Abstract
Background
Erythrocyte mass contributes to maintaining systemic oxygen delivery and blood viscosity, with the latter being one of the determinants of blood pressure. However, the physiological response to blood pressure changes under anaemic conditions remain unknown.
Methods and Findings
We show that anaemia decreases blood pressure in human patients and mouse models. Analyses of pathways related to blood pressure regulation demonstrate that anaemia enhances the expression of the gene encoding the vasopressor substance renin in kidneys. Although kidney juxtaglomerular cells are known to continuously produce renin, renal interstitial fibroblasts are identified in the present study as a novel site of renin induction under anaemic hypotensive conditions in mice and rats. Notably, some renal interstitial fibroblasts are found to simultaneously express renin and the erythroid growth factor erythropoietin in the anaemic mouse kidney. Antihypertensive agents but not hypoxic stimuli induced interstitial renin expression, suggesting that blood pressure reduction triggers interstitial renin induction in anaemic mice. The interstitial renin expression was also detected in injured fibrotic kidneys of the mouse and human, and the renin-expressing interstitial cells in murine fibrotic kidneys were identified as myofibroblasts originating from renal interstitial fibroblasts. Since the elevated expression levels of renin in fibrotic kidneys along with progression of renal fibrosis were well correlated to the systemic blood pressure increase, the renal interstitial renin production seemed to affect systemic blood pressure.
Interpretation
Renal interstitial fibroblasts function as central controllers of systemic oxygen delivery by producing both renin and erythropoietin.
Funding
Grants-in-Aid from Japan Society for the Promotion of Science (JSPS) KAKENHI (17K19680, 15H04691, and 26111002) and the Takeda Science Foundation.
Keywords: Erythropoiesis, Hypoxia, Renal anaemia, Renin-angiotensin system
Research in context.
Evidence before this study: Red blood cells (erythrocytes) are essential for oxygen delivery to organs and occupy almost half of the blood volume in healthy humans. A decrease in circulating erythrocytes (anaemia) causes not only a systemic oxygen shortage (hypoxia), but also a reduction in blood viscosity, which is a critical factor for blood pressure maintenance. However, the impact of anaemia on blood pressure has been poorly studied. Renin is indispensable for blood pressure maintenance, and erythropoiesis requires erythropoietin (Epo). Renin and Epo are produced in the kidneys by juxtaglomerular (JG) cells and interstitial fibroblasts, respectively.
Added value of this study: By analysing data from anaemic patients and a variety of animal models, we demonstrate herein that anaemia causes hypotension. Additionally, we show that anaemic hypotension induces renin production at the transcriptional level in a large number of renal interstitial fibroblasts and a small number of JG cells. In the anaemic mouse kidney, we find renal interstitial fibroblasts simultaneously expressing renin and Epo. Furthermore, we find that the renal interstitial production of renin is activated by hypotension alone and not by hypoxic stimuli. Thus, this study identifies renal interstitial fibroblasts as a novel major site of renin induction in response to the blood pressure decrease.
Implications of all the available evidence: Renal interstitial fibroblasts are proposed to function as central controllers of erythrocyte circulation by regulating the expression of the genes encoding renin and Epo. Against the high demand of renin under hypotension conditions, renal interstitial fibroblasts initiate renin production to support JG cells, which have been considered the major site of renin production. The renin induction in renal interstitium is suggested to be involved in any types of anaemia, amongst which iron-deficiency anaemia is a common disease in women of childbearing age. Additionally, renal interstitial renin likely contributes to blood pressure disorders associated with kidney disease, from which more than 10% of the population worldwide suffers.
Alt-text: Unlabelled box
1. Introduction
Red blood cells (erythrocytes) are essential for the delivery of oxygen to every organ, and a decrease in circulating erythrocytes (anaemia) causes a whole-body oxygen shortage (systemic hypoxia). Additionally, since erythrocytes occupy almost half of the blood volume in healthy humans, anaemia reduces blood viscosity, resulting in a decrease in systemic blood pressure [1,2]. Moreover, anaemic hypoxia causes peripheral vasodilation and cardiac exhaustion, both of which also contribute to the blood pressure decrease [3]. Therefore, anaemia is believed to delay the circulation of the decreased numbers of erythrocytes, and the combined effect of erythrocyte loss and anaemic hypotension potentially aggravates systemic hypoxia. However, the impact of anaemia on blood pressure is poorly studied, even though anaemia, such as iron-deficiency anaemia, is a common disease in women of childbearing age.
Erythrocyte production (erythropoiesis) in the bone marrow is activated by the erythroid growth factor erythropoietin (Epo), which is produced by interstitial fibroblasts in the kidneys referred to as renal Epo-producing (REP) cells [4]. Because Epo is essential for erythropoiesis, deletion of the Epo gene from the mouse genome results in embryonic death due to severe anaemia [5,6]. REP cells lose the ability to produce Epo in kidney disease, which affects more than 10% of the worldwide population and results in a significant economic burden associated with kidney replacement therapies, such as haemodialysis and kidney transplantation. Therefore, chronic kidney disease (CKD) results in the loss of Epo production in the majority of REP cells, and renal anaemia due to relative Epo deficiency or Epo hyporesponsiveness is a frequent complication of CKD [7]. For more than 30 years, recombinant human Epo and its derivatives have exhibited excellent efficacy in the treatment of renal anaemia as erythropoiesis-stimulating agents (ESAs) [8]. To maintain oxygen homoeostasis, REP cells produce and secrete Epo in a hypoxia-inducible manner. Epo gene transcription is the rate-limiting step for Epo secretion by REP cells [4] and is governed by the transcription factor HIF2α, a member of the hypoxia-inducible transcription factors (HIFs) [7,9]. Under normal air conditions, HIFs are inactivated by prolyl hydroxylase domain proteins (PHDs), which require oxygen for their catalytic activity [7]. When hypoxia inhibits PHD function, PHD-mediated inactivation of HIFs is inhibited, and HIFs induce the expression of various target genes, including the Epo gene [7]. Very recently, PHD inhibitory compounds (PHDIs) were launched as new treatments for renal anaemia in Japan and China [7,10].
Blood pressure is mainly regulated by the renin-angiotensin system (RAS), in which angiotensinogen (AGT) is converted to the effective vasopressor factor angiotensin II (AngII) by renin and angiotensin-I converting enzyme (ACE) [11]. AGT processing principally occurs in the blood because the genes for AGT, renin, and ACE are primarily expressed in the liver, kidneys, and lungs, respectively. In addition to systemic RAS, tissue-specific RAS (local RAS) with local high concentrations of RAS components has been identified in many organs, including the kidney, heart, brain, and liver [12,13]. AngII binds to its specific receptors and induces vasoconstriction. In addition, AngII signalling causes the adrenal cortex to secrete the mineralocorticoid aldosterone, which is an inducer of Na+ reabsorption in the distal tubules of the kidneys, thereby increasing the body fluid volume. The abnormal activation of RAS, such as the overproduction of renin due to kidney disease, is one of the causes of hypertension (renal hypertension), and AngII receptor blockers (ARBs) are widely used for the treatment of hypertension [14]. Renin secretion from the kidneys is controlled by a strict multistep system and strongly impacts elevations in blood pressure. Renin is mainly secreted by a limited cell population, i.e., juxtaglomerular (JG) cells, which are localized around afferent arterioles in the kidneys [15]. Although the mechanisms controlling renin secretion from JG cells are unclear, it is known that a decrease in blood pressure induces renin release from abundant granules of JG cells. Noradrenalin released from the sympathetic nerves via the β1 receptor in JG cells also stimulates renin release from renin granules [15].
To elucidate the influence of anaemia on blood pressure and the mechanism underlying the response to anaemic hypotension, here we investigate the impact of anaemia on RAS. Particularly, the roles of the renal interstitial fibroblasts, which produce Epo to activate erythropoiesis, during adaptation to anaemic hypotension are explored using samples derived from humans and rodents. Additionally, the contribution of renal interstitial fibroblasts, which are transformed into proliferative myofibroblasts in injured kidneys, to blood pressure regulation under pathologic conditions is investigated.
2. Materials and methods
2.1. Cohort study
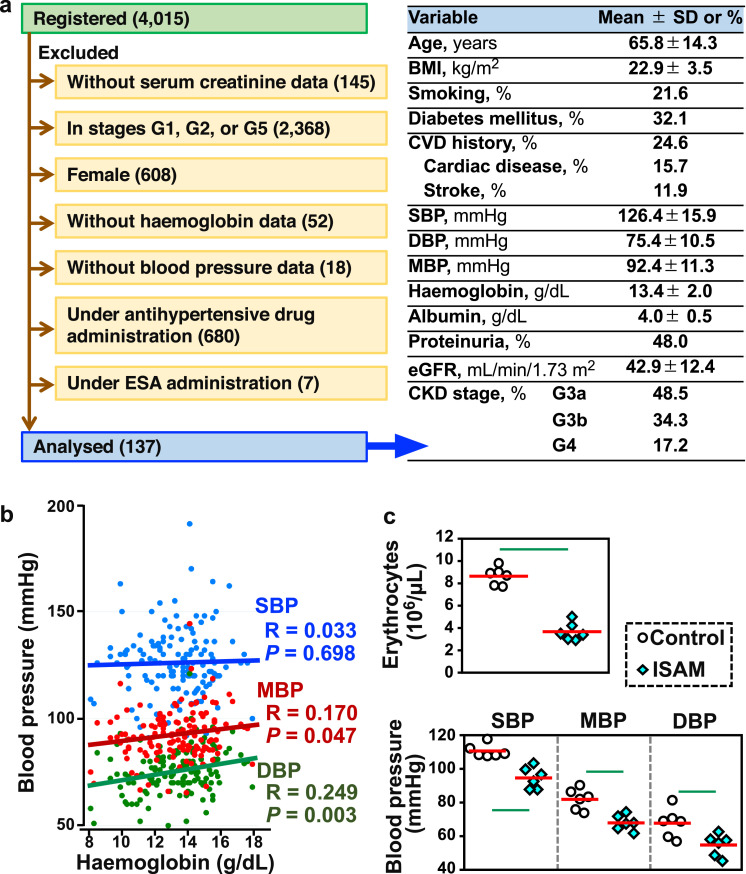
The Gonryo study involved CKD outpatients treated in 11 hospitals offering specialized nephrology services between May 2006 and November 2008 in Miyagi, Japan. The Gonryo study, in which 4015 patients provided informed consent for participation, was approved by the Institutional Review Boards of the Tohoku University School of Medicine (approval number 2006–10/UMIN000011211) [16]. To examine the correlation between haemoglobin levels and blood pressure, data from female patients and the cases administered ESA or antihypertensive agents were excluded from the cohort. Then, data from 137 male patients in CKD stages G3a, G3b, and G4 (as estimated from the glomerular filtration rate [eGFR], which was between 15 and 59 mL/min/1.73 m2 as calculated by the Japanese formula) were selected [17]. The baseline data for the 137 patients are shown in Fig. 1a.
Fig. 1.
Chronic anaemia lowers blood pressure in humans and mice. (a) The baseline data of patients in the Gonryo cohort, which were analysed in this study. To investigate the relationship between haemoglobin and blood pressure values, this study analysed data for 137 male CKD patients in CKD stage G3a, G3b, or G4 without administration of antihypertensive agents or erythropoiesis-stimulating agents (ESA). BMI, body mass index; CVD, cardiovascular disease. (b) Correlation of haemoglobin concentrations with mean (red) and diastolic (green) blood pressures (MBP and DBP) in 137 CKD patients. Systolic blood pressure (SBP, blue) was not statistically correlated with haemoglobin concentrations. A linear regression analysis was performed to estimate the correlation using the non-parametric Spearman rank test. (c) Erythrocyte count, SBP, MBP, and DBP in ISAM and control mice at 11 weeks of age. Red bars indicate mean values. Green lines indicate P<0.01 in the Wilcoxon-Mann-Whitney test. n = 5 and 6, for erythrocyte count and blood pressure, respectively.
2.2. Animals
All animal experiments were approved by the Animal Care Committee of Tohoku University (approval number 2017-MdA-090). Lines of inherited super anaemic mutant (ISAM) mice (EpoGFP/GFP:TgEpo3’ genotype) and ISAM-REC mice (EpoGFP/GFP:TgEpo3’:Rosa26LSL-tdTomato/wt: TgEpoCre) on a C57BL/6 background were established and maintained in-house [18]. Littermate EpoGFP/wt mice were used as normal control mice for the ISAM mice. Wild-type male mice (C57BL/6 and DBA/2) and male rats (Slc:SD, weighing approximately 300 g) were purchased from Japan SLC. The inbred male spontaneously hypertensive rats (SHR/Izm) and their control rats (Wistar-Kyoto rat, WKY/Izm) were also purchased from Japan SLC and analysed at 12 weeks of age [19]. Female transgenic mice overexpressing the human REN gene were crossed with male transgenic mice overexpressing the human AGT gene, and pregnancy-associated hypertension (PAH) developed 16.5 days after fertilization (embryonic day 16.5 [E16.5]) in the pregnant female mice due to the overproduction of human AngII, which is produced by maternal renin and embryonic AGT [20,21]. Human REN transgenic female mice crossed with male REN transgenic mice were used as normal pregnant controls. All mouse experiments except the PAH model used male mice at 12–14 weeks of age, and all animals were housed under a 12:12-hour day-night cycle with constant access to water and food (Oriental Yeast, containing 1.9 g/kg of sodium, 2.4 g/kg of magnesium, and 9.0 g/kg of potassium).
2.3. Blood pressure measurement
The systolic, mean, and diastolic blood pressures were recorded using a tail-cuff apparatus (BP-98a, Softron), by which blood pressure less than 60 mmHg is undetectable. We performed the blood pressure measurement once a day starting 2 days before the evaluation day to allow the mice to become accustomed to the measurement protocols [20].
2.4. Blood analyses
Approximately 300 µL of peripheral blood was collected from the heart or submandibular vein into a 1.5-mL tube containing 5.0 µL of 0.5 M EDTA. The blood indices were measured using an automatic blood analyser (Nihon Koden). Blood concentrations of aldosterone were measured using an aldosterone ELISA kit (ENZO Life Sciences).
2.5. Reverse transcription-quantitative PCR (RT-qPCR)
Using ISOGEN (Nippon Gene), 3 μg of total RNA was extracted from the tissue specimens. Subsequently, cDNA was synthesized using SuperScript III and random primers (Invitrogen). Quantitative PCR was performed using a LightCycler 96 System (Roche Diagnostics) with gene-specific primers (Table S1). The mRNA expression levels of the housekeeping Hprt gene were used as the internal standard.
2.6. Immunoblotting
The proteins in the mouse plasma (4 µL) were transferred to nitrocellulose membranes (Bio-Rad) after electrophoresis on 10% polyacrylamide gels, and the membranes were incubated with a goat anti-mouse renin antibody (R&D Systems, AF4277) diluted (1:1000) with Signal Enhancer HIKARI (Nacalai Tesque) at 4 °C overnight. Horseradish peroxidase (HRP)-conjugated anti-goat IgG (Abcam) was used with chemiluminescent detection reagents (GE Healthcare) to detect the signals using a C-DiGit blot scanner system (Li-COR).
2.7. Formalin-fixed paraffin-embedded organ sections
Kidneys fixed in formalin (Nacalai Tesque) overnight at 4 °C were embedded in paraffin and sliced into thin sections (3-μm or 8-μm thickness). A 2100-Retriever (Aptum Biologics) was used to retrieve the sections.
2.8. in situ hybridization (ISH)
Retrieved paraffin sections were subjected to ISH using an RNAscope Kit (Advanced Cell Diagnostics) in accordance with the manual. RNAscope 2.5 HD Detection Reagents-BROWN and RNAscope 2.5 HD Duplex Detection Reagents (Advanced Cell Diagnostics) were used for the single and double staining, respectively. The probes used in this study are listed in Table S1. Counterstaining was performed using Mayer's haematoxylin stain solution (Merck) and Carracci haematoxylin stain solution (Muto) for single and double staining, respectively.
2.9. Immunohistochemistry
The retrieved paraffin sections were incubated with a goat anti-mouse renin antibody (R&D Systems, AF4277) diluted (1:1000) or rabbit anti-human platelet-derived growth factor receptor β (PDGFRβ; Abcam, ab32570) diluted (1:1000) with Signal Enhancer HIKARI Solution B (Nacalai Tesque) at 4 °C overnight after incubation with a blocking reagent (Vector laboratories). After the sections were washed with PBS-T, they were incubated with an ImmPRESS Excel Amplified HRP Polymer-conjugated secondary antibody (anti-goat IgG for renin or anti-rabbit IgG for PDGFRβ, Vector laboratories) at room temperature for 2 h followed by colour development with a peroxidase colour development solution (Vector laboratories). Mayer's haematoxylin (Merck) was used for counterstaining.
2.10. C.E.R.A. administration
C.E.R.A. (continuous Epo receptor activator also known as epoetin beta pegol or methoxy polyethylene glycol-epoetin beta, Chugai Pharmaceuticals, 3 μg/kg) was subcutaneously injected into the ISAM mice [22]. The mice were analysed 6 h after injection in the single-injection group or one week after the final injection in the 4-times-weekly injection group (Groups 6H and 28D, respectively). Mice injected with PBS-T were used as vehicle-injected controls.
2.11. Analyses of sorted cells
For preparation of the single-cell suspensions from kidneys, the kidneys were dissected and digested with collagenase II (Worthington). Cells positive for GFP and/or tdTomato were isolated from the cell suspension of 8 mice using a FACS Jazz cell sorter with FACS Diva software (Becton Dickinson). The total RNA from the isolated cells (1 × 105 cells for each fraction) was purified with an RNeasy Micro Kit (Qiagen) and reverse-transcribed with SenscriptRT (Qiagen). Triplicate quantitative PCR was performed as described above.
2.12. Haemorrhagic anaemia model
The mice were bled (approximately 300 µL) from the submandibular vein daily for 4 days (Day 0 to Day 4) and analysed a day after the final phlebotomy (Day 5).
2.13. Haemolytic anaemia model
The mice and rats were peritoneally injected with phenylhydrazine (Wako, 60 mg/kg) daily for 3 days (Day 0 to Day 2) and analysed 2 days after the final phlebotomy (Day 5). Mice injected with PBS-T were used as vehicle-injected controls.
2.14. Hypoxic exposure
The control EpoGFP/wt mice were exposed to 6% oxygen air, which was regulated by a ProOx oxygen controller (BioSpherix) and a Nilox nitrogen generator (Sanyo Electronic Industries), for 48 h after being preconditioned with 10% oxygen air for 2 h.
2.15. PHDI treatment
PHDI GSK360A (Toronto Research Chemicals) was dissolved in 5% glucose with 32.5 mmol/L sodium hydroxide and injected into wild-type C57BL/6 mice at a dose of 50 mg/kg. Buffer-injected mice were used as control mice, and both the PHDI-injected and vehicle-injected mice were analysed 6 h after the injection [23,24].
2.16. Administration of antihypertensive agents
The ARB losartan (60 mg/kg, Wako) was intraperitoneally injected into wild-type DBA/2 mice (Japan SLC) daily for 4 days (Day 0 to Day 3), and the treated mice and vehicle (PBS)-injected mice were analysed 24 h after the final injection (Day 4). For administration with the vasodilator hydralazine (Sigma), wild-type C57BL/6 mice (Japan SLC) freely drank hydralazine-containing water (5 mg/L) for 4 days until the analyses.
2.17. Kidney injury
To induce renal fibrosis, wild-type C57BL/6 male mice (Japan SLC) were subjected to unilateral ureteral obstruction (UUO), in which the left ureter was cut between 2 ligatures made by 4–0 Polysorb (Syneture) at the level of the lower pole of the kidney [25]. Injured (left) and contralateral (right) kidneys were analysed at 3, 14, and 21 days after UUO surgery. Myofibroblasts were detected in tissue sections from injured kidneys by means of immunofluorescence with Cy3-conjugated anti-human α-smooth muscle actin (αSMA) antibody (mouse IgG2a, Sigma, C6198) [25].
2.19. Human kidney sections
Formalin-fixed paraffin-embedded kidney sections from patients suffering from chronic tubulointerstitial nephritis were purchased from OriGene (CS700464 and CS705284 for Patient 1 and Patient 2, respectively). The detailed information on the patients is provided in Table S2.
2.19. Statistical analysis
Statistical significance was defined when the P-value was <0.05. The correlations were calculated by the non-parametric Spearman rank test using Stata software version 13.1 (Stata Corp LP). Mann-Whitney U tests and one-way analysis of variance (ANOVA) followed by Tukey-Kramer honestly significant difference tests were used to compare 2 groups and multiple groups, respectively.
3. Results
3.1. Anaemia and hypotension are correlated in humans and mice
To determine whether anaemia is associated with hypotension, we analysed haemoglobin and blood pressure values of 137 male CKD patients (in CKD stages G3a, G3b, and G4) from the Gonryo cohort [16] after excluding the data from patients under ESA or antihypertensive agent administration (Fig. 1a). As expected [1,2], the haemoglobin concentration and mean/diastolic blood pressure (MBP and DBP) were positively correlated (Fig. 1b). Although systolic blood pressure (SBP) was not correlated with haemoglobin values, the MBP and DBP data suggested that anaemia might decrease blood viscosity and pressure. Subsequently, blood pressure in a genetically modified mouse line exhibiting adult-onset Epo-deficiency anaemia, referred to as ISAM mice and described previously [18], was analysed when the mice were 11 weeks of age. Erythrocyte concentrations of the ISAM mice had decreased to approximately 30% of the normal mouse values at 4 weeks of age and remained there throughout life (Fig. 1c) [18,22]. The blood pressure data revealed that the systolic, mean, and diastolic blood pressures of the ISAM mice were significantly lower than those of the normal control mice (Fig. 1c). These data demonstrated that chronic pure red cell aplasia correlates to hypotension, most likely due to decreased blood viscosity.
3.2. Chronic anaemia enhances Ren1 mRNA expression in the kidney
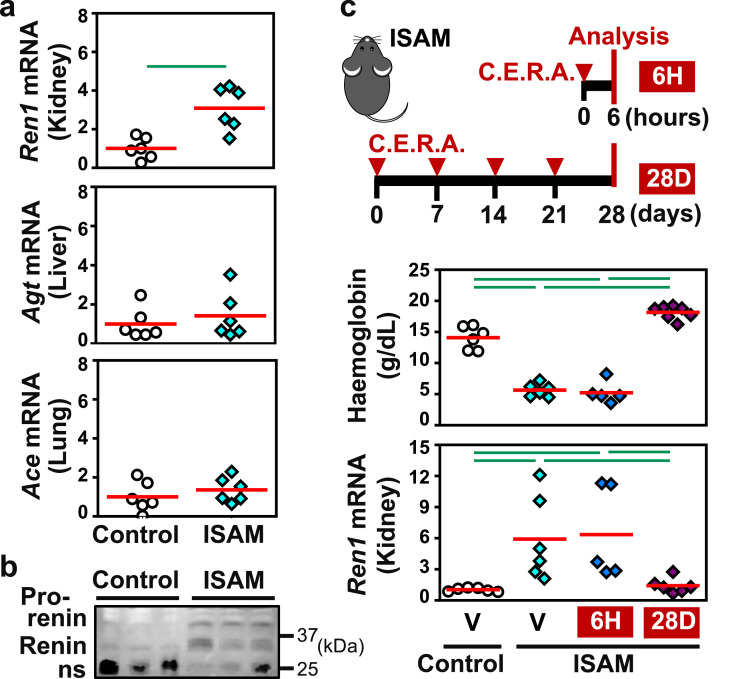
The impact of anaemic hypotension on RAS was investigated by measuring the mRNA levels of the major RAS component genes, including Ren1 in the kidneys, Agt in the liver, and Ace in the lungs. Amongst the studied RAS components, only Ren1 exhibited an mRNA level that was significantly increased in the ISAM mice compared with the control mice; the mRNA levels of the remaining RAS components were unaltered in ISAM mice relative to the controls (Fig. 2a). Immunoblotting experiments using mouse plasma revealed high concentrations of both the renin precursor (prorenin) and mature renin in ISAM plasma (Fig. 2b and Fig. S1). No differences in Agt and Ace mRNA expression in kidney were observed between the mouse genotypes. Therefore, we propose that anaemic hypotension activates systemic RAS rather than local RAS.
Fig. 2.
Chronic anaemia induces renal renin expression in mice. (a) RT-qPCR analyses were performed to determine the relative mRNA expression of the Ren1, Agt, and Ace genes in the kidney, liver, and lung, respectively, which were obtained from ISAM and control mice at 11 weeks of age. (b) Immunoblotting of control and ISAM plasma with an anti-renin antibody detected renin, its precursor (prorenin), and nonspecific bands (ns), which were probably immunoglobulin. (c) Blood haemoglobin concentrations and renal Ren1 mRNA expression levels in control and ISAM mice treated with C.E.R.A. or vehicle (V). Two schemes of C.E.R.A. administration, e.g., 6H and 28D, are shown in the top panel. The average expression levels (red bars) in the control mice were set as 1 for each gene expression analysis (a and c). Green lines indicate P<0.01 in the Wilcoxon-Mann-Whitney test (a) or Tukey-Kramer HSD test (c). n = 5–7 per group (a and c).
Since Epo reagents reverse anaemia in ISAM mice [22], we examined whether the correction of anaemia reduced renal renin production to normal levels in mice with identical genotypes. A single injection of the long-acting Epo reagent C.E.R.A. restored the haemoglobin concentration in the blood of ISAM mice to the normal concentration in one week [22]. The mice were treated with C.E.R.A. 4 times weekly (Fig. 2c). After the treatment, the mice recovered from anaemia for 4 weeks (5 weeks after the first C.E.R.A. injection), their blood pressures normalized (Fig. S2), and the Ren1 mRNA levels in the kidneys decreased to the levels observed in the control mice (Fig. 2c). Since Epo is clinically known to have hypertensive effects [26], we examined the direct effects of C.E.R.A. administration by analysing ISAM mice before the increase in haemoglobin concentrations. Both blood pressure and renal Ren1 mRNA expression levels in ISAM mice showed no significant differences from control levels 6 h after the first C.E.R.A. injection (Fig. 2c), suggesting that Epo did not directly regulate blood pressure and renal renin production. These data demonstrate that chronic anaemia induces the expression of the Ren1 gene in the kidney and provokes systemic RAS to prevent severe hypotension.
3.3. Renal interstitium is the renin induction site under chronic anaemia
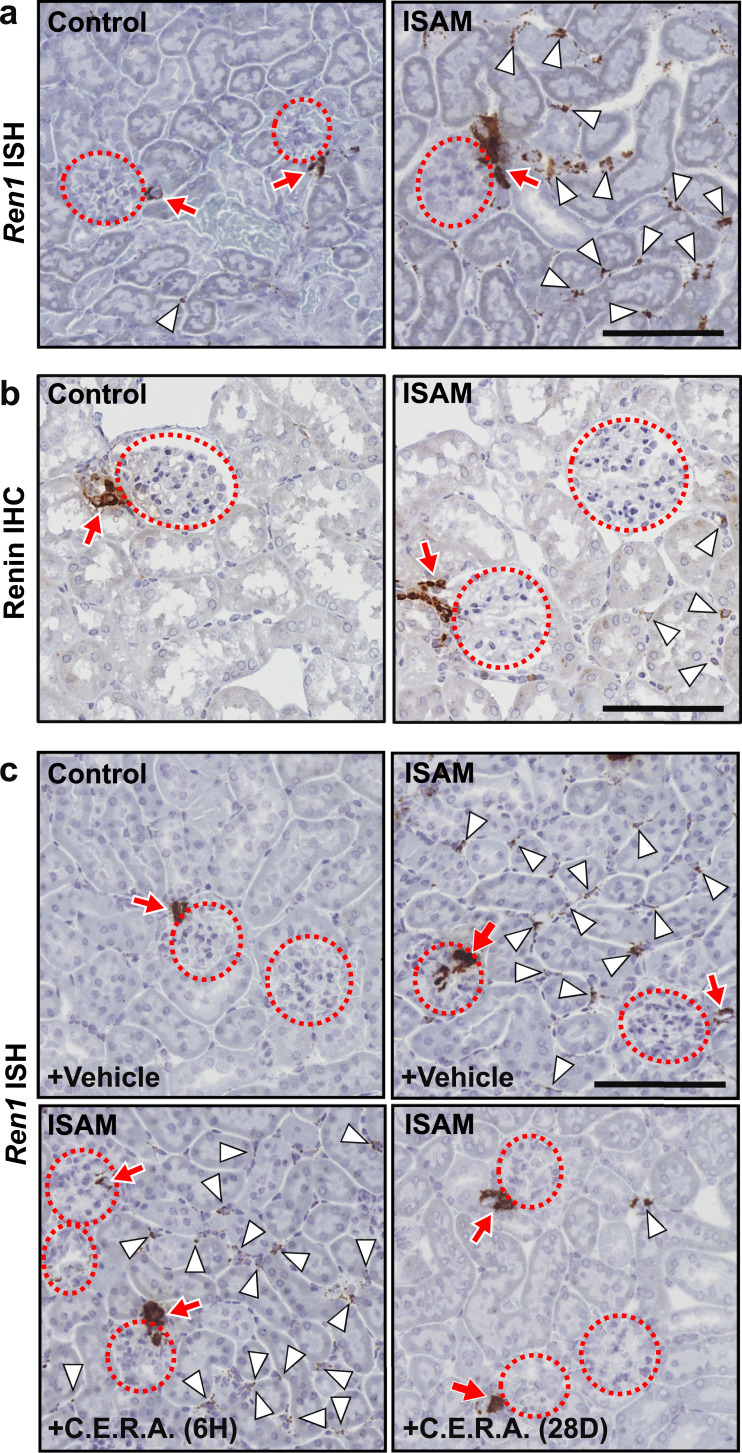
To identify the cells responsible for renal renin production induced by anaemic hypotension, we performed ISH of Ren1 mRNA. As expected, Ren1 mRNA expression was consistently detected in JG cells in both the ISAM and control mice (arrows in Fig. 3a). Unexpectedly, Ren1 mRNA expression was detected in many peritubular interstitial cells in the ISAM kidneys (arrowheads in Fig. 3a). The anaemic hypotension-inducible production of renin in the interstitial cells was confirmed by immunohistochemistry with antibodies against renin (Fig. 3b and Fig. S3a). The staining intensity of renin immunohistochemistry in the interstitial cells was weaker than in JG cells, whereas the staining intensity of Ren1 ISH was comparable between these cell types (Fig. 3a and b). These observations suggest that renin produced in the interstitial cells is not stored but is immediately secreted; in contrast, renin produced in JG cells is stored in renin granules until secretion [15].
Fig. 3.
Representative histological images of renal interstitial production of renin in anaemic mice. (a and b) ISH (a) and immunohistochemistry (IHC, b) were performed to detect Ren1 mRNA and renin protein, respectively. (c) ISH of Ren1 mRNA in the kidney sections from control and ISAM mice treated with vehicle or C.E.R.A. (6H and 28D, see Fig. 2c). Dashed circles, arrows, and arrowheads indicate glomeruli, JG cells, and interstitial cells, respectively. Scale bars represent 100 μm.
Consistent with the reduction in renal Ren1 mRNA expression in the ISAM mice that recovered from anaemia after long-term Epo administration (Fig. 2c), interstitial Ren1 gene expression in the ISAM mice mostly disappeared after correction of the anaemia (Fig. 3c). No effects of short-term (6 h) Epo treatment on interstitial Ren1 gene expression were observed (Fig. 3c). The Ren1 mRNA expression profile in the JG cells was not influenced by Epo administration (arrows in Fig. 3c). Interstitial renin expression was also detected in a small population of renal interstitial cells in the control mice (arrowhead in Fig. 3a,), demonstrating that renal interstitial cells could express the Ren1 gene and that the interstitial cell population expressing renin expanded during anaemia.
3.4. Chronic anaemia induces Epo and Ren1 coexpression in renal fibroblasts
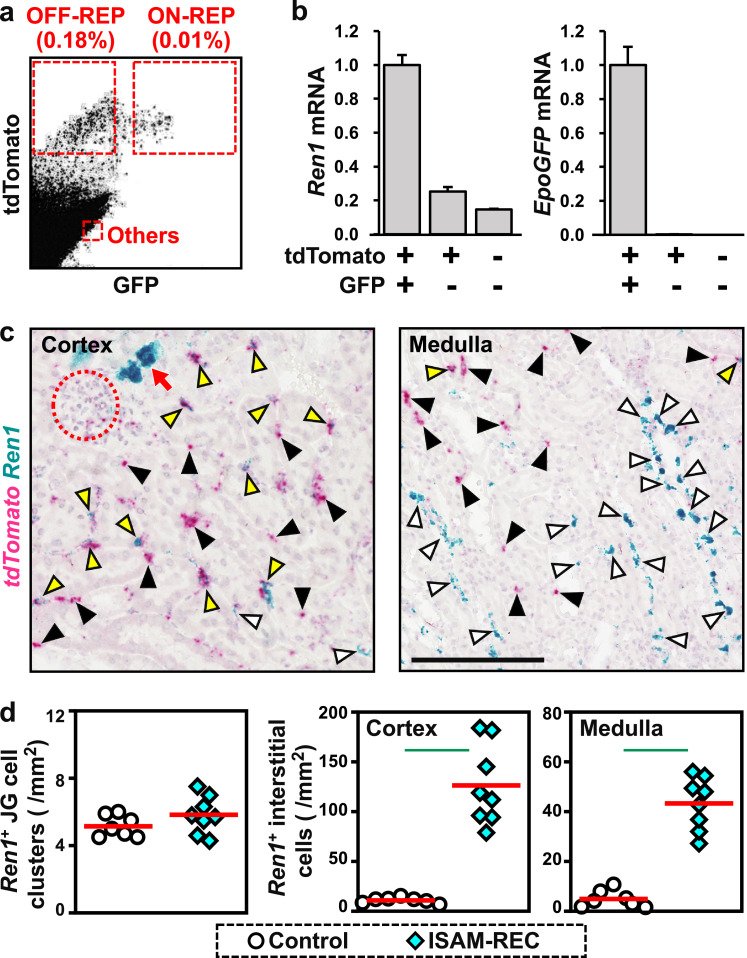
Epo-producing fibroblasts, REP cells, are located in the renal interstitium [4,18]. We investigated whether REP cells express the Ren1 gene in response to anaemic hypotension. In the ISAM-REC mice, almost all REP cells are permanently labelled with tdTomato fluorescence, which is expressed by the reporter transgene to detect cells undergoing Cre-mediated recombination, because EpoCre transgene expression is highly enhanced by chronic anaemia in ISAM mice [18]. Additionally, the endogenous Epo gene is homozygously replaced with GFP (green fluorescent protein) cDNA (the EpoGFP gene) in ISAM-REC mice [18]. The REP cells that displayed active or inactive Epo gene transcription were separately isolated from the ISAM-REC mouse kidneys with a cell sorter as tdTomato+/EpoGFP+ “ON-REP” cells and tdTomato+/EpoGFP– “OFF-REP” cells, respectively (Fig. 4a) [18].
Fig. 4.
Detection of cells coexpressing renin and Epo. (a) Flow cytometry of GFP and tdTomato expression in a renal cell suspension from ISAM-REC mice. (b) Relative expression of Ren1 and EpoGFP mRNA in isolated cell fractions (tdTomato+/GFP+, ON-REP; tdTomato+/GFP–, OFF-REP; and tdTomato–GFP–, others in a). (c) Representative images of double ISH detecting tdTomato (red) and Ren1 (blue) mRNA in sections of the renal cortex and medulla from the ISAM-REC mice. Dotted circles and red arrows indicate glomeruli and JG cells, respectively. White, black, and yellow arrowheads indicate interstitial fibroblasts positive for Ren1 (blue), tdTomato (red), and both (purple), respectively. The scale bar represents 100 μm. (d) Quantification of ISH images. Red bars indicate mean values. Green lines indicate P<0.01 in the Wilcoxon-Mann-Whitney test. n = 7 and 8, for control and ISAM-REC mice, respectively.
While EpoGFP mRNA was exclusively detected in the sorted ON-REP cells, Ren1 mRNA expression was detectable in all 3 cell fractions and tended to be high in the ON-REP fraction and low in the tdTomato– non-REP cell fraction containing JG cells as a minor cell population (Fig. 4b). These data demonstrated that at least some REP cells, which are fibroblasts located mainly in the peritubular interstitium of the renal cortex, express the Ren1 gene under chronic anaemia conditions. Additionally, the observation of Ren1 gene expression in ON-REP cells of the ISAM-REC mice suggests that some interstitial fibroblasts simultaneously produce Epo and renin in anaemic mouse kidneys. The double staining to detect tdTomato and Ren1 mRNA expression in the ISAM-REC kidneys by ISH confirmed that the Ren1 gene was expressed by REP cells labelled with tdTomato mRNA expression (yellow arrowheads in Fig. 4c). Thus, the renal interstitial cells expressing the Ren1 gene in anaemic mice are fibroblasts, including REP cells, but not endothelial and myeloid lineage cells [27].
The ISH data on Ren1 mRNA expression in the anaemic and control mice demonstrated that anaemia turned on Ren1 expression in many interstitial fibroblasts widely distributed in both the renal cortex and medulla. In contrast, the numbers of Ren1+ JG cell clusters were unaffected by anaemia (Fig. 4d). In the renal cortex, in which most interstitial fibroblasts are REP cells [4,18], more than 85% of Ren1+ interstitial cells were positive for tdTomato expression, while approximately 30% of tdTomato+ cells expressed Ren1 mRNA (Table S3). These data indicated that a part of REP cells, a major fraction of renal cortical interstitial fibroblast, were responsible for renin induction in response to anaemia. However, a minor portion of Ren1+ cells overlapped with tdTomato+ REP cells in the renal medulla, which contained a few REP cells (Table S3) [4,18]. In the medulla as well as the cortex, Ren1+ interstitial cells were PDGFRβ+ fibroblasts (Fig. S3b). We thus propose that the kidney increases renin production levels under anaemic conditions by activating Ren1 gene expression, principally in interstitial fibroblasts.
3.5. Acute anaemia results in severe hypotension and induces fibroblastic renin
Although anaemia is believed to reduce blood pressure through decreasing blood viscosity [1,2], the possibility that hearts are fatigued because of a long-term insufficient oxygen supply due to chronic anaemia could not be excluded. In fact, the cardiac deterioration of chronic anaemia patients is due to the long-term shortage of oxygen, and cardiomegaly has been observed in ISAM mice [18,22]. Additionally, it was unclear whether the induction of renal interstitial renin contributed to the increasing blood pressure in ISAM mice because these mice exhibit hypotension regardless of elevations in renal renin production.
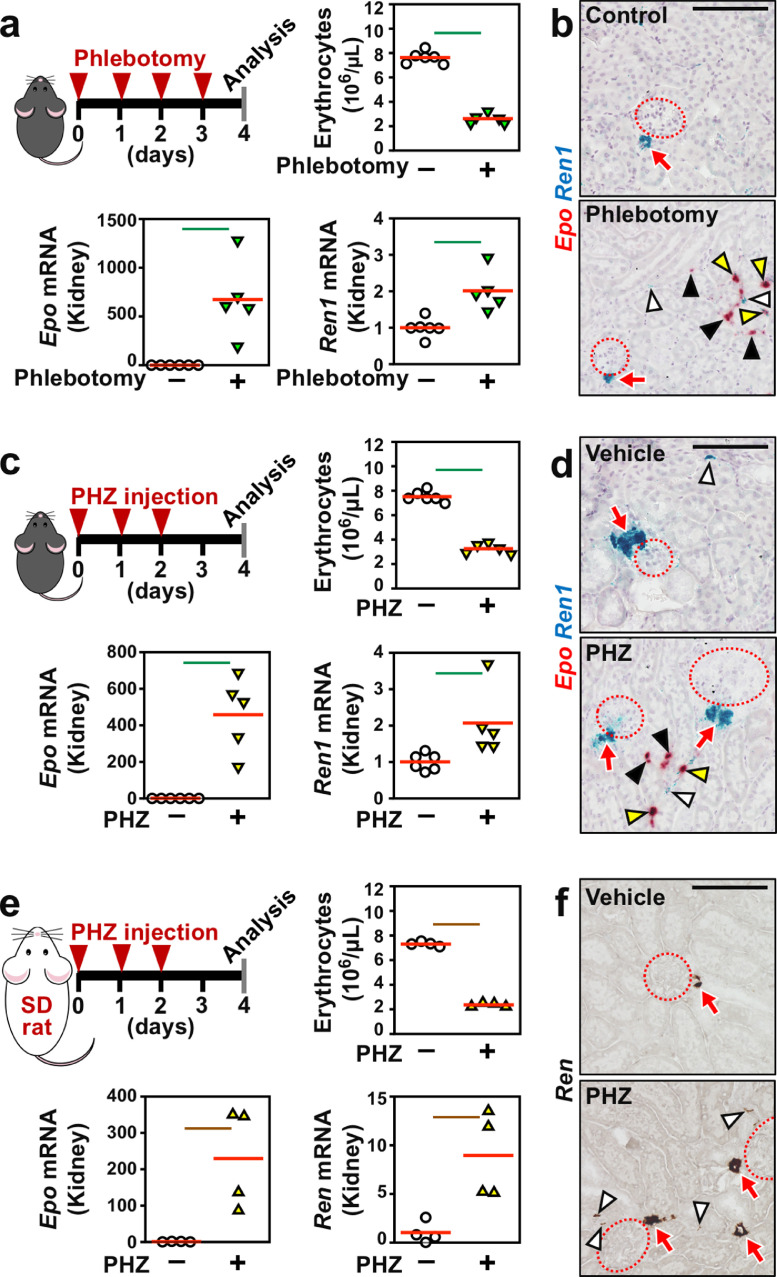
We investigated whether acute anaemia resulted in hypotension and renal interstitial renin induction. Bleeding anaemia was induced in wild-type mice by daily phlebotomy for 4 days. The erythrocyte concentrations were significantly reduced 24 h after the final phlebotomy (Fig. 5a). At this time, the blood pressures were below the quantifiable level, and the Ren1 and Epo mRNA levels were upregulated by acute bleeding anaemia (Fig. 5a). The ISH data demonstrated that Ren1 mRNA expression in the renal interstitium was induced by acute bleeding anaemia, consistent with the Ren1 mRNA expression profile (Fig. 5a and b).
Fig. 5.
Acute anaemia induces Ren1 mRNA expression in the renal interstitium. (a) Epo and Ren1 mRNA expression levels were elevated in kidneys from wild-type C57BL/6 mice by phlebotomy-induced haemorrhagic anaemia. The experimental scheme, erythrocyte counts, and Epo and Ren1 mRNA levels are shown. (b) Representative images of ISH of Epo (red) and Ren1 (blue) mRNA in kidney sections from control and haemorrhagic mice. (c) Epo and Ren1 mRNA expression levels were elevated in kidneys from wild-type C57BL/6 mice by phenylhydrazine (PHZ)-induced haemolytic anaemia. The experimental scheme, erythrocyte counts, and Epo and Ren1 mRNA levels are shown. (d) Representative images of ISH of Epo (red) and Ren1 (blue) mRNA in kidney sections from control and haemolytic mice. (e) Epo and Ren1 mRNA expression levels were elevated in kidneys from wild-type Slc:SD rats by PHZ-induced haemolytic anaemia. The experimental scheme, erythrocyte counts, and Epo and Ren1 mRNA levels are shown. (f) Representative images of ISH of Ren1 mRNA (brown) in kidney sections from control and haemolytic rats. The average expression levels (red bars) in the control mice were set as 1 for each gene expression analysis (a, c, and e). Green and brown lines indicate P<0.01 and P<0.05, respectively, in the Wilcoxon-Mann-Whitney tests (a, c, and e). n = 4–6 per group. Dotted circles and red arrows indicate glomeruli and JG cells, respectively (b, d, and f). White, black, and yellow arrowheads indicate interstitial fibroblasts positive for Ren1 (b, d, and f), Epo (b and d), and both (b and d), respectively. Scale bars represent 100 μm (b, d, and f).
Phenylhydrazine injections resulted in acute haemolytic anaemia and hypotension (undetectable blood pressure) in wild-type mice (Fig. 5c). Acute haemolytic anaemia, the severity of which was comparable with that of the ISAM mice, increased Epo and Ren1 mRNA expression in the renal interstitium (Fig. 5c, d, and Fig. S4a). In agreement with the induction levels of Ren1 mRNA in kidneys in the haemolytic anaemia model, which were lower than in the chronic Epo-deficiency anaemia model (Fig. 2a and Fig. 5c), the increase in Ren1+ interstitial cells by haemolytic anaemia was reduced compared with than the chronic anaemia in the ISAM mice (Fig. 5d and Fig. S4a). In the chronic anaemia model as well, the numbers of Ren1+ JG cell clusters were unchanged by the acute haemolytic anaemia model (Fig. 5d and Fig. S4a). Thus, renal interstitial fibroblasts commonly produce renin under acute and chronic anaemia conditions. Because renin induction was expected to increase adrenal secretion of aldosterone, one of the end products of RAS, blood concentrations of aldosterone were measured in the anaemic mouse models. All 3 models tended to exhibit elevated aldosterone levels compared with the control non-anaemic mice, and the induction levels were comparable amongst the anaemia models (Fig. S5). These data suggested that anaemic conditions commonly induced renin production in the renal interstitial fibroblasts and activated RAS.
Since the data obtained from the acute anaemia mouse models accurately recapitulated the data obtained from the ISAM mice with chronic anaemia, rats were used as a second species to confirm the renal interstitial renin induction by anaemic hypotension. Phenylhydrazine injection reduced the erythrocyte concentration in the peripheral blood of rats and induced the expression of the Epo (Epo) and renin (Ren) genes in the rat kidney (Fig. 5e). As expected, the renal interstitium was identified by ISH as a site of renin induction in the anaemic rats (Fig. 5f). However, the increase in Ren1-expressing fibroblast counts in anaemic rats was lower than in anaemic mice (Fig. S4a–c). This difference in the renal interstitial renin expression between species seemed to be related to the difference in the renin expression profile in JG cells. In mice, more than 60% of the JG cell clusters were positive for Ren1 mRNA expression even in a normal situation, and JG cells competent for renin induction in an anaemic situation were limited in numbers (Fig. S4b). Conversely, less than 20% of the JG cell clusters expressed Ren mRNA in rats, and anaemic stimuli significantly elevated the numbers of Ren-expressing JG cell clusters (Fig. S4b). Thus, since the majority of JG cells were able to induce renin production in response to anaemia in rats, renal interstitial fibroblasts of rats did not have to activate their renin production, whereas anaemic mice required renin production in renal interstitial fibroblasts to compensate for the shortage of renin production in JG cells.
Because renal interstitial cells coexpressing Ren1 and Epo were repetitively detected under anaemic conditions (Fig. 5b, d, and Fig. S6), interstitial renin-producing cells could be considered fibroblastic cells, which can produce both Epo and renin in an anaemia-inducible manner. The finding of milder hypotension in the chronic anaemia models compared with the acute anaemia models indicates that the continuous induction of renal interstitial renin production significantly contributed to ameliorating anaemic hypotension in the chronic phase of severe anaemia. Thus, blood cell loss decreased blood pressure, and renal interstitial fibroblasts, in addition to JG cells, produced renin to maintain the erythrocyte circulation during anaemic hypotension.
3.6. Hypoxia signalling is not involved in anaemia-inducible renin production
Since anaemia systemically depletes the oxygen supply, the effects of hypoxia signalling on the anaemia-inducible production of renin in renal interstitial fibroblasts were examined. In mice undergoing hypoxia (6% oxygen) for 48 h, Epo mRNA expression was induced in the renal interstitium, while erythropoiesis was not stimulated within this short period as previously reported (Fig. S7a) [28]. The hypoxic conditions did not affect Ren1 mRNA expression in the whole kidney or the renal interstitium (Fig. S7a), indicating that hypoxia was not involved in anaemia-inducible renin production in the kidney.
We further investigated the effects of hypoxic signalling on renal interstitial renin regulation using the PHDI GSK360A, which pharmacologically activates hypoxic signalling [23,24]. Consistent with our recent report [23], renal interstitial Epo mRNA expression was strongly induced 6 h after GSK360A injection into mice (Fig. S7b). However, consistent with the data from the hypoxic mice, no change in renal Ren1 gene expression was observed following GSK360A injection (Fig. S7b). These data indicated that hypoxic signalling in anaemic mice was unrelated to renin induction in renal interstitial fibroblasts.
3.7. Renin expression in renal interstitia depends on blood pressure changes
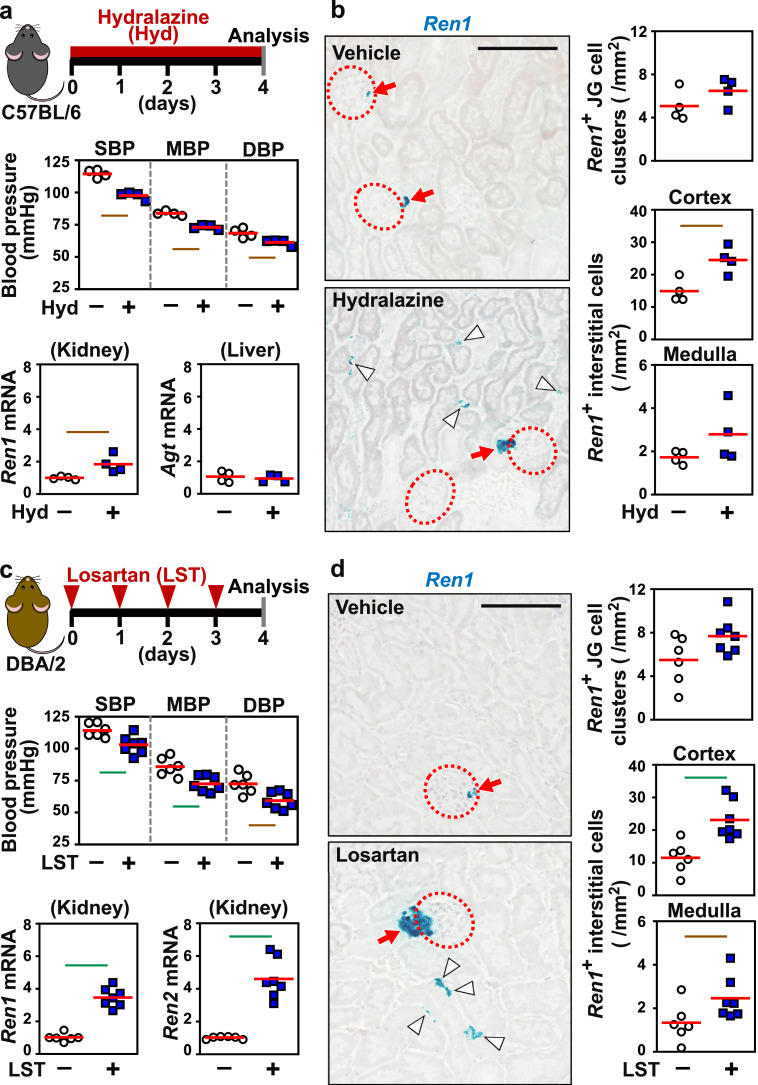
Since hypoxic signalling was excluded as a candidate trigger of interstitial renin induction by anaemia, the impact of hypotension on interstitial renin production was investigated. To induce hypotension without anaemia in mice, we first provided wild-type C57BL/6 mice with drinking water containing the vasodilator hydralazine for 4 days (Fig. 6a). As a result, mouse blood pressure significantly decreased, and the expression level of renal Ren1 mRNA increased (Fig. 6a). However, the Agt and Ace mRNA levels in the liver and lung, respectively, were unchanged after hydralazine treatment (Fig. 6a and Fig. S8a). The ISH data indicated that Ren1 mRNA expression was significantly induced in the interstitia of the renal cortex, while the induction levels were very low or insignificant in the medullary interstitia and JG cell clusters (Fig. 6b).
Fig. 6.
Renal interstitial renin is induced by antihypertensive agents. (a) Vasodilator (hydralazine, Hyd) administration causes hypotension and renal renin induction in wild-type C56BL/6 mice. The experimental scheme, blood pressure, Ren1 mRNA levels in kidney, and Agt mRNA levels in liver are shown. (b) Representative images and quantification of ISH of Ren1 mRNA in kidney sections of mice freely drinking Hyd for 4 days. (c) Injection of an AngII receptor blocker reagent (losartan, LST) into wild-type DBA/2 mice resulted in hypotension and the induction of the genes encoding renin (Ren1 and Ren2). The experimental scheme, blood pressure, and Ren1 and Ren2 mRNA levels in the kidney are shown. (d) Representative images and quantification of ISH of Ren1 mRNA in kidney sections of mice after LST administration. The average expression levels (red bars) in the control mice were set as 1 for each gene expression analysis (a and c). n = 4–7 per group. Dotted circles and red arrows indicate glomeruli and JG cells, respectively (b and d). White arrowheads indicate interstitial fibroblasts positive for Ren1 (blue in b and d). Scale bars represent 100 μm (b and d). Green and brown lines indicate P<0.01 and P<0.05, respectively, in the Wilcoxon-Mann-Whitney test. .
Next, losartan [29], an ARB, was injected into mice to investigate the effects of hypotension and the AngII signal on renin induction in renal interstitial fibroblasts. In this experiment, we analysed mice of the DBA/2 strain, which have 2 adjacent renin genes (Ren1 and Ren2) [30], whereas the C57BL/6 strain has the Ren1 gene only, and examined the expression of both genes after ARB treatment. As expected, ARB treatment decreased blood pressure without anaemia and increased the mRNA expression of both Ren1 and Ren2 in the DBA/2 mouse kidneys (Fig. 6c). The mRNA expression levels of Agt and Ace in the liver and lung, respectively, were unaffected by ARB treatment (Fig. S8b). The ISH data indicated that ARB induced Ren1 mRNA expression in the renal interstitium, especially in the renal cortex, rather than in the renal medulla (Fig. 6d). JG cells consistently expressed Ren1 mRNA regardless of losartan injection. These findings demonstrated that hypotension alone activated the expression of the Ren1 gene in renal interstitial fibroblasts and that the Ren2 gene also responded to hypotension in mouse strains harbouring the gene.
Furthermore, we tested whether hypertension could reduce interstitial renin production. For the hypertension model, we adopted a mouse model of PAH, in which human AngII is genetically overproduced during late pregnancy [20, 21]. The Ren1 mRNA levels in whole kidney from the hypertensive mice were significantly lower than those in whole kidney from the control mice, whereas the hepatic Agt mRNA levels were unaffected by hypertension (Fig. S9a). ISH of mouse Ren1 mRNA revealed that the renin-producing cell counts tended to be reduced in JG cells and medullary interstitial cells under hypertension, with a significant decrease observed in cortical interstitial cells (Fig. S9b). Thus, renal interstitial fibroblasts regulated renin production in response to not only a decrease in blood pressure but also hypertension.
3.8. Renal interstitial renin is induced in injured kidneys of mice, rat, and humans
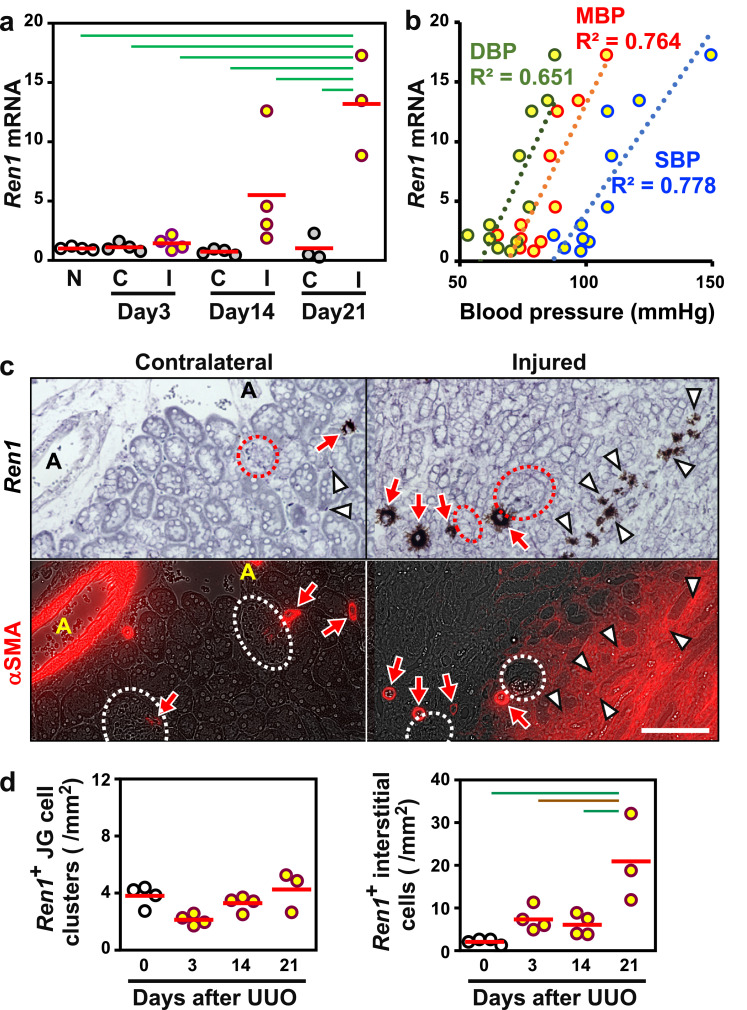
Renal interstitial fibroblasts are transformed into myofibroblasts and proliferate to promote renal fibrosis in injured kidneys [4,25]. We next investigated the renin expression profile in injured kidneys. The Ren1 mRNA expression in whole kidneys gradually increased with the progression of renal fibrosis induced by ureteral obstruction in mice (Fig. 7a). The mRNA expression levels in injured kidneys were well correlated to the systemic blood pressure (Fig. 7b), indicating that renin induction in fibrotic kidneys contributed to the increase in systemic blood pressure.
Fig. 7.
Renal interstitial renin is induced by renal fibrosis and related to the increase in systemic blood pressure. (a) Ren1 mRNA levels in injured (I) and contralateral (C) kidneys of mice subjected to UUO for 3, 14, and 21 days. The average expression level (red bar) in non-treated kidneys (N) was set as 1. (b) Scatter plots of the relationship between systemic blood pressures (DBP, green; MBP, red; SBP, blue) and Ren1 mRNA levels in injured kidneys. R2 values for the relation are shown. (c) Representative images of serial sections stained with Ren1 ISH (upper, brown) and αSMA IHC (lower, red) demonstrate that interstitial cells positive for Ren1 mRNA expression (white arrowheads) are αSMA-positive myofibroblasts in injured kidneys 21 days after UUO surgery. Ren1+ JG cells (red arrows) next to glomeruli (dotted circles) and renal arterioles (A) are also positive for αSMA. The scale bar represents 100 μm. (d) Quantification of Ren1 ISH in injured kidney sections from mice after UUO surgery. n = 3–4 per group. Green and brown lines indicate P<0.01 and P<0.05, respectively, in the Tukey-Kramer HSD test (a and d).
Tissue section analyses demonstrated that αSMA+ myofibroblasts that emerged in tubulointerstitia of injured kidneys were responsible for the renin induction (Fig. 7c). In agreement with the Ren1 mRNA expression levels in whole kidneys, the numbers of Ren1+ interstitial cells were increased with the progression of renal fibrosis, while Ren1 mRNA expression in JG cell clusters was unaffected by injury (Fig. 7d and Fig. S10). These data indicated that overproduction of renin in myofibroblasts, which originate from renal interstitial fibroblasts including REP cells under nephritic conditions, contributed to systemic hypertension in fibrotic kidneys.
The spontaneously hypertensive rat (SHR) is a rat strain that is widely used as a model for essential hypertension caused by multiple unidentified genetic variations with elevated expression of the Ren gene in kidneys [19]. Ren mRNA expression levels were higher in kidneys of SHR than their normotensive Wistar-Kyoto control rats (WKY) as previously reported (Fig. S11) [19]. ISH data demonstrated that Ren expression was activated in interstitial cells as well as JG cells of SHR kidneys (Fig. S11). Thus, we propose that renal interstitial renin production contributes to the hypertensive phenotype of SHR.
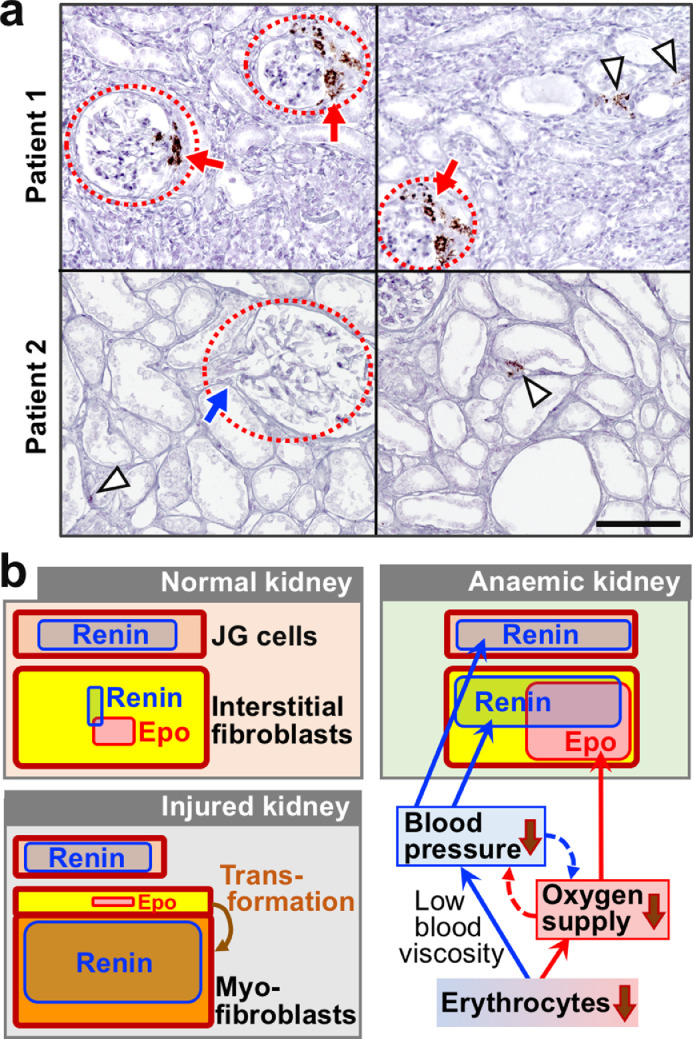
Since the pathological significance of renal interstitial renin production was observed, we finally investigated renin expression in kidney sections of human patients suffering from chronic tubulointerstitial nephritis (Table S2). ISH of REN mRNA revealed that renin was expressed in interstitial cells as well as JG cells of both nephritis patients examined (Fig. 8a). Taken together, kidney injury activated renal interstitial renin production in mice and humans, and the pathologic overproduction of renin potentially increased systemic blood pressure. Indeed, cases have been reported in which renal hypertension develops as a complication of kidney disease (Fig. 8b) [14].
Fig. 8.
Renal interstitial cells express renin in kidneys of CKD patients, and a schematic diagram of Epo and renin production in kidneys. (a) Representative images of ISH of REN (brown) in kidney sections of patients suffering from chronic tubulointerstitial nephritis. Arrowheads and dotted circles are interstitial fibroblasts positive for REN mRNA expression and glomeruli, respectively. Red and blue arrows indicate JG cells that are positive and negative for REN mRNA expression, respectively. The scale bar is 100 μm. (b) A schematic diagram of Epo and renin production in the kidney under normal, anaemic, and injured conditions. Renal interstitial fibroblasts (yellow) maintain the systemic oxygen supply under anaemic conditions through inducing Epo and renin production in response to hypoxia and hypotension, respectively. In injured kidneys, most interstitial fibroblasts are transformed into myofibroblasts (orange), which lose the ability to produce Epo and enhance renin production.
4. Discussion
We hypothesized that anaemia lowers blood pressure because erythrocyte loss reduces blood viscosity. A positive correlation was experimentally demonstrated between erythrocyte concentration and blood pressure in humans and mice. Thus, we propose that hypotension and erythrocyte loss in anaemic patients synergistically reduce the oxygen supply to peripheral organs through reducing the circulation rates of erythrocytes, the concentrations of which are decreased in anaemia. Additionally, we discovered that production of the major vasopressor renin is induced mainly in renal interstitial fibroblasts to maintain blood pressure under anaemic conditions rather than JG cells, with the latter being well-known renin-producing cells, (Fig. 8b). Moreover, we concluded that renal interstitial fibroblasts that inductively produce both renin and Epo play a central role in maintaining the systemic oxygen supply through blocking the pathological interaction between hypoxia and hypotension under anaemic conditions.
JG cells are known as the principal renin-producing cells, and renin secretion by these cells is controlled by renin release from granules that store renin [11,12]. The renin release from these granules is stimulated by paracrine signalling from specific renal tubular cells (macula densa) that sense decreases in Cl– flow in the thick ascending limb of Henle's loop [15]. The released renin activates local RAS to constrict the efferent arteriole around the glomeruli. According to previous reports by our group and others, this macula densa mechanism fine-tunes the glomerular filtration rate and systemic blood pressure homoeostasis [31]. Although a small number of smooth muscle cells adjacent to JG cells express the Ren1 gene in a hypotension-inducible manner (the “recruitment phenomenon”) according to our ISH data, the release of renin from granules is thought to be the rate-limiting step of renin secretion from JG cells [11,15]. This study identified renal interstitial fibroblasts as the major sites of renin induction under hypotensive conditions. Since Ren1 mRNA expression is detectable in a few renal interstitial fibroblasts under normal conditions, the transcriptional ON/OFF switching of the gene(s) for renin in response to hypotension is the rate-limiting step of renin secretion in the renal interstitium. Thus, the regulatory mechanisms of renin production for blood pressure homoeostasis differ between JG cells and interstitial fibroblasts in the kidney. Additionally, we propose that the widely distributed interstitial fibroblasts may complement the shortage of renin produced by the limited numbers of JG cells to help address the high demand for renin in severe hypotension.
Although this is the first report to show hypotension-dependant renin production in renal interstitial fibroblasts, renin production in renal interstitial cells of Agt knockout mice has been previously reported [32]. Regarding the relationship between JG cells and renal interstitial fibroblasts, some reports have proposed that JG cells and renal interstitial fibroblasts share a developmental origin and cellular features [33,34]. Indeed, genetic activation of HIF2α in JG cells induces ectopic Epo gene expression [33,34]. Additionally, JG cells and interstitial fibroblasts commonly bind capillaries between tubules in the kidney [9,35]. Therefore, interstitial renin production may be stimulated by paracrine signals from collecting duct cells that correspond to the macula densa cells of JG cells.
We found that some renal interstitial fibroblasts simultaneously coproduce renin and Epo in anaemic mouse kidneys. Anaemia induces systemic hypoxia and hypotension; the former exclusively activates Epo production, while the latter stimulates renin production in renal interstitial fibroblasts. Therefore, the triggers for Epo and renin production are mutually independent in a renal interstitial fibroblast. Although various studies have investigated the transcriptional regulatory mechanism of the Ren1 gene in JG cells [36], [37], [38], the triggers of renin induction have been poorly elucidated. A previous report using transgenic mouse models demonstrated that the 4.9-kb Ren1 gene promoter is sufficient for Ren1 gene induction in the recruitment phenomenon [36]. However, the promoter region cannot drive hypotension-inducible gene expression in renal interstitial fibroblasts, suggesting that the Ren1-gene regulatory mechanism in recruited smooth muscle cells is different from that in interstitial fibroblasts. Additionally, researchers have suggested that the Ren1 gene regulatory elements for interstitial expression are not included in the 4.9-kb upstream region. In fact, Ren1 gene enhancers were shown to be scattered in the genomic region extending 20 kb upstream from the transcriptional start site [37].
We hypothesized that inducible renin production in renal interstitial fibroblasts is important for maintaining blood pressure homoeostasis, whereas overproduction of renin provokes pathologic hypertension. CKD often causes hypertension due to renin overproduction [39,40], and renal interstitial fibroblasts are the origin of myofibroblasts, which emerge and expand in fibrotic kidneys in CKD patients [25,41]. In fact, renal myofibroblasts were found to strongly express renin in fibrotic kidneys of mice subjected to UUO, and the increased levels of renin expression correlated well to systemic blood pressure. Although further studies are required, these observations led to the hypothesis that renin is produced by expanded myofibroblasts in fibrotic kidneys and causes renal hypertension (Fig. 8b).
The present study examined the renin production of renal interstitial fibroblasts, which were previously believed to produce only Epo. These two roles of renal interstitial fibroblasts seem to cooperatively maintain the oxygen supply to peripheral organs through controlling the mass and circulation of erythrocytes. In addition, as we previously reported that AngII activates Epo production in the kidney [42,43], renin produced in renal interstitial fibroblasts by anaemic hypotension stimuli may enhance the renal interstitial Epo production induced by anaemic hypoxia through AngII signalling. In fact, the frequent use of ACE inhibitors or ARB is considered to be related to anaemia [44]. Thus, renal interstitial renin is likely important not only for blood pressure maintenance but also for Epo induction under anaemic conditions.
Author contributions
K.M. and N.S. designed the study. K.M., T.N., S.S., T.Y., K.S., K.K., M.N., and N.S. performed the experiments and analysed the data. K.K., M.Y., and N.S. provided the gene-modified mouse lines and reagents. K.M. and N.S. constructed the figures and wrote the manuscript. M.M., S.I., M.Y., and N.S. developed the project. All authors discussed the results and suggested revisions.
Declaration of Competing Interests
The authors declare no competing interests.
Acknowledgments
This work was partially supported by Grants-in-Aid from MEXT/JSPS KAKENHI (17K19680, 15H04691, and 26111002) and the Takeda Science Foundation. The funders played no role in the design of the study, data collection, analysis, decision to publish, or preparation of the manuscript. We thank Atsuko Konuma, Mizuho Tanno, Atsushi Ishida, Shunsuke Imai, Tomoyo Arai, Yumako Ito, Chika Tanaka, Hayato Yamaki, and Keiko Taguchi (Tohoku University) for their technical help and scientific advice. We also thank the Biomedical Research Core and Centre for Laboratory Animal Research of Tohoku University Graduate School of Medicine for technical support.
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103209.
Appendix. Supplementary materials
References
- 1.Whittaker S.R., Winton F.R. The apparent viscosity of blood flowing in the isolated hindlimb of the dog, and its variation with corpuscular concentration. J Physiol. 1933;78:339–369. doi: 10.1113/jphysiol.1933.sp003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pop G.A., Duncker D.J., Gardien M. The clinical significance of whole blood viscosity in (cardio)vascular medicine. Neth Heart J. 2002;10:512–516. [PMC free article] [PubMed] [Google Scholar]
- 3.Metivier F., Marchais S.J., Guerin A.P., Pannier B., London G.M. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transpl. 2000;15:14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki N., Yamamoto M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016;468:3–12. doi: 10.1007/s00424-015-1740-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu H., Liu X., Jaenisch R., Lodish H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki N., Hirano I., Pan X., Minegishi N., Yamamoto M. Erythropoietin production in neuroepithelial and neural crest cells during primitive erythropoiesis. Nat Commun. 2013;4:2902. doi: 10.1038/ncomms3902. [DOI] [PubMed] [Google Scholar]
- 7.Schödel J., Ratcliffe P.J. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. 2019;15:641–659. doi: 10.1038/s41581-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 8.Brier M.E., Gaweda A.E., Aronoff G.R. Personalized anemia management and precision medicine in ESA and iron pharmacology in end-stage kidney disease. Semin Nephrol. 2018;38:410–417. doi: 10.1016/j.semnephrol.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Souma T., Nezu M., Nakano D. Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. J Am Soc Nephrol. 2016;27:428–438. doi: 10.1681/ASN.2014121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanghani N.S., Haase V.H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chron Kidney Dis. 2019;26:253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparks M.A., Crowley S.D., Gurley S.B., Mirotsou M., Coffman T.M. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H., Nangaku M., Navar L.G., Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 13.Simões E., Silva A.C., Miranda A.S., Rocha N.P., Teixeira A.L. Renin angiotensin system in liver diseases: friend or foe? World J Gastroenterol. 2017;23:3396–3406. doi: 10.3748/wjg.v23.i19.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito S. Cardiorenal connection in chronic kidney disease. Clin Exp Nephrol. 2012;16:8–16. doi: 10.1007/s10157-011-0493-2. [DOI] [PubMed] [Google Scholar]
- 15.Friis U.G., Madsen K., Stubbe J. Regulation of renin secretion by renal juxtaglomerular cells. Pflugers Arch. 2013;465:25–37. doi: 10.1007/s00424-012-1126-7. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T., Nakayama M., Miyazaki M. Relationship between low blood pressure and renal/cardiovascular outcomes in Japanese patients with chronic kidney disease under nephrologist care: the Gonryo study. Clin Exp Nephrol. 2015;19:878–886. doi: 10.1007/s10157-015-1084-4. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki S., Souma T., Hirano I. A mouse model of adult-onset anaemia due to erythropoietin deficiency. Nat Commun. 2013;4:1950. doi: 10.1038/ncomms2950. [DOI] [PubMed] [Google Scholar]
- 19.Chen L., Fukuda N., Shimizu S. Role of complement 3 in renin generation during the differentiation of mesenchymal stem cells to smooth muscle cells. Am J Physiol Cell Physiol. 2020;318:C981–C990. doi: 10.1152/ajpcell.00461.2019. [DOI] [PubMed] [Google Scholar]
- 20.Nezu M., Souma T., Yu L. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal. 2017;10:eaam5711. doi: 10.1126/scisignal.aam5711. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto E., Ishida J., Sugiyama F., Horiguchi H., Murakami K., Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274:995–998. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki N., Sasaki Y., Kato K. Efficacy estimation of erythropoiesis-stimulating agents using erythropoietin-deficient anemic mice. Haematologica. 2016;101:e356–e360. doi: 10.3324/haematol.2015.140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki N., Matsuo-Tezuka Y., Sasaki Y. Iron attenuates erythropoietin production by decreasing hypoxia-inducible transcription factor 2α concentrations in renal interstitial fibroblasts. Kidney Int. 2018;94:900–911. doi: 10.1016/j.kint.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Sato K., Hirano I., Sekine H. An immortalized cell line derived from renal erythropoietin-producing (REP) cells demonstrates their potential to transform into myofibroblasts. Sci Rep. 2019;9:11254. doi: 10.1038/s41598-019-47766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souma T., Yamazaki S., Moriguchi T. Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol. 2013;24:1599–1616. doi: 10.1681/ASN.2013010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R. Mechanisms and mediators of hypertension induced by erythropoietin and related molecules. Nephrol Dial Transpl. 2018;33:1690–1698. doi: 10.1093/ndt/gfx324. [DOI] [PubMed] [Google Scholar]
- 27.Pan X., Suzuki N., Hirano I., Yamazaki S., Minegishi N., Yamamoto M. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS ONE. 2011;6:e25839. doi: 10.1371/journal.pone.0025839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tojo Y., Sekine H., Hirano I. Hypoxia signaling cascade for erythropoietin production in hepatocytes. Mol Cell Biol. 2015;35:2658–2672. doi: 10.1128/MCB.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia H., Feng Y., Obr T.D., Hickman P.J., Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen P.B., Yang T., Huang Y., Mizel D., Briggs J., Schnermann J. Plasma renin in mice with one or two renin genes. Acta Physiol Scand. 2004;181:431–437. doi: 10.1111/j.1365-201X.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 31.Itoh S., Carretero O.A., Murray R.D. Renin release from isolated afferent arterioles. Kidney Int. 1985;27:762–767. doi: 10.1038/ki.1985.77. [DOI] [PubMed] [Google Scholar]
- 32.Berg A.C., Chernavvsky-Sequeira C., Lindsey J., Gomez R.A., Sequeira-Lopez M.L. Pericytes synthesize renin. World J Nephrol. 2013;2:11–16. doi: 10.5527/wjn.v2.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerl K., Miquerol L., Todorov V.T. Inducible glomerular erythropoietin production in the adult kidney. Kidney Int. 2015;88:1345–1355. doi: 10.1038/ki.2015.274. [DOI] [PubMed] [Google Scholar]
- 34.Kurt B., Gerl K., Karger C., Schwarzensteiner I., Kurtz A. Chronic hypoxia-inducible transcription factor-2 activation stably transforms juxtaglomerular renin cells into fibroblast-like cells in vivo. J Am Soc Nephrol. 2015;26:587–596. doi: 10.1681/ASN.2013111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martini A.G., Danser A.H.J. Vol. 24. High Blood Press Cardiovasc Prev; 2017. pp. 231–242. (Juxtaglomerular cell phenotypic plasticity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez M.F., Medrano S., Brown E.A. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J Clin Invest. 2018;128:4787–4803. doi: 10.1172/JCI121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ushiki A., Matsuzaki H., Fukamizu A., Tanimoto K. Homeostatic response of mouse renin gene transcription in a hypertensive environment is mediated by a novel 5′ enhancer. Mol Cell Biol. 2018;38 doi: 10.1128/MCB.00566-17. e00566-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saleem M., Hodgkinson C.P., Xiao L. Sox6 as a new modulator of renin expression in the kidney. Am J Physiol Renal Physiol. 2020;318:F285–F297. doi: 10.1152/ajprenal.00095.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckalew V.M., Jr, Berg R.L., Wang S.R., Porush J.G., Rauch S., Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of diet in renal disease study group. Am J Kidney Dis. 1996;28:811–821. doi: 10.1016/s0272-6386(96)90380-7. [DOI] [PubMed] [Google Scholar]
- 40.Bakris G.L., Ritz E. The message for World Kidney Day 2009: hypertension and kidney disease: a marriage that should be prevented. Kidney Int. 2009;75:449–452. doi: 10.1038/ki.2008.694. [DOI] [PubMed] [Google Scholar]
- 41.Asada N., Takase M., Nakamura J. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato H., Ishida J., Imagawa S. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005;19:2023–2025. doi: 10.1096/fj.05-3820fje. [DOI] [PubMed] [Google Scholar]
- 43.Kato H., Ishida J., Matsusaka T. Erythropoiesis and blood pressure are regulated via AT1 receptor by distinctive pathways. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0129484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritz E., Haxsen V. Diabetic nephropathy and anaemia. Eur J Clin Invest. 2005;35:66–74. doi: 10.1111/j.1365-2362.2005.01544.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.