman.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 109.Lurie D.I. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J Exp Neurosci. 2018;12 doi: 10.1177/1179069518793639. (1179069518793639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nchako E., Bussell S., Nesbeth C., Odoh C. Barriers to the availability and accessibility of controlled medicines for chronic pain in Africa. Int Health. 2018;10:71–77. doi: 10.1093/inthealth/ihy002. [DOI] [PubMed] [Google Scholar]
- 111.Yuan Q.-L., Guo T.-M., Liu L., Sun F., Zhang Y.-G. Traditional Chinese medicine for neck pain and low Back pain: a systematic review and meta-analysis. PLOS ONE. 2015;10(2) doi: 10.1371/journal.pone.0117146. [DOI] [PMC free article] [PubMed] [Google Scholar]