Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid mediator that has been implicated in the pathophysiology of kidney disease. However, few studies have attempted to measure changes in the levels of various LPA species in the kidney after the development of renal disease. The present study measured the renal LPA levels during the development of kidney disease in rat models of hypertension, diabetes, and obstructive nephropathy using liquid chromatography/mass spectrometry/mass spectrometry. LPA levels (sum of 16:0, 18:0, 18:1, 18:2, and 20:4 LPA) were higher in the renal cortex of hypertensive Dahl salt-sensitive (Dahl S) rats fed a high-salt diet than those in normotensive rats fed a low-salt diet (296.6 ± 22.9 vs. 196.3 ± 8.5 nmol/g protein). LPA levels were elevated in the outer medulla of the kidney of streptozotocin-induced type 1 diabetic Dahl S rats compared with control rats (624.6 ± 129.5 vs. 318.8 ± 17.1 nmol/g protein). LPA levels were also higher in the renal cortex of 18-month-old, type 2 diabetic nephropathy (T2DN) rats with more severe renal injury than in 6-month-old T2DN rats (184.9 ± 20.9 vs. 116.9 ± 6.0 nmol/g protein). LPA levels also paralleled the progression of renal fibrosis in the renal cortex of Sprague-Dawley rats after unilateral ureteral obstruction (UUO). Administration of an LPA receptor antagonist, Ki16425, reduced the degree of renal fibrosis in UUO rats. These results suggest that the production of renal LPA increases during the development of renal injury and contributes to renal fibrosis.
SIGNIFICANCE STATEMENT
The present study reveals that the lysophosphatidic acid (LPA) levels increase in the kidney in rat models of hypertension, diabetes, and obstructive nephropathy, and administration of an LPA receptor antagonist attenuates renal fibrosis. Therapeutic approaches that target the formation or actions of renal LPA might be renoprotective and have therapeutic potential.
Introduction
Diabetes and hypertension are the leading causes of chronic kidney disease (CKD). The Global Burden of Disease data base estimates that 276 million people had CKD in 2016 (Xie et al., 2018). Patients with CKD are at high risk for cardiovascular death and are more likely to develop the end-stage renal disease (ESRD) (Tonelli et al., 2006; Gansevoort et al., 2013). Although therapeutic interventions, such as inhibition of the renin-angiotensin system and tight control of blood glucose and blood pressure, slow the progression of renal disease, the prevalence of ESRD continues to rise (Sharma and Sarnak, 2017). Therefore, there is an unmet need for novel and effective therapies to arrest the progression of CKD.
Lysophosphatidic acid (LPA) is a bioactive phospholipid that regulates multiple cell functions, including cell proliferation, migration, differentiation, and survival (Aikawa et al., 2015). LPA consists of a glycerol backbone with a phosphate group at the sn-3 position and a single fatty acyl chain at the sn-1 or sn-2 position. There are various species of LPA that differ in the length and degree of saturation of the fatty acyl chains. Previous studies have demonstrated that various LPA species have different biologic effects (Bandoh et al., 2000; Hayashi et al., 2001). To date, two major pathways of LPA production are known (Aoki et al., 2008; Nakanaga et al., 2010). Extracellular LPA is generated from lysophosphatidylcholine by the phospholipase D activity of autotaxin. Intracellular LPA is generated from phosphatidic acid by phospholipase A1 or phospholipase A2. On the other hand, the main pathway for LPA degradation involves its dephosphorylation by lipid phosphate phosphatases (lipid phosphate phosphatases 1 to 3) (Benesch et al., 2016). The local LPA concentration in tissue is tightly controlled by a balance of production and degradation of LPA through these enzymes. LPA acts through specific G protein–coupled receptors. So far, at least six LPA receptors have been identified (LPA1 to LPA6) (Mutoh et al., 2012).
LPA exerts various biologic effects on different renal cell types, including proximal tubular cells, mesangial cells, and fibroblasts (Inoue et al., 1995, 1997; Fang et al., 2000; Geng et al., 2012). Several investigators have suggested that LPA stimulates mesangial cell proliferation and contraction and contributes to the pathogenesis of glomerular disease (Inoue et al., 1997, 1999). Others have reported that the expression of LPA1 is increased in the kidney of unilateral ureteral obstruction (UUO) mice, and genetic deletion or pharmacological inhibition of the LPA1 decreased renal interstitial fibrosis (Pradère et al., 2007; Swaney et al., 2011). More recently, the dual LPA1/3 antagonist, BMS002, was reported to reduce some of the features of diabetic nephropathy, including albuminuria, glomerulosclerosis, renal fibrosis, and the fall in glomerular filtration rate (GFR) in type 2 diabetic db/db mice (Zhang et al., 2017). These observations suggest the involvement of LPA in the pathophysiology of various forms of renal disease.
LPA is present in various tissues and biologic fluids, including the kidney, blood, and urine (Meleh et al., 2007; Grove et al., 2014). LPA is rapidly released from activated platelets and injured cells after inflammation, thrombosis, and tissue injury. They are proposed to contribute to the inflammatory and proliferative responses to injury (Eichholtz et al., 1993; Dohi et al., 2012). In this regard, it is reported that the concentration of LPA in the effluent from the pelvis of a ligated kidney is higher than that in the urinary bladder of UUO-treated rats (Tsutsumi et al., 2011). More recently, Mirzoyan et al. (2016) reported that urinary LPA concentrations increased after five-sixths nephrectomy in mice when compared with sham control animals. The elevated concentration of LPA was significantly correlated with albuminuria and the degree of renal tubulointerstitial fibrosis (Mirzoyan et al., 2016). A recent clinical study has also found that urinary LPA concentrations are increased in patients with diabetes and were associated with albuminuria (Saulnier-Blache et al., 2017). These findings indicate that renal LPA production is altered during the development of renal injury, and increases in renal LPA may contribute to the pathophysiology of renal disease. However, little information is available regarding changes in the levels of various LPA species in the kidney in different models of renal disease. Therefore, this study examined the renal LPA levels during the development of renal injury in rat models of hypertension, diabetes, and obstructive nephropathy using liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS). In addition, to determine whether renal LPA contributes to renal injury, we examined the effect of an LPA receptor antagonist on renal fibrosis after UUO in Sprague-Dawley (SD) rats.
Materials and Methods
General.
Experiments were performed using male SD, Dahl salt-sensitive (Dahl S), and type 2 diabetic nephropathy (T2DN) rats. The SD rats were purchased from Charles River Laboratories (Wilmington, MA). The Dahl S and T2DN rats were obtained from inbred colonies maintained at the University of Mississippi Medical Center. The animals were housed in the Animal Care Facility at the University of Mississippi Medical Center, which is approved by the American Association for the Accreditation of Laboratory Animal Care. The rats had free access to food and water throughout the study. Unless specifically mentioned, the rats were fed a normal rodent diet containing 1% NaCl (Harlan Teklad 8640; Harlan Laboratories, Madison, WI). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Hypertensive Model.
These experiments were performed on 12-week-old Dahl S rats maintained on a low-salt (LS) diet containing 0.3% NaCl (Harlan Teklad 7034; Harlan Laboratories) from weaning. The rats were divided into two groups. One group was kept on an LS diet, whereas the other was switched to a high-salt (HS) diet containing 8.0% NaCl (Harlan Teklad TD.92012; Harlan Laboratories). Blood pressure was measured using a tail-cuff device (Hatteras Instruments, Cary, NC). Twenty-four-hour urine samples were collected in metabolic cages to measure protein excretion. Urinary protein concentration was determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA). At the end of the experiment, the kidneys were collected for measurement of LPA.
Diabetic Models.
Nine-week-old Dahl S rats maintained on a normal salt diet containing 1% NaCl from weaning were treated with an intravenous injection of streptozotocin (STZ) (50 mg/kg; Sigma-Aldrich, St. Louis, MO) to induce type 1 diabetes, and a long-acting insulin implant was placed subcutaneously (2 U/day; Linshin Canada, ON, Canada) to maintain blood glucose levels around 400 mg/dl. Age-matched Dahl S rats served as nondiabetic controls. Four weeks after induction of diabetes, blood samples were collected from the tail vein for measurement of blood glucose and glycosylated hemoglobin (HbA1c). Urine samples were collected in metabolic cages to measure protein excretion. At the end of the experiment, the kidneys were collected for measurement of the LPA.
Six (6M)- and 18 months (18M)-old T2DN rats were used as a model of type 2 diabetes that develops progressive proteinuria and diabetic nephropathy (Nobrega et al., 2004; Kojima et al., 2013a,b). Blood samples were collected for measurement of plasma glucose and HbA1c levels. Plasma glucose and HbA1c levels were measured using a glucometer (Bayer HealthCare, Mishawaka, IN) and a rapid HbA1c device (Bayer HealthCare, Sunnyvale, CA). Twenty-four-hour urine samples were collected via metabolic cages to study protein excretion, and the kidneys were collected for measurement of LPA.
Obstructive Nephropathy Model.
These experiments were performed on 7-week-old SD rats. The rats were anesthetized with isoflurane and placed on a heated surgical table to maintain body temperature at 37°C. A midline abdominal incision was made, and the left ureter was ligated at two points and cut between the ligatures. The abdominal incision was closed, and the rats were allowed to recover. Four days after ligation, the rats were anesthetized with isoflurane, and the left kidney was collected for measurement of LPA. Age-matched SD rats were studied as a control.
Measurement of Renal LPA Concentrations.
Samples of the renal cortex and outer medulla (approximately 20 mg) were homogenized in 1.2 ml of saline containing a phosphatase inhibitor cocktail (Calbiochem, San Diego, CA). The homogenate was transferred into a glass tube and acidified with acetic acid (Sigma-Aldrich) to pH 4. After the addition of 10 ng of 17:0 LPA (Avanti Polar Lipids, Alabaster, AL) as an internal standard, the samples were extracted with 1-butanol (EM Science, Cherry Hill, NJ). After centrifugation, the organic phase was transferred to a new glass tube. The aqueous phase was then re-extracted with water-saturated 1-butanol. The organic phase was combined with the initial fraction and dried under nitrogen. The extracted samples were analyzed by LC/MS/MS to determine the concentration of the various species of LPA, as described below.
LC/MS/MS Analysis.
LPA standards, 16:0, 18:0, and 18:1 LPA, were purchased from Avanti Polar Lipids, Inc. Other standards, 18:2 and 20:4 LPA, were purchased from Echelon Biosciences Inc. (Salt Lake City, UT). The stock solutions of LPA standards and the internal standard 17:0 LPA were prepared by dissolving the lipids in methanol, and the stock solutions were stored at −20°C. LPA was analyzed using a Dionex Ultimate 3000 LC system (Sunnyvale, CA) coupled to a QTRAP 4000 triple quadrupole-linear ion-trap mass spectrometer with a Turbo V ion-Source interface (AB Sciex, Foster City, CA). The samples were separated using an Atlantis T3 (3 μm), 2.1 × 75–mm analytical column proceeded by an Atlantis T3 (3 μm), 2.1 × 10–mm guard cartridge from Waters Corporation (Milford, MA). Mobile phase (MP)-A contained 58% methanol, 41.8% water, 0.2% formic acid, and 5 mM ammonium formate. MP-B consisted of 99% methanol, 0.8% water, 0.2% formic acid, and 5 mM ammonium formate. The flow rate was set at 0.4 ml/min. The initial mobile phase consisted of 25% MP-B for 1.5 minutes. The gradient was then ramped from 25% to 100% MP-B over 3.3 minutes and held there for 15.7 minutes. The MP was then returned to 25% MP-B for equilibration. The column oven temperature was at 50°C, and the autosampler temperature was maintained at 12°C. The dried extracted samples were reconstituted in 200 μl of the mobile phase, and 10 μl were injected. For MS/MS analysis, a QTRAP 4000 triple quadrupole-linear ion trap mass spectrometer with a Turbo V ion-Source interface (AB Sciex) was operated in electrospray ionization negative ion multiple reaction monitoring (MRM) mode. The mass spectrometry parameters for the LPAs were as follows: Ion Spray Voltage: −4500 V, Source Temperature: 400°C, Collision Gas: HIGH, Curtain Gas: 20 psi, Nebulizer Gas: 40 psi, and Heater Source Gas: 60 psi. For the MRM experiment of LPAs, the (M-1) - adduct ions were followed. Each LPA MRM parameter was optimized using direct infusions of standard LPAs (16:0, 18:0, 18:1, 18:2, and 20:4 LPA) in water-methanol-formic acid (1/1/0.1, v/v/v) solution. The MRM modes followed (Q1 ion/Q3 fragment m/z pairs in parentheses) for each LPA species were: 16:0 (409.2/153), 18:0 (437.4/153), 18:1 (435.3/153), 18:2 (433.2/153), and 20:4 (457.2/153) as well as internal standard 17:0 (423.2/153). Values are expressed as the amount of the LPAs per gram of the tissue protein. The sum of all measured LPA species represents the total LPA tissue concentration.
Assay Validation.
The linearity of the assay for each LPA species was assessed by analyzing the calibration curves. Calibration curves were generated by serial dilution into methanol over the range of 0.001–3 μM of each LPA species and 1 ng of the 17:0 LPA internal standard. The peak area ratio for each standard was calculated by dividing measured value by the peak area of 1 ng of the internal standard. The linearity of representative standard curves is presented in Supplemental Fig. 1. Quality control samples were prepared by dissolving stock solution of LPA standards in saline at final concentrations of 0.4, 1, and 2 μM since a kidney matrix in the absence of LPA was not available. The intra-assay and interassay precisions were determined using the coefficients of variation, and the intra-assay and interassay accuracies were defined as follows: (the measured values)/(the expected values) × 100. Intra-assay precision and accuracy were calculated using each concentration of the quality control samples on the same day. Interassay accuracy and variations were calculated from measurements of the same standards over three separate days. Intra-assay and interassay precisions and accuracies for LPA species were shown in Supplemental Table 1.
Effect of Ki16425 on Renal Injury in Obstructive Nephropathy Rats.
Vehicle (polyethylene glycol 400/corn oil/water, 1/1/0.5, v/v/v) or Ki16425 (100 mg/kg; NCE Biomedical, Wuhan, China) was administered subcutaneously twice daily for seven consecutive days beginning on day 1 of the UUO surgery. Ki16425 is reported to be an LPA1 and LPA3 antagonist (Ohta et al., 2003). Vehicle-treated sham-operated rats served as a control. Seven days after UUO, the rats were anesthetized with isoflurane, and the ligated kidney was collected for histologic analysis. The kidneys were fixed in 10% formalin, and paraffin sections (3 µm) were prepared and stained with Masson’s trichrome stain. Ten randomly chosen cortical and medullary fields (×100 total magnification) were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon Instruments Inc., Melville, NY). The degree of renal fibrosis was assessed by measuring the percentage of blue staining of collagen and fibronectin in the section using the NIS Elements D 3.0 software (Nikon Instruments Inc.).
Immunohistochemistry.
Kidneys were fixed in formalin, and 3-µm-thick paraffin sections were prepared and mounted on slides. The slides were deparaffinized in xylene, rehydrated through a decreasing ethanol gradient, and rinsed in PBS. The slides were pretreated with proteinase K (Dako, Carpinteria, CA) for 10 minutes and exposed to a blocking solution (Dako) for 30 minutes at room temperature. The slides were incubated with a monoclonal CD68 antibody (1:100; AbD Serotec, Raleigh, NC) overnight at 4°C, rinsed in PBS, and then incubated with a secondary antibody conjugated with Alexa Fluor 488 (1:200; Jackson ImmunoResearch, West Grove, PA) for 1 hour. After three rinses in PBS, they were then counterstained with 0.001% Evans Blue (Sigma-Aldrich), rinsed in distilled water, and mounted with fluorescent mounting medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were obtained using a Nikon Eclipse 55i microscope equipped with a 540-nm excitation filter and a 590-nm emission filter and a Nikon DS-Fi1 color camera.
Statistical Analysis.
Data were presented as mean ± S.E.M. The significance of differences in mean values between two groups was determined using an unpaired t test. The significance of the differences in mean values between multiple groups was determined using an ANOVA followed by a Holm-Sidak test for preplanned comparisons using the SigmaPlot 11 software (Systat Software, San Jose, CA).
Results
Blood Pressure and Renal Injury in Dahl S Rats Fed an LS or HS Diet.
A comparison of blood pressure and renal injury in Dahl S rats fed either an LS or HS diet for 4 weeks is presented in Table 1. Systolic blood pressure in Dahl S rats fed an HS diet was about 20 mm Hg higher than the corresponding value measured in Dahl S rats fed an LS diet. The degree of proteinuria was 3-fold greater in Dahl S rats fed an HS diet than in Dahl S rats fed an LS diet.
TABLE 1.
Metabolic parameters of Dahl S rats fed an LS or HS diet
Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group.
| Dahl-LS (5) | Dahl-HS (5) | |
|---|---|---|
| Body weight, g | 390.4 ± 10.9 | 355.0 ± 3.9* |
| Kidney weight, g | 1.23 ± 0.05 | 1.54 ± 0.04* |
| Systolic blood pressure, mm Hg | 182.6 ± 3.9 | 202.9 ± 5.6* |
| Urine flow rate, ml/day | 22.2 ± 8.0 | 97.2 ± 3.9* |
| Proteinuria, mg/day | 120.8 ± 22.0 | 330.6 ± 66.9* |
P < 0.05 vs. corresponding values measured in Dahl S rats fed an LS diet.
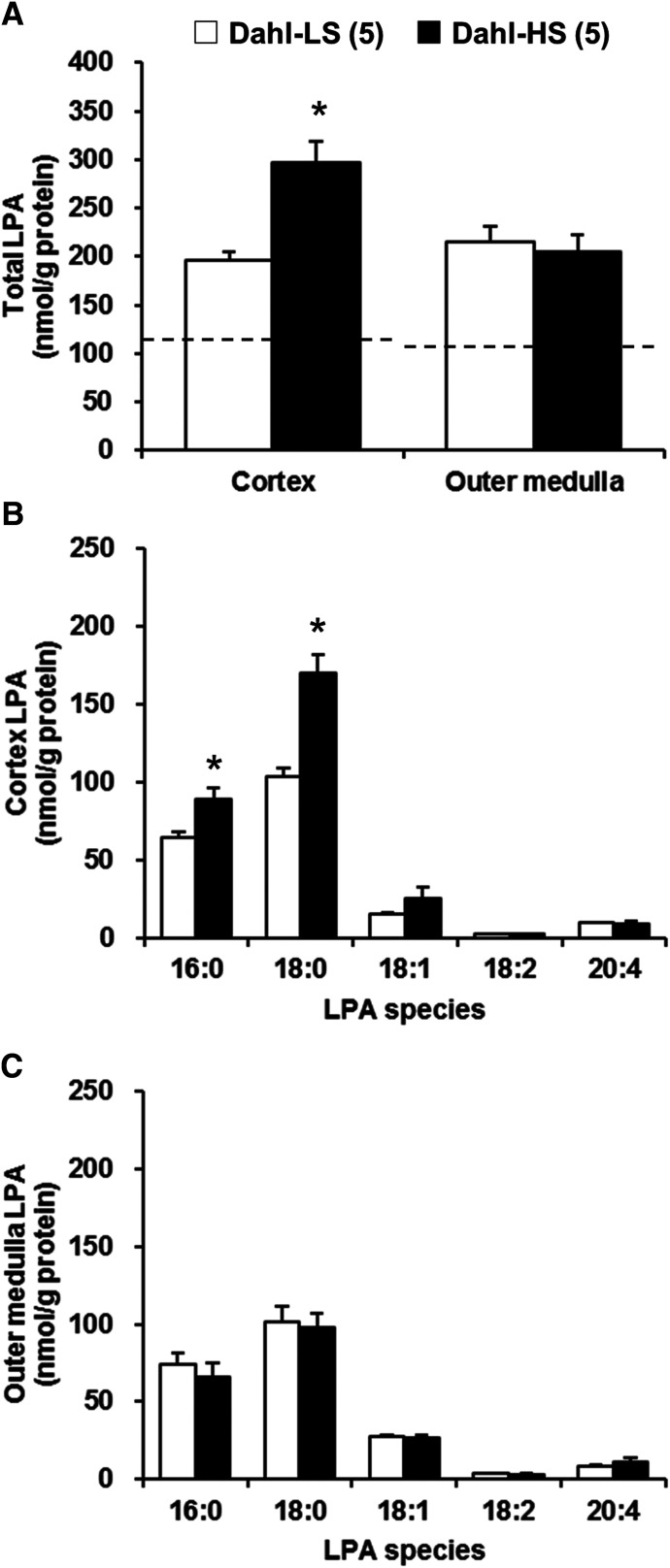
Comparison of Renal LPA Levels in Dahl S Rats Fed an LS and HS Diet.
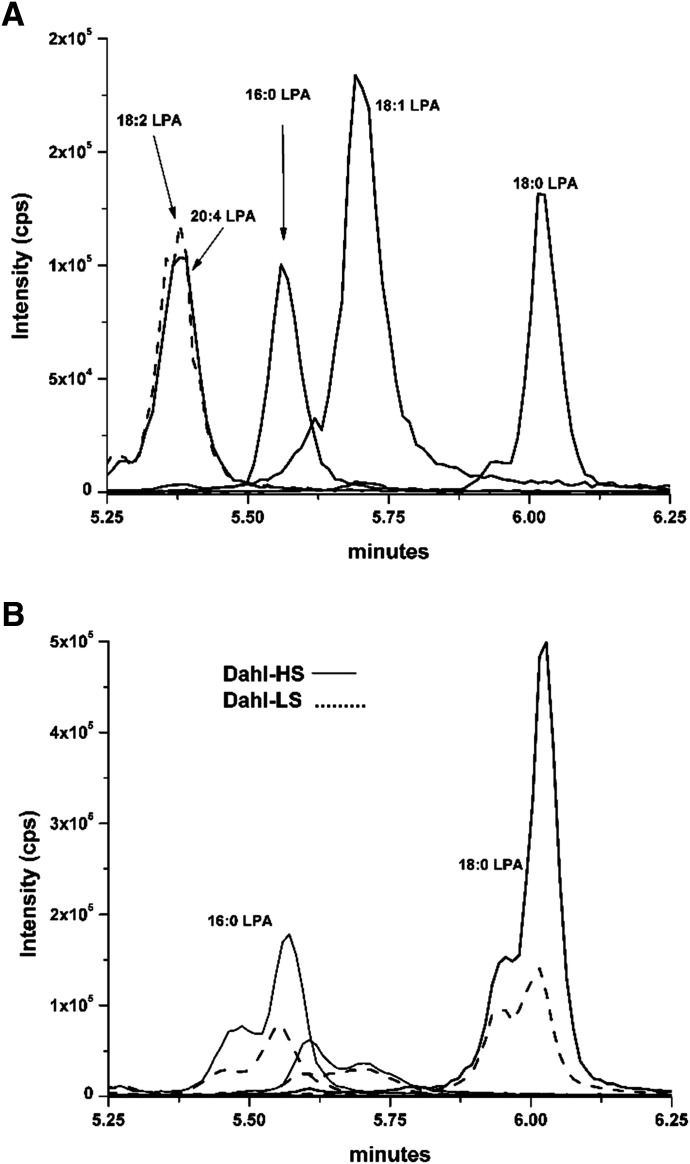
A representative chromatogram showing the various LPA standards and a typical renal cortex LPA profile for Dahl S rats fed an LS or HS diet is presented in Fig. 1. A comparison of the renal LPA levels in the renal cortex and outer medulla of the Dahl S rats fed either an LS or HS diet for 4 weeks is presented in Fig. 2. The baseline levels of total LPA in the renal cortex and outer medulla of Dahl S rats fed an LS diet were above the normal level (Fig. 2A, dashed line). The levels of total LPA in the renal cortex were significantly higher in Dahl S rats fed an HS diet than in those fed an LS diet (296.6 ± 22.9 vs. 196.3 ± 8.5 nmol/g protein). In contrast, there were no significant differences in the total LPA levels in the renal outer medulla in Dahl S rats fed an LS or HS diet (Fig. 2A). The most abundant LPA species in the renal cortex and outer medulla was 18:0, which was followed by 16:0 LPA (Fig. 2, B and C). The levels of 16:0 and 18:0 LPA in the renal cortex were significantly elevated in Dahl S rats fed an HS diet in comparison with those fed an LS diet (Fig. 2B).
Fig. 1.
Liquid chromatography/mass spectrometry separation and peak height comparisons after 1-ng injections of various LPA standards (A). LPAs of 18:2 (dashed line) and 20:4 that overlap in retention time were distinguishable based on their unique MRMs (433.2/153 and 457/153, respectively) (A). Comparison of a typical LPA profiles of extracts of the renal cortex of Dahl S rats fed an LS or HS diet (B).
Fig. 2.
Comparison of renal LPA levels in Dahl S rats fed an LS or HS diet for 4 weeks. Total LPA levels in renal cortex and outer medulla (A). The molecular species composition of LPA in renal cortex (B) and outer medulla (C). Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group. For comparison, the dashed line represents the total LPA levels measured in the kidneys of control SD rats fed a normal salt diet. The SD data are replotted from Fig. 5. *P < 0.05 vs. the corresponding value measured in Dahl S rats fed an LS diet.
Blood Glucose and Renal Injury in Control and STZ-Induced Diabetic Dahl S Rats.
A comparison of blood glucose and renal injury in control and STZ-induced diabetic Dahl S rats is presented in Table 2. After 4 weeks of STZ injection, blood glucose levels (485.4 ± 62.3 vs. 88.0 ± 10.5 mg/dl) and HbA1c concentrations (11.5% ± 0.6% vs. 4.9% ± 0.2%) were significantly elevated in STZ-induced diabetic Dahl S rats in comparison with control Dahl S rats. Urine output in STZ-induced diabetic Dahl S rats was significantly higher than the corresponding value measured in control Dahl S rats. The degree of proteinuria was 4-fold greater in STZ-treated diabetic Dahl S rats than in control rats.
TABLE 2.
Metabolic parameters of control and STZ-treated diabetic Dahl S (Dahl-STZ) rats
Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group.
| Dahl S (5) | Dahl-STZ (5) | |
|---|---|---|
| Body weight, g | 398.6 ± 20.8 | 337.4 ± 16.6 |
| Kidney weight, g | 1.32 ± 0.08 | 1.81 ± 0.06* |
| Blood glucose, mg/dl | 88.0 ± 10.5 | 485.4 ± 62.3* |
| HbA1c, % | 4.9 ± 0.2 | 11.5 ± 0.6* |
| Urine flow rate, ml/day | 7.4 ± 0.9 | 101.6 ± 9.1* |
| Proteinuria, mg/day | 126.2 ± 18.4 | 495.3 ± 65.4* |
P < 0.05 vs. corresponding values measured in control Dahl S rats.
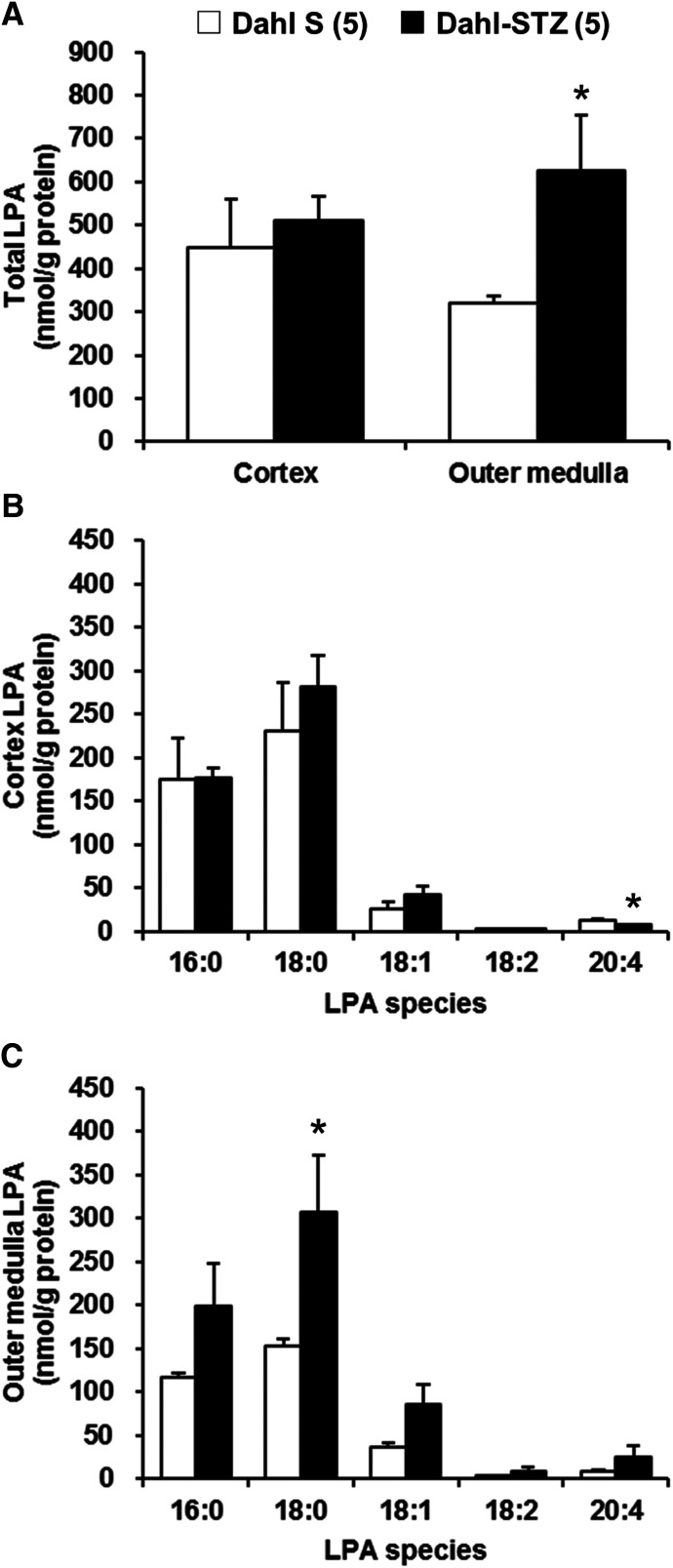
Comparison of Renal LPA Levels in Control and STZ-Induced Diabetic Dahl S Rats.
A comparison of the renal LPA levels in the renal cortex and outer medulla of control and STZ-treated diabetic Dahl S rats is presented in Fig. 3. There were no differences in the total LPA levels in the renal cortex in control and STZ-induced diabetic Dahl S rats (Fig. 3A). The total LPA levels in the renal outer medulla were markedly increased in STZ-induced diabetic Dahl S rats in comparison with control rats (624.6 ± 129.5 vs. 318.8 ± 17.1 nmol/g protein) mainly due to an elevation in 18:0 LPA (Fig. 3, A and C). Saturated LPAs (16:0 and 18:0 LPA) were the predominant species in the renal cortex and outer medulla of control and STZ-induced diabetic Dahl S rats (Fig. 3, B and C). The level of 18:0 LPA in renal outer medulla was significantly increased in STZ-induced diabetic Dahl S rats compared with that seen in control Dahl S rats (Fig. 3C).
Fig. 3.
Comparison of renal LPA levels in control and STZ-induced diabetic Dahl S rats. Total LPA levels in renal cortex and outer medulla (A). The molecular species composition of LPA in renal cortex (B) and outer medulla (C). Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group. *P < 0.05 vs. the corresponding value measured in control Dahl S rats.
Blood Glucose and Renal Injury in 6M- and 18M-Old T2DN Rats.
A comparison of blood glucose concentration and proteinuria in 6M- and 18M-old T2DN rats is presented in Table 3. Both 6M- and 18M-old T2DN rats were diabetic, and blood glucose and HbA1c levels were elevated to >200 mg/dl and >8%, respectively. Proteinuria was 6-fold greater in 18M-old T2DN rats than in 6M-old T2DN rats (325.0 ± 35.5 vs. 55.5 ± 15.8 mg/day).
TABLE 3.
Metabolic parameters of 6M- and 18M-old T2DN rats
Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group.
| 6M T2DN (5) | 18M T2DN (10) | |
|---|---|---|
| Body weight, g | 388.4 ± 13.5 | 429.3 ± 11.2* |
| Kidney weight, g | 1.42 ± 0.05 | 1.91 ± 0.08* |
| Blood glucose, mg/dl | 367.6 ± 61.4 | 216.2 ± 21.9* |
| HbA1c, % | 10.4 ± 1.0 | 8.1 ± 0.5* |
| Urine flow rate, ml/day | 41.0 ± 15.4 | 34.6 ± 5.0 |
| Proteinuria, mg/day | 55.5 ± 15.8 | 325.0 ± 35.5* |
P < 0.05 vs. corresponding values measured in 6M-old T2DN rats.
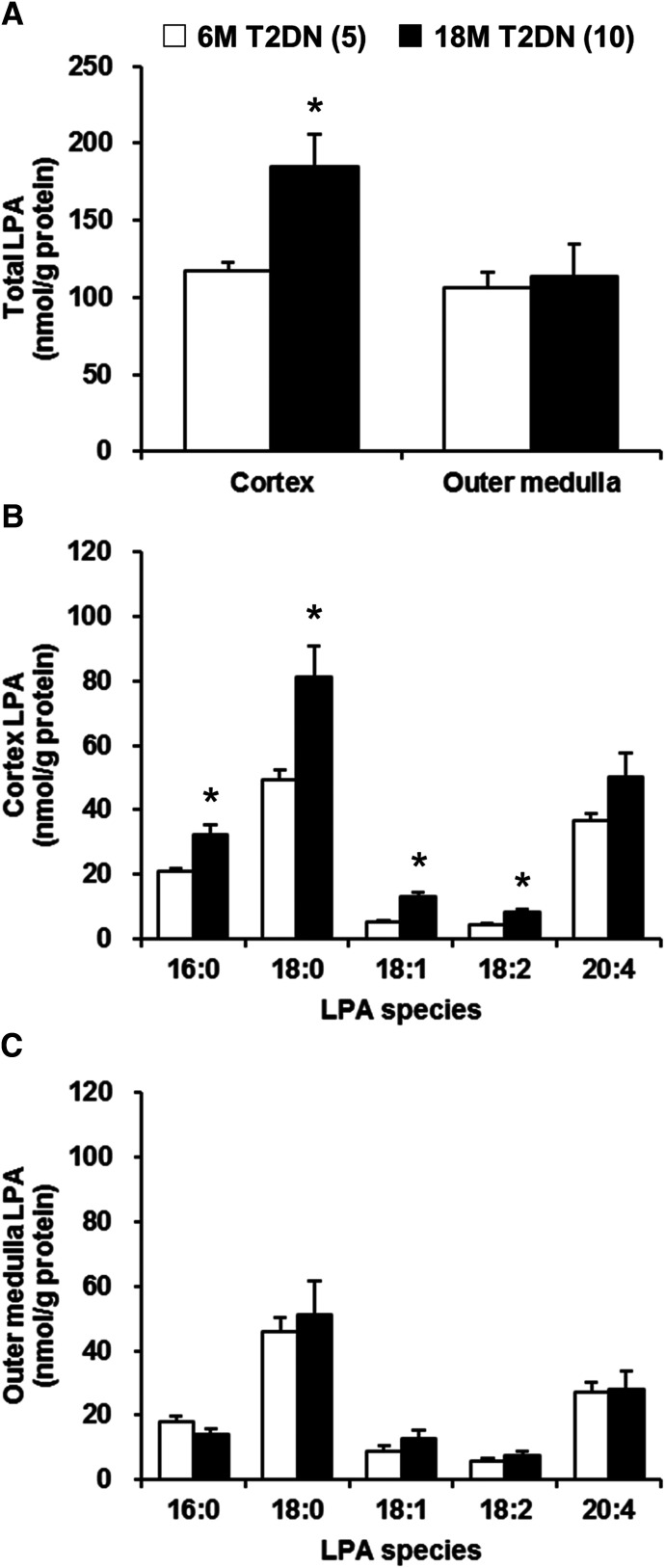
Comparison of Renal LPA Levels in 6M- and 18M-Old T2DN Rats.
A comparison of the renal LPA levels in the renal cortex and outer medulla of 6M- and 18M-old T2DN rats is presented in Fig. 4. The total LPA levels in the renal cortex were significantly higher in 18M-old T2DN rats than in 6M-old T2DN rats (184.9 ± 20.9 vs. 116.9 ± 6.0 nmol/g protein). There were no significant differences in the total LPA levels in the renal outer medulla between the 6M- and 18M-old T2DN rats (Fig. 4A). The most abundant LPA species in the renal cortex and outer medulla of the 6M- and 18M-old T2DN rats was 18:0, which was followed by 20:4 LPA (Fig. 4, B and C). There was a major elevation in 18:0 LPA levels in the renal cortex and lesser increases in 16:0, 18:1, and 18:2 LPA in the 18M-old T2DN rats.
Fig. 4.
Comparison of renal LPA levels in 6M- and 18M-old T2DN rats. Total LPA levels in renal cortex and outer medulla (A). The molecular species composition of LPA in renal cortex (B) and outer medulla (C). Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group. *P < 0.05 vs. the corresponding value measured in 6M-old T2DN rats.
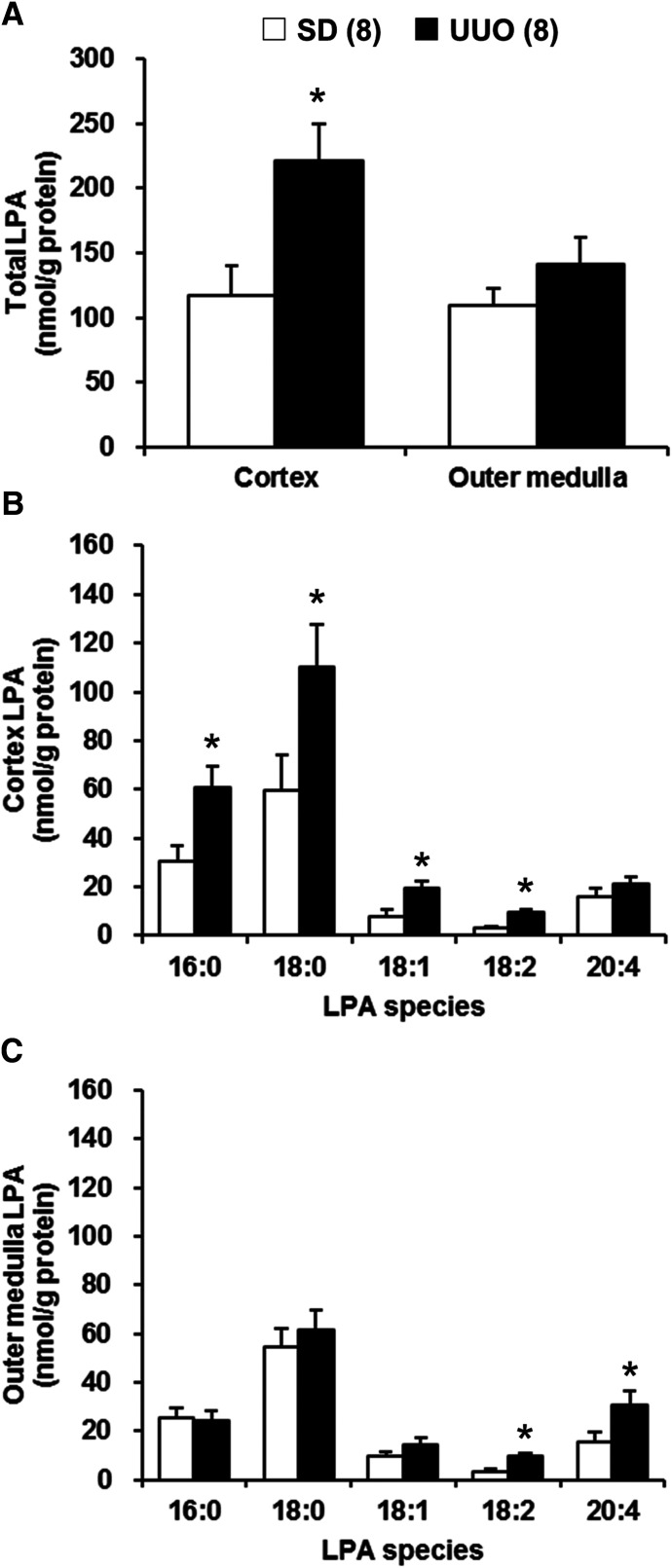
Comparison of Renal LPA Levels in Control and UUO SD Rats.
A comparison of renal LPA levels in the renal cortex and outer medulla in control and UUO SD rats is presented in Fig. 5. The levels of total LPA in the renal cortex were significantly higher in UUO than in control SD rats. In contrast, there were no significant differences in the total LPA levels in the renal outer medulla of control and UUO SD rats (Fig. 5A). The most abundant LPA species in the renal cortex and outer medulla of the control and UUO SD rats was 18:0 LPA, which was followed by 16:0 and 20:4 LPA (Fig. 5, B and C). The levels of 16:0, 18:0, 18:1, and 18:2 LPA were significantly elevated in the renal cortex of UUO in comparison with control SD rats (Fig. 5B). The levels of 18:2 and 20:4 LPA in renal outer medulla were also significantly higher in UUO than in control SD rats (Fig. 5C).
Fig. 5.
Comparison of renal LPA levels in control and UUO SD rats. Total LPA levels in renal cortex and outer medulla (A). The molecular species composition of LPA in renal cortex (B) and outer medulla (C). Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of rats studied per group. *P < 0.05 vs. the corresponding value measured in control SD rats.
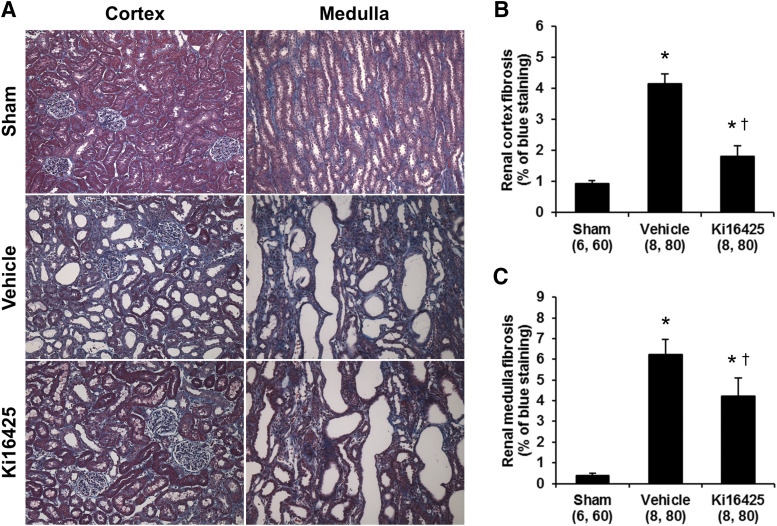
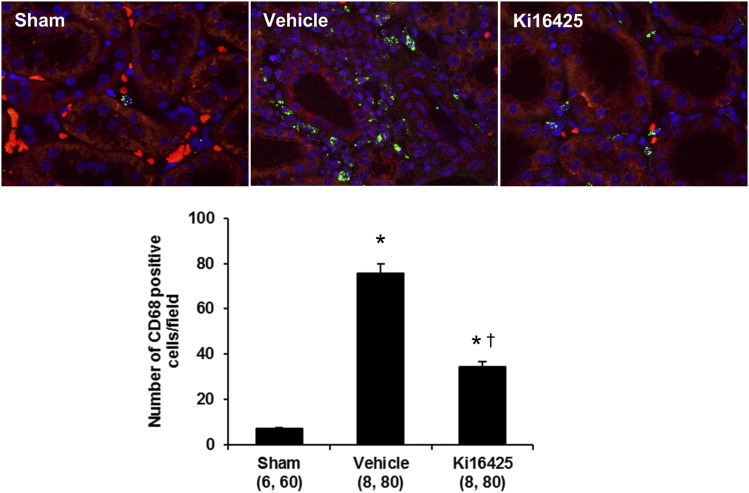
Effects of Ki16425 on Renal Injury in UUO Rats.
To examine whether elevated renal LPA levels contribute to renal injury, we studied the effect of LPA receptor antagonist Ki16425 on renal fibrosis in UUO rats. The effects of Ki16425 on renal fibrosis in UUO rats are presented in Fig. 6. The degree of fibrosis in the renal cortex and medulla were greater in the vehicle-treated UUO than in sham-operated rats (Fig. 6A). The percentage of cortical (Fig. 6B) and medullary (Fig. 6C) areas stained with Mason’s trichrome were 4- and 12-fold greater in vehicle-treated UUO than in sham-operated rats. Chronic treatment with Ki16425 markedly reduced cortical and medullary fibrosis by 66% and 32%, respectively, compared with the levels measured in vehicle-treated UUO rats. We also examined immune cell infiltration in the kidney of UUO rats. The number of CD68 positive immune cells in the cortical region was significantly higher in the vehicle-treated UUO than in sham-operated rats (Fig. 7). Treatment with Ki16425 significantly decreased the number of CD68 positive cells in the kidney of UUO rats.
Fig. 6.
Effects of Ki16425 (200 mg/kg per day) on renal fibrosis in UUO rats. Representative images of the renal cortex and medulla stained with Masson’s trichrome in sham and UUO rats treated with vehicle or Ki16425 (A). Quantitative analysis of blue staining area was performed on 10 random nonoverlapping fields in the renal cortex (B) and medulla (C). Original magnification, 100×. Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of areas scored and the number of rats studied per group. *P < 0.05 vs. the corresponding value measured in sham rats. †P < 0.05 vs. the corresponding value measured in vehicle-treated UUO rats.
Fig. 7.
Effects of Ki16425 on immune cell infiltration in UUO rats. Representative images of the renal cortex were stained with CD68 antibody (green) in sham and UUO rats treated with vehicle or Ki16425. The tissues were counterstained with 4′,6-diamidino-2-phenylindole to visualize nuclei (blue) and 0.001% Evans blue (red) to quench green autofluorescence and visualize tubular structure. The number of CD68 positive immune cells in the renal cortex was counted in 10 random nonoverlapping fields per rat. Original magnification, 200×. Mean ± S.E.M. are presented. Numbers in parentheses indicate the number of areas scored and the number of rats studied per group. *P < 0.05 vs. the corresponding value measured in sham rats. †P < 0.05 vs. the corresponding value measured in vehicle-treated UUO rats.
Discussion
The present study determined whether the renal LPA levels increase during the development of renal injury in rat models of hypertension, diabetes, and obstructive nephropathy using LC/MS/MS. Additionally, the possible role of renal LPA in renal fibrosis was investigated in UUO rats using an LPA receptor antagonist. The Dahl S rat is a well established genetic model of salt-sensitive hypertension that rapidly develops severe progressive proteinuria, glomerulosclerosis, interstitial fibrosis, and a decline in GFR when fed an HS diet (Chen et al., 1993; Dahly et al., 2002; Dahly-Vernon et al., 2005). The present results indicate that Dahl S rats fed an HS diet developed hypertension and renal injury, as indicated by an elevation in blood pressure and proteinuria as compared with Dahl S rats maintained on an LS diet. We found that total LPA levels increased to a much greater extent in the renal cortex of Dahl S rats fed an HS diet than that seen in Dahl S rats fed an LS diet. These findings suggest that elevated levels of LPA may contribute to the development of hypertension-related renal injury in Dahl S rats. We also found that the baseline levels of total LPA were higher in control Dahl S rats fed a normal salt diet in the diabetic studies than those seen in Dahl S rats fed an LS diet. Although it is possible that moderate increases in salt intake may directly affect renal LPA levels, a more likely explanation is that the difference reflects the degree of renal injury and inflammation since Dahl S rats develop more hypertension and proteinuria with age when maintained on a normal versus LS diet (Chen et al., 2013; McPherson et al., 2020).
We next evaluated the renal LPA levels in type 1 and 2 models of diabetic nephropathy. STZ-induced diabetic Dahl S rat is a type 1 diabetic model that has been reported to develop all the features of diabetic nephropathy including hyperfiltration, thickening of basement membranes, mesangial matrix expansion, progressive proteinuria, glomerulosclerosis, interstitial fibrosis, and a more than 50% decline in GFR (Slaughter et al., 2013; Kojima et al., 2015). We found that blood glucose, HbA1c, and proteinuria were significantly higher in STZ-induced diabetic Dahl S rats compared with age-matched controls. LPA levels increased significantly in the renal outer medulla of STZ-induced diabetic Dahl S rats in comparison with the levels seen in age-matched controls.
The T2DN rat is a genetic model of diabetic nephropathy that was created by introducing the mitochondrial genome of the Fawn Hooded Hypertensive rat into the genetic background of the Goto-Kakizaki rat that develops type 2 diabetes but not progressive renal disease. Previous studies have indicated that T2DN rats develop diabetes at 3 months of age and diabetic nephropathy, including thickening of glomerular basement membrane, focal glomerulosclerosis, interstitial fibrosis, and a fall in GFR, as they increase in age from 6 to 18 months (Kojima et al., 2013a,b). The results of the present study indicated that LPA levels were elevated in the renal cortex of 18M-old T2DN rats with more severe renal injury than in 6M-old T2DN rats. These findings suggest that elevations in renal LPA may contribute to the development of renal injury associated with both type 1 and 2 models of diabetic nephropathy. The differences between the two models were that LPA levels increased in the renal outer medulla in STZ-induced diabetic Dahl S rats, whereas LPA levels increased in the renal cortex in the 18M-old T2DN rats. STZ-induced diabetic Dahl S rat is an accelerated type 1 diabetic model that develops renal injury and decline in GFR over a span of 12 weeks, whereas the T2DN rat is a genetic model of type 2 diabetes that slowly develops CKD between 12 and 18 months of age. Although the reason for the difference remains to be determined, one possible explanation for the differences may be due to either the type of diabetes or the progression of diabetic nephropathy.
We also examined the renal LPA levels in UUO rats, which is an animal model typically used to study renal fibrosis, inflammation, and extracellular matrix accumulation. The present results indicate that total LPA levels were elevated in the renal cortex of UUO rats compared with control SD rats. These results are consistent with the previous findings that the release of LPA increased in the kidney explants isolated from UUO mice (Pradère et al., 2007). Taken together, the present results suggest that the formation and/or the release of LPA increases in the kidney during the development of renal injury. Consistent with this suggestion, previous investigators reported that the levels of LPA were elevated in the renal effluent collected from the pelvis of UUO rats, and this was associated with an increase in the renal expression of LPA-producing enzymes, acylglycerol kinase, and autotaxin (Tsutsumi et al., 2011). Moreover, the expression of autotaxin has been reported to be elevated in the kidney of patients with diabetes and animal models of diabetic nephropathy (Zhang et al., 2017).
In the setting of renal injury, LPA may exert a host of biologic effects on a variety of renal cell types, including proximal tubular cells, mesangial cells, and fibroblasts (Inoue et al., 1997; Fang et al., 2000; Geng et al., 2012). Recently, LPA has been implicated in the pathogenesis of tissue fibrosis in the lung, liver, and kidney (Pradère et al., 2008; Ikeda and Yatomi, 2012; Shea and Tager, 2012). Renal fibrosis is a final common pathway underlying the progression of CKD to ESRD (Hodgkins and Schnaper, 2012). Previous studies indicated that there were no significant changes in plasma LPA levels in UUO rats (Tsutsumi et al., 2011). Therefore, to examine whether the elevation of renal LPA contributes to renal injury, we studied the effect of LPA receptor antagonist, Ki16425, on renal fibrosis in UUO rats. We found that Ki16425 attenuated the progression of renal fibrosis and CD68 positive immune cell infiltration after UUO, suggesting that increases in renal LPA may at least in part contribute to the renal inflammation and fibrosis in UUO rats. UUO model reflects important aspects of renal inflammation and fibrosis that are a prominent feature of CKD. However, UUO model lacks the functional readouts of CKD in that serum creatinine levels are normal, and there is little if any proteinuria because contralateral nonobstructed kidney is normal and injured kidney has no urine output. Therefore, additional future studies will be needed to determine whether Ki16425 has the potential to improve renal function in chronic models of hypertension-induced CKD or diabetic nephropathy.
The origin of renal LPA and the species formed is not well understood. This is the first study to profile the formation of various species of LPA in the renal cortex and outer medulla in various models of renal disease. The results indicate that the most dominant species of LPA found in the renal cortex and outer medulla was 18:0 LPA in all of the disease models studied. This finding is in line with previous studies that found the predominant LPA species in renal effluent of UUO rats was 18:0 LPA (Tsutsumi et al., 2011). On the other hand, several studies indicate that the predominant LPA species that circulate in the plasma of rats are the unsaturated LPA species: 18:2 and 20:4 LPA (Ino et al., 2012; Saga et al., 2014). These findings together with our new results suggest that elevations of renal LPA levels are due to local LPA production in the kidney rather filtration and uptake of LPA from the plasma since the composition of LPA species in the kidney and plasma is markedly different.
Previous studies have indicated that LPA species that differ in the length and degree of saturation of the fatty acyl chain have distinct biologic effects (Bandoh et al., 2000; Yoshida et al., 2003). In this study, we found that saturated LPAs were the species that were increased in the kidney of rats with renal disease. Although it is unclear how signaling of saturated LPA species differs from that of unsaturated species, our findings suggest that one should focus on signaling pathways and receptors that mediate the responses to saturated LPAs in the search for new potential therapeutics to prevent renal disease. In contrast, previous studies indicated that LPA receptors have a relatively higher affinity for unsaturated LPA species than for saturated species (Tigyi, 2010). Therefore, further studies are needed to determine the significance of elevation of saturated LPA species in renal disease.
In summary, the present study indicates that the renal formation of LPA, especially saturated species, increases during the development of renal injury in rat models of hypertension and diabetes and after obstructive nephropathy. In addition, administration of an LPA receptor antagonist attenuated the progression of renal injury in UUO rat model of renal fibrosis. These results suggest that the increases in renal LPA production may contribute to the pathophysiology of CKD and that therapeutic approaches that target the renal formation of LPA or block the LPA receptor might be renoprotective and have therapeutic potential.
Acknowledgments
The authors thank Naoki Kojima and Yoshikazu Muroya for their assistance and helpful comments for this project. The authors thank Christine A. Purser for the LC/MS/MS analysis.
Abbreviations
- CKD
chronic kidney disease
- Dahl S
Dahl salt-sensitive
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- HbA1c
glycosylated hemoglobin
- HS
high-salt
- LC/MS/MS
liquid chromatography/mass spectrometry/mass spectrometry
- LPA
lysophosphatidic acid
- LS
low-salt
- 18M
18 months
- 6M
six months
- MP
mobile phase
- SD
Sprague-Dawley
- STZ
streptozotocin
- T2DN
type 2 diabetic nephropathy
- UUO
unilateral ureteral obstruction
Authorship Contributions
Participated in research design: Hirata, Smith, Takahashi, Miyata, Roman.
Conducted experiments: Hirata.
Performed data analysis: Hirata, Roman.
Wrote or contributed to the writing of the manuscript: Hirata, Roman.
Footnotes
T.H., T.T., and N.M. are employees of Taisho Pharmaceutical Co., Ltd. that develops drugs for a wide range of diseases, including cardiovascular diseases. R.J.R. and S.V.S. are employees of the University of Mississippi Medical Center and receive funding from National Institutes of Health to explore the genetic basis of renal and cerebrovascular disease. This work was partially supported by funds provided by Taisho Pharmaceutical Co., Ltd., Saitama, Japan and National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL36279] and National Institute of Diabetes and Digestive and Kidney Diseases [Grant RO1-DK104184].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aikawa S, Hashimoto T, Kano K, Aoki J. (2015) Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem 157:81–89. [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Okudaira S. (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781:513–518. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. (2000) Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett 478:159–165. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Venkatraman G, Bekele RT, Brindley DN. (2016) Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J Biomed Res 30:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Geurts AM, Jacob HJ, Fan F, Roman RJ. (2013) Heterozygous knockout of transforming growth factor-β1 protects Dahl S rats against high salt-induced renal injury. Physiol Genomics 45:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, St John PL, Kirk KA, Abrahamson DR, Sanders PW. (1993) Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest 68:174–184. [PubMed] [Google Scholar]
- Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. (2002) Antihypertensive effects of chronic anti-TGF-β antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283:R757–R767. [DOI] [PubMed] [Google Scholar]
- Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. (2005) Transforming growth factor-β, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45:643–648. [DOI] [PubMed] [Google Scholar]
- Dohi T, Miyauchi K, Ohkawa R, Nakamura K, Kishimoto T, Miyazaki T, Nishino A, Nakajima N, Yaginuma K, Tamura H, et al. (2012) Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin Chim Acta 413:207–212. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. (1993) The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J 291:677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu S, LaPushin R, Lu Y, Furui T, Penn LZ, Stokoe D, Erickson JR, Bast RC, Jr, Mills GB. (2000) Lysophosphatidic acid prevents apoptosis in fibroblasts via G(i)-protein-mediated activation of mitogen-activated protein kinase. Biochem J 352:135–143. [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. (2013) Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382:339–352. [DOI] [PubMed] [Google Scholar]
- Geng H, Lan R, Singha PK, Gilchrist A, Weinreb PH, Violette SM, Weinberg JM, Saikumar P, Venkatachalam MA. (2012) Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am J Pathol 181:1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, Hudson BG, Caprioli RM. (2014) Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res 55:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. (2001) Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res 89:251–258. [DOI] [PubMed] [Google Scholar]
- Hodgkins KS, Schnaper HW. (2012) Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol 27:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Yatomi Y. (2012) Autotaxin in liver fibrosis. Clin Chim Acta 413:1817–1821. [DOI] [PubMed] [Google Scholar]
- Ino M, Shimizu Y, Tanaka T, Tokumura A. (2012) Alterations of plasma levels of lysophosphatidic acid in response to fasting of rats. Biol Pharm Bull 35:2059–2063. [DOI] [PubMed] [Google Scholar]
- Inoue CN, Epstein M, Forster HG, Hotta O, Kondo Y, Iinuma K. (1999) Lysophosphatidic acid and mesangial cells: implications for renal diseases. Clin Sci (Lond) 96:431–436. [PubMed] [Google Scholar]
- Inoue CN, Forster HG, Epstein M. (1995) Effects of lysophosphatidic acid, a novel lipid mediator, on cytosolic Ca2+ and contractility in cultured rat mesangial cells. Circ Res 77:888–896. [DOI] [PubMed] [Google Scholar]
- Inoue CN, Ko YH, Guggino WB, Forster HG, Epstein M. (1997) Lysophosphatidic acid and platelet-derived growth factor synergistically stimulate growth of cultured rat mesangial cells. Proc Soc Exp Biol Med 216:370–379. [DOI] [PubMed] [Google Scholar]
- Kojima N, Slaughter TN, Paige A, Kato S, Roman RJ, Williams JM. (2013a) Comparison of the development diabetic induced renal disease in strains of Goto-Kakizaki rats. J Diabetes Metab 9 (Suppl 9):S9-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Williams JM, Slaughter TN, Kato S, Takahashi T, Miyata N, Roman RJ. (2015) Renoprotective effects of combined SGLT2 and ACE inhibitor therapy in diabetic Dahl S rats. Physiol Rep 3:e12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. (2013b) Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson KC, Shields CA, Poudel B, Johnson AC, Taylor L, Stubbs C, Nichols A, Cornelius DC, Garrett MR, Williams JM. (2020) Altered renal hemodynamics is associated with glomerular lipid accumulation in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol 318:F911–F921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleh M, Pozlep B, Mlakar A, Meden-Vrtovec H, Zupancic-Kralj L. (2007) Determination of serum lysophosphatidic acid as a potential biomarker for ovarian cancer. J Chromatogr B Analyt Technol Biomed Life Sci 858:287–291. [DOI] [PubMed] [Google Scholar]
- Mirzoyan K, Baïotto A, Dupuy A, Marsal D, Denis C, Vinel C, Sicard P, Bertrand-Michel J, Bascands JL, Schanstra JP, et al. (2016) Increased urinary lysophosphatidic acid in mouse with subtotal nephrectomy: potential involvement in chronic kidney disease. J Physiol Biochem 72:803–812. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Rivera R, Chun J. (2012) Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol 165:829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanaga K, Hama K, Aoki J. (2010) Autotaxin--an LPA producing enzyme with diverse functions. J Biochem 148:13–24. [DOI] [PubMed] [Google Scholar]
- Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. (2004) Initial characterization of a rat model of diabetic nephropathy. Diabetes 53:735–742. [DOI] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, et al. (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64:994–1005. [DOI] [PubMed] [Google Scholar]
- Pradère JP, Gonzalez J, Klein J, Valet P, Grès S, Salant D, Bascands JL, Saulnier-Blache JS, Schanstra JP. (2008) Lysophosphatidic acid and renal fibrosis. Biochim Biophys Acta 1781:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradère JP, Klein J, Grès S, Guigné C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, et al. (2007) LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 18:3110–3118. [DOI] [PubMed] [Google Scholar]
- Saga H, Ohhata A, Hayashi A, Katoh M, Maeda T, Mizuno H, Takada Y, Komichi Y, Ota H, Matsumura N, et al. (2014) A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension. PLoS One 9:e93230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier-Blache JS, Feigerlova E, Halimi JM, Gourdy P, Roussel R, Guerci B, Dupuy A, Bertrand-Michel J, Bascands JL, Hadjadj S, et al. (2017) Urinary lysophopholipids are increased in diabetic patients with nephropathy. J Diabetes Complications 31:1103–1108. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sarnak MJ. (2017) Epidemiology: the global burden of reduced GFR: ESRD, CVD and mortality. Nat Rev Nephrol 13:447–448. [DOI] [PubMed] [Google Scholar]
- Shea BS, Tager AM. (2012) Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc 9:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter TN, Paige A, Spires D, Kojima N, Kyle PB, Garrett MR, Roman RJ, Williams JM. (2013) Characterization of the development of renal injury in Type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 305:R727–R734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, et al. (2011) Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther 336:693–700. [DOI] [PubMed] [Google Scholar]
- Tigyi G. (2010) Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol 161:241–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. (2006) Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17:2034–2047. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T, Adachi M, Nikawadori M, Morishige J, Tokumura A. (2011) Presence of bioactive lysophosphatidic acid in renal effluent of rats with unilateral ureteral obstruction. Life Sci 89:195–203. [DOI] [PubMed] [Google Scholar]
- Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. (2018) Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94:567–581. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. (2003) Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation 108:1746–1752. [DOI] [PubMed] [Google Scholar]
- Zhang MZ, Wang X, Yang H, Fogo AB, Murphy BJ, Kaltenbach R, Cheng P, Zinker B, Harris RC. (2017) Lysophosphatidic acid receptor antagonism protects against diabetic nephropathy in a type 2 diabetic model. J Am Soc Nephrol 28:3300–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]