Abstract
In the landscape of cancer immunotherapy, immune cell engagers (ICEs) are rapidly emerging as a feasible and easy-to-deliver alternative to adoptive cell therapy for the antitumor redirection of immune effector cells. Even if in hematological malignancies this class of new therapeutics already hit the clinic, the development of ICEs in solid tumors still represents a challenge. Considering that ICEs are a rapidly expanding biotechnology in cancer therapy, we designed this review as a primer for clinicians, focusing on the major obstacles for the clinical implementation and the most translatable approaches proposed to overcome the limitations in solid tumors.
Key words: immunotherapy, BiTEs, immune cell engagers, solid tumors, T cell redirection
Highlights
-
•
Immune cell engagers (ICEs) are molecules able to redirect immune effector cells against cancer cells.
-
•
ICEs can be used for the redirection of T cells, natural killer (NK) cells and cytotoxic/phagocytic cells.
-
•
ICEs have the potential for enabling a feasible and effective redirection of effector cells in patients with solid tumors.
-
•
ICEs design, antigen specificity and heterogeneity and immunosuppressive microenvironment are major issues in solid tumors.
Introduction
Immunotherapy emerged as a revolutionary weapon in the treatment of hematological and solid malignancies in the last decade.1 Emblematic is the case of immune checkpoint inhibitors (ICIs), which have shown impressive clinical responses in certain histologies.2,3 Notably, the antitumor immune response unleashed by ICIs largely relies on tumor immunogenicity, whereas not all the tumors may present appropriately immunogenic characteristics able to trigger a cell-mediated antitumor response.4,5 Additionally, certain tumor types employ different strategies to overcome the immune response, as in the case of the loss/reduction in expression of class I major histocompatibility complex (MHC) molecules or alterations in the antigen processing machinery that prevent the appropriate antigen presentation to antitumor T cells.6 Immune cell engagers (ICEs) have been specifically developed with the aim of redirecting immune cells against surface tumor-associated antigens (TAAs) for MHC-independent cancer cell elimination and generation of immune responses against poorly immunogenic tumors. Beyond adoptive cell therapy that requires an expensive and complex process of cell manufacturing,7 ICEs could be seen as a feasible promise for the next generation immunotherapy. In this review we provide a concise outlook on ICEs with a focus on solid tumors, illustrate the available clinical data and discuss the challenges still open and the emerging advances.
Design and mechanism of action of ICEs
ICEs are molecules able to redirect immune effector cells (regardless of their antigen specificity) against cancer cells with the aim of triggering an efficient tumor cell killing.8 Most ICEs are trans-binding bispecific antibodies (bsAbs) usually consisting of two linked single-chain fragment variables (scFvs) that originate from different monoclonal antibodies: one scFv recognizes a surface TAA, whereas the other is specific for a certain membrane molecule expressed on effector immune cells.9 The scFv with specificity for the effector immune cell must be also able to trigger an appropriate signal transduction cascade to activate the killing machinery. The compact structure resulting from the link of these two different scFvs allows the formation of the immune synapsis between tumor and immune cells and eventually leads to tumor cell elimination. In order to avoid the risk of an uncontrolled triggering and subsequent toxicity, the activation of effector cells takes place only when both bsAbs ‘arms’ are engaged with their respective target antigens.10
The so-called ‘bystander killing’ effect (i.e. the killing of nearby target-negative cells via the release of molecules that diffuse locally upon ICEs-mediated effector cell activation in the presence of target-positive cells) also contributes to the antitumor activity of ICEs. Recent insights suggest that the diffusion of released cytokines leads to the upregulation of cell surface molecules (e.g. ICAM-1 and FAS) on bystander cells. The expression of these molecules makes bystander cells susceptible to effector cell-mediated killing even in the absence of a regular cytolytic synapse.11 Notably, this phenomenon is of particular interest in solid tumors that are characterized by a marked heterogeneity of TAA expression and by an immune suppressive tumor microenvironment (TME): a bystander killing effect can indeed mitigate antigen escape and contribute to eliminate the pro-tumoral cellular compartment of TME.
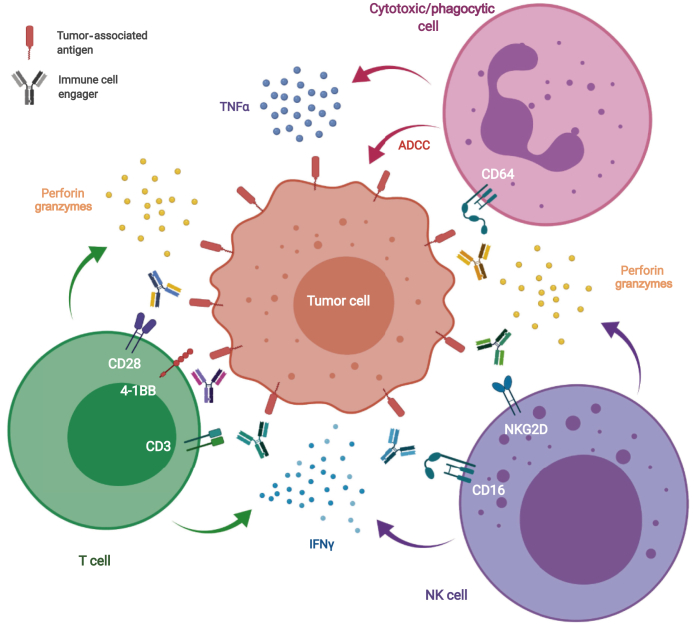
Based on the type of immune effector engaged, ICEs developed up-to-now can be divided into three main categories: T cell, natural killer (NK) cell and cytotoxic/phagocytic cell engagers (Figure 1).
Figure 1.
Mechanism of action of immune cells engagers.
Immune cell engagers are able to redirect immune effector cells and create an artificial immune synapse between tumor cells (targeting a tumor-associated antigen) and T cells (engaging CD3 or costimulatory molecules such as CD28 or 4-1BB), NK cells (engaging CD16 or NKG2D) and cytotoxic/phagocytic cells (engaging CD64).
IFN, interferon; NK, natural killer; TNF, tumor necrosis factor alpha.
T cell engagers
Bispecific T cell engagers (BiTes) are the most common class of ICEs and consist of a TAA-targeting scFv linked with an scFv usually activating a specific chain of the CD3 complex (mainly the CD3ε chain) that is associated with the T cell receptor (TCR) complex and participate in TCR-mediated signaling.12 By this approach, T cells are physically redirected against tumor cells and at the same time activated. The formation of this ‘artificial’ immunological synapse is accompanied by the redistribution of signaling and secretory granule proteins in T cells, leading to the release of perforin and granzyme.13 Such contact-dependent cytotoxicity is likely the main mechanism for BiTes-induced direct killing of tumor cells, as EDTA chelation of Ca2+ (required for perforin multimerization and pore formation) leads to the complete inhibition of target cell apoptosis.14 Activation of T cells also results in the secretion of cytokines and T-cell proliferation, which may be required to sustain a durable antitumor immune response.15 Together with canonical cytotoxic T cells (CD8+ T cells), also CD4+ T cells, γδ T cells and NK T cells (NKT cells) can be activated by and contribute to the antitumor activity of BiTes specific for the CD3 complex.16, 17, 18 A co-stimulation molecule (e.g. CD28 or 4-1BB) can also be exploited as a target to engage activated T cells, as showed using a trispecific antibody engaging CD3 and CD28 on T cells,19 or 4-1BB engaging molecules.20,21
NK cell engagers
NK cells are cytotoxic innate lymphoid cells capable of recognizing viral infected or transformed cells by a set of germline-encoded receptors, and are characterized by the lack of TCR and CD3 molecules and by the expression of CD56 (also known as neural cell adhesion molecule) and CD16 (also known as FcγRIII).22 NK cells activity is balanced by specific membrane receptors with activating (e.g. natural cytotoxicity receptors, like CD16) or inhibitory (e.g. inhibitory killer immunoglobulin-like receptors) functions.23 CD16 is the most implemented NK cell target for the development of ICEs in the format of bsAbs. Data from preclinical studies showed that CD16-directed ICEs are able to activate NK cells and induce TAA-specific cytotoxicity with cytokine and chemokine production.24 CD16-directed ICEs have shown antitumor activity in hematological malignancies and AFM13 (a CD30xCD16 bispecific compound) is currently in phase II clinical development for the treatment of Hodgkin's lymphoma.25 In solid tumors, CD16-directed ICEs have preclinically been shown to induce effective responses in several solid tumor models.26,27 Another approach to exploit NK cytotoxicity using ICEs involves the engagement of the activating NKG2D receptor. In preclinical models, NKG2D-directed bsAbs demonstrated activity both in vitro and in vivo against carcinoembryonic antigen (CEA)- and human epidermal growth factor receptor 2 (HER2)-positive tumors,26 and a CD24xNKG2D bsAb demonstrated in vivo activity in a model of hepatocellular carcinoma in combination with sorafenib.28
Cytotoxic/phagocytic cell engagers
Cytotoxic/phagocytic immune cells (i.e. monocytes, macrophages, dendritic cells and cytokine-activated neutrophils) can be engaged via the non-ligand binding site of the high-affinity receptor for immunoglobulin G (FcγRI, also known as CD64) which is selectively expressed by these immune cells.29 Chemically linked bispecific molecules engaging CD64 and targeting a TAA are able to trigger antibody-dependent cell-mediated cytotoxicity and cytotoxic lysis of tumor cells as shown in preclinical models of solid tumors targeting HER2 and epithelial cell adhesion molecule (EpCAM).30,31
Clinical experiences with ICEs in solid tumors
ICEs demonstrated impressive clinical results in hematological malignancies, as demonstrated by the success of blinatumomab, a BiTE targeting CD19 and engaging CD3, which led to Food and Drug Administration and European Medicines Agency (EMA) approval for the treatment of adults and pediatric patients with certain relapsed or refractory acute lymphoblastic leukemia (ALL).32, 33, 34 Unfortunately, translating these results in solid tumor patients is still challenging (Table 1).29,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 Emblematic is the case of cytotoxic/phagocytic cell engagers. Despite the encouraging activity observed in tumor models, the clinical activity of HER2xCD64 and epidermal growth factor receptor (EGFR)xCD64 molecules in early phase clinical trials was scant, probably because of the high bsAb concentration and high effector-to-target cell ratio required for effective tumor cell elimination.62 The first large-scale evidence about the clinical activity of a BiTE in solid tumors came with the approval of catumaxomab by the EMA in 2009 for the intraperitoneal treatment of malignant ascites in adult patients with EpCAM-positive carcinoma. EMA approval was based on the positive results of a large phase II/III trial in terms of time-to-next-paracentesis and signs and symptoms of ascites.36,46 However, when attempts were made to shift from locoregional to systemic administrations, the results were not encouraging. A phase I study, which aimed to demonstrate the safety and tolerability of intravenous (i.v.) infusion of catumaxomab, revealed dose-dependent hepatotoxicity of different grades, with one patient experiencing fulminant fatal acute liver failure which led to the early termination of the study. The severe adverse events were attributed to the off-target binding of a catumaxomab active Fc region to Fcγ receptors expressed by Kupffer cells in the liver, inducing local cytokine release and T cell-mediated hepatotoxicity.63 Solitomab (MT110, AMG110), another EpCAMxCD3 BiTE, has been investigated in 65 patients with relapsed/refractory advanced-stage solid cancers in a phase I dose-escalation study, with administration by continuous i.v. infusion. The treatment was associated with dose-limiting toxicities in 15 patients, including elevation of serum liver enzymes in 8 patients and severe diarrhea in 6 patients (with one fatal outcome) which precluded dose escalation to potentially therapeutic levels.49 Regardless of the Fc/Fcγ receptors interaction, the toxicity observed with the systemic administration of EpCAM-targeted BiTEs can be explained by EpCAM expression in both non-malignant and malignant epithelial cells,64 thus lowering the specificity for cancer cells and the therapeutic window. More encouraging results emerged in a phase I clinical trial evaluating the safety of ertumaxomab, an HER2xCD3 BiTE, in patients with metastatic, HER2-positive breast cancer. Five out of 15 assessable patients showed a clinical benefit, including 1 complete response, 2 partial responses and 2 disease stabilizations.51 Fourteen patients with HER2-positive advanced solid tumors were enrolled in another phase I trial with ertumaxomab. Clinical benefit was seen in 3 out of 11 assessable patients, including 1 partial response and 2 disease stabilizations.50 Pasotuxizumab is a prostate-specific membrane antigen (PSMA)xCD3 BiTE investigated in a phase I trial. An interim analysis showed the ability of pasotuxizumab to reduce serum prostate-specific antigen levels and the numbers of circulating tumor cells.61 At the higher dose levels investigated, one patient achieved a long-lasting near complete response as assessed by PSMA-position emission tomography.
Table 1.
Safety and activity data from published studies on ICEs in solid tumors
| Drug name | ICE design | Phase | Condition | Number of patients treated | Administration | Toxicity | Objective responses | Reference |
|---|---|---|---|---|---|---|---|---|
| Catumaxomab | EpCAMxCD3 | I | Solid tumors | 16 | i.v. (8 h) | Liver toxicity, fatal acute hepatic failure | Not reported | 35 |
| Catumaxomab | EpCAMxCD3 | I | Lung cancer | 21 | i.v. (6 h) | Liver toxicity, lymphopenia | Not reported | 36 |
| Catumaxomab | EpCAMxCD3 | I/II | Epithelial tumors | 24 | i.p. (3 h) | Liver toxicity, lymphopenia, anemia, pleural empyema | 1 CR 4 PR |
37 |
| Catumaxomab | EpCAMxCD3 | I/II | Colon, gastric, pancreatic cancer | 24 | i.p. (6 h) | SIRS, pulmonary edema, liver toxicity, lymphopenia | 1 CR 3 PR |
38 |
| Catumaxomab | EpCAMxCD3 | I/II | Ovarian cancer | 23 | i.p. (6 h) | Liver toxicity, lymphopenia, hypercalcemia | Not reported | 39 |
| Catumaxomab | EpCAMxCD3 | II | Solid tumors | 13 | i.p. (6 h) | Transient increases in liver enzymes | Not reported | 40 |
| Catumaxomab | EpCAMxCD3 | IIa | Ovarian cancer | 45 | i.p. (6 h) | Anemia, erythema induratum, liver toxicity | 1 PR | 41 |
| Catumaxomab | EpCAMxCD3 | II | Gastric cancer | 54 | Intraoperative i.p. bolus + i.p. (3 h) | Not applicable | Not reported | 42 |
| Catumaxomab | EpCAMxCD3 | II | Gastric cancer | 64 | Intraoperative i.p. bolus + i.p. (3 h) | Hypotension, liver toxicity, SIRS, abdominal pain, hypertension | 3 CR 13 PR |
43 |
| Catumaxomab | EpCAMxCD3 | II | Ovarian cancer | 32 | i.p. (3 h) | Liver toxicity, dehydration, abdominal pain, chills, fatigue, nausea, vomiting | Not reported | 44 |
| Catumaxomab | EpCAMxCD3 | II | Ovarian cancer | 41 | Intraoperative i.p. bolus + i.p. (3 h) | Liver toxicity, pleural effusion, pulmonary embolism | Not reported | 45 |
| Catumaxomab | EpCAMxCD3 | II/III | Epithelial cancers | 258 | i.p. (6 h) | Liver toxicity, ileus, gastric hemorrhage, lymphopenia, anemia | Not reported | 46 |
| Catumaxomab | EpCAMxCD3 | IIIb | Epithelial cancers | 219 | i.p. (3 h) | Liver toxicity, fatigue, nausea, pyrexia, abdominal pain | Not reported | 47 |
| Catumaxomab | EpCAMxCD3 (rechallenge) | I/II | Epithelial cancers | 8 | i.p. (3 h) | Liver toxicity, fatigue, nausea, pyrexia, abdominal pain | Not reported | 48 |
| Solitomab (MT110, AMG110) | EpCAMxCD3 | I | Solid tumors | 65 | i.p. (3 h) | Liver toxicity, diarrhea, lipase increase, peripheral edema | 1 PR | 49 |
| Ertumaxomab | HER2xCD3 | I | Solid tumors | 14 | i.v. (3 h) | Infusion reaction, CRS, cephalgia, tumor pain, hypertension | 1 PR | 50 |
| Ertumaxomab | HER2xCD3 | I | Breast cancer | 17 | i.v. (6 h) | Hypotension and ARDS, SIRS, liver toxicity | 1 CR 2 PR |
51 |
| MDX-H210 | HER2xCD64 | I | Solid tumors | 13 | i.v. (2 h) | Fever, chills, rigor | 1 PR | 52 |
| MDX-H210 | HER2xCD64 | I | Solid tumors | 24 | i.v. (2 h) | Neutropenia, thrombocytopenia, monocytopenia | Not reported | 53 |
| MDX-H210 | HER2xCD64 | I | Prostate cancer | 7 | i.v. (2 h) | Flu-like symptoms, hematological, liver toxicity | Not reported | 29 |
| MDX-H210 | HER2xCD64 | I | Breast cancer | 30 | i.v. (2 h) | Tumor pain, hypotension, fever, chills, liver toxicity | Not reported | 54 |
| MDX-H210 | HER2xCD64 | I | Breast cancer | 23 | i.v. (8 h) | Diarrhea, infusion reaction | Not reported | 55 |
| MDX-H210 | HER2xCD64 | Ia/Ib | Breast and ovarian cancer | 15 | i.v. (2 h) | Hypotension, nausea | 2 PR | 56 |
| MDX-H210 | HER2xCD64 | II | Prostate cancer | 25 | i.v. | Flu-like symptoms, infusion reaction | Not reported | 57 |
| 2B1 | HER2xCD16 | I | Solid tumors | 15 | i.v. (1 h) | Hypotension, leucopenia, thrombocytopenia | 1 PR | 58 |
| AMG211, MEDI-565 | CEAxCD3 | I | Gastrointestinal cancer | 39 | i.v. (3 h) | Hypoxia, diarrhea, CRS, liver toxicity | Not reported | 59 |
| RO6958688 | CEAxCD3 | Ia | Solid tumors | 36 | i.v. | Infusion reaction, pyrexia, diarrhea, hypoxia, colitis | 2 PR | 60 |
| RO6958688 | CEAxCD3 | Ib | Solid tumors | 10 | i.v. | Infusion reaction, pyrexia, diarrhea, hypoxia, colitis | 2 PR | 60 |
| Pasotuxizumab, BAY2010112 | PSMAxCD3 | I | Prostate cancer | 16 | i.v. | Lymphopenia, infections, CRS, fatigue | 1 PR | 61 |
ARDS, acute respiratory distress syndrome; CEA, carcinoembryonic antigen; CR, complete response; CRS, cytokine release syndrome; EpCAM, epithelial cell adhesion molecule; HER2, human epidermal growth factor receptor 2; ICE, immune cell engager; i.p., intraperitoneal; i.v., intravenous; PR, partial response; PSMA, prostate-specific membrane antigen; SIRS, systemic inflammatory response syndrome.
Going back from bed to benchside: improving ICEs
In recent years, major efforts have been made to improve the toxicity profile and clinical activity of ICEs, and several ongoing clinical trials are actually recruiting patients (Table 2), portraying a promising future for ICE-based cancer immunotherapy.
Table 2.
Ongoing clinical studies with ICEs in solid tumors registered on ClinicalTrials.gov
| Identifier | Drug name | ICE design | Condition | Phase | Note | Status |
|---|---|---|---|---|---|---|
| NCT03927573 | GEM3PSCA | PSMAxCD3 | Solid tumors | I | Recruiting | |
| NCT04104607 | CC-1 | PSMAxCD3 | Prostate cancer | I | Recruiting | |
| NCT04496674 | CC-1 | PSMAxCD3 | Squamous NSCLC | I/II | Recruiting | |
| NCT03792841 | AMG160 | PSMAxCD3 | Prostate cancer | I | ± Pembrolizumab | Recruiting |
| NCT03983395 | ISB1302 | HER2xCD3 | Breast cancer | I/II | Recruiting | |
| NCT03406858 | - | HER2xCD3 | Prostate cancer | II | + Pembrolizumab | Recruiting |
| NCT03661424 | - | HER2xCD3 | Breast cancer | I | Recruiting | |
| NCT03272334 | - | HER2xCD3 | Breast cancer | I/II | + Pembrolizumab | Recruiting |
| NCT03330561 | PRS-343 | HER2x4-1BB | Solid tumors | I | Recruiting | |
| NCT03269526 | - | EGFRxCD3 | Pancreatic cancer | I/II | Recruiting | |
| NCT03344250 | - | EGFRxCD3 | Glioblastoma | I | + Temozolomide | Recruiting |
| NCT03296696 | AMG596 | EGFRvIIIxCD3 | Glioblastoma | I | Active, non-recruiting | |
| NCT04009460 | ES101 | PD-L1x4-1BB | Solid tumors | I | Recruiting | |
| NCT03922204 | MCLA-145 | PD-L1x4-1BB | Solid tumors | I | Recruiting | |
| NCT03809624 | INBRX-105 | PD-L1x4-1BB | Solid tumors | I | Recruiting | |
| NCT03484962 | - | MUC1xCD3 | Hepatocellular carcinoma | II | + Activated cytokines-induced killer cells | Recruiting |
| NCT03564340 | REGN4018 | MUC16xCD3 | Ovarian cancer | I/II | ± Cemiplimab | Recruiting |
| NCT04117958 | AMG199 | MUC17xCD3 | Gastric cancer | I | Recruiting | |
| NCT04260191 | AMG910 | CLDN18.2xCD3 | Gastric cancer | I | Recruiting | |
| NCT03319940 | AMG757 | DLL1xCD3 | SCLC | I | ± Pembrolizumab | Recruiting |
| NCT04221542 | AMG 509 | STEAP1xCD3 | Prostate cancer | I | Recruiting | |
| NCT03531632 | MGD007 | gpA33xCD3 | Colorectal cancer | I/II | + MGA012 | Active non recruiting |
| NCT03860207 | Hu3F8-BsAb | GD2xCD3 | Solid tumors | I/II | Recruiting | |
| NCT04424641 | GEN1044 | 5T4xCD3 | Solid tumors | I/II | Not yet recruiting | |
| NCT03411915 | XmAb18087 | SSTR2xCD3 | NET and GIST | I | Recruiting | |
| NCT04128423 | AMV564 | CD33xCD3 | Solid tumors | I | Recruiting |
EGFR, epidermal growth factor receptor; GIST, gastrointestinal stromal tumor; HER2, human epidermal growth factor receptor 2; ICE, immune cell engager; NET, neuroendocrine tumor; PD-L1, programmed death-ligand 1; PSMA, prostate-specific membrane antigen; SCLC, small cell lung cancer.
Fine tuning of ICEs design
As mentioned before, the systemic administration of catumaxomab was severely limited by toxicity.63 Of note, catumaxomab is a trifunctional ICE: beyond the binding with EpCAM on malignant cells and CD3 on T cells, this bsAb is able to recruit accessory cells via binding of its Fc region to Fcγ receptors, further promoting antibody-dependent cell-mediated cytotoxicity in target cells, but also causing hyperactivation of FcγR-expressing cells, as in the case of Kupffer cells in the liver. Since the high toxicity rate in case of systemic administration, catumaxomab has not been widely adopted and most of the subsequent BiTEs were designed with engineered Fc domains to reduce Fcγ receptor binding or as bsAbs fragments lacking the Fc region.65 With few exceptions, nowadays most BiTEs incorporate monovalent CD3-binding scFvs (i.e. with affinity for one epitope of CD3), since bivalent/multivalent anti-CD3 scFvs (i.e. with affinity for more than one epitope of CD3) were linked to an excess of activation-induced cell death in effector cells (limiting the efficacy) and TAA-independent immune effectors activation (increasing the toxicity).66 For example, side-effects in cynomolgus monkeys were dependent on the affinity of the anti-CD3 scFv part of a full-length CLL1xCD3 bsAb, with the high-affinity variant being poorly tolerated because of extensive cytokine release.67 Moreover, high affinity for CD3 was also linked to a reduced systemic exposure and shifted biodistribution from tumors to CD3-rich tissues (e.g. spleen and lymph nodes), as demonstrated in vivo in different tumor models.68,69
Conversely, a high valency of the anti-TAA scFv is generally desirable, since it leads to increased binding avidity and antitumor activity, as shown in a functional comparison using anti-GD2 BiTEs.70
A limitation that emerged from early clinical trials with certain ICEs was the development of anti-ICE antibodies that seriously affected the clinical development of the drugs.71 This problem became evident in a phase I study that tested MEDI-565, a CEAxCD3 BiTE.59 The study was discontinued after the observation of anti-MEDI-565 antibodies production in all patients treated at high doses. Current strategies are focused on improving the design of ICEs to limit their immunogenicity, for example using humanized scFvs.72
The very small size of certain BiTEs (due to the lack of the Fc domain) is responsible for a short half-life and the requirement for continuous infusion.12 Several strategies have been explored to overcome this issue, such as conjugation with an engineered IgG Fc domain, human serum albumin or polyethylene glycol.73 Moreover, Leconet et al.74 developed an injectable in situ biodegradable polymer-based protein delivery system that was successfully designed to prolong the in vivo elimination half-life and the antitumor activity of a BiTE targeting PSMA in prostate cancer.74
Choosing the right TAA
High tumor specificity is a fundamental prerequisite for a TAA to be an appropriate ICE target. The toxicity observed with the systemic administration of catumaxomab was, at least in part, driven by the expression of the targeted TAA (i.e. EpCAM) in normal tissues (e.g. colon, liver). In addition, the density, size and mobility of TAAs on the membrane of tumor cells are crucial determinants for the choice of a good TAA.75, 76, 77 All these properties have been taken into consideration to explore ‘highly-fit’ TAAs as novel targets. For example, EGFRvIII is a mutated variant of EGFR (deletion of exons 2-7) expressed solely in malignant cells of glioblastoma. An EGFRvIIIxCD3 BiTE demonstrated a potent in vivo antitumor effect against glioblastoma models,77 and a phase I clinical trial is being conducted in patients with EGFRvIII-positive glioblastoma (NCT03296696).78 Glypican-3 (GPC3) is a heparan sulfate chain proteoglycan expressed in fetal tissues (e.g. liver, lung, kidney and placenta) but not detected in normal postnatal tissues due to methylation-induced epigenetic silencing.79,80 GPC3 is expressed in a wide range of solid tumors (including hepatocellular carcinoma, melanoma, ovarian clear cell carcinoma and lung squamous cell carcinoma).80 Ishiguro et al.81 reported that ERY974, a GPC3xCD3 BiTE, was highly effective in vivo in immunogenic and non-immunogenic tumor models expressing GPC3, and the results of a phase I clinical trial of ERY974 in solid tumors are eagerly awaited (NCT02748837).82 The disialoganglioside GD2 is another interesting oncofetal surface antigen expressed by several solid tumors.83 A preclinical study exploring hu3F8-BsAb (a GD2xCD3 BiTE in which the IgG backbone was aglycosylated to prevent Fcγ receptor-mediated toxicity) showed high tumor killing potency, absence of neurotoxicity and ability to drive circulating T cells into solid tumors.84 These encouraging preclinical results warranted the initiation of a clinical trial in patients with relapsed/refractory neuroblastoma, osteosarcoma and other solid tumors (NCT03860207). A promising non-conventional TAA is TRAIL-R2 (also known as DR5), a member of the tumor necrosis factor α superfamily. Indeed, a TRAIL-R2xCD3 BiTE demonstrated preclinical antitumor activity both in vitro in melanoma and breast cancer and ex vivo in a model of ascitic fluid freshly isolated from ovarian cancer patients.85,86 Actually, several other promising targets are being studied, including SSTR2 in gastrointestinal stromal tumors and neuroendocrine tumors (NCT03411915), MUC-1 in hepatocellular carcinoma (NCT03484962), MUC-17 in gastric cancer (NCT04117958) and STEAP-1 in prostate cancers (NCT04221542).
Overcoming resistance to ICEs
Studies into potential mechanisms of resistance to BiTes indicated that the reduction in expression of target antigens on cancer cells is a cause of tumor immune escape. CD19-negative relapses have been observed in ALL patients treated with blinatumomab, preventing further activity of the BiTE.87 To obviate this hurdle, multispecific antibodies were developed to concurrently recognize multiple antigens on the surface of cancer cells, as in the case of the tetraspecific molecules FL518 and CRTB6 targeting EGFR, HER2, HER3 and vascular endothelial growth factor, that demonstrated the ability to avoid tumor antigen escape.88
The upregulation of inhibitory immune checkpoints by tumor and TME cells represents another mechanism of resistance to ICEs. Following BiTE-mediated effector activation and interferon-γ release, programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) are up-regulated in T cells and cancer/TME cells, respectively, whereas combining BiTE treatment with the inhibition of the PD-1/PD-L1 axis was shown to enhance the antitumor efficacy of BiTEs in preclinical models.89,90 Consistently, preliminary clinical data showed an enhanced activity and a manageable safety profile for the CEAxCD3 BiTE cibisatamab when combined with the anti-PD-L1 antibody atezolizumab in patients with metastatic colorectal cancer,60 similarly to what was observed in patients with ALL treated with blinatumomab in combination with the anti-PD-1 antibody nivolumab.91 A different approach to deal with the overexpression of inhibitory immune checkpoints in the TME is the development of immune-checkpoint targeting BiTEs. The proof-of-concept for this type of T cell engager has been developed in a preclinical model of melanoma using a PD-L1xCD3 BiTE able to redirect T and NKT cells towards PD-L1-positive cells with a promising antitumor activity, especially when combined with the inhibition of other inhibitory immune checkpoints.92 The direct attack of TME immunosuppressive cells, such as myeloid-derived suppressor cells, is under study using a CD33xCD3 BiTE (AMV564, NCT04128423). Inhibitory signals can be also converted into activating signals, for example using a simultaneous multiple interaction T cell engaging (SMITE) strategy. The SMITE approach consists of the combination of several BiTEs at a time, with each BiTe binding cancer cells and either CD3 or CD28 to provide T cell costimulation. A SMITE system encompassing a PD-L1xCD28 BiTE can convert inhibitory PD-L1 signals into positive costimulatory signals through engagement of CD28 on T cells that are activated via the second TAAxCD3 BiTE.93
Future perspectives and conclusions
Given the complex nature of solid tumors, exploiting the synergy between ICEs and other innovative therapeutics, such as oncolytic viruses (OVs) and chimeric antigen receptor (CAR) T cells, may be an ideal strategy to improve the efficacy of cancer immunotherapy. OVs are viruses able to selectively enter, replicate in and destroy cancer cells inducing systemic antitumor immune responses.94 OVs can be genetically engineered to produce and deliver a functional BiTE, as in the case of ICOVIR-15K-BiTE, an oncolytic adenovirus engineered to express an EGFRxCD3 BiTE. Preclinical evidence showed that tumor-infiltrating T cells could be more effectively activated by ICOVIR-15K-BiTE compared with the OV ICOVIR-15K alone.95 Other preclinical studies reported on the antitumor activity of the oncolytic group B adenovirus EnAdenotucirev engineered to express an EpCAMxCD3 BiTE,96 and the ability of an oncolytic adenovirus engineered to express a FAPxCD3 BiTe to cause virolysis of cancer cells together with a depletion of the immunosuppressive cancer-associated fibroblasts.97 With the aim of creating a synergizing system between ICEs and adoptive cell therapy, Choi et al.98 designed a single-gene-modified T cell product for the treatment of glioblastoma, namely, CART.BiTE, consisting of an anti-EGFRvIII CAR T cell product modified to deliver an EGFRxCD3 BiTE. This novel design allowed dealing with heterogeneous EGFRvIII tumor expression resulting in promising in vivo antitumor activity and overcoming the drawbacks of their parental biotechnologies, such as the necessity of continuous injection for BiTEs or antigen escape for CAR T cells.
In conclusion, ICEs have the potential to enable a large-scale level strategy of immune effector cells redirection in patients with solid tumors, sparing the major infrastructural and pharmacoeconomic obstacles that characterize adoptive cell therapy. However, further preclinical and clinical research efforts are needed to obviate technical issues, overcome resistance and eventually let ICEs hit the clinic in solid tumors.
Acknowledgments
Funding
None declared.
Disclosure
MDN holds a patent application as an inventor on bispecific antibodies for use in cancer immunotherapy (US patent application number 15/740560). The remaining authors have no potential conflicts of interest that might be relevant to the contents of this manuscript.
References
- 1.Fucà G., de Braud F., Di Nicola M. Immunotherapy-based combinations: an update. Curr Opin Oncol. 2018;30(5):345–351. doi: 10.1097/CCO.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 2.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst R.S., Soria J.C., Kowanetz M. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh P.C., Harview C.L., Yearley J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aptsiauri N., Ruiz-Cabello F., Garrido F. The transition from HLA-I positive to HLA-I negative primary tumors: the road to escape from T-cell responses. Curr Opin Immunol. 2018;51:123–132. doi: 10.1016/j.coi.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brischwein K., Schlereth B., Guller B. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43(8):1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Choi B.D., Cai M., Bigner D.D., Mehta A.I., Kuan C.T., Sampson J.H. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther. 2011;11(7):843–853. doi: 10.1517/14712598.2011.572874. [DOI] [PubMed] [Google Scholar]
- 10.Bargou R., Leo E., Zugmaier G. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 11.Ross S.L., Sherman M., McElroy P.L. Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing. PLoS One. 2017;12(8):e0183390. doi: 10.1371/journal.pone.0183390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu M., Wu B., Brandl C. Blinatumomab, a bispecific T-cell engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. doi: 10.1007/s40262-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 13.Offner S., Hofmeister R., Romaniuk A., Kufer P., Baeuerle P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43(6):763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Haas C., Krinner E., Brischwein K. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214(6):441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen H.H., Kim T., Song S.Y. Naïve CD8(+) T cell derived tumor-specific cytotoxic effectors as a potential remedy for overcoming TGF-β immunosuppression in the tumor microenvironment. Sci Rep. 2016;6:28208. doi: 10.1038/srep28208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kischel R., Hausmann S., Baeuerle P., Kufer P. Abstract #3252: effector memory T cells make a major contribution to redirected target cell lysis by T cell-engaging BiTE antibody MT110. Cancer Res. 2009;69(suppl 9):3252. [Google Scholar]
- 17.Quintarelli C., Orlando D., Boffa I. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology. 2018;7(6):e1433518. doi: 10.1080/2162402X.2018.1433518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Ruiz E., Etxeberria I., Rodriguez-Ruiz M.E., Melero I. Anti-CD137 and PD-1/PD-L1 antibodies en route toward clinical synergy. Clin Cancer Res. 2017;23(18):5326–5328. doi: 10.1158/1078-0432.CCR-17-1799. [DOI] [PubMed] [Google Scholar]
- 19.Wu L., Seung E., Xu L. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Cancer. 2020;1(1):86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 20.Hinner M.J., Aiba R.S.B., Jaquin T.J. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin Cancer Res. 2019;25(19):5878–5889. doi: 10.1158/1078-0432.CCR-18-3654. [DOI] [PubMed] [Google Scholar]
- 21.Claus C., Ferrara C., Xu W. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11(496):eaav5989. doi: 10.1126/scitranslmed.aav5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 23.Lanier L.L. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleason M.K., Verneris M.R., Todhunter D.A. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothe A., Sasse S., Topp M.S. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godbersen C., Coupet T.A., Huehls A.M. NKG2D ligand-targeted bispecific T-cell engagers lead to robust antitumor activity against diverse human tumors. Mol Cancer Ther. 2017;16(7):1335–1346. doi: 10.1158/1535-7163.MCT-16-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothe A., Jachimowicz R.D., Borchmann S. The bispecific immunoligand ULBP2-aCEA redirects natural killer cells to tumor cells and reveals potent anti-tumor activity against colon carcinoma. Int J Cancer. 2014;134(12):2829–2840. doi: 10.1002/ijc.28609. [DOI] [PubMed] [Google Scholar]
- 28.Han Y., Sun F., Zhang X. CD24 targeting bi-specific antibody that simultaneously stimulates NKG2D enhances the efficacy of cancer immunotherapy. J Cancer Res Clin Oncol. 2019;145(5):1179–1190. doi: 10.1007/s00432-019-02865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwaab T., Lewis L.D., Cole B.F. Phase I pilot trial of the bispecific antibody MDXH210 (anti-Fc gamma RI X anti-HER-2/neu) in patients whose prostate cancer overexpresses HER-2/neu. J Immunother. 2001;24(1):79–87. doi: 10.1097/00002371-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer C., Strauss G., Lindner M., Marmé A., Deo Y.M., Moldenhauer G. Efficient carcinoma cell killing by activated polymorphonuclear neutrophils targeted with an Ep-CAMxCD64 (HEA125x197) bispecific antibody. Cancer Immunol Immunother. 2002;51(11-12):621–629. doi: 10.1007/s00262-002-0326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keler T., Graziano R.F., Mandal A. Bispecific antibody-dependent cellular cytotoxicity of HER2/neu-overexpressing tumor cells by Fc gamma receptor type I-expressing effector cells. Cancer Res. 1997;57(18):4008–4014. [PubMed] [Google Scholar]
- 32.Przepiorka D., Ko C.W., Deisseroth A. FDA approval: blinatumomab. Clin Cancer Res. 2015;21(18):4035–4039. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 33.Feucht J., Kayser S., Gorodezki D. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topp M.S., Gökbuget N., Stein A.S. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 35.Mau-Sørensen M., Dittrich C., Dienstmann R. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother Pharmacol. 2015;75(5):1065–1073. doi: 10.1007/s00280-015-2728-5. [DOI] [PubMed] [Google Scholar]
- 36.Sebastian M., Passlick B., Friccius-Quecke H. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56(10):1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebastian M., Kiewe P., Schuette W. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM x Anti-CD3): results of a phase 1/2 study. J Immunother. 2009;32(2):195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 38.Ströhlein M.A., Lordick F., Rüttinger D. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie. 2011;34(3):101–108. doi: 10.1159/000324667. [DOI] [PubMed] [Google Scholar]
- 39.Burges A., Wimberger P., Kümper C. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13(13):3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 40.Ruf P., Kluge M., Jäger M. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br J Clin Pharmacol. 2010;69(6):617–625. doi: 10.1111/j.1365-2125.2010.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumann K., Pfisterer J., Wimberger P. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecol Oncol. 2011;123(1):27–32. doi: 10.1016/j.ygyno.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Atanackovic D., Reinhard H., Meyer S. The trifunctional antibody catumaxomab amplifies and shapes tumor-specific immunity when applied to gastric cancer patients in the adjuvant setting. Hum Vaccin Immunother. 2013;9(12):2533–2542. doi: 10.4161/hv.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bokemeyer C., Stein A., Ridwelski K. A phase II study of catumaxomab administered intra- and postoperatively as part of a multimodal approach in primarily resectable gastric cancer. Gastric Cancer. 2015;18(4):833–842. doi: 10.1007/s10120-014-0423-6. [DOI] [PubMed] [Google Scholar]
- 44.Berek J.S., Edwards R.P., Parker L.P. Catumaxomab for the treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: a phase II study. Int J Gynecol Cancer. 2014;24(9):1583–1589. doi: 10.1097/IGC.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 45.Sehouli J., Reinthaller A., Marth C. Intra- and postoperative catumaxomab in patients with epithelial ovarian cancer: safety and two-year efficacy results from a multicentre, single-arm, phase II study. Br J Cancer. 2014;111(8):1519–1525. doi: 10.1038/bjc.2014.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heiss M.M., Murawa P., Koralewski P. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127(9):2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sehouli J., Pietzner K., Wimberger P. Catumaxomab with and without prednisolone premedication for the treatment of malignant ascites due to epithelial cancer: results of the randomised phase IIIb CASIMAS study. Med Oncol. 2014;31(8):76. doi: 10.1007/s12032-014-0076-7. [DOI] [PubMed] [Google Scholar]
- 48.Pietzner K., Vergote I., Santoro A. Re-challenge with catumaxomab in patients with malignant ascites: results from the SECIMAS study. Med Oncol. 2014;31(12):308. doi: 10.1007/s12032-014-0308-x. [DOI] [PubMed] [Google Scholar]
- 49.Kebenko M., Goebeler M.E., Wolf M. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7(8):e1450710. doi: 10.1080/2162402X.2018.1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haense N., Atmaca A., Pauligk C. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016;16:420. doi: 10.1186/s12885-016-2449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiewe P., Hasmüller S., Kahlert S. Phase I trial of the trifunctional anti-HER2 x anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12(10):3085–3091. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 52.Posey J.A., Raspet R., Verma U. A pilot trial of GM-CSF and MDX-H210 in patients with erbB-2-positive advanced malignancies. J Immunother. 1999;22(4):371–379. doi: 10.1097/00002371-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Lewis L.D., Beelen A.P., Cole B.F. The pharmacokinetics of the bispecific antibody MDX-H210 when combined with interferon gamma-1b in a multiple-dose phase I study in patients with advanced cancer. Cancer Chemother Pharmacol. 2002;49(5):375–384. doi: 10.1007/s00280-002-0424-8. [DOI] [PubMed] [Google Scholar]
- 54.Repp R., van Ojik H.H., Valerius T. Phase I clinical trial of the bispecific antibody MDX-H210 (anti-FcgammaRI x anti-HER-2/neu) in combination with Filgrastim (G-CSF) for treatment of advanced breast cancer. Br J Cancer. 2003;89(12):2234–2243. doi: 10.1038/sj.bjc.6601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pullarkat V., Deo Y., Link J. A phase I study of a HER2/neu bispecific antibody with granulocyte-colony-stimulating factor in patients with metastatic breast cancer that overexpresses HER2/neu. Cancer Immunol Immunother. 1999;48(1):9–21. doi: 10.1007/s002620050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valone F.H., Kaufman P.A., Guyre P.M. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol. 1995;13(9):2281–2292. doi: 10.1200/JCO.1995.13.9.2281. [DOI] [PubMed] [Google Scholar]
- 57.James N.D., Atherton P.J., Jones J., Howie A.J., Tchekmedyian S., Curnow R.T. A phase II study of the bispecific antibody MDX-H210 (anti-HER2 x CD64) with GM-CSF in HER2+ advanced prostate cancer. Br J Cancer. 2001;85(2):152–156. doi: 10.1054/bjoc.2001.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiner L.M., Clark J.I., Davey M. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res. 1995;55(20):4586–4593. [PubMed] [Google Scholar]
- 59.Pishvaian M., Morse M.A., McDevitt J. Phase 1 dose escalation study of MEDI-565, a bispecific T-cell engager that targets human carcinoembryonic antigen, in patients with advanced gastrointestinal adenocarcinomas. Clin Colorectal Cancer. 2016;15(4):345–351. doi: 10.1016/j.clcc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Tabernero J., Melero I., Ros W. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC) J Clin Oncol. 2017;35(suppl 15):3002. [Google Scholar]
- 61.Hummel H.-D., Kufer P., Grüllich C. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting Bispecific T cell Engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2019;37(suppl 15):5034. [Google Scholar]
- 62.Chames P., Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1(6):539–547. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borlak J., Länger F., Spanel R., Schöndorfer G., Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget. 2016;7(19):28059–28074. doi: 10.18632/oncotarget.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnell U., Cirulli V., Giepmans B.N. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828(8):1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Labrijn A.F., Janmaat M.L., Reichert J.M., Parren P.W.H.I. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 66.Kuhn C., Weiner H.L. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8(8):889–906. doi: 10.2217/imt-2016-0049. [DOI] [PubMed] [Google Scholar]
- 67.Leong S.R., Sukumaran S., Hristopoulos M. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129(5):609–618. doi: 10.1182/blood-2016-08-735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mandikian D., Takahashi N., Lo A.A. Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol Cancer Ther. 2018;17(4):776–785. doi: 10.1158/1535-7163.MCT-17-0657. [DOI] [PubMed] [Google Scholar]
- 69.Suurs F.V., Lorenczewski G., Stienen S. Biodistribution of a CD3/EpCAM bispecific T-cell engager is driven by the CD3 arm. J Nucl Med. 2020;61:1594–1601. doi: 10.2967/jnumed.120.241877. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed M., Cheng M., Cheung I.Y., Cheung N.K. Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody. Oncoimmunology. 2015;4(4):e989776. doi: 10.4161/2162402X.2014.989776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azinovic I., DeNardo G.L., Lamborn K.R. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother. 2006;55(12):1451–1458. doi: 10.1007/s00262-006-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J., Han D., Shi S. A novel fully human antibody targeting extracellular domain of PSMA inhibits tumor growth in prostate cancer. Mol Cancer Ther. 2019;18(7):1289–1301. doi: 10.1158/1535-7163.MCT-18-1078. [DOI] [PubMed] [Google Scholar]
- 73.Strohl W.R., Strohl L.M., editors. Therapeutic Antibody Engineering. Woodhead Publishing; Sawston, UK: 2012. 4 - Fundamental technologies for antibody engineering; pp. 57–595. [Google Scholar]
- 74.Leconet W., Liu H., Guo M. Anti-PSMA/CD3 bispecific antibody delivery and antitumor activity using a polymeric depot formulation. Mol Cancer Ther. 2018;17(9):1927–1940. doi: 10.1158/1535-7163.MCT-17-1138. [DOI] [PubMed] [Google Scholar]
- 75.Pfosser A., Brandl M., Salih H., Grosse-Hovest L., Jung G. Role of target antigen in bispecific-antibody-mediated killing of human glioblastoma cells: a pre-clinical study. Int J Cancer. 1999;80(4):612–616. doi: 10.1002/(sici)1097-0215(19990209)80:4<612::aid-ijc21>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 76.Ruf P., Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98(8):2526–2534. doi: 10.1182/blood.v98.8.2526. [DOI] [PubMed] [Google Scholar]
- 77.Choi B.D., Pastan I., Bigner D.D., Sampson J.H. A novel bispecific antibody recruits T cells to eradicate tumors in the ‘immunologically privileged’ central nervous system. Oncoimmunology. 2013;2(4):e23639. doi: 10.4161/onci.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenthal M., Balana C., Linde M.E.V. Novel anti-EGFRvIII bispecific T cell engager (BiTE) antibody construct in glioblastoma (GBM): trial in progress of AMG 596 in patients with recurrent or newly diagnosed disease. J Clin Oncol. 2019;37(suppl 15):TPS2071. [Google Scholar]
- 79.Nakatsura T., Nishimura Y. Usefulness of the novel oncofetal antigen glypican-3 for diagnosis of hepatocellular carcinoma and melanoma. BioDrugs. 2005;19(2):71–77. doi: 10.2165/00063030-200519020-00001. [DOI] [PubMed] [Google Scholar]
- 80.Nakatsura T., Yoshitake Y., Senju S. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306(1):16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 81.Ishiguro T., Sano Y., Komatsu S.I. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9(410):eaal4291. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 82.Ogita Y., Weiss D., Sugaya N. A phase 1 dose escalation (DE) and cohort expansion (CE) study of ERY974, an anti-Glypican 3 (GPC3)/CD3 bispecific antibody, in patients with advanced solid tumors. J Clin Oncol. 2018;36(suppl 15):TPS2599. [Google Scholar]
- 83.Nazha B., Inal C., Owonikoko T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H., Cheng M., Guo H., Chen Y., Huse M., Cheung N.K. Retargeting T cells to GD2 pentasaccharide on human tumors using Bispecific humanized antibody. Cancer Immunol Res. 2015;3(3):266–277. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satta A., Grazia G., Caroli F. A bispecific antibody to link a TRAIL-based antitumor approach to immunotherapy. Front Immunol. 2019;10:2514. doi: 10.3389/fimmu.2019.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Satta A., Mezzanzanica D., Caroli F. Design, selection and optimization of an anti-TRAIL-R2/anti-CD3 bispecific antibody able to educate T cells to recognize and destroy cancer cells. MAbs. 2018;10(7):1084–1097. doi: 10.1080/19420862.2018.1494105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braig F., Brandt A., Goebeler M. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–104. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 88.Hu S., Fu W., Xu W. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res. 2015;75(1):159–170. doi: 10.1158/0008-5472.CAN-14-1670. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Piskol R., Ybarra R. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11(508):eaax8861. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 90.Scott E.M., Jacobus E.J., Lyons B. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J Immunother Cancer. 2019;7(1):320. doi: 10.1186/s40425-019-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webster J., Luskin M.R., Prince G.T. Blinatumomab in combination with immune checkpoint inhibitors of PD-1 and CTLA-4 in adult patients with relapsed/refractory (R/R) CD19 positive B-cell acute lymphoblastic leukemia (ALL): preliminary results of a phase I study. Blood. 2018;132(suppl 1):557. [Google Scholar]
- 92.Horn L.A., Ciavattone N.G., Atkinson R. CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1. Oncotarget. 2017;8(35):57964–57980. doi: 10.18632/oncotarget.19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Correnti C.E., Laszlo G.S., de van der Schueren W.J. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32(5):1239–1243. doi: 10.1038/s41375-018-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adair R.A., Roulstone V., Scott K.J. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4(138):138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fajardo C.A., Guedan S., Rojas L.A. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77(8):2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 96.Freedman J.D., Hagel J., Scott E.M. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol Med. 2017;9(8):1067–1087. doi: 10.15252/emmm.201707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freedman J.D., Duffy M.R., Lei-Rossmann J. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78(24):6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 98.Choi B.D., Yu X., Castano A.P. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]