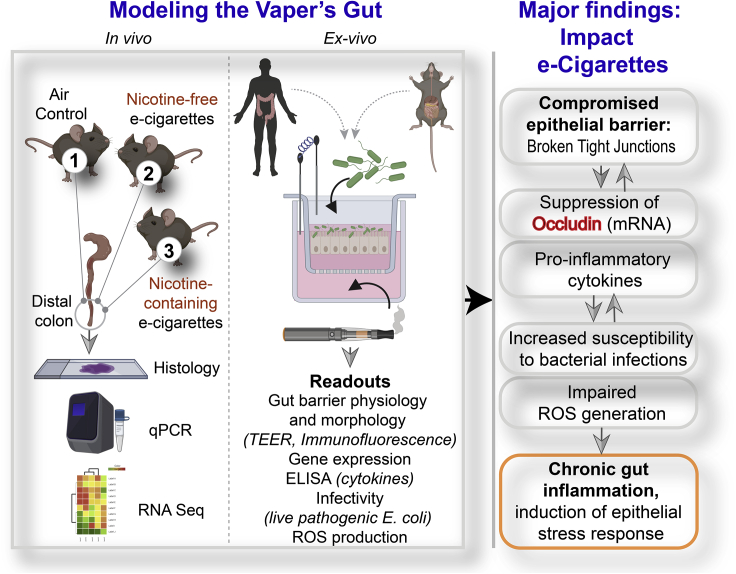
Summary
E-cigarette usage continues to rise, yet the safety of e-cigarette aerosols is questioned. Using murine models of acute and chronic e-cigarette aerosol inhalation, murine colon transcriptomics, and murine and human gut-derived organoids in co-culture models, we assessed the effects of e-cigarette use on the gut barrier. Histologic and transcriptome analyses revealed that chronic, but not acute, nicotine-free e-cigarette use increased inflammation and reduced expression of tight junction (TJ) markers. Exposure of murine and human enteroid-derived monolayers (EDMs) to nicotine-free e-cigarette aerosols alone or in co-culture with bacteria also causes barrier disruption, downregulation of TJ protein, and enhanced inflammation in response to infection. These data highlight the harmful effects of “non-nicotine” component of e-cigarettes on the gut barrier. Considering the importance of an intact gut barrier for host fitness and the impact of gut mucosal inflammation on a multitude of chronic diseases, these findings are broadly relevant to both medicine and public health.
Subject areas: Biological Sciences, Physiology, Toxicology, Microbiology, Microbiome, Transcriptomics
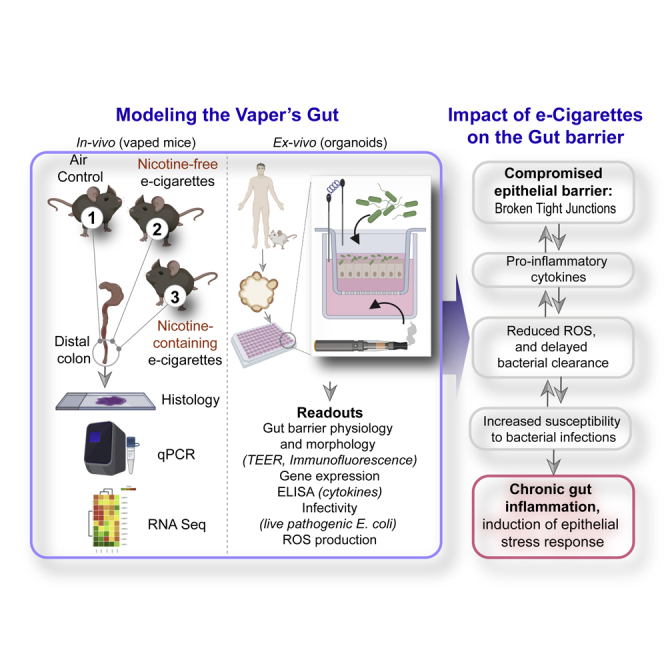
Graphical abstract
Highlights
-
•
Chronic vaping disrupts the gut barrier and triggers inflammation
-
•
Transcriptome studies reveal the broad impact of e-cig on gut health
-
•
Enteroid monolayers reveal that e-liquid, not nicotine, is the culprit
-
•
Chronic exposure to e-cig increases susceptibility to bacterial infection
Biological Sciences; Physiology; Toxicology; Microbiology; Microbiome; Transcriptomics
Introduction
Electronic nicotine delivery systems, commonly referred to as e-cigarettes and vaping devices, were introduced to the international market in 2007 (Etter and Bullen, 2011). Since then, e-cigarettes have become widely popular in the United States (CDC; and United-States, 2016), primarily among the nation's youth. A large amount of research surrounding its regulation and consumption has been focused on the addictive nicotine component in these devices; however, recent studies have increasingly begun to scrutinize the harmful potential of the chemicals in the e-liquids, e.g., propylene glycol (PG), glycerol (VG), flavorings, and contaminants (Alasmari et al., 2017, 2019; Bozier et al., 2020; Khlystov and Samburova, 2016; Perez and Crotty Alexander, 2020; Yu et al., 2016). The PG:VG ratio is a key determinant of the amount of vapor that is optimal for maintaining the flavor, whereas the composition of the e-liquid determines the exact proportions of the ∼50–150 chemicals that are generated via heat-mediated pyrolysis and chemical decomposition (Margham et al., 2016). Despite the presence of these wide ranges of multiple chemicals, few regulations control the chemical composition of e-cigarettes, and they remain popular as a risk-free alternative to combustible cigarettes.
This concept of risk-free use has recently been challenged. For example, morbidity and mortality among teenagers and young adults owing to e-cigarette or vaping product use-associated lung injury (King et al., 2020) created a public health crisis in 2019–2020; a single (non-nicotine) chemical in the e-liquids, i.e., vitamin E acetate was found to be the culprit (Blount et al., 2020). In vitro and ex vivo studies have already shown that e-liquids induce inflammatory responses and alter innate immune defenses in myeloid and primary airway epithelial cells (Muthumalage et al., 2017; Scott et al., 2018; Wu et al., 2014). Mice exposed to e-cigarette aerosols for 2 weeks were found to have impaired pulmonary bacterial and viral clearance (Hwang et al., 2016; Sussan et al., 2015), which suggested increased susceptibility to influenza and coronavirus infections in particular. Evidence of pulmonary and systemic inflammation has also been found in the serum (Singh et al., 2019) and bronchoalveolar lavage (Song et al., 2020) samples from human e-cigarette-users, with elevated biomarkers of inflammation, e.g., interleukin (IL)-1β, IL-6, IL-8, IL-13, and interferon (IFN)-γ. Furthermore, mechanisms that link the use of e-cigarettes to an increased risk of cancers (Tang et al., 2019) or of cancer progression (Mravec et al., 2020) have been proposed, and e-cigarettes have been shown to induce DNA damage (independent of nicotine [Yu et al., 2016]) while reducing repair pathways (Lee et al., 2018). In light of these studies, an NIH-funded panel declared that all chemical components of e-cigarette and vaping aerosols have the potential to adversely affect health (Crotty Alexander et al., 2020), both in the heart and lungs and throughout the body (Crotty Alexander et al., 2020).
Here we set out to assess the impact of e-cigarette aerosol inhalation (with or without nicotine) on the gastrointestinal tract. The gut is resident to diverse microbiota, and its handling of and response to the same is known to regulate several chronic diseases such as inflammatory bowel diseases (IBDs), obesity, cardiovascular diseases, cancers, and rheumatoid arthritis (Gilbert et al., 2016). Studies on human subjects have shown that e-cigarette use significantly modulates the oral microbiome (Pushalkar et al., 2020) but not the gut microbiome (Stewart et al., 2018). Studies in mice, however, have shown that e-cigarettes alter epithelial mucus profiles and microbial diversity (Allais et al., 2016) and that such changes may induce inflammation and decrease the integrity of the gut barrier (Fricker et al., 2018). Although e-cigarette use is long suspected to negatively impact human gastrointestinal physiology, based on the numerous digestive symptoms reported by vapers (Hua et al., 2013), little to nothing is known as to how e-cigarette use may impact the human gut barrier. Using a combination of mouse models, transcriptomics, and murine and human gut-derived organoids as ex vivo near-physiologic model systems, here we expose the hitherto unknown effects of e-cigarettes on the gastrointestinal tract and provide insights into the potential long-term effects of e-cigarettes on health.
Results
Daily e-cigarette aerosol inhalation drives inflammation in the colon and reduces the expression of genes related to barrier function
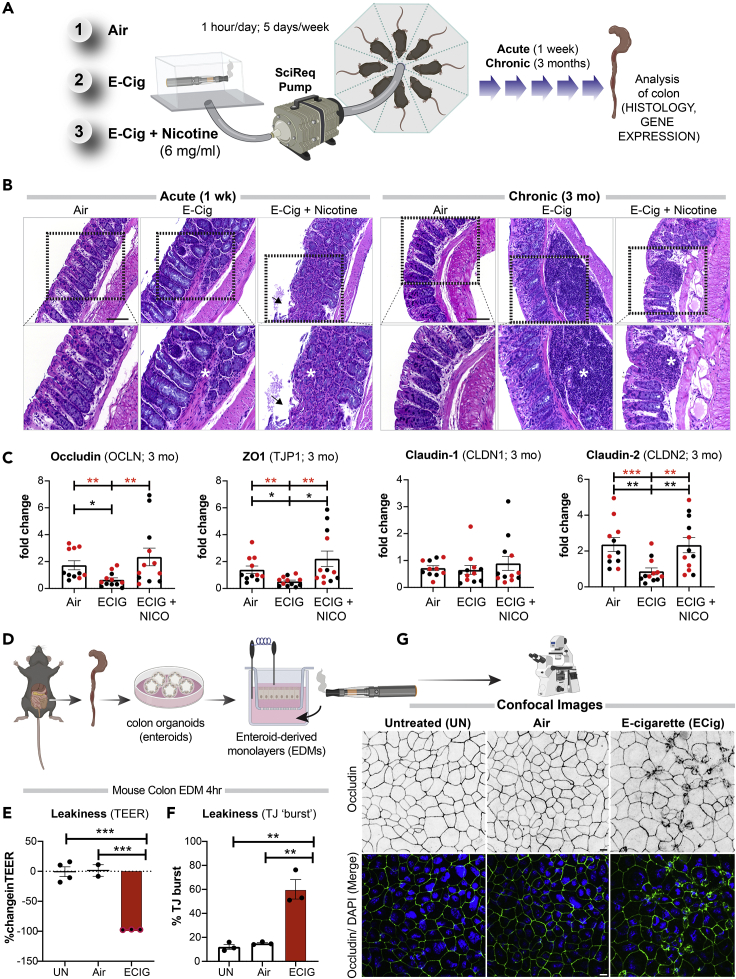
To establish the in vivo effect of inhalation of these aerosols on the colon, the distal colon was harvested from mice exposed daily to e-cigarette aerosols (1 h/day) at two time points: 1 week (resembling acute exposure) and 3 months (resembling chronic exposure) (Figure 1A). Because the most common chemicals in e-cigarette aerosols are nicotine and humectants (propylene glycol and vegetable glycerin), we utilized nicotine-free and nicotine-containing (6 mg/mL) e-liquids with a 70:30 ratio of propylene glycol and vegetable glycerol (PG:VG) within a KangerSubtank attached to a box Mod e-device and used exposure chambers with room air for controls (Figure 1A). This low concentration of nicotine (6 mg/mL) was selected on the basis of several published works (ranging typically between ∼6 and 9 mg/mL) and the amount present in the most popular brands (Cox et al., 2016; Dawkins et al., 2016; Stewart et al., 2018). The ratio of 70:30 PG:VG was chosen because it is the most commonly used and preferred ratio as per consumer experience (Smith et al., 2020). H&E staining of distal colons from mice acutely exposed to nicotine-free (vehicle only) e-cigarette aerosols (e-cig) for 1 week demonstrated small, infrequent patches of leukocyte infiltration in the submucosal layers (Figure 1B, left; asterisk). Acute exposure to nicotine-containing aerosols (e-cig + nicotine) was associated with infrequent patches of epithelial erosions. By contrast, chronic e-cig exposure over 3 months led to large submucosal inflammatory infiltrates within the colon (Figure 1B, right). No inflammatory infiltrates were seen in air controls, and only smaller and infrequent infiltrates were present in colons of mice chronically exposed to nicotine-containing e-cig (e-cig + nicotine).
Figure 1.
Electronic cigarettes trigger inflammation in the murine distal colon and disrupt the integrity of the murine gut barrier
(A) Schematic displays the key aspects of the murine model of vaping used in this study. Mice were exposed to air (negative control), nicotine-free e-cig (e-cig alone; MOD brand), or nicotine-containing e-cig (e-cig + 6 mg/mL nicotine) for 1 week or 3 months.
(B) Hematoxylin-eosin staining of distal colons after 1 week (left) or 3 months (right) of exposure to e-cig. Asterisks, inflammatory infiltrates; arrowheads, epithelial erosions.
(C) Bar graphs display the relative levels of expression of genes in the colon (black dots, male mice; red dots, female mice) that encode proteins that regulate epithelial tight junctions. Data are displayed as mean ± SEM. Statistical significance was estimated using either one-way ANOVA with Tukey's test (black ∗) or Mann-Whitney's test (red ∗); ∗p<0.05, ∗∗p <0.01 and ∗∗∗p <0.001.
(D) Schematic displays the key aspects of ex vivo disease modeling to interrogate the impact of vaping on the murine colonic epithelial barrier.
(E) Bar graphs display the percent change in TEER. Data are displayed as mean ± SEM (n = 3–4 independent experiments). UN, normal media; Air, air-infused media.
(F and G) EDMs were treated as indicated, fixed and stained for occludin (green) and DAPI (blue, nuclei), and analyzed by confocal microscopy. Bar graphs in (F) display the percent increase in the tight junction (TJ) “bursts” (indicative of disrupted TJs). Data are displayed as mean ± SEM (n = 3 fields/condition; 40–50 tricellular TJs/field). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗∗p <0.01. Confocal microscopic images in (G) are representative of EDMs, either untreated (UN) or after 4 h of treatment with air or e-cig-infused media. Scale bar, 10 μm.
Markers of gut epithelial tight junctions (TJs), e.g., occludin (OCLN), zonula occludens (ZO)-1 (TJP1), and Claudin-2 (CLDN2) had significantly reduced gene expression in mice chronically exposed to nicotine-free aerosols (e-cig) compared with air controls (Figure 1C). The fact that e-cig exposure affects the levels of Claudin-2, a major regulator of TJ-specific obliteration of the intercellular space (Kubota et al., 1999), but not its counterpart Claudin-1 (Figure 1C), which is specialized for TJ integrity in the skin epidermis, indicates that the effects of e-cig on TJs may be gut specific. No reduction was observed in any of the barrier function genes in mice exposed to nicotine-containing e-cigarettes. No significant differences were observed in the levels of pro-inflammatory cytokines MCP1 or IL-8 in the chronically exposed mice (Figure S1). Finally, no statistically significant differences were observed in the transcript levels of TJ markers and pro-inflammatory cytokines in acute exposures between any conditions (Figure S1).
These findings indicate that chronic, but not acute, exposure to aerosols of nicotine-free e-cigarettes is sufficient to trigger inflammation in the gut and that such inflammation is associated with reduced expression of markers of epithelial TJs. Findings also suggest that concomitant exposure to nicotine may ameliorate both phenotypes.
E-cigarettes disrupt the integrity of the gut barrier
An intact gut barrier is an important first-line defense. To determine if the observed decrease in colon TJ markers in mice exposed to e-cigs is a direct consequence of circulating chemicals inhaled in the aerosols on the gut epithelial barrier, we used an ex vivo near-physiologic model system called the “gut-in-a-dish” (Figure 1D) (Ghosh et al., 2020). In this model, crypt-derived stem cells isolated from mouse colon (see the Transparent methods section) were used to generate organoids and later differentiated into polarized enteroid-derived monolayers (EDMs). The EDMs are also widely believed to be a model that is superior to cultured colon cancer cell lines (e.g., Caco2) because (1) they have been validated as model systems that closely resemble the physiologic gut lining in which all cell types (enterocytes, goblet, paneth enteroendocrine, and tuft cells) are proportionately represented (Foulke-Abel et al., 2014; Mahe et al., 2013; Miyoshi and Stappenbeck, 2013; Noel et al., 2017); (2) they are not transformed and yet allow culturing over several passages so that they can be used to ensure reproducibility of meaningful functional studies; and (3) because they preserve dimensionality, i.e., apicobasal polarity with functional tight junctions that can recreate the gut barrier ex vivo.
To dissect how e-cig-derived chemicals in the systemic circulation impact the gut barrier, we exposed the basolateral surfaces of the murine colonic EDMs to e-cigarette aerosol-infused media. As negative controls, we exposed the EDMs either to normal growth media (“UN”) or to air-infused media (“Air”). We analyzed the integrity of the gut barrier using two readouts (Figure 1D): (1) paracellular permeability, as reflected by low trans-epithelial electrical resistance (TEER) and (2) molecular characterization of epithelial TJs by looking at the localization of occludin; this integral membrane protein allows us to not just visualize but also quantify the degree of TJ disruption. Exposure to nicotine-free e-cigarette aerosol media caused a significant drop in TEER (∼% change value of −97.3 ± 0.3%) compared with untreated (UN, −0.5 ± 8.1%) and air-treated (1.4 ± 9.7%) controls (Figure 1E). Findings indicate a significant increase in paracellular permeability upon exposure to e-cig when compared with untreated (p = 0.0002) and air-treated (p = 0.0004) controls (Figure 1E). In e-cig-exposed EDMs, confocal microscopy showed a significantly increased “burst” tricellular TJs (these are specialized regions of the TJ where three or more cells come in contact [Furuse et al., 2014], and are also the regions where TJ disruption can be visualized/assessed first [Ghosh et al., 2020]) by ∼60.1 ± 8.1% when compared with untreated (p = 0.0010) and air-treated (p = 0.0015) controls (Figures 1F and 1G). These findings show that chemicals contained within the most basic e-cigarette aerosols have a direct disruptive effect on the epithelial barrier. Because the chemicals used to make the e-liquids and e-cig aerosols used in these studies (propylene glycol and glycerol) are found in >99% of all e-cigarettes, these data broadly apply to e-cigarettes and vaping devices.
Chronic exposure to inhaled e-cigarette aerosols induces stress responses in the colon
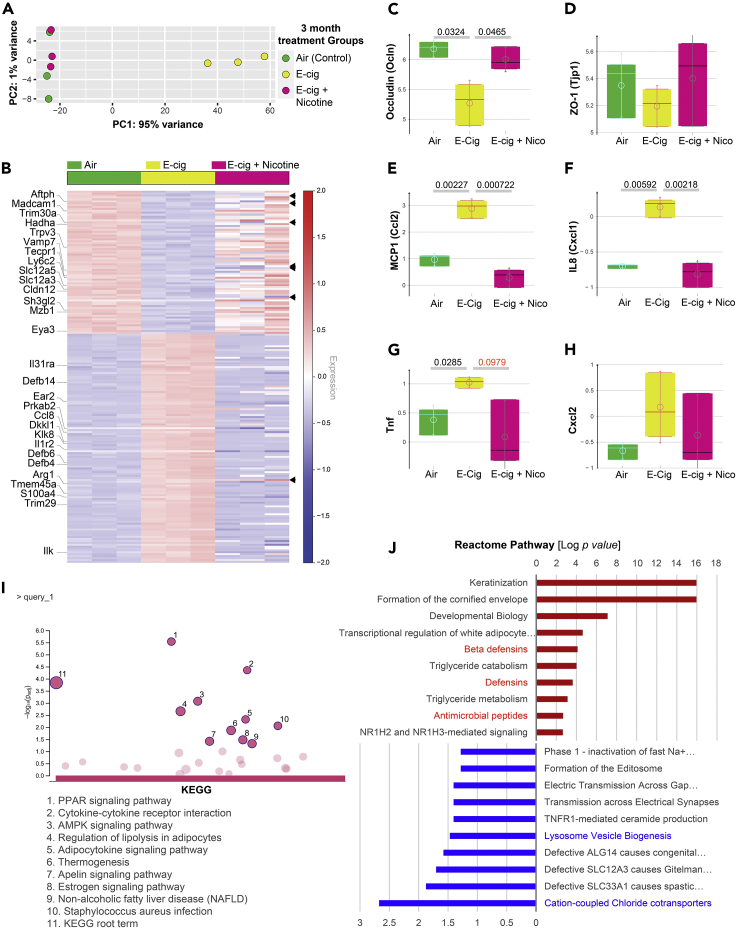
To determine the global impact of e-cigarettes on the gut, we next carried out RNA sequencing (RNA-seq) on the distal colons. Acute exposure to nicotine-free e-cigarettes did not significantly change gene expression in the colon (Figure S2A), whereas chronic exposure was associated with significant changes (Figure 2A). A differential expression analysis showed that chronic exposure to nicotine-free e-cigarettes was associated with a significant upregulation of 120 genes and downregulation of 75 genes (with a 30% false discovery rate, FDR) (Figure 2B; Table S1). Barring a handful of genes (arrowheads, Figures 2B and S2B), most of these differences were abolished when mice were exposed to nicotine-containing e-cigarettes (Figure 2B). TJ markers occludin and ZO1 were downregulated by nicotine-free, but not nicotine-containing, e-cigarettes (Figures 2C and 2D). Multiple pro-inflammatory cytokines were either elevated significantly (MCP1, IL-8, and TNF-α) or showed an increasing trend but did not reach significance (Cxcl2) (Figures 2E–2H). These RNA-seq findings are in agreement with our prior observations by histology (Figure 1B) and the targeted analyses of TJ markers by qPCR (Figure 1C), in that colons of mice exposed to nicotine-free, but not nicotine-containing, e-cig have impaired TJ markers and are inflamed.
Figure 2.
Nicotine-free, but not nicotine-containing, e-cigarettes trigger wide-ranging changes in gene expression in the colon
(A) Principal component analysis (PCA) of the topmost variable genes in the distal colons of the three groups of mice, all exposed to air or e-cig as indicated for 3 months. PCA identified air control and nicotine-containing e-cig group as similar to each other, but distinctly different from the e-cig alone group along the first principal component.
(B) Heatmap visualization of the differentially expressed genes between the three groups of mice. Each row represents one of the genes, whereas columns represent expression averages of replicates for each investigated group. Red color indicates relative over-expression, whereas blue color indicates relative under-expression. Top genes in the Reactome pathway analyses are marked on the left side. Arrowheads on the right side indicate a few genes that remained altered in both e-cig alone and e-cig + nicotine groups (see Figure S2B).
(C–H) Whisker plots display the levels of expression of the genes, as determined by RNA-seq encoding tight junction markers (occludin, C; ZO1, D; and pro-inflammatory cytokines (MCP1), E; IL-8, F; TNF-α, G; Cxcl2, H).
(I and J) KEGG (I) and Reactome (J) pathway analyses of the list of differentially expressed genes (see Table S1) reveal the most up or downregulated pathways. Red color indicates upregulation, whereas blue color indicates the downregulation of gene expression. No significant enrichment of pathways was seen in the list of downregulated genes by KEGG analyses.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (Figure 2I) and Gene Ontology (GO) (Figure S3) analyses revealed that the most enriched disease-related pathways were those that are involved in cellular sensing and response to external stress and stimuli (peroxisome proliferator-activated receptors [PPARs] and 5′ AMP-activated protein kinase [AMPK] signaling), cell death and programmed cell death, defense response to other organisms, metabolism (lipolysis, adipocytokine, and thermogenesis), and inflammation (cytokine and receptors). With regard to cytokine signaling, we noted that il31ra (subunit for IL6R), il1r2 (decoy receptor for IL1R1), ccl8 (a chemoattractant), ear2 (chemoattractant), and ilk (activator of nuclear factor κB [NF-κB]) were upregulated, whereas trim30a (a suppressor of NF-κB) and madcam1 (a cell-adhesion molecule required for leukocyte trafficking) were downregulated. No KEGG pathways were significantly enriched among the downregulated genes. A Reactome pathway (Figure 2J) analysis on the same gene sets showed enrichment of anti-microbial peptides, specifically beta-defensins (Defb4, 6 and 14), and de-enrichment of regulators of lysosome biogenesis (sh3gl2, eya3, vamp7) and chloride transporters (slc12a3, slc12a5). Besides these statistically enriched pathways and processes, it is noteworthy that several cancer-related genes (dkkl1, trim29, s100a4, tmem45a, and klk8) were also upregulated (Figure 2B), and as expected, an enrichment of pathways for differentiation program in the colon (e.g., multiple Keratins; Figure 2J). Many of these differentiation-related genes continued to remain high despite nicotine (Figure S2B). These findings suggest the upregulation of two opposing programs in the colon that tightly regulate oncogenesis in the colon.
Chronic (repetitive) exposure to e-cigarettes disrupts the integrity of the human gut barrier and triggers inflammation
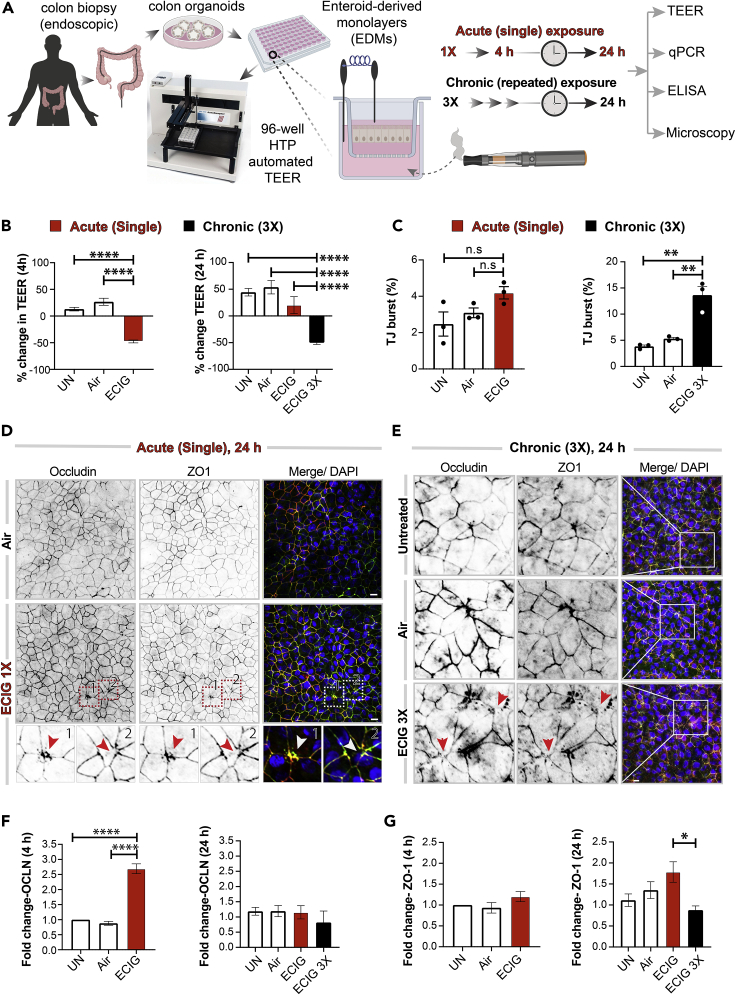
To translate the relevance of our findings in mice colon to the human gut, we generated organoids from colonic and ileal biopsies obtained from healthy human subjects (three independent donors, age range 29–71 years). These organoids were subsequently differentiated into EDMs and exposed either acutely (single exposure) or chronically (repeated exposures x3, each 4 h apart) prior to analyzing them at 24 h for barrier integrity and markers of inflammation using multiple modalities (see Figure 3A). An acute single exposure to e-cig was associated with a significant drop in TEER (−48.1 ± 5.5%) at 4 h (Figure 3B, left). However, much of that initial drop at 4 h was virtually reversed after 24 h to levels that were similar to untreated or air-treated control EDMs (UN = 41.7 ± 8.0%, Air = 49.1 ± 14.5% and e-cig = 23.6 ± 18.5% (Figure 3B, right). When the EDMs were subjected to 3x e-cig exposures, which is a more physiologic exposure based on human use patterns, the drop in TEER was sustained at 24 h (−59.96 ± 3.82%; Figure 3B, right).
Figure 3.
Chronic exposure to e-cigarettes disrupts the integrity of the human gut barrier, triggers inflammation
(A) Schematic displays the key aspects of ex vivo disease modeling to interrogate the impact of vaping on the human colonic epithelial barrier. Two modes of exposures (acute and chronic) were modeled and a variety of functional readouts were analyzed. UN, normal media; Air, air-infused media.
(B) Bar graphs display percent change in transepithelial electrical resistance (TEER) over time. Data are displayed as mean ± SEM (n = 3). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗∗∗∗p <0.0001.
(C–E) EDMs were treated as indicated prior to fixation, and then stained for tight junction (TJ) marker occludin (green), ZO1 (red), and DAPI (blue, nuclei) and analyzed by confocal microscopy. Bar graphs in (C) display the percent increase in tight junction (TJ) “bursts.” Data are displayed as mean ± SEM (n = 3 fields/condition). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗∗p <0.01; n.s., not significant. Confocal microscopic images representative of EDMs after 24 h of treatment with either a single (D) or repeated (3x; E) exposures to air (control) or nicotine-free e-cig vapor-infused media are shown. Scale bar, 10 μm. Insets “1” and “2” in (D) are displayed below as magnified panels and the arrowheads (red/white) in (D) and (E) point to examples of burst TJs.
(F and G) Bar graphs display the relative fold change in mRNA expression of tight junction markers (Occludin and ZO1) in human EDMs after acute (4 h) or chronic (24 h) exposure of e-cig vapor-infused media. Data are displayed as mean ± SEM (n = 3). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗p<0.05 and ∗∗∗∗p <0.0001.
We also confirmed that the observed drop in TEER in the colon-derived EDMs was also associated with a loss of structural integrity of the TJs. An acute single exposure was associated with only infrequent aberrant tricellular TJ morphology and a statistically insignificant “burst” appearance at 24 h (Figure 3C, left; Figure 3D, arrowheads). However, repeated 3x exposures resulted in a significant ∼3-fold increase in the percentage of burst TJs (Figure 3C, right; Figure 3E, arrowheads). The levels of transcripts of the membrane-integral TJ marker, occludin, increased after acute exposure (Figure 3F, left) but returned to normal levels at 24 h (Figure 3F, right; Figure S4). The levels of the peripheral TJ marker ZO1 was unchanged at 4 h after an acute exposure (Figure 3G, left), while there was a significant drop in gene expression of ZO1 after chronic repetitive multiple exposure (Figure 3G, right).
Because prior studies have implicated loss of epithelial barrier integrity as permissive to inflammation (Ghosh et al., 2020), we next investigated inflammatory gene expression in the e-cigarette-exposed EDMs by qPCR. Compared with untreated or air-treated controls, chronic repetitive exposure to e-cigarettes (but not single acute exposure) increased the expression of transcripts for all the pro-inflammatory cytokines tested (Figures 4A–4D). ELISA studies conducted on EDM supernatants confirmed that the protein levels of the pro-inflammatory cytokine IL-8 were significantly elevated (Figure 4E).
Figure 4.
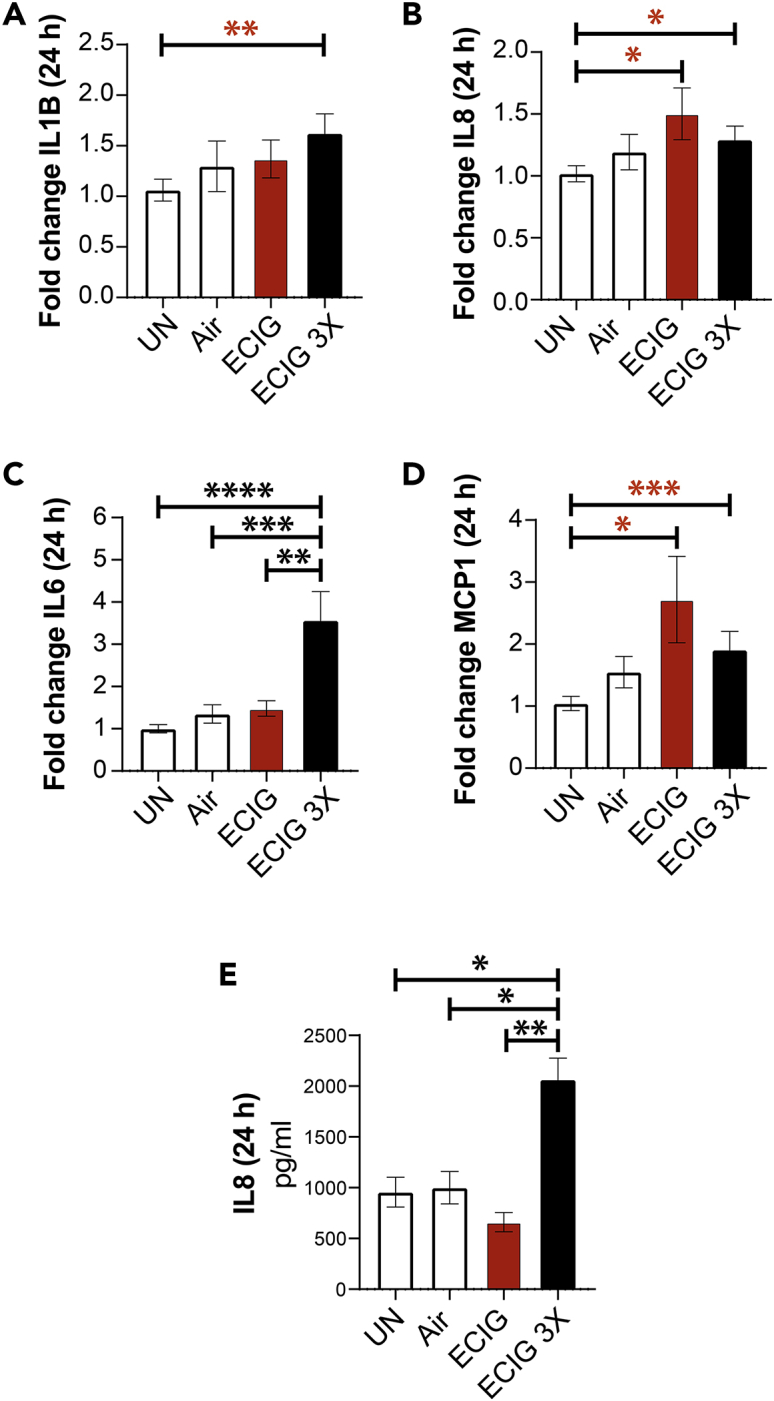
Chronic exposure to nicotine-free e-cigarette induces the expression of pro-inflammatory cytokines in the human colonic epithelium
(A–D) Bar graphs display the relative fold change in the levels of mRNA for pro-inflammatory cytokines. Data are shown as mean ± SEM (n = 3). UN, normal media; Air, air-infused media. Statistical significance was estimated using either one-way ANOVA with Tukey's test (black) or Mann-Whitney test (red); ∗p<0.05, ∗∗p <0.01, and ∗∗∗p <0.001. (E) Bar graphs display the concentration of IL-8 released in the basolateral compartment of polarized EDMs after exposure to e-cig vapor-infused media. Data are shown as mean ± SEM (n = 3 independent experiments). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗p<0.05 and ∗∗p <0.01.
Similar findings were also observed in the case of human ileum-derived EDMs. TEER dropped significantly at 4 h after both single and 3x exposures (Figures S5A–S5C; ∼55% drop compared with control EDMs). The patterns of change in occludin and ZO1 were mirrored in the case of human ileum-derived EDMs (Figures S5D–S5G). A single acute exposure had little or no effect on cytokine transcripts (Figures S5H, S5J, S5L, and S5N), whereas chronic repetitive e-cigarette exposure caused increased IL-1B (Figure S5I), IL-6 (Figure S5K), IL-8 (Figure S5M), and MCP1 (Figure S5O).
Taken together, these physiologic (TEER), morphologic (burst appearance of TJs), and transcriptomic (qPCR assessment of markers of TJ transcripts) readouts are all in agreement, i.e., exposure to nicotine-free e-cigarette aerosols causes epithelial barrier dysfunction in the human gut. They also demonstrate that chronic (repetitive), but not acute (single), exposure is necessary for such disruption and that such disruption is associated with the induction of pro-inflammatory cytokines.
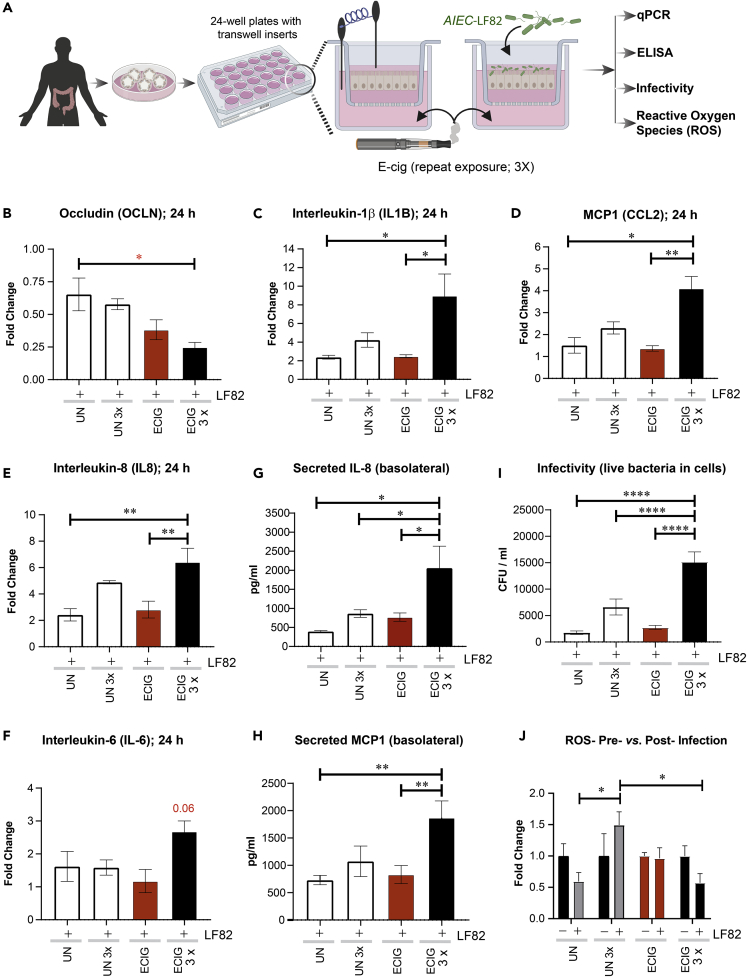
Chronic (repetitive) exposure of the gut epithelium to e-cigarette aerosols accentuates inflammatory responses to infections
Because the gut epithelial barrier of those who vape is concomitantly exposed to chemical components of e-cigarettes as well as luminal microbes, we exposed EDMs simultaneously to both stressors. First, we exposed the basolateral side of EDMs grown on Transwells to e-cigarette-infused media (mimicking the absorption of core chemicals contained within nicotine-free vaping aerosols into the blood stream and diffusion into tissues) and subsequently challenged the apical surface with live pathogenic microbes (to simulate luminal microbes) (Figure 5A). As before (in Figure 3), EDMs were either treated acutely (single exposure) or chronically (repeated exposures x3, each 4 h apart) with e-cig-infused media (ECIG X3). In the negative controls, which were exposed only to media (UN), the media were changed every 4 h (UNX3), just like the e-cig condition. We used the adherent invasive E. coli (AIEC)-LF82, a pathogen that was originally isolated from a patient with IBD (Boudeau et al., 1999). Compared with untreated controls, EDMs repeatedly exposed to e-cigarette aerosol media had a significant drop in the levels of occludin mRNA (Figure 5B, left) and significant increases in the levels of transcripts of inflammatory cytokines IL-1B, IL-8, and MCP1 (Figures 5C–5E). Levels of IL-6 also trended up but fell short of statistical significance (Figure 5F). ELISA studies confirmed that EDMs repeatedly exposed to e-cigarette aerosol media also secreted higher amounts of IL-8 (Figure 5G) and MCP1 (Figure 5H). Unlike the EDMs that were repeatedly exposed to e-cigarettes (3x), those exposed only once did not have a significant reduction in occludin (Figure 5B, left) or induction of proinflammatory cytokines (Figures 5C–5H). These findings indicate that exposure to the common core chemical components of e-cigarette aerosols is sufficient to make the gut hyperreactive to microbes and that repetitive exposure is necessary for such hyperresponsiveness.
Figure 5.
Chronic exposure to nicotine-free e-cigarette induces the expression of pro-inflammatory cytokines in the human colonic epithelium
(A) Schematic displays the overall experimental design for assessing how e-cig affects the gut epithelial response to infectious pathogenic microbes, e.g., E. coli strain AIEC-LF-82.
(B–F) Bar graphs display the relative fold change (compared to media control) in the levels of mRNA for pro-inflammatory cytokines. Data are shown as mean ± SEM (n = 3 independent experiments). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗p<0.05, ∗∗p <0.01.
(G and H) Bar graphs display the concentration of IL-8 (G) and MCP-1 (H) released in the basolateral compartment of polarized EDMs after exposure to e-cig vapor-infused media. Data are shown as mean ± SEM (n = 3 independent experiments). Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗p<0.05 and ∗∗p <0.01.
(I) Bar graphs display the bacterial load internalized in EDMs pretreated as indicated with or without single or repeated exposure to e-cig vapor-infused media and then exposed to pathogenic AIEC-LF82 for 3 h. Data are expressed as the number of internalized bacteria to the infected control EDMs (untreated; i.e., not exposed to e-cig) and is represented as the mean ± SEM of three separate experiments. Statistical significance was estimated using one-way ANOVA with Tukey's test;∗∗∗∗ p< 0.0001.
(J) Bar graphs display cellular accumulation of ROS, as determined by measuring the levels of oxidized DNA in the supernatant in the basolateral compartment of polarized EDMs after exposure to the indicated treatments. Data are represented as the mean ± SEM of three separate experiments. Statistical significance was estimated using one-way ANOVA with Tukey's test; ∗p<0.05.
Because cytokine production by epithelial cells after an infection is a culmination of multiple events during epithelial sensing and signaling that are triggered by microbes, we next asked how exposure to e-cigarettes impacts some of the early steps, i.e., infectivity of the gut epithelium and epithelial reaction to such infection by production of reactive oxygen species (ROS); the latter serves as a critical second messenger that modulates innate immune signaling in the gut epithelium (Jones et al., 2012). EDMs exposed repeatedly to e-cig (3x) showed a statistically significant higher number of internalized bacteria compared with control EDMs after 3 h of infection, demonstrating decreased host defenses with higher infectivity of gut epithelium after e-cigarette exposure (Figure 5I). Finally, we found that repeated exposures of e-cigarette aerosol media (3x) followed by infection of EDMs were associated with a reduction in ROS (Figure 5J). Unlike the chronically exposed EDMs, those exposed only once did not show a significant increase in infectivity, nor did they show a significant reduction in ROS production (Figures 5I and 5J).
Taken together, these findings indicate that chronic repetitive exposure to e-cigarettes alters the gut epithelial cell response to infection with pathogenic microbes, characterized by higher infectivity and induction of pro-inflammatory cytokines and a failure to induce protective ROS. Because the overall composition of the gut microbes does not appear to be significantly altered among the subjects who consume e-cigarettes (Stewart et al., 2018), our findings show that e-cigarettes may impair gut homeostasis primarily via modulation of host responses to microbes.
Discussion
E-cigarettes trigger gut inflammation
The major discovery we report in this work is that chronic repetitive, but not acute, exposure to e-cigarette aerosols disrupts the gut epithelial barrier, increases the susceptibility of the gut lining to bacterial infections, and triggers gut inflammation (Right; Figure 6). We also show the components in the e-liquid as the major culprit (expanded upon later in “Discussion”). We established causality by using near physiologic ex vivo murine and human gut models (Left; Figure 6); the minimalistic nature of the polarized enteroid monolayer system and our ability to manipulate it in a physiologically relevant manner allowed us to pinpoint the target cell for e-cig-induced injury as the gut epithelial cell. It is possible that the barrier-disruptive injury is a direct consequence of the heat-decomposed chemical components in the e-cig vapor or is caused indirectly via secondary metabolites or the cytokines generated from EDMs. By using invasive E. coli in co-culture studies with EDMs, we also determine that handling of microbes by the gut epithelium was fundamentally impaired upon chronic and repetitive exposure to e-cig, resulting in higher infectivity and inflammation. These findings are in keeping with prior studies showing higher infectivity and inflammation in the epithelial lining of the oral mucosa (Pushalkar et al., 2020) and the lung (Crotty Alexander et al., 2018; Madison et al., 2019; Miyashita et al., 2018).
Figure 6.
Summary of findings
Left: Schematic summarizes the various in vivo and ex vivo murine and human models used (top) and the readouts assessed (bottom) in this work to study the impact of vaping on the gut barrier. Right: The major findings and conclusions from this study showing how e-cig vapors can trigger distinct events (from top to bottom), such as epithelial barrier disruption, which is permissive to altered gene expression (lower occludin and higher cytokines), and heightened susceptibility for and response to bacterial infections, culminating in chronic inflammation and epithelial stress response. Potential feedforward loops that could set up a vicious loop of mucosal injury are indicated using bidirectional arrows.
E-cigarettes broadly impact gut health
Our RNA-seq studies showed three major inter-related themes of altered transcriptional programs. The first are the pathways concerning cellular response to stress and stimuli comprising prominent inductions of genes that participate within the PPAR and AMPK signaling pathways. The second are the pathways concerning mucosal response to infection and inflammation, with prominent induction of genes that encode the anti-microbial peptide β-defensins and downregulation of multiple genes that modulate lysosomal biogenesis and Tecpr1, which is necessary and sufficient for autophagic clearance of microbes (Ogawa et al., 2011). The upregulation of stress response genes and the very specific pattern of upregulation of β-isoform of defensins are not unique to the gut; transcriptomic analyses on human bronchial epithelium have documented the same previously (Shen et al., 2016). The third and final theme is that of a balanced upregulation of genes that support pro- and anti-oncogenic pathways and processes, the most prominent of which were genes involved in cellular differentiation, i.e., multiple keratins. Because a sufficient amount of keratin is needed for efficient stress protection in the colonic epithelia and because keratins play an essential role of maintaining the epithelial barrier and its downregulation in intestinal tissue has been correlated with the progression of IBD (Asghar et al., 2015; Dong et al., 2017), our findings suggest that the three themes of altered gene expression may be inter-related consequences of epithelial stress response to chronic stimuli (simultaneous exposure to e-cig and microbes) and inflammation.
E-liquid, not nicotine, is the culprit
Despite a vast array of literature pointing toward nicotine as a major source of ill-effects in people who smoke and vape, our RNA-seq studies using murine models of vaping unexpectedly but decisively revealed that it is the e-liquid component in e-cigarettes that induces broad and sweeping changes in gene expression in the gut. Virtually all changes in the distal colon were reversed by a concomitant co-administration of nicotine. These findings are in keeping with the barrier-tightening effect and anti-inflammatory effect of nicotine that have been demonstrated previously (Eliakim and Karmeli, 2003; Geng et al., 1996; Harries et al., 1982; Lashner et al., 1990; Madretsma et al., 1996; Prytz et al., 1989; Suenaert et al., 2000, 2003; Sykes et al., 2000; Van Dijk et al., 1995; Wang et al., 2012, 2016; Zhang and Petro, 1996). For example, exposure of epithelial monolayers to nicotine and its metabolites at concentrations corresponding to those reported in the blood of smokers significantly improves TJ integrity, and thus decreases epithelial gut permeability (Wang et al., 2012). Similarly, studies in humans (Prytz et al., 1989; Suenaert et al., 2000) have shown that nicotine does tighten the gut and that such effect may only be seen upon chronic, but not acute, exposures (Harries et al., 1982; Lashner et al., 1990; Suenaert et al., 2003). As for mechanism(s) behind such tightening, an upregulation of TJ markers (Wang et al., 2012, 2016), most prominently that of occludin, has been reported. Furthermore, nicotine has been generally found to serve as a protective factor for the development and progression of ulcerative colitis (Sykes et al., 2000), a condition that is characterized by leakiness of the gut barrier and chronic inflammation in the gut lining. When we tested the impact of e-cig on the gut barrier, we found that the nicotine-free vapors could disrupt the epithelial barriers in both murine (Figure S6) and human (Figures S7A–S7F) EDMs to a similar extent as vapors that contained nicotine. These findings are in keeping with other published work showing that it is the e-cig liquid alone (with PG/VG) that is sufficient for disrupting the proteome of bronchial epithelial cells (Ghosh et al., 2018) and suppressing bronchial epithelial cell ciliary motility (Clapp et al., 2019). The findings are also in keeping with the fact that nicotine is known to exert its anti-inflammatory action via nicotinic acetylcholinergic receptors (nAChRs) on monocytes (Madretsma et al., 1996; Sykes et al., 2000) and T cells (Geng et al., 1996; Zhang and Petro, 1996), presumably through the direct activation of the cholinergic anti-inflammatory pathway, which involves inhibition of NF-κB signaling (Wang et al., 2003). That we see nicotine's anti-inflammatory action in mice (Figures 1 and 2) but do not see the same in EDMs (Figures S6 and S7) suggests that the observed anti-inflammatory action in vivo might be epithelium independent, but it is the e-cig base formulation containing PV/VG that is necessary and sufficient to destroy the gut barrier.
Translational relevance of findings
The gut is a complex environment; the gut mucosal barrier serves as the final frontier between the largest immune system in the body and trillions of microbes, diverse microbial products, food antigens, and toxins in the lumen. A compromised gut barrier allows microbes and antigens to leak through and encounter the host immune system, thereby generating inflammation and systemic endotoxemia. The compromised gut barrier is believed to be a major pathophysiologic component and a contributor to the initiation and/or progression of various chronic diseases, including, but not limited to, metabolic endotoxemia, type II diabetes, fatty liver disease, obesity, atherosclerosis, and IBDs. In documenting the harmful effects of e-cigarettes on the gut barrier, our study not just highlights the potential effects of e-cigarettes on the gastrointestinal tract but also provides insights into the potential long-term effects of e-cigarettes on health.
Limitations of the study
The safety of flavors and other additives (i.e., cannabinoids, THC) was not assessed here. In the absence of regulations over e-liquid contents and compositions and the plethora of flavors there is to test (thousands), scaling up to power such a study is not trivial. Another limitation is that the enteroid system is a minimalistic model and lacks critical components (e.g., immune and non-immune cells present under normal physiologic conditions) that are important for setting up a vicious cycle of inflammation in the gut and further damage of the epithelial lining. Thus, it is possible that the phenotypes observed in our EDM-based assays underestimate the full extent of injury and inflammation due to e-cigarettes. In fact, we believe that the missing immune cells in these assays could explain why there is an apparent discrepancy in the effects of e-cig and nicotine combinations observed in vivo versus those observed in vitro on EDMs. Finally, although we reconstitute the EDM model with live microbes (the single pathogenic strain of AIEC-LF82), the full impact of e-cigarettes in the setting of the polymicrobial gut luminal milieu was not estimated here. Ongoing work is investigating several of these outstanding aspects.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Pradipta Ghosh (prghosh@health.ucsd.edu).
Materials availability
EDMs generated in this study will be made available on request, but we may require a payment and/or a completed Materials Transfer Agreement if there is potential for commercial application.
Data and code availability
This study has generated RNA-seq data of colon isolated from e-cigarette-treated mice. The RNA-seq datasets (metadata, RAW data, and processed data) generated in this work has been deposited at the NCBI GEO and can be queried using GSE161521.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This work was supported by awards from the Tobacco-Related Disease Research Program (TRDRP): 28IP-0024 (to P.G. and S.D.), 30IP-0965 and 26IP-0040 (to L.C.A.), the National Institutes of Health (NIH) grants DK107585 (to S.D.), AI141630 (to P.G.), R01HL147326 (to L.E.C.-A.) and R00-CA151673 (to D.S). S.D. was also supported by a DiaComp Pilot and Feasibility award (Augusta University). P.G., S.D., and D.S were supported by NIH/National Center for Advancing Translational Sciences (NCATS) award UG3TR002968 and the Leonna M Helmsley Charitable Trust. S.-R.I. was supported by the NIH Diversity Supplement award. J.E. was supported by a Postdoctoral Fellowship from the American Cancer Society (PF-18-101-01-CSM). L.E.C.-A. was also supported by the American Heart Association (grant-in-aid 16BGIA27790079) and an ATS Foundation Award for Outstanding Early Career Investigators. This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929). In addition, a P30 grant (NIH/NIDDK, P30DK120515) subsidized the RNA-seq and histology work showcased here. We are grateful to the HUMANOID CoRE for providing the media for the organoid culture.
Author contributions
A.S., J.L., A.G.F., A.M., T.K., I.M.S., and S.-R.I. designed and performed the experiments; R.F.P. and D.S. performed the RNA-seq analysis; J.E. performed and supervised the confocal microscopy experiments. A.S., J.L., A.G.F., P.G., and S.D. analyzed the data and generated the first draft of the manuscript; P.G. and S.D. wrote the manuscript. L.E.C.-A. provided expertise and access to the e-cigarette-infused media and e-cig treated murine colonic specimens; P.G., L.E.C.-A., and S.D. supervised the project.
Declaration of interests
S.D. and P.G. have patents on methodology to prepare enteroid monolayers and functional assays related to the gut barrier. The authors have declared that no other conflict of interest exists.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102035.
Contributor Information
Laura E. Crotty-Alexander, Email: lca@ucsd.edu.
Pradipta Ghosh, Email: prghosh@health.ucsd.edu.
Soumita Das, Email: sodas@health.ucsd.edu.
Supplemental information
References
- Alasmari F., Crotty Alexander L.E., Hammad A.M., Bojanowski C.M., Moshensky A., Sari Y. Effects of chronic inhalation of electronic cigarette vapor containing nicotine on neurotransmitters in the frontal cortex and striatum of C57BL/6 mice. Front Pharmacol. 2019;10:885. doi: 10.3389/fphar.2019.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F., Crotty Alexander L.E., Nelson J.A., Schiefer I.T., Breen E., Drummond C.A., Sari Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and alpha-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog.Neuropsychopharmacol. Biol. Psychiatry. 2017;77:1–8. doi: 10.1016/j.pnpbp.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allais L., Kerckhof F.M., Verschuere S., Bracke K.R., De Smet R., Laukens D., Van den Abbeele P., De Vos M., Boon N., Brusselle G.G. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ.Microbiol. 2016;18:1352–1363. doi: 10.1111/1462-2920.12934. [DOI] [PubMed] [Google Scholar]
- Asghar M.N., Silvander J.S., Helenius T.O., Lahdeniemi I.A., Alam C., Fortelius L.E., Holmsten R.O., Toivola D.M. The amount of keratins matters for stress protection of the colonic epithelium. PLoS One. 2015;10:e0127436. doi: 10.1371/journal.pone.0127436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount B.C., Karwowski M.P., Shields P.G., Morel-Espinosa M., Valentin-Blasini L., Gardner M., Braselton M., Brosius C.R., Caron K.T., Chambers D. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Glasser A.L., Masseret E., Joly B., Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozier J., Chivers E.K., Chapman D.G., Larcombe A.N., Bastian N., Masso-Silva J.A., Byun M.K., McDonald C.F., Alexander Crotty L.E., Ween M.P. The evolving landscape of electronic cigarettes: a systematic review of recent evidence. Chest. 2020;157:1362–1390. doi: 10.1016/j.chest.2019.12.042. [DOI] [PubMed] [Google Scholar]
- CDC, P H S, United-States O o t S G . National Center for Chronic Disease Prevention and Health Promotion; 2016. Office on Smoking and Health. E-Cigarette Use AmongYouth and Young Adults: AReport of the Surgeon General Atlanta. [Google Scholar]
- Clapp P.W., Lavrich K.S., van Heusden C.A., Lazarowski E.R., Carson J.L., Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L470–L486. doi: 10.1152/ajplung.00304.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S., Kosmider L., McRobbie H., Goniewicz M., Kimber C., Doig M., Dawkins L. E-cigarette puffing patterns associated with high and low nicotine e-liquid strength: effects on toxicant and carcinogen exposure. BMC Public Health. 2016;16:999. doi: 10.1186/s12889-016-3653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty Alexander L.E., Drummond C.A., Hepokoski M., Mathew D., Moshensky A., Willeford A., Das S., Singh P., Yong Z., Lee J.H. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul.Integr.Comp. Physiol. 2018;314:R834–R847. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty Alexander L.E., Ware L.B., Calfee C.S., Callahan S.J., Eissenberg T., Farver C., Goniewicz M.L., Jaspers I., Kheradmand F., King T.E., Jr. NIH workshop report: E-cigarette or vaping product use associated lung injury (EVALI): Developing a research agenda. Am. J. Respir. Crit. Care Med. 2020;202:795–802. doi: 10.1164/rccm.201912-2332WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L.E., Kimber C.F., Doig M., Feyerabend C., Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 2016;233:2933–2941. doi: 10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- Dong X., Liu Z., Lan D., Niu J., Miao J., Yang G., Zhang F., Sun Y., Wang K., Miao Y. Critical role of Keratin 1 in maintaining epithelial barrier and correlation of its down-regulation with the progression of inflammatory bowel disease. Gene. 2017;608:13–19. doi: 10.1016/j.gene.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F. Divergent effects of nicotine administration on cytokine levels in rat small bowel mucosa, colonic mucosa, and blood. Isr. Med. Assoc. J. 2003;5:178–180. [PubMed] [Google Scholar]
- Etter J.F., Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Foulke-Abel J., In J., Kovbasnjuk O., Zachos N.C., Ettayebi K., Blutt S.E., Hyser J.M., Zeng X.L., Crawford S.E., Broughman J.R. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp. Biol. Med. (Maywood) 2014;239:1124–1134. doi: 10.1177/1535370214529398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M., Goggins B.J., Mateer S., Jones B., Kim R.Y., Gellatly S.L., Jarnicki A.G., Powell N., Oliver B.G., Radford-Smith G. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018;3:e94040. doi: 10.1172/jci.insight.94040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Izumi Y., Oda Y., Higashi T., Iwamoto N. Molecular organization of tricellular tight junctions. Tiss.Barr. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Savage S.M., Razani-Boroujerdi S., Sopori M.L. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- Ghosh A., Coakley R.C., Mascenik T., Rowell T.R., Davis E.S., Rogers K., Webster M.J., Dang H., Herring L.E., Sassano M.F. Chronic E-cigarette exposure alters the human bronchial epithelial proteome. Am. J. Respir. Crit. Care Med. 2018;198:67–76. doi: 10.1164/rccm.201710-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Swanson L., Sayed I.M., Mittal Y., Lim B.B., Ibeawuchi S.R., Foretz M., Viollet B., Sahoo D., Das S. The stress polarity signaling (SPS) pathway serves as a marker and a target in the leaky gut barrier: implications in aging and cancer. Life Sci. Alliance. 2020;3:e201900481. doi: 10.26508/lsa.201900481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J.A., Quinn R.A., Debelius J., Xu Z.Z., Morton J., Garg N., Jansson J.K., Dorrestein P.C., Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- Harries A.D., Baird A., Rhodes J. Non-smoking: a feature of ulcerative colitis. Br. Med. J. 1982;284:706. doi: 10.1136/bmj.284.6317.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M., Alfi M., Talbot P. Health-related effects reported by electronic cigarette users in online forums. J. Med. Internet Res. 2013;15:e59. doi: 10.2196/jmir.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.H., Lyes M., Sladewski K., Enany S., McEachern E., Mathew D.P., Das S., Moshensky A., Bapat S., Pride D.T. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016;94:667–679. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Mercante J.W., Neish A.S. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr. Med. Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A., Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ. Sci. Technol. 2016;50:13080–13085. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- King B.A., Jones C.M., Baldwin G.T., Briss P.A. The EVALI and youth vaping epidemics - implications for public health. N. Engl. J. Med. 2020;382:689–691. doi: 10.1056/NEJMp1916171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K., Furuse M., Sasaki H., Sonoda N., Fujita K., Nagafuchi A., Tsukita S. Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr. Biol. 1999;9:1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- Lashner B.A., Hanauer S.B., Silverstein M.D. Testing nicotine gum for ulcerative colitis patients.Experience with single-patient trials. Dig. Dis. Sci. 1990;35:827–832. doi: 10.1007/BF01536795. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Park S.H., Weng M.W., Wang H.T., Huang W.C., Lepor H., Wu X.R., Chen L.C., Tang M.S. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. U S A. 2018;115:E1560–E1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison M.C., Landers C.T., Gu B.H., Chang C.Y., Tung H.Y., You R., Hong M.J., Baghaei N., Song L.Z., Porter P. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin.Invest. 2019;129:4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madretsma G.S., Donze G.J., van Dijk A.P., Tak C.J., Wilson J.H., Zijlstra F.J. Nicotine inhibits the in vitro production of interleukin 2 and tumour necrosis factor-alpha by human mononuclear cells. Immunopharmacology. 1996;35:47–51. doi: 10.1016/0162-3109(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Mahe M.M., Aihara E., Schumacher M.A., Zavros Y., Montrose M.H., Helmrath M.A., Sato T., Shroyer N.F. Establishment of gastrointestinal epithelial organoids. Curr.Protoc. Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margham J., McAdam K., Forster M., Liu C., Wright C., Mariner D., Proctor C. Chemical composition of aerosol from an E-cigarette: aquantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016;29:1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- Miyashita L., Suri R., Dearing E., Mudway I., Dove R.E., Neill D.R., Van Zyl-Smit R., Kadioglu A., Grigg J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018;51:1701592. doi: 10.1183/13993003.01592-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Stappenbeck T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B., Tibensky M., Horvathova L., Babal P. E-cigarettes and cancer risk. Cancer Prev. Res. (Phila) 2020;13:137–144. doi: 10.1158/1940-6207.CAPR-19-0346. [DOI] [PubMed] [Google Scholar]
- Muthumalage T., Prinz M., Ansah K.O., Gerloff J., Sundar I.K., Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2017;8:1130. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel G., Baetz N.W., Staab J.F., Donowitz M., Kovbasnjuk O., Pasetti M.F., Zachos N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017;7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Yoshikawa Y., Kobayashi T., Mimuro H., Fukumatsu M., Kiga K., Piao Z., Ashida H., Yoshida M., Kakuta S. A Tecpr1-dependent selective autophagy pathway targets bacterial pathogens. Cell Host Microbe. 2011;9:376–389. doi: 10.1016/j.chom.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Perez M., Crotty Alexander L.E. Why is vaping going up in flames? Ann. Am. Thorac.Soc. 2020;17:545–549. doi: 10.1513/AnnalsATS.201910-802PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prytz H., Benoni C., Tagesson C. Does smoking tighten the gut? Scand. J. Gastroenterol. 1989;24:1084–1088. doi: 10.3109/00365528909089259. [DOI] [PubMed] [Google Scholar]
- Pushalkar S., Paul B., Li Q., Yang J., Vasconcelos R., Makwana S., Gonzalez J.M., Shah S., Xie C., Janal M.N. Electronic cigarette aerosol modulates the oral microbiome and increases risk of infection. iScience. 2020;23:100884. doi: 10.1016/j.isci.2020.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., Lugg S.T., Aldridge K., Lewis K.E., Bowden A., Mahida R.Y., Grudzinska F.S., Dosanjh D., Parekh D., Foronjy R. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73:1161–1169. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Wolkowicz M.J., Kotova T., Fan L., Timko M.P. Transcriptome sequencing reveals e-cigarette vapor and mainstream-smoke from tobacco cigarettes activate different gene expression profiles in human bronchial epithelial cells. Sci. Rep. 2016;6:23984. doi: 10.1038/srep23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.P., Lawyer G., Muthumalage T., Maremanda K.P., Khan N.A., McDonough S.R., Ye D., McIntosh S., Rahman I. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. 2019;5 doi: 10.1183/23120541.00182-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.T., Heckman B.W., Wahlquist A.E., Cummings K.M., Carpenter M.J. The impact of E-liquid propylene glycol and vegetable glycerin ratio on ratings of subjective effects, reinforcement value, and use in current smokers. Nicotine Tob. Res. 2020;22:791–797. doi: 10.1093/ntr/ntz130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.A., Reisinger S.A., Freudenheim J.L., Brasky T.M., Mathe E.A., McElroy J.P., Nickerson Q.A., Weng D.Y., Wewers M.D., Shields P.G. Effects of electronic cigarette constituents on the human lung: a pilot clinical trial. Cancer Prev. Res. (Phila) 2020;13:145–152. doi: 10.1158/1940-6207.CAPR-19-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.J., Auchtung T.A., Ajami N.J., Velasquez K., Smith D.P., De La Garza R., 2nd, Salas R., Petrosino J.F. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ. 2018;6:e4693. doi: 10.7717/peerj.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaert P., Bulteel V., Den Hond E., Geypens B., Monsuur F., Luypaerts A., Ghoos Y., Rutgeerts P. In vivo influence of nicotine on human basal and NSAID-induced gut barrier function. Scand. J. Gastroenterol. 2003;38:399–408. doi: 10.1080/00365520310000834. [DOI] [PubMed] [Google Scholar]
- Suenaert P., Bulteel V., Den Hond E., Hiele M., Peeters M., Monsuur F., Ghoos Y., Rutgeerts P. The effects of smoking and indomethacin on small intestinal permeability. Aliment.Pharmacol.Ther. 2000;14:819–822. doi: 10.1046/j.1365-2036.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- Sussan T.E., Gajghate S., Thimmulappa R.K., Ma J., Kim J.H., Sudini K., Consolini N., Cormier S.A., Lomnicki S., Hasan F. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes A.P., Brampton C., Klee S., Chander C.L., Whelan C., Parsons M.E. An investigation into the effect and mechanisms of action of nicotine in inflammatory bowel disease. Inflamm. Res. 2000;49:311–319. doi: 10.1007/s000110050597. [DOI] [PubMed] [Google Scholar]
- Tang M.S., Wu X.R., Lee H.W., Xia Y., Deng F.M., Moreira A.L., Chen L.C., Huang W.C., Lepor H. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc. Natl. Acad. Sci. U S A. 2019;116:21727–21731. doi: 10.1073/pnas.1911321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk J.P., Madretsma G.S., Keuskamp Z.J., Zijlstra F.J. Nicotine inhibits cytokine synthesis by mouse colonic mucosa. Eur. J. Pharmacol. 1995;278:R11–R12. doi: 10.1016/0014-2999(95)00211-3. [DOI] [PubMed] [Google Scholar]
- Wang B., Wu Z., Ji Y., Sun K., Dai Z., Wu G. L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J. Nutr. 2016;146:501–508. doi: 10.3945/jn.115.224857. [DOI] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhao J.X., Hu N., Ren J., Du M., Zhu M.J. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J. Gastroenterol. 2012;18:2180–2187. doi: 10.3748/wjg.v18.i18.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Jiang D., Minor M., Chu H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V., Rahimy M., Korrapati A., Xuan Y., Zou A.E., Krishnan A.R., Tsui T., Aguilera J.A., Advani S., Crotty Alexander L.E. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Petro T.M. The effect of nicotine on murine CD4 T cell responses. Int. J. Immunopharmacol. 1996;18:467–478. doi: 10.1016/s0192-0561(96)00054-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study has generated RNA-seq data of colon isolated from e-cigarette-treated mice. The RNA-seq datasets (metadata, RAW data, and processed data) generated in this work has been deposited at the NCBI GEO and can be queried using GSE161521.