Abstract
Background
Previous systematic reviews showed additional benefit of adjuvant bisphosphonates (BP) in the treatment of periodontitis. In contrast, it is unclear the effect of BP in patients with diabetes and smokers, its pooled effect when administered locally or systemically is also unknown.
Objectives
This study aimed to systematically review the literature about the use of BP as adjuvant to nonsurgical scaling and root planning (SRP).
Methodology
This study followed the PRISMA guideline. This study included randomized clinical trials that administered locally or systemically BPs as adjuvant for periodontal treatment. Five databases were used. Meta-analyses were performed, using the pooled mean differences (MD) for clinical attachment level (CAL) and probing pocket depth (PPD). Standard mean difference (SMD) was used for radiographic assessment (RADIO). Subgroup analyses were performed for locally delivered meta-analyses, considering diabetes and smoking exposure.
Results
Thirteen studies were included. It was showed MD of 1.52 mm (95%CI: 0.97–2.07) and 1.44 mm (95%CI: 1.08–1.79) for PPD reduction and CAL gain, respectively, for locally delivered BP. BP was not able to provide significant improvements in smokers (subgroup analysis) when considering CAL (MD: 1.37; 95%CI: −0.17–2.91) and PPD (MD: 1.35; 95%CI: −0.13–2.83). Locally delivered BP also improved significantly the RADIO assessments (SMD: 4.34; 95%CI: 2.94–5.74). MD for systemically administered BP was 0.40 mm (95%CI: 0.21–0.60), 0.51 mm (95%CI: 0.19–0.83) and 1.05 (95%CI: 0.80–1.31) for PPD, CAL and RADIO, respectively.
Conclusion
The administration of BP in adjunct to SRP may result in additional clinical effects.
Keywords: Alendronate, Diphosphonates, Periodontal therapy, Periodontitis
1. Introduction
Periodontitis is caused by subgingival bacterial communities, composing a biofilm.1 These bacteria cause tissue rupture and may trigger destructive host immune responses, leading to degradation of periodontal tissues and tooth loss in more advanced cases.1 The treatment of periodontitis uses as a basis the removal of the pathogenic subgingival microbiota by scaling and root planing (SRP), along with the control of supragingival biofilm and periodic periodontal maintenance.2 Literature shows that SRP is an effective method for the treatment of periodontitis.3 However, some factors, such as smoking,4 diabetes mellitus,5 immunosuppression,6 and local factors (furcation areas and root depressions) might impair periodontal healing after SRP. In consequence, there is a necessity for additional therapeutic interventions, such as surgical approaches or the application of adjuvant substances.
In this context, the use of drugs, administered orally or locally, has gained space in the literature, as modulators of the host response.7, 8, 9 Therefore, it may be hypothesized that the use of antiresorptive drugs may provide an alternative adjuvant therapy effective for periodontitis. Among the antiresorptive drugs, bisphosphonates (BP) are largely used. BPs exert a potent inhibitory effect on bone resorption,10,11 as these drugs present a high affinity to bone tissue and bind strongly to hydroxyapatite crystals, especially on the remodeling surface.10,12 Consequently, this leads to increased bone mineral density13 and induction of osteoblasts to bone deposition.12 In addition, these drugs are deposited in the mineralized bone matrix.14 A in vitro study also demonstrated that BPs also present anti-inflammatory properties, inhibiting pro-inflammatory factors of immune system cells.15
Although bone resorption is a physiological process, which aims to increase serum calcium, the pathological resorption leads to bone fractures.16 In this way, BPs are largely used for the treatment of osteoporosis and other chronic bone diseases, such as Paget’s diseases of bone and bone metastases.17
Some reviews have been published in the literature on adjunctive effect of BPs in periodontal treatment, demonstrating beneficial effects of this drug in periodontal tissues.12,18,19 However, there are still unanswered questions about the efficacy of BPs on periodontal parameters, especially in diabetic and smoker individuals. Additionally, in recent years, several clinical trials have been published on this topic. It is also important to know the pooled effect BP when administered systemically and locally delivered, which is not provided by the previously published systematic reviews.
Therefore, this study aimed to systematically review the literature about the adjunct effect of BPs to nonsurgical mechanical periodontal therapy on clinical periodontal parameters compared to mechanical periodontal therapy alone or associated with placebo. This study presented the following null hypothesis: no additional significant improvement in clinical attachment level (CAL) (primary outcome), probing pocket depth (PPD), bleeding on probing (BOP), and radiographic assessment (RADIO) (secondary outcomes) would be detected in individuals with periodontitis that received BP and SRP in comparison to SRP alone or associated with placebo.
2. Methods
2.1. Focused question
The present study followed the PRISMA guideline for systematic review.20 This study had the following focused question: “In adults patients with periodontitis, does the adjuvant use of BP in nonsurgical mechanical periodontal therapy promote additional improvements in periodontal clinical parameters, such as and clinical attachment level, probing pocket depth and radiographic assessment when compared to nonsurgical mechanical periodontal therapy alone or associated with placebo?”
The PICO question comprised patients with periodontitis (any grade and any stage) (P), nonsurgical mechanical periodontal treatment with adjuvant use of BP (I), compared to nonsurgical mechanical periodontal treatment alone or in association with placebo (C), and CAL, PPD, BOP and RADIO alterations (O).
2.2. Search strategy
Five databases, MEDLINE-Pubmed, Web of Science, Scopus, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL), were searched to detect potentially relevant randomized clinical trial, involving adults with periodontitis. The literature search was performed up to November 22nd, 2020. In MEDLINE-Pubmed, the search strategy is described below:
#1 - periodontal disease[Title/Abstract] OR periodontal diseases[MeSH Terms] OR periodontal treatment[Title/Abstract] OR periodontal therapy[Title/Abstract] OR subgingival curettage[MeSH Terms] OR periodontal intervention[Title/Abstract] OR periodontium[MeSH Terms] OR periodontics[MeSH Terms] OR wound healing[MeSH Terms] OR periodontal repair[Title/Abstract] OR periodontal regeneration[Title/Abstract] OR periodontitis[Title/Abstract].
#2 – Diphosphonates[MeSH Terms] OR Diphosphonates[Title/abstract] OR bisphosphonate[Title/abstract] OR alendronate[Title/abstract] OR neridronate[Title/abstract] OR Pamidronate[Title/abstract] OR Olpadronate[Title/abstract] OR Ibandronate[Title/abstract] OR Risedronate[Title/abstract] OR Zoledronate[Title/abstract].
#3 - #1 and #2.
The abovementioned search strategy was adjusted and used in all other databases. It was also performed a hand search in the following journals: Journal of Periodontal Research, Journal of Clinical Periodontology and Journal of Periodontology. The reference of every selected study and related reviews were also searched for eligibility.12,18,19 Clinical trials database (clinicaltrials.gov) was searched for grey literature using an adaptation of the abovementioned search strategy.
2.3. Selection criteria and risk of bias assessment
Title, abstract and the full-text reading were individually screened for eligibility by two researchers (FWMGM and BFS). For the screening of title/abstract, the kappa index between researchers was 0.96. Meanwhile, the kappa index for full-text reading was 0.94. To both phases, any discrepancies was solved by extensive discussion between the researchers. A third researcher was required only when a consensus was not possible (TMM).
In order to be included, the studies needed to fulfill all the following criteria:
-
•
Randomized clinical trials, involving adults of at least 18 years old.
-
•
Individuals with a diagnosis of periodontitis.
-
•
The test group was composed by individuals receiving nonsurgical periodontal therapy and adjuvant administration of any BP in any administration route.
-
•
The control group was composed by individuals receiving nonsurgical periodontal therapy alone or in association with a placebo.
-
•
Studies with a minimum follow-up of 3-months.
-
•
The study needed to present at least two assessments (at baseline and last follow-up) of the following periodontal parameters: CAL, PPD or BOP. Studies that performed any oral radiographic analyses were also included.
No restriction about the systemic status of the included individuals was imposed. In contrast, it was excluded reviews, letters to the editor, case reports, observational studies, in vitro studies, and animal model studies. If more than one adjuvant therapy was applied in the test group, the study was excluded. No restriction to language or date of publication were imposed.
The risk of bias assessment was performed using the tool developed by the Cochrane collaboration.21 The seven criteria of this tool were assessed independently by two researchers (FWMGM and TMM). Low risk of bias was attributed when the study provided sufficient information. High risk of bias was indicated when the study did not perform the assessed criteria. When both low or high risk of bias were not possible to be assessed, we attributed an unclear risk of bias.
2.4. Data extraction
Two researchers (FWMGM and BFS) performed data extraction independently in a spreadsheet in Excel developed for this study. In this process, a third researcher (TMM) was involved if any discrepancy was detected. The spreadsheet contained the following variables: authors, year of publication, country, study design, follow-up, number of individuals in each experimental group, the BP used in the test group, dosage, administration route, periodontal diagnosis and treatment protocol, systemic condition, smoking exposure, mean age, number of man and women in each group, how the radiographic analyses was performed, and the evaluation of the periodontal parameters in each experimental period.
In case of any missing data, corresponding authors of the included studies were contacted by e-mail to provide further information. None of the contacted authors replied our request.
2.5. Statistical analysis
We performed separate meta-analyses for locally delivered and systemic administered BPs. It was calculated the mean difference (MD) between baseline and 6-months after therapy for PPD and CAL parameters, for both types of administration. In the locally delivered meta-analyses, subgroup analyses, considering the systemically health individuals, those with diabetes and smoking exposure, were also performed.
For the RADIO assessment in the studies the used systemic BPs, it was also calculated the MD between baseline and 6-months after therapy. Moreover, in the studies that used locally delivered BPs, different RADIO assessment was performed. Therefore, the standard mean difference (SMD) was calculated. In addition, to all meta-analyses performed (CAL, PPD, and RADIO) using locally delivered BPs, publication bias was assessed by funnel plot analysis and the Egger’s test. Publication bias analysis was performed in RStudio (version 1.3).
The RevMan 5.3 software was used to performed all meta-analyses, using a random effects model. The heterogeneity was assessed by the Q test and quantified with I2 statistics. Overall quality of the evidence was applied using the GRADE approach.22 This analysis was performed for each outcome included in the meta-analyses.
3. Results
3.1. Studies selection
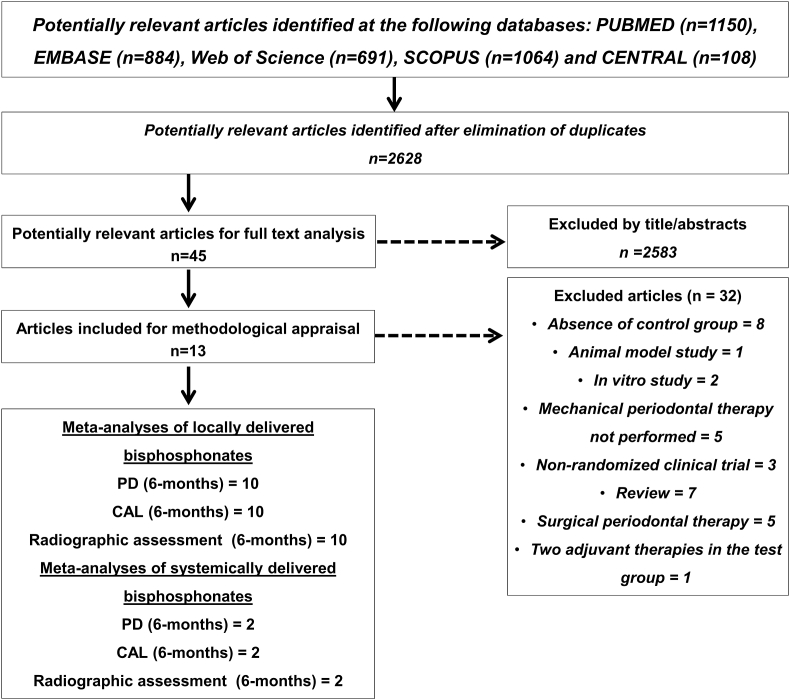
Fig. 1 shows the flowchart of the studies inclusion during the review. Among the 2628 studies initially screened, 13 were included.11,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 The main reasons for exclusion are reported in Fig. 1. For a better understanding and comparison between the selected studies, the main characteristics and results of the included studies are demonstrated in Table S1.
Fig. 1.
Flowchart of the studies during the review.
3.2. Characteristics of included studies
All selected studies were published between 2001 and 2019. The samples were separated between a test group, which received SRP and adjuvant use of BP, and a control group, which did not receive BP treatment. The sample size ranged from 9 to 25 and from 8 to 34 individuals, for test and control groups, respectively. All studies included individuals with at least 18 years old, and 11 studies included both male and female. One study included only postmenopausal women30 and another only male participants.33 Most of the studies included systemically healthy and non-smokers individuals with periodontitis, but two studies evaluated patients with type 2 diabetes.26,29 Three studies evaluated the effects of adjuvant BP in smokers.11,33,34
All participants included in the studies were treated with nonsurgical mechanical periodontal therapy. According to BP treatment, three studies used systemic administration,11,29,30 while others studies used BP locally delivered23, 24, 25, 26, 27, 28,31, 32, 33, 34 Among the studies using systemic BP, one of them used intramuscular application of neridronate 12.5mg/2 ml (once a week during 12 weeks)11 and two studies used oral administration of alendronate 10 mg/day (once a day during 6 months).29,30 Alendronate gel (1%) was used in all studies with local application of BP, except for one study that used local application of zolendronate gel 0.05% (20 μL).24 All the included studies that used BP locally delivered, applied the gel only once immediately after SRP, except for one study that administered the gel 4 weeks after SRP.24
3.3. Risk of bias assessment
None study fulfilled all criteria with low risk of bias (Fig. 2). No studies provided explanation of how allocation concealment was performed. The majority of the studies had low risk of bias for random sequence generation.24, 25, 26, 27, 28,31, 32, 33, 34 Only one study presented high risk of bias,29 and one RCT had unclear risk of bias for blinding of outcome assessment.34 Additionally, only two studies had high risk of bias for incomplete outcome data.24,34
Fig. 2.
Risk of bias of the randomized clinical trials included studies.
3.4. Qualitative results – bisphosphonates used systemically
Regarding the comparison within groups, all the included studies presented significant improvements in all groups after therapy in terms of periodontal parameters. In the follow-up visits (3 and 6 months after baseline), two studies demonstrated significant decrease in PPD and intrabony defect (IBD) in the groups in which BP was used as adjuvant (p < 0.05).29,30 Meanwhile, CAL gain favoring the group that used oral alendronate was demonstrated in only one study.29 This study included only patients with type 2 diabetes.
The study that administered nidronate intramuscularly demonstrated no statistically significant differences between groups regarding all the evaluated periodontal parameters.11 Subgroup analyses, considering initial moderate and deep probing depth, showed the same trend of results.
3.5. Qualitative results – bisphosphonates used locally
In those studies, the maximum follow-up period ranged from 6 months23,24,26,27,31, 32, 33, 34 to 12 months.25 Regarding the studies that used alendronate gel 1% as adjuvant therapy, all of them showed significant improvements in the periodontal parameters 3–12 months after therapy, which include reduction of PPD and BOP, CAL gain, and decrease in IBD.
Among those studies, a greater reduction of PPD, favoring the group that used alendronate, was demonstrated in seven studies.25, 26, 27, 28,31, 32, 33 Meanwhile, two studies did not demonstrate significant differences between groups for the reduction of PPD.23,34 All studies showed greater gain of CAL in the groups that used alendronate, except for one study.34
Different radiographic analyses were performed among the included studies. Three studies measured the distance between cementoenamel junction to the base of the bone defect.23,24,34 The distance from the alveolar crest to the base of the bone defect was assessed in five studies.26,28,31, 32, 33 Moreover, the distance of the furcation fornix and the base of the bone defect was measured in two studies.25,27 All studies demonstrated greater resolution of the IBD or bone fill in the groups that use alendronate, except for one study,23 which demonstrated similar bone fill between groups. The study that administrated zolendronate gel 0.05%24 found significant improvements only in the group that used the BP.
3.6. Meta-analyses for alterations in clinical attachment level
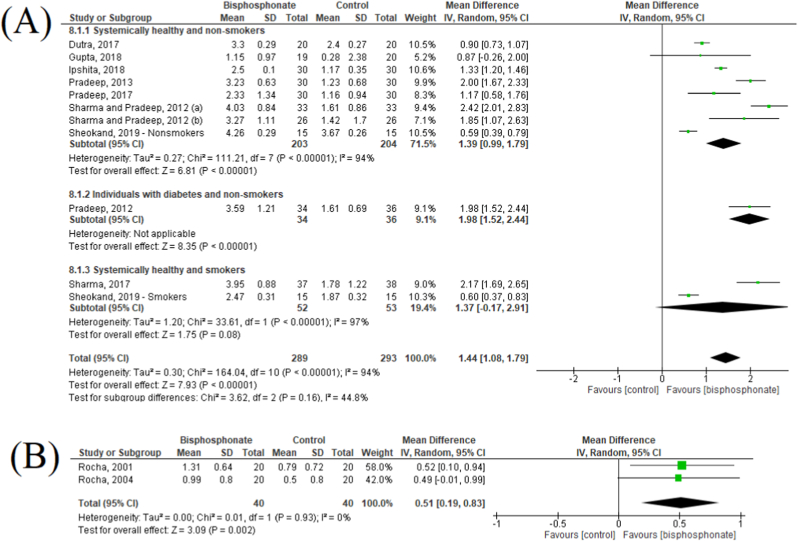
Fig. 3A presented the meta-analysis for CAL alteration between baseline and 6-months after therapy. Ten studies were included in this analysis.23, 24, 25, 26, 27, 28,31, 32, 33, 34 Overall, it was showed a pooled MD of 1.44 mm (95%CI: 1.08–1.79), favoring the groups that used locally delivered BPs. In the subgroup analyses, similar results were found for systemically healthy non-smokers or with diabetes (MD: 1.39; 95%CI: 0.99–1.79 and MD: 1.98; 95%CI: 1.52–2.44, respectively). In contrast, the studies that included only smokers showed no statistically significant difference between groups (MD: 1.37; 95%CI: −0.17 – 2.91). Regarding publication bias analysis, despite the high asymmetry detected in the funnel plot, Egger’s test shows a p = 0.22 (Fig. 4A).
Fig. 3.
Forest plot of clinical attachment level gain after 6-months of follow-up in the locally (A) and systemically delivered bisphosphonates (B).
Fig. 4.
Funnel plot of the publication of bias of clinical attachment level gain (A), probing pocket depth reduction (B) and radiographic assessment (C).
The meta-analysis for CAL alteration in the studies that used systemic BPs is showed in Fig. 3B. Only two studies were included in this analysis.29,30 It was showed a pooled MD of 0.51 mm (95%CI: 0.19–0.83), favoring the test group. This analysis showed no heterogeneity (I2: 0%, P = 0.93).
Meta-analyses for alterations in probing pocket depth.
Fig. 5A shows the alteration of PPD between baseline and 6-months in the studies that used locally delivered BPs. Ten studies were included in this analysis.23, 24, 25, 26, 27, 28,31, 32, 33, 34 A statistically significant greater PPD reduction was detected in the test group (MD: 1.52; 95%CI: 0.97–2.07). In the subgroup analyses, a similar trend of results was detected for non-smokers, either systemically healthy or with diabetes. In contrast, no significant difference between groups was detected for smokers (MD: 1.35–95%CI: −0.13 – 2.83). The funnel plot for this meta-analysis is provided in Fig. 4B, and it shows a statistically significant publication bias (Egger’s test, p = 0.01).
Fig. 5.
Forest plot of probing pocket depth reduction after 6-months of follow-up in the locally (A) and systemically delivered bisphosphonates (B).
Two studies were included in the analysis of PPD alteration in the studies that used systemic BPs.29,30 This analysis showed a pooled MD of 0.40 mm (95%CI: 0.21–0.60), favoring the test groups, was demonstrated (Fig. 5B). No heterogeneity was also detected (I2: 0%; P = 0.94).
Meta-analyses for alterations in the radiographic analyses.
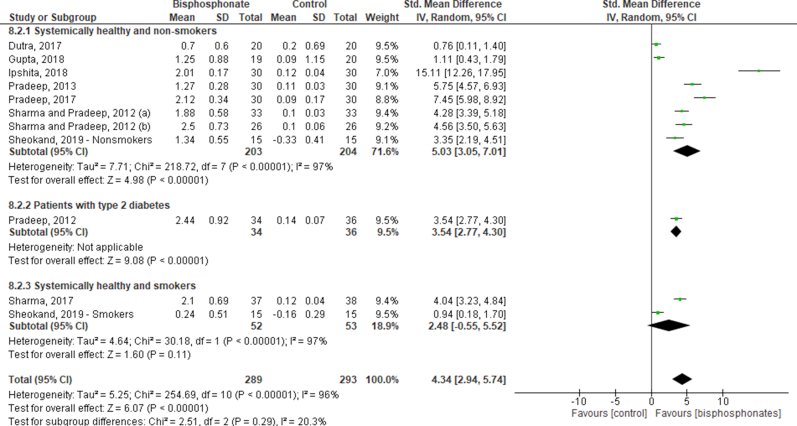
Fig. 6A shows the meta-analysis for RADIO in the locally delivered BPs. Ten studies were also included in this analysis.23, 24, 25, 26, 27, 28,31, 32, 33, 34 The overall analysis showed a significant improvement in the RADIO assessment, favoring the test group (SMD: 4.34; 95%CI: 2.94–5.74). Different radiographic assessments were performed in the studies: the distance between the cementoenamel junction to the base of the defect, distance from the alveolar crest to the base of the defect (intrabony defect – IBD), distance from the furcation fornix to the base of the defect. These different analyses composed the subgroups, and the same trend of results was reported in all subgroups analyses. In addition, publication bias analysis is provided in Fig. 4C. This analysis also showed publication bias (Egger’s test, p < 0.001).
Fig. 6.
Forest plot of radiographic assessment resolution after 6-months of follow-up, considering how the radiographies were measured in the locally (A) and the systemically delivered bisphosphonates (B).
The meta-analysis for RADIO assessment in the studies that used systemic BPs is reported in Fig. 6B, which included two studies.29,30 Both studies evaluated the IBD, and showed a pooled MD of 1.05 mm (95%CI: 0.80–1.31), favoring the BP group.
3.7. Quality of evidence at the review level
The GRADE for both primary and secondary outcomes performed in the meta-analyses is presented in Table 1. To all outcomes assessed, the quality of evidence was rated as very low.
Table 1.
Summary of the quality assessment to all outcomes included in the meta-analyses.
| Certainty assessment |
Summary of findings |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° of patients |
||||||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Comparison | Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Clinical attachment gain (locally delivered bisphosphonates) | ||||||||||||
| 10 | Randomized trials | Very seriousa | Very seriousb | Not serious | Very seriousc | None | 289 | 293 | – | MD 1.44 higher (1.08 higher to 1.79 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
| Clinical attachment gain (systemically delivered bisphosphonates) | ||||||||||||
| 2 | Randomized trials | Very seriousa | Not serious | Not serious | Very seriousc | None | 40 | 40 | - | MD 0.51 higher (0.19 higher to 0.83 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
| Probing depth reduction (locally delivered bisphosphonates) | ||||||||||||
| 10 | Randomized trials | Very seriousa | Very seriousb | Not serious | Very seriousc | None | 289 | 293 | - | MD 1.52 higher (0.97 higher to 2.07 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
| Probing depth reduction (systemically delivered bisphosphonates) | ||||||||||||
| 2 | Randomized trials | Very seriousa | Not serious | Not serious | Very seriousc | None | 40 | 40 | - | MD 0.40 higher (0.21 higher to 0.60 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
| Radiographic analysis (locally delivered bisphosphonates) | ||||||||||||
| 10 | Randomized trials | Very seriousa | Very seriousb | Not serious | Very seriousc | None | 289 | 293 | - | SMD 4.34 higher (2.93 higher to 5.74 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
| Radiographic analysis (systemically delivered bisphosphonates) | ||||||||||||
| 2 | Randomized trials | Very seriousa | Not serious | Not serious | Very seriousc | None | 40 | 40 | - | MD 1.05 higher (0.80 higher to 1.31 higher) |
⊕◯◯◯VERY LOW |
CRITICAL |
Legend: CI: Confidence interval; MD: Mean difference; SMD: Standard mean difference.
Explanations: a. At least one study presented a high risk of bias in at least one criteria. b. A high heterogeneity was detected. c. There is a high variability in the results found.
4. Discussion
The present study aimed to systematically review the literature about the adjuvant effect of BPs in the nonsurgical treatment of periodontitis. Overall, it was showed that both locally delivered and systemically BPs may present greater PPD reduction and CAL gain. However, additional benefit may not be observed for smokers regarding PPD and CAL. Moreover, significant improvements in the radiographic analysis were observed favoring the groups that used BP.
BPs were synthesized for the first time in 1800 but were only used in medicine after the 1960s. Initially, they were used for industrial matters, mainly as anticorrosive and anti-fouling agents.35 They are presented in two forms, with nitrogen in their composition, such as alendronate, ibandronate, pamidronate, risedronate and zolendronate, or without nitrogen, such as etidronate and tiludronate.36 When comparing their forms, those that contain nitrogen in their formula have a greater affinity for bone or circulating calcium molecules.36 It is observed that those without nitrogen have fewer adverse effects when compared to the nitrogen-containing BPs, which can cause gastrointestinal disorders, ocular lesions and mandibular and maxillaries osteonecrosis.15,37
The mechanism of action of this drug happens in three correlated levels (tissue, cellular and molecular). At the tissue level, the action is characterized by a reduction of bone turnover due to the decrease in osteoclastic quantity and activity. At the cellular level, there is inhibition in cell recruitment, adhesion, and activity, including higher apoptosis. Finally, at the molecular level, it is believed that the drug interferes in cell transduction, which is the communication between the cells. However, this last mechanism is not fully understood.38 As a result, BP promotes a reduced rate of bone removal.39
Regarding the pharmacological routes, they may be administrated orally or intravenously. Studies that evaluated the consequences of systemic administration of BPs demonstrated that this drug is effective to prevent alveolar bone loss during experimental periodontitis.15,40 Similar results were also observed in humans, as the present study demonstrated the oral administration of alendronate promote significantly greater PPD reduction and CAL gain. Despite of that, it must be highlighted that higher adverse events were reported in these groups, which may limit their clinical applicability in the clinical setting.
One important side effect of the BP administration is the osteonecrosis of the jaws (ONJ). The previous or current use of BP or other antiangiogenics/antiresorptive drugs are important for the proper diagnosis,41 but the pathogenesis of ONJ is not fully understood. The literature hypothesized that relatively high vascularity, bone turnover and remodeling, due to continuous mechanical stress, make the jaws more vulnerable to necrosis.41 Despite this knowledge, none of the included studies reported ONJ as an adverse event after 3–12 months of follow-up. It must be highlighted that none of the included studies clearly stated ONJ occurrence as an outcome.
In order to avoid the abovementioned side effects, the literature has used locally delivered drugs.42 In the present study, no study showed side effects when BP was locally delivered. The results favoring the BP groups may be explained by their anti-inflammatory action.43 According to the literature, BPs led to a significant decrease in inflammation and serum level of bone metabolism markers, with consequent improvement in periodontal clinical parameters. A reduction in the inflammatory infiltrate, along with fewer neutrophil recruitment, myeloperoxidase activity, inflammatory mediators, matrix metalloproteinases and collagenase, gelatinase and elastase may explain the anti-inflammatory effect. Clinically, the anti-inflammatory effect of BPs was marked by a reduction in gingival bleeding rates.44
When radiographic analyses were considered, groups that used BPs showed greater resolution of the bone defects. These results may be explained by the capacity of BPs to inhibit bone resorption. Consequently, these compounds began to be largely used in several diseases, such as hypercalcemia of malignancy, postmenopausal osteoporosis, corticosteroid-induced osteoporosis and pain associated with bone metastasis.45,46 BPs are able to inhibit osteoclast differentiation,47 reduce bone resorption48 and induce apoptosis of osteoclasts, suggesting that bone cells are affected directly by these drugs.49
Additionally, literature shows that some BPs, such as disodium clodronate, etidronate, and tiludronate, present anti-inflammatory activity as they were capable to inhibit the release of proinflammatory cytokines (interleukin-1, interleukin-6, and tumor necrosis factor) and nitric oxide from macrophages.50, 51, 52 The greater resolution of bone defects demonstrated by BPs may be explained by the affinity of BP for binding to the hydroxyapatite crystals of bone, promoting differentiation of osteoblast. The results of the present study are in accordance with previous findings that demonstrated that BP (alendronate) was effective in reducing alveolar bone loss in an animal model.53, 54, 55
In the present study, only one RCT had high risk of bias29 and another had an unclear risk of bias for blinding of outcome assessment.34 Additionally, only two studies had high risk of bias for incomplete outcome data.24,34 In general, the articles analyzed in the present study demonstrated a low risk of bias, which may allow a higher internal validity of the findings.
In the subgroup analyses performed, in the present study, the adjuvant use of locally-delivered BP showed no significant difference for PPD reduction and CAL gain, when compared to the use of placebo, among smokers. The existing literature reports smoking as a major risk factor for periodontitis, increasing its prevalence, extent and severity.56,57 Furthermore, smokers are also associated with poorer response to periodontal treatment.58 Mineral contents of bone tissue may also be interfered by smoking exposure, reducing an accelerated periodontal bone height reduction57,59 and higher bone fractures in elderly women.60 Moreover, lower serum bicarbonate levels were detected in smokers, which may explain the present results.61 It must be highlighted that higher occurrence of ONJ may be expected among smokers.62 Moreover, when radiographic analyses were performed accordingly to systemic conditions and smoking exposure, as observed for the other periodontal parameters, BPs promote significant improvements in non-smokers, whether they are systemically healthy or with diabetes. The same trend of results was not detected for smokers (Figure S1). Based on the present results, adjuvant administration of BPs may not be indicated for smokers with periodontitis.
Two previous systematic reviews have been published about the adjuvant effect of BPs in the periodontal treatment.12,19 Further studies were published in this field, which indicated the necessity to update the mentioned systematic reviews. Both systematic reviews showed significant improvements in the periodontal parameters. However, in their quantitative analyses, all administration routes of BPs were gathered in the same analyses. The literature shows that different patterns of periodontal response may be expected when drugs are administrated locally or by other administration routes.63,64 In this sense, separate analysis may be performed for the different administration routes. Additionally, the mentioned studies failed to analyze the effect of BP in individuals with type 2 diabetes and smokers. All those characteristics were considered when performed the quantitative analyses of the present study.
Conversely, the present systematic review shows some limitations. High heterogeneities were detected in all meta-analyses performed for the locally-delivered BPs, which may limits the external validity of the data presented. Low heterogeneity was detected in the BPs administrated orally, but only two studies were included in these analyses. Additionally, the maximum follow-up detected was only 12-months. Therefore, further randomized clinical trials, with longer follow-up periods, involving non-smokers and the adjuvant use of locally-delivered BPs may be necessary. In these studies, the inclusion of ONJ occurrence must be included as an outcome.
5. Conclusion
It was concluded that administration of BP promotes significant improvements in the periodontal parameters. When considering the different administration routes, locally-delivered BP may be preferable due to its lower incidence of side effects. No significant improvements are expected in smokers after adjuvant use of BP in the periodontal therapy regarding PPD reduction and CAL gain. Studies with longer follow-up periods are necessary in order to increase the clinical applicability of BPs in the periodontal treatment.
Declaration of competing interest
The authors report no conflict of interest related to this study. This study was self-funded.
Acknowledgements
The authors report no conflict of interest related to this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. All other funding was self-supported by the authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2021.01.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Forest plot of radiographic assessment resolution after 6-months of follow-up, considering the diabetes and smoking exposures in the locally delivered bisphosphonates.
References
- 1.Armitage G.C., Robertson P.B. The biology, prevention, diagnosis and treatment of periodontal diseases: scientific advances in the United States. J Am Dent Assoc. 2009;140(Suppl 1):36S–43S. doi: 10.14219/jada.archive.2009.0356. [DOI] [PubMed] [Google Scholar]
- 2.Kaldahl W.B., Kalkwarf K.L., Patil K.D. A review of longitudinal studies that compared periodontal therapies. J Periodontol. 1993;64:243–253. doi: 10.1902/jop.1993.64.4.243. [DOI] [PubMed] [Google Scholar]
- 3.Cobb C.M. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 4.Javed F., Al-Rasheed A., Almas K., Romanos G.E., Al-Hezaimi K. Effect of cigarette smoking on the clinical outcomes of periodontal surgical procedures. Am J Med Sci. 2012;343:78–84. doi: 10.1097/MAJ.0b013e318228283b. [DOI] [PubMed] [Google Scholar]
- 5.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 6.Garcia V.G., Fernandes L.A., de Almeida J.M. Comparison between laser therapy and non-surgical therapy for periodontitis in rats treated with dexamethasone. Laser Med Sci. 2010;25:197–206. doi: 10.1007/s10103-009-0678-z. [DOI] [PubMed] [Google Scholar]
- 7.Assem N.Z., Alves M.L.F., Lopes A.B., Gualberto EC Junior, Garcia V.G., Theodoro L.H. Antibiotic therapy as an adjunct to scaling and root planing in smokers: a systematic review and meta-analysis. Braz Oral Res. 2017;31:e67. doi: 10.1590/1807-3107BOR-2017.vol31.0067. [DOI] [PubMed] [Google Scholar]
- 8.Szulc M., Zakrzewska A., Zborowski J. Local drug delivery in periodontitis treatment: a review of contemporary literature. Dent Med Probl. 2018;55:333–342. doi: 10.17219/dmp/94890. [DOI] [PubMed] [Google Scholar]
- 9.Muniz F.W.M.G., Taminski K., Cavagni J., Celeste R.K., Weidlich P., Rösing C.K. The effect of statins on periodontal treatment-a systematic review with meta-analyses and meta-regression. Clin Oral Invest. 2018;22:671–687. doi: 10.1007/s00784-018-2354-9. [DOI] [PubMed] [Google Scholar]
- 10.Russell R.G., Watts N.B., Ebetino F.H., Rogers M.J. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 11.Graziani F., Cei S., Guerrero A. Lack of short-term adjunctive effect of systemic neridronate in non-surgical periodontal therapy of advanced generalized chronic periodontitis: an open label-randomized clinical trial. J Clin Periodontol. 2009;36:419–427. doi: 10.1111/j.1600-051X.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 12.Akram Z., Abduljabbar T., Kellesarian S.V., Hassan M.I.A., Javed F., Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br J Clin Pharmacol. 2017;83:444–454. doi: 10.1111/bcp.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen M.R. The effects of bisphosphonates on jaw bone remodeling, tissue properties, and extraction healing. Odontology. 2011;99:8–17. doi: 10.1007/s10266-010-0153-0. [DOI] [PubMed] [Google Scholar]
- 14.Tower R.J., Campbell G.M., Müller M., Will O., Glüer C.C., Tiwari S. Binding kinetics of a fluorescently labeled bisphosphonate as a tool for dynamic monitoring of bone mineral deposition in vivo. J Bone Miner Res. 2014;29:1993–2003. doi: 10.1002/jbmr.2224. [DOI] [PubMed] [Google Scholar]
- 15.Furlaneto F.A., Nunes N.L., Oliveira Filho I.L. Effects of locally administered tiludronic acid on experimental periodontitis in rats. J Periodontol. 2014;85:1291–1301. doi: 10.1902/jop.2014.130581. [DOI] [PubMed] [Google Scholar]
- 16.Coughlan T., Dockery F. Osteoporosis and fracture risk in older people. Clin Med. 2014;14:187–191. doi: 10.7861/clinmedicine.14-2-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaz I., Zegarra P., González-Enríquez J. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21:1943–1951. doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 18.Badran Z., Kraehenmann M.A., Guicheux J., Soueidan A. Bisphosphonates in periodontal treatment: a review. Oral Health Prev Dent. 2009;7:3–12. [PubMed] [Google Scholar]
- 19.Chen J., Chen Q., Hu B., Wang Y., Song J. Effectiveness of alendronate as an adjunct to scaling and root planing in the treatment of periodontitis: a meta-analysis of randomized controlled clinical trials. J Periodontal Implant Sci. 2016;46:382–395. doi: 10.5051/jpis.2016.46.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Dutra B.C., Oliveira A.M.S.D., Oliveira P.A.D. Effect of 1% sodium alendronate in the non-surgical treatment of periodontal intraosseous defects: a 6-month clinical trial. J Appl Oral Sci. 2017;25:310–317. doi: 10.1590/1678-7757-2016-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A., Govila V., Pant V.A. A randomized controlled clinical trial evaluating the efficacy of zoledronate gel as a local drug delivery system in the treatment of chronic periodontitis: a clinical and radiological correlation. Natl J Maxillofac Surg. 2018;9:22–32. doi: 10.4103/njms.NJMS_12_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ipshita S., Kurian I.G., Dileep P., Kumar S., Singh P., Pradeep A.R. One percent alendronate and aloe vera gel local host modulating agents in chronic periodontitis patients with class II furcation defects: a randomized, controlled clinical trial. J Investig Clin Dent. 2018;9:e12334. doi: 10.1111/jicd.12334. [DOI] [PubMed] [Google Scholar]
- 26.Pradeep A.R., Sharma A., Rao N.S., Bajaj P., Naik S.B., Kumari M. Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double-masked controlled clinical trial. J Periodontol. 2012;83:1322–1328. doi: 10.1902/jop.2012.110292. [DOI] [PubMed] [Google Scholar]
- 27.Pradeep A.R., Kumari M., Rao N.S., Naik S.B. 1% alendronate gel as local drug delivery in the treatment of Class II furcation defects: a randomized controlled clinical trial. J Periodontol. 2013;84:307–315. doi: 10.1902/jop.2012.110729. [DOI] [PubMed] [Google Scholar]
- 28.Pradeep A.R., Kanoriya D., Singhal S., Garg V., Manohar B., Chatterjee A. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent. 2017;8(3) doi: 10.1111/jicd.12215. [DOI] [PubMed] [Google Scholar]
- 29.Rocha M., Nava L.E., Vázquez de la Torre C., Sánchez-Márin F., Garay-Sevilla M.E., Malacara J.M. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo-controlled trial. J Periodontol. 2001;72:204–209. doi: 10.1902/jop.2001.72.2.204. [DOI] [PubMed] [Google Scholar]
- 30.Rocha M.L., Malacara J.M., Sánchez-Marin F.J., Vazquez de la Torre C.J., Fajardo M.E. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol. 2004;75:1579–1585. doi: 10.1902/jop.2004.75.12.1579. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A., Pradeep A.R. Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial. J Periodontol. 2012;83:11–18. doi: 10.1902/jop.2011.110091. [DOI] [PubMed] [Google Scholar]
- 32.Sharma A., Pradeep A.R. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83:19–26. doi: 10.1902/jop.2011.110206. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A., Raman A., Pradeep A.R. Role of 1% alendronate gel as adjunct to mechanical therapy in the treatment of chronic periodontitis among smokers. J Appl Oral Sci. 2017;25:243–249. doi: 10.1590/1678-7757-2016-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheokand V., Chadha V., Palwankar P. The comparative evaluation of 1% alendronate gel as local drug delivery system in chronic periodontitis in smokers and non smokers: randomized clinical trial. J Oral Biol Craniofac Res. 2019;9:198–203. doi: 10.1016/j.jobcr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell R.G. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- 36.Polymeri A.A., Kodovazenitis G.J., Polymeris A.D., Komboli M. Bisphosphonates: clinical applications and adverse events in dentistry. Oral Health Prev Dent. 2015;13:289–299. doi: 10.3290/j.ohpd.a34370. [DOI] [PubMed] [Google Scholar]
- 37.Kolur T., Nair S.C., Kumar B. Osteonecrosis of maxilla secondary to bisphosphonate therapy: a case report. J Maxillofac Oral Surg. 2015;14(Suppl 1):52–56. doi: 10.1007/s12663-011-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green J., Clézardin P. The molecular basis of bisphosphonate activity: a preclinical perspective. Semin Oncol. 2010;37(Suppl 1):S3–S11. doi: 10.1053/j.seminoncol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Hunter S.A., Orheim R., Sazon M., Newman H., Woll J.E., Bergevin M. Demineralization removes residual alendronate in allograft bone procured from donors with a history of bisphosphonate use. J Periodontol. 2011;82:281–286. doi: 10.1902/jop.2010.100236. [DOI] [PubMed] [Google Scholar]
- 40.Bargman R., Posham R., Boskey A.L., DiCarlo E., Raggio C., Pleshko N. Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta. Osteoporos Int. 2012;23:1141–1150. doi: 10.1007/s00198-011-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruggiero S.L., Dodson T.B., Fantasia J. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Da Rocha H.A., Silva C.F., Santiago F.L., Martins L.G., Dias P.C., Magalhães D. Local drug delivery systems in the treatment of periodontitis: a literature review. J Int Acad Periodontol. 2015;17:82–90. [PubMed] [Google Scholar]
- 43.Shinoda H., Takeyama S., Suzuki K., Murakami S., Yamada S. Pharmacological topics of bone metabolism: a novel bisphosphonate for the treatment of periodontitis. J Pharmacol Sci. 2008;106:555–558. doi: 10.1254/jphs.fm0070272. [DOI] [PubMed] [Google Scholar]
- 44.Palomo L., Bissada N.F., Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause. 2005;12:685–690. doi: 10.1097/01.gme.0000184421.50696.34. [DOI] [PubMed] [Google Scholar]
- 45.Weinreb M., Quartuccio H., Seedor J.G. Histomorphometrical analysis of the effects of the bisphosphonate alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res. 1994;29:35–40. doi: 10.1111/j.1600-0765.1994.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 46.Landesberg R., Eisig S., Fennoy I., Siris E. Alternative indications for bisphosphonate therapy. J Oral Maxillofac Surg. 2009;67(5 SUPPL):27–34. doi: 10.1016/j.joms.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Löwik C.W., van der Pluijm G., van der Wee-Pals L.J., van Treslong-De Groot H.B., Bijvoet O.L. Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. J Bone Miner Res. 1988;3:185–192. doi: 10.1002/jbmr.5650030210. [DOI] [PubMed] [Google Scholar]
- 48.Sahni M., Guenther H.L., Fleisch H., Collin P., Martin T.J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993;91:2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito M., Amizuka N., Nakajima T., Ozawa H. Ultrastructural and cytochemical studies on cell death of osteoclasts induced by bisphosphonate treatment. Bone. 1999;25:447–452. doi: 10.1016/s8756-3282(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 50.Giuliani N., Pedrazzoni M., Passeri G., Girasole G. Bisphosphonates inhibit IL-6 production by human osteoblast-like cells. Scand J Rheumatol. 1998;27:38–41. doi: 10.1080/030097498441155. [DOI] [PubMed] [Google Scholar]
- 51.Makkonen N., Salminen A., Rogers M.J. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. Eur J Pharmaceut Sci. 1999;8:109–118. doi: 10.1016/s0928-0987(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 52.Mönkkönen J., Similä J., Rogers M.J. Effects of tiludronate and ibandronate on the secretion of proinflammatory cytokines and nitric oxide from macrophages in vitro. Life Sci. 1998;62:PL95–PL102. doi: 10.1016/s0024-3205(97)01178-8. [DOI] [PubMed] [Google Scholar]
- 53.Binderman I., Adut M., Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J Periodontol. 2000;71:1236–1240. doi: 10.1902/jop.2000.71.8.1236. [DOI] [PubMed] [Google Scholar]
- 54.Kaynak D., Meffert R., Günhan M., Günhan O., Ozkaya O. A histopathological investigation on the effects of the bisphosphonate alendronate on resorptive phase following mucoperiosteal flap surgery in the mandible of rats. J Periodontol. 2000;71:790–796. doi: 10.1902/jop.2000.71.5.790. [DOI] [PubMed] [Google Scholar]
- 55.Yaffe A., Golomb G., Breuer E., Binderman I. The effect of topical delivery of novel bisacylphosphonates in reducing alveolar bone loss in the rat model. J Periodontol. 2000;71:1607–1612. doi: 10.1902/jop.2000.71.10.1607. [DOI] [PubMed] [Google Scholar]
- 56.Susin C., Oppermann R.V., Haugejorden O., Albandar J.M. Periodontal attachment loss attributable to cigarette smoking in an urban Brazilian population. J Clin Periodontol. 2004;31:951–958. doi: 10.1111/j.1600-051x.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 57.Bergström J., Eliasson S., Preber H. Cigarette smoking and periodontal bone loss. J Periodontol. 1991;62:242–246. doi: 10.1902/jop.1991.62.4.242. [DOI] [PubMed] [Google Scholar]
- 58.Johnson G.K., Guthmiller J.M. The impact of cigarette smoking on periodontal disease and treatment. Periodontol. 2000;44:178–194. doi: 10.1111/j.1600-0757.2007.00212.x. 2007. [DOI] [PubMed] [Google Scholar]
- 59.Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. doi: 10.1007/s10266-004-0043-4. [DOI] [PubMed] [Google Scholar]
- 60.Thorin M.H., Wihlborg A., Åkesson K., Gerdhem P. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int. 2016;27:249–255. doi: 10.1007/s00198-015-3290-z. [DOI] [PubMed] [Google Scholar]
- 61.Chen W., Melamed M.L., Abramowitz M.K. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis. 2015;65:240–248. doi: 10.1053/j.ajkd.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nisi M., La Ferla F., Karapetsa D. Risk factors influencing BRONJ staging in patients receiving intravenous bisphosphonates: a multivariate analysis. Int J Oral Maxillofac Surg. 2015;44:586–591. doi: 10.1016/j.ijom.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Chambrone L., Vargas M., Arboleda S. Efficacy of local and systemic antimicrobials in the non-surgical treatment of smokers with chronic periodontitis: a systematic review. J Periodontol. 2016;87:1320–1332. doi: 10.1902/jop.2016.160268. [DOI] [PubMed] [Google Scholar]
- 64.Jepsen K., Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000;71:82–112. doi: 10.1111/prd.12121. 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.