Abstract
Objectives:
To elucidate the risk factors for hospital admission among COVID-19 patients with type 2 diabetes mellitus (T2DM).
Methods:
This retrospective study was conducted at the Prince Sultan Military Medical City, Riyadh, Saudi Arabia between May 2020 and July 2020. Out of 7,260 COVID-19 patients, 920 were identified as T2DM. After the exclusion process, 806 patients with T2DM were included in this analysis. Patients’ data were extracted from electronic medical records. A logistic regression model was performed to estimate the risk factors of hospital admission.
Results:
Of the total of 806 COVID-19 patients with T2DM, 48% were admitted in the hospital, 52% were placed under home isolation. Older age between 70-79 years (OR [odd ratio] 2.56; p=0.017), ≥80 years (OR 6.48; p=0.001) were significantly more likely to be hospitalized compared to <40 years. Similarly, patients with higher HbA1c level of ≥9% compared to <7%; (OR 1.58; p=0.047); patients with comorbidities such as, hypertension (OR 1.43; p=0.048), cardiovascular disease (OR 1.56; p=0.033), cerebrovascular disease (OR 2.38; p=0.016), chronic pulmonary disease (OR 1.51; p=0.018), malignancy (OR 2.45; p=0.025), chronic kidney disease (CKD) IIIa, IIIb, IV (OR 2.37; p=0.008), CKD V (OR 5.07; p=0.007) were significantly more likely to be hospitalized. Likewise, insulin-treated (OR 1.46; p=0.03) were more likely to require hospital admission compared to non-insulin treated patients.
Conclusion:
Among COVID-19 patients with diabetes, higher age, high HbA1c level, and presence of other comorbidities were found to be significant risk factors for the hospital admission.
Keywords: COVID-19, diabetes mellitus, coronavirus, hospitalization, hospital admission
Since January 2020, there has been an unprecedented global healthcare crisis, which has been precipitated by the speedy evolution of the coronavirus 2019 (COVID-19) outbreak induced by severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) that was reported in Wuhan, China on December 2019.1 The rapid escalation in the number of cases prompted the World Health Organization (WHO) to proclaim a Public Health Emergency of International Concern on January 2020.2 As of August 2020, it has been found that 227 countries and territories worldwide have been affected by the COVID-19, including Saudi Arabia.3
From the data being received, it appears that patients with diabetes mellitus (DM) reveal a higher degree of symptom severity induced by the viral infection. When patients having diabetes develop a viral infection, treatment becomes more challenging because of variations in the blood glucose levels and, likely, the diabetes-related complications that may be present.4 Patients with diabetes having COVID-19 have been observed to have a tendency to experience greater symptom severity and have a higher mortality rate and hospitalization than other patients. Moreover, patients with diabetes and COVID-19 exhibit high risk for developing serious complications, including respiratory tract infections, multi-organ failure, and finally death.5 It is believed that multiple factors, the impaired immune response, in particular, are responsible for the heightened risk in COVID-19-infection among patients with diabetes.6,7 Besides, the high glucose levels are noted to damage the defense mechanisms of the immune system in such patients and trigger a few diabetes-related problems including nerve damage; the impaired blood flow escalates the susceptibility of the patient to infection.7,8 Also, many patients with type 2 diabetes mellitus (T2DM) are overweight, and obesity has been identified as yet another risk factor for those patients with severe COVID-19 infection, necessitating hospitalization.7,8
The observational data received recently suggests that those experiencing metabolic comorbidities, along with COVID-19, are at high risk of hospitalization and mortality. However, based on the present information, the incidence and mortality of COVID-19 in patients with diabetes differed in different countries and population groups.9 Geographical perspective, the Kingdom of Saudi Arabia is a major sovereign state in Western Asia and ranks as the second-largest in the Arab world, and the fifth-largest in Asia, with a population exceeding 34.8 million people. The International Diabetes Federation (IDF) Diabetes Atlas, in its 9th edition (2019) reports that 18.3% (4,275,200) of the Saudi adult population suffers from diabetes, with several more being listed as pre-diabetic.10 Therefore, the risk of COVID-19 among patients with diabetes signals greater alarm in Saudi Arabia. A comprehensive understanding of the clinical characteristics of COVID-19 among patients with diabetes will facilitate to recognizing those at high-risk, and identifying the factors related to an increased risk of hospital admission and directing the future management of patients with diabetes, in Saudi Arabia. Therefore, the objective of the present study was to describe the risk factors for hospital admission among COVID-19 patients with diabetes in Saudi Arabia.
Methods
This retrospective study was conducted between May 2020 and July 2020. Among the 7,260 COVID-19 patients who visited Prince Sultan Military Medical City (PSMMC), Riyadh, Saudi Arabia, we identified a total of 920 patients with DM. After the exclusion process, a final count of 806 patients were included in this study. In our study, the patients had a clear diagnosis of diabetes, according to the documentation entered by their physicians in the electronic medical records of the PSMMC. The study protocol was approved by the Research and Ethics Committee of PSMMC, Riyadh, Saudi Arabia.
This study included patients of both genders who had been diagnosed with diabetes. The exclusion criteria for this study were pregnant women with gestational diabetes, those identified with type 1 diabetes mellitus (T1DM) and diabetes diagnosed at the time of a confirmed COVID-19 test.
Measurements and definitions
The COVID-19 diagnosis was made in accordance with the interim guidance of the World Health Organization. The nasopharyngeal and reverse transcription polymerase chain reaction standard method of detection of SARS-CoV-2 were employed.
Home isolation
Only patients confirmed as asymptomatic or experiencing mild symptoms and clinically stable (with no oxygen requirements/and no evidence of pneumonia) were permitted to home isolation).11
Hospital admission severity criteria: i) Mild to moderate: patients who exhibited distinct upper respiratory and constitutional symptoms or patients who revealed early clinical or radiological pneumonia. ii) Severe: patients with ≥1 of the listed symptoms, respiratory rate ≥30/min, blood oxygen saturation ≤93%, partial pressure of oxygen/fraction of inspired oxygen ratio <300 or lung infiltrates >50% of the lung field from between 24-48 hours, and iii) Critical: patients who revealed ≥1 of the following symptoms such as acute respiratory distress syndrome (ARDS), sepsis, altered consciousness, multi-organ failure or cytokine release syndrome.11
The electronic medical records for relevant medical laboratory data were reviewed for all the patients with T2DM admitted into the hospital, as well as for those under home isolation. Information pertaining to patient age, gender, body mass index (BMI), duration of diabetes, and Hemoglobin A1c (HbA1c) value was gathered. Further data on the presence of other comorbidities such as hypertension, cardiovascular disease (CVD), cerebrovascular disease, chronic pulmonary disease (CPD), chronic kidney disease (CKD), and malignancy, as well as the records of diabetes treatment, were collected for all the patients included in this study.
Statistical analysis
Data analysis was carried out using Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA, United States) and the IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA). The continuous variables were represented as mean ± SD, while the categorical variables are shown as frequencies and percentages. Besides the descriptive analysis, the Chi-square test (for categorical variables) was performed to identify the variables associated to the dependent variable. A logistic regression model was performed to estimate the risk factors of hospital admission. A p-value of <0.05 was considered statistically significant.
Results
The flowchart shows the patients selected for this study. Among the total of 7,260 COVID-19 patients, 920 (12.67%) T2DM patients were identified, and after the exclusion process, the final number of 806 patients were included in this study for analysis (Figure 1).
Figure 1.
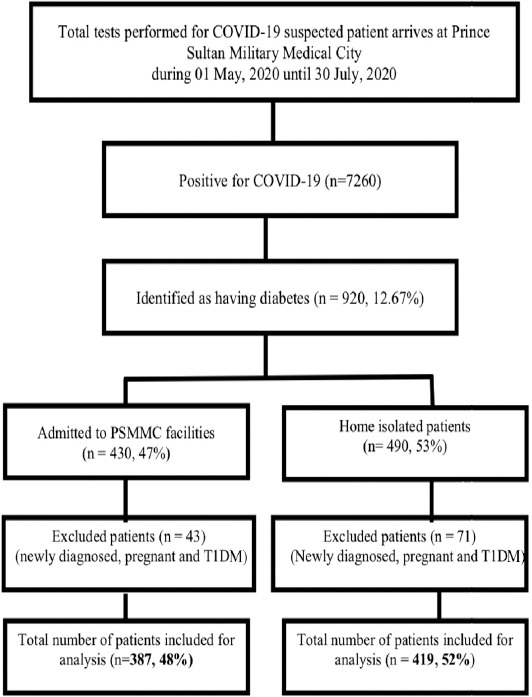
Selection criteria of the study population. T1DM: type 1 diabetes mellitus, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
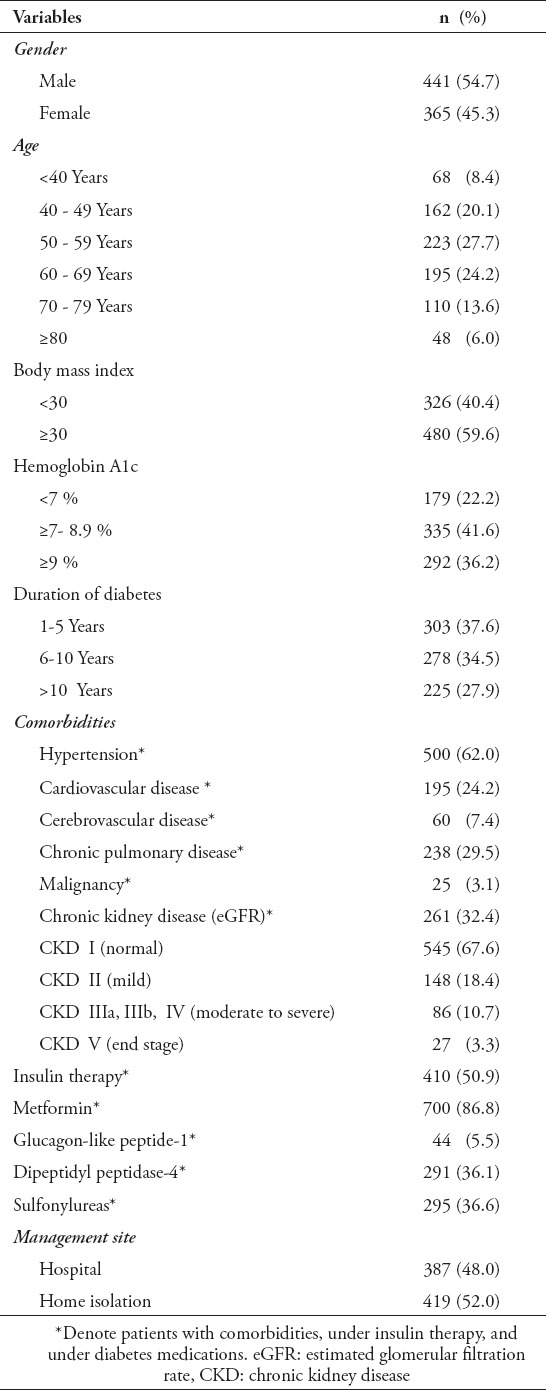
In Table 1, the demographic and mean values of the variables in the study are listed. Males (54.7%) were more susceptible to acquire COVID-19 infection than females (45.3%). The individuals in the study cohort had a mean age of 57.6 ± 13.9 (mean ± SD), BMI of 31.8 ± 6.16, HbA1c of 8.46 ± 1.96 and the duration of the diabetes was 7.27 ± 4 years. More numbers of the study population (n=223) fell into the age category of 50-59 (27.7%) years, 480 (59.6%) had a BMI ≥ 30, 335 (41.6%) had HbA1c values ≥ 7- 8.9 %, and 303 (37.6%) had diabetes for 1-5 years. The study showed that 500 (62%) of the patients experienced hypertension, 195 (24.2%) had CVD, 60 (7.4%) had cerebrovascular disease, while 238 (29.5%) had CPD, and 25 (3.1%) had a malignancy. Among the patients having kidney disease, 148 (18.4%) had CKD II, 86 (10.7%) had CKD IIIa, IIIb, IV, and 27 (3.3%) had end-stage renal failure. The study had a population of 410 (50.9%) on insulin therapy, 700 (86.8%) on metformin, 44 (5.5%) on glucagon-like peptide-1 (GLP-1), 291 (36.1%) on dipeptidyl peptidase-4 (DPP-4) and 295 (36.6%) on sulfonylureas. Among the 806 patients with DM, 387 required hospitalization, while 419 (52%) developed a mild COVID-19 infection, which could be managed under conditions of home isolation, according to the local guidelines.
Table 1.
Demographic and clinical characteristics of the study population (N=806)
In Table 2, the demographic and clinical factors linked to the hospitalization of those with diabetes and COVID-19 are shown. Significant association were evident between hospital admission and home isolation on age (p=0.0001), HbA1c values (p=0.042), duration of diabetes (p=0.017), as well as for patients with hypertension (p=0.0001), CVD (p=0.0001), cerebrovascular disease (p=0.0001), CPD (p=0.001), malignancy (p=0.001), patients on insulin therapy (p=0.0001), metformin (p=0.001), DPP-4 (p=0.049), as well as the severity of the kidney disease (p=0.0001).
Table 2.
Demographic and clinical variables associated with hospitalization among COVID-19 patients with diabetes.
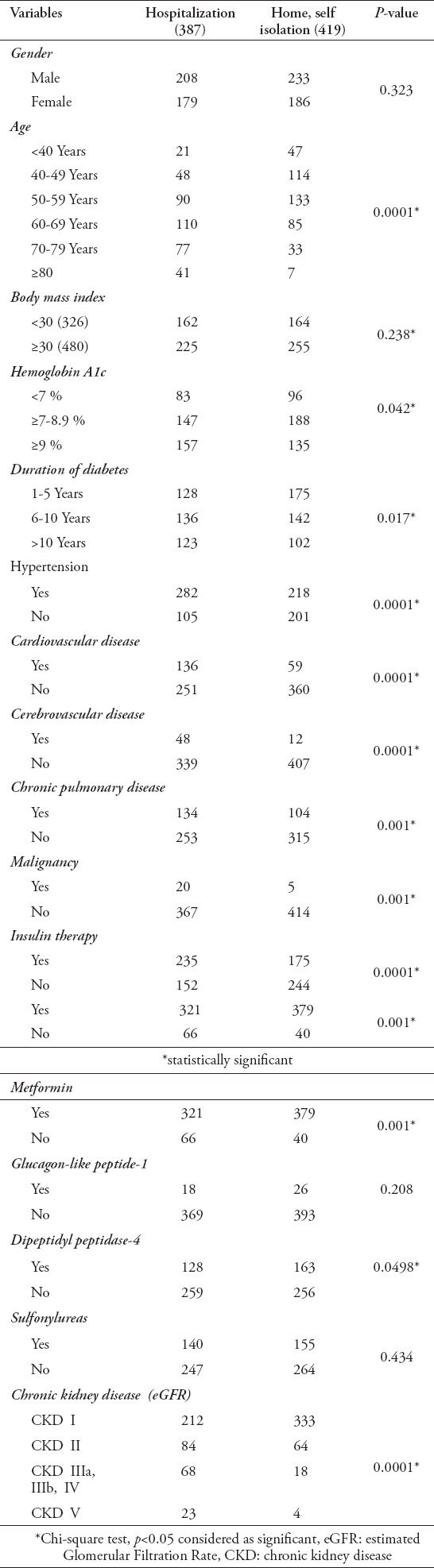
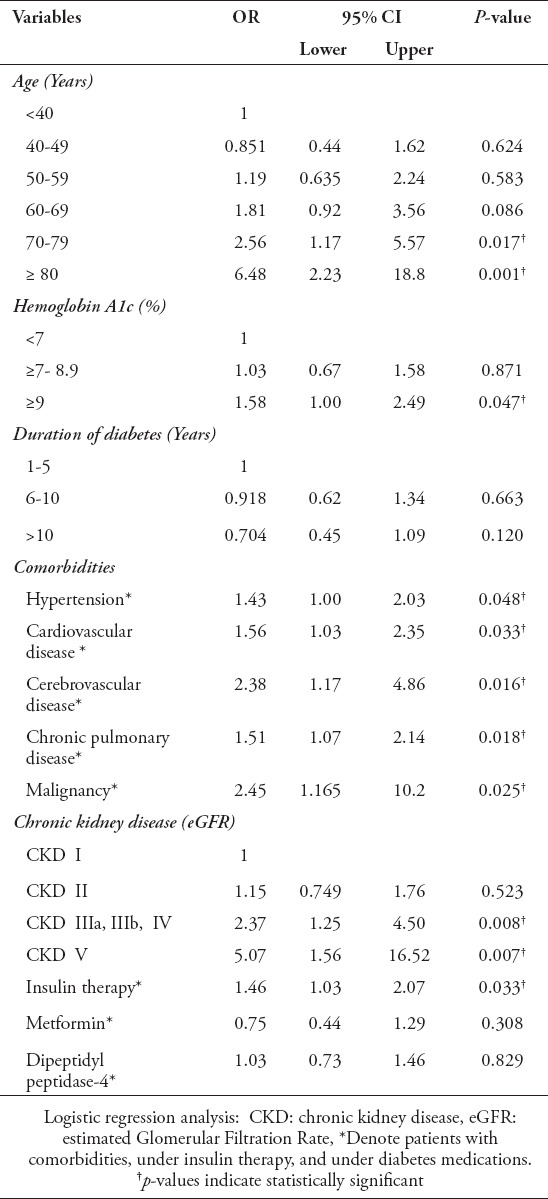
Table 3 shows the results of the regression analyses with 95% confidence interval for significant confounders for hospital admission of COVID-19 patients with diabetes. The factors that were independently associated with hospitalization included older age, 70-79 years (Odds ratio [OR] 2.56; p=0.017), ≥80 years (OR 6.48; p=0.001) compared to <40 years; the HbA1c values of ≥9% (OR 1.58; p=0.047) too were independently associated with hospital admission, as compared to HbA1c values of <7%. Unlike patients without comorbidities, patients with hypertension (OR 1.43; p=0.048), CVD (OR 1.56; p=0.033), cerebrovascular disease (OR 2.38; p=0.016), CPD (OR 1.51; p=0.018), and malignancy (OR 2.45; p=0.025) were found independently associated with hospitalization. Similarly, patients without kidney disease, those with CKD IIIa, IIIb, IV (OR 2.37; p=0.008), and patients with end-stage renal failure (OR 5.07; p=0.007) were independently associated with hospital admission. Compared to patients not requiring insulin therapy, patients on insulin therapy revealed a higher risk for hospital admission (OR 1.46; p=0.003).
Table 3.
Results of regression analyses with 95% confidence interval for significant confounders for hospitalization of COVID-19 patients with diabetes.
Discussion
Despite the paucity of information regarding the influence exerted by the current COVID-19 pandemic on patients with diabetes, a few recent studies have implied the presence of a bidirectional relationship between DM and COVID-19. Also, diabetes has been recognized as a significant risk factor for hospitalization, as well as severe illness and even death among COVID-19 infected patients.5,12 However, all patients with DM are not equally susceptible to an increased risk for COVID-19-induced severity and mortality. Specific clinical and biological features distinguish those high-risk phenotypes within the people with DM, and these important prognostic markers require clear categorization.13 In the present study, we aimed is to describe the risk factors for hospital admission among COVID-19 patients with T2DM in Saudi Arabia. In the current study, we identified 920 (12.7%) COVID-19 patients in all, who also had diabetes. However, from the data obtained, different studies showed that the incidence of diabetes among COVID-19 patients differs in different countries and population groups. From a retrospective study performed using 191 adult patients admitted to 2 hospitals in Wuhan, China, 19% of them had diabetes, and the nonsurvivors significantly had a higher probability of having diabetes than did the survivors (31% vs 14%).14 The most extensive study to date has been conducted in Spain. It reported data for 121,263 COVID-19 patients, showing approximately 10% prevalence of diabetes in this group.15 In a meta-analysis of 9 observational studies of patients hospitalized with COVID-19, the pooled prevalence of diabetes was found to be 9%.16 However, another review stated that the prevalence of diabetes varied from 7 to 21%.17 It is noteworthy that a higher number of COVID-19 patients with diabetes in our hospital were male (54.7%). This finding concurs with the result from an earlier study that men with COVID-19, more than women, are at greater risk for developing severe outcomes like hospital admission and even death.18
In prior disease epidemics, a higher risk of viral infection was noted in patients with diabetes, reported especially in those advanced in years. Several datasets from China, Italy and the USA have consistently stated that the clinical course of COVID-19 is serious and intensive in older patients (>70 years of age) and in those with pre-existing comorbidities, DM in particular.5,19,20 One study listed age as one of the risk factors for poor outcomes with COVID-19, in those 80 years of age and above, who possess 12-times more risk than those in the 50- to 59-year age category.21 In the present study, the results indicated that the higher age group, the 70- to 79-year-olds (OR 2.56; p=0.017) and those ≥80 years (OR 6.48; p=0.001) were more susceptible, and ran a higher risk of hospitalization than those in the <40-year age group. From the data available, it appears that age is connected to worse outcomes in those with COVID-19. Therefore, the hypothesis proposed is that this relationship is greater in patients with diabetes, particularly because the incidence of diabetes rises with age, and peaks in those above 65 years of age. Such persons, exceeding 65 years of age, have a higher tendency to experience an extended duration of diabetes and are more prone to develop diabetic complications. Also, diabetes and advanced age are frequently correlated to comorbidities like cardiovascular disease, hypertension, and obesity.22 The present study also demonstrated that 62% of the patients had hypertension, 24.2% had CVD, 60 (7.4%) had cerebrovascular disease, 29.5% had CPD, and 3.1% had a malignancy. Among those patients experiencing kidney disease, 148 (18.4%) had CKD II, 86 (10.7%) had CKD IIIa, IIIb, IV and 27 (3.3%) had end-stage renal failure. When compared with patients having no comorbidities, those suffering from hypertension (OR 1.43; p=0.048), CVD (OR 1.56; p=0.033), cerebrovascular disease (OR 2.38; p=0.016) and CPD (OR 1.51; p=0.018) were independently associated to hospitalization. Likewise, compared to patients with no kidney disease, those with CKD IIIa, IIIb, IV (OR 2.37; p=0.008), and those in CKD V (OR 5.07; p=0.007) independently concurred with hospital admission. An earlier retrospective analysis also reiterated that when patients with diabetes contracted COVID-19, they had a higher incidence of hypertension (56.9%), CVD (20.9%), and cerebrovascular disease (7.8%).23 Similar results were found for 136 patients with diabetes among the 904 patients with COVID-19. In the COVID-19 patients, those having diabetes more frequently also had hypertension, CVD and CKD.24 Some studies also found that patients with malignancy were especially vulnerable to COVID-19 because they generally had multiple risk factors for this infection, such as advanced age.25 In the present study, we also found malignancy (OR 2.45; p=0.025) was independently associated with the hospitalization of the patients.
From the results of the present study, the HbA1c level ≥ 9% (OR 1.58; p=0.047) was found to be independently associated with hospital admission as compared to HbA1c level <7%. This evidently showed that diabetes, especially those of the poorly-controlled group, relates to a significantly higher risk of severe COVID-19, requiring hospitalization. The lack of data on the glycemic control in the hospitalized COVID-19 patients with diabetes and acute hyperglycemia continues to be of concern.26 However, the studies available at present indicated that even optimal glycemic control, avoidance of acute hyperglycemia, hypoglycemia, and glycemic variability could cause a significant improvement in the outcomes of the COVID-19 patients with diabetes.27 In a retrospective study carried out in the USA on 451 patients with COVID-19 and diabetes or hyperglycemia, it appeared that patients with uncontrolled hyperglycemia endured longer hospital stays and experienced higher mortality rate in the hospital compared with those whose blood sugar levels were under control.28 Another study revealed that uncontrolled diabetes with HbA1c >9% corresponded to an approximately 60% greater risk of pneumonia-related hospitalization in the course of the bacterial infection.29
Individuals with insulin-dependent diabetes contracting COVID-19 were at particularly high risk for low outcome rates; therefore, those needing treatment with insulin are a population significant for being high risk for developing COVID-19-related complications and urgent hospitalization. The findings of the present study showed that patients given insulin therapy, in comparison to those without such treatment, had a greater risk of hospitalization (OR 1.46; p=0.003). A prior study on examining individuals with insulin-dependent diabetes diagnosed with COVID-19, reported that in all, 27.6% of those on insulin therapy were admitted to the intensive care unit as against 1.8% of those not requiring insulin for diabetes control. Also, they found that a higher number of those on insulin-treatment succumbed to COVID-19 (51.7%), in comparison to the number of deaths of persons not needing insulin therapy (3.6%). Further, patients with diabetes on insulin treatment also revealed a notably higher incidence of respiratory failure, acute cardiac and kidney disease due to COVID-19. It is thus evident that patients with diabetes necessitating insulin therapy may have a higher risk of the rate of disease progression and thus a poorer prognosis after contracting the COVID-19 and thus raise the risk of hospital admission.30 It is noteworthy that patients with poor diabetes control can experience acute hyperglycemic crises, such as diabetic ketoacidosis or a hyperosmolar hyperglycemic state, which could also be precipitated by COVID-19, resulting in disastrous outcomes.31 Therefore, these patients must be given aggressive treatment actively along with intravenous fluids. According to the treatment guidelines, these patients should be started on insulin therapy also, very early in the fight against the virus.
Study limitations
The retrospective nature of the study, the limited social, demographic factors investigated, and conducted at a single center were the limitations of the study. Further research studies on the topic are necessary to overcome the limitations cited.
In conclusion, our findings are evident that patients who are higher age, with higher HbA1c levels and comorbidities, had a higher tendency for needing hospitalization. A thorough understanding of the clinical characteristics of diabetes with COVID-19 will facilitate, recognizing those at high risk, identifying the factors associated with a greater risk of hospital admission and directing the future management of COVID-19 in patients with diabetes. In those hospitalized COVID-19 patients with diabetes, stringent glucose monitoring is crucial to optimize the clinical outcomes. Patients experiencing poor glycemic control and having diabetes-related comorbidities require prompt referrals to a hospital at the onset of the very first symptoms of the COVID-19 infection. This will ensure early diagnosis and treatment and impede the rate of disease progression from reaching the more severe phases of this disease.
Acknowledgment
The authors gratefully GobalEdico (www.globaledico.com) for English language editing.
Footnotes
References
- 1.Ahmad T, Haroon Baig M, Hui J. Coronavirus disease 2019 (COVID-19) pandemic and economic impact. Pak J Med Sci. 2020;36:S73–S78. doi: 10.12669/pjms.36.COVID19-S4.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 as a Public Health Emergency of International Concern (PHEIC) under the IHR. [Accessed 2020 August 12]. Updated 2020. Available from URL: https://extranet.who.int/sph/covid-19-public-health-emergency-international-concern-pheic-under-ihr .
- 3.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins Whiting School of Engineering (JHU). ArcGIS. Johns Hopkins University. [Accessed 2020 July 16]. Updated 2020. Available from URL: https://systems.jhu.edu/
- 4.The International Diabetes Federation (IDF) COVID-19 and diabetes. [Accessed 2020 August 13]. Updatde 2020. Available from URL: https://www.idf.org/aboutdiabetes/what-is-diabetes/covid-19-and-diabetes/1-covid-19-and-diabetes.html .
- 5.Azar WS, Njeim R, Fares AH, Azar NS, Azar ST, El Sayed M, et al. COVID-19 and diabetes mellitus:how one pandemic worsens the other. Rev Endocr Metab Disord. 2020:1–13. doi: 10.1007/s11154-020-09573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Hussain A, Misra A. Diabetes and COVID-19:evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74:864–870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong IK, Ho Yoon K, Kyu Lee M. Diabetes and COVID-19:global and regional perspectives. Diabetes Res Clin Pract. 2020;166:108303. doi: 10.1016/j.diabres.2020.108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkhadir J. COVID-19 and diabetes from IDF MENA region. Diabetes Res Clin Pract. 2020;166:108277. doi: 10.1016/j.diabres.2020.108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katulanda P, Dissanayake HA, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Yogendranathan N, et al. Prevention and management of COVID-19 among patients with diabetes:an appraisal of the literature. Diabetologia. 2020;63:1440–1452. doi: 10.1007/s00125-020-05164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Diabetes Federation (IDF) Diabetes Atlas 19th Edition. [Accessed 2020 August 7]. Updated 2019. Available from URL: https://diabetesatlas.org/en/resources/
- 11.Ministry of Health. Saudi MOH Protocol for Patients Suspected of/Confirmed with COVID-19. [Accessed 2020 August 12]. Updated 2020 July 31. Available from URL: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf .
- 12.Rajpal A, Rahimi L, Ismail-Beigi F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J Diabetes. 2020:1–14. doi: 10.1111/1753-0407.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koliaki C, Tentolouris A, Eleftheriadou I, Melidonis A, Dimitriadis G, Tentolouris N. Clinical management of diabetes mellitus in the era of COVID-19:practical issues, peculiarities and concerns. J Clin Med. 2020;9:2288. doi: 10.3390/jcm9072288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preito-Alhambra D, Ballo E, Coma E, Mora N, Aragon M, PratsUribe A, et al. Hospitalization and 30-day fatality in 121,263 COVID-19 outpatient cases. medRxiv. 2020:1–19. [Google Scholar]
- 16.Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus:A meta-analysis. Diabetes Res Clin Pract. 2020;164:108200. doi: 10.1016/j.diabres.2020.108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AK, Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19:A narrative review. Diabetes Res Clin Pract. 2020;165:108266. doi: 10.1016/j.diabres.2020.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19:focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Open SAFELY Collaborative. Williamson E, Walker AJ, Bhaskaran K, Bacon S, Bates C, et al. Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. J Chem Inf Model. 2019;53:1689–1699. [Google Scholar]
- 22.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes:understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:e5. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China:a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–13407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 25.Tartarone A, Lerose R. COVID-19 and cancer care:what do international guidelines say? Med Oncol. 2020;37:80. doi: 10.1007/s12032-020-01406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadokostaki E, Tentolouris N, Liberopoulos E. COVID-19 and diabetes:What does the clinician need to know? Prim Care Diabetes. 2020;14:558–563. doi: 10.1016/j.pcd.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceriello A, Standl E, Catrinoiu D, Itzhak B, Lalic NM, Rahelic D, et al. Akbar DH, editor. Issues for the management of people with diabetes and COVID-19 in ICU. Bacterial pneumonia:comparison between diabetics and non-diabetics. Cardiovasc Diabetol. 2020; 19: 114.E28. Acta Diabetol. 2001;38:77–82. doi: 10.1186/s12933-020-01089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbar DH. Bacterial pneumonia:comparison between diabetics and non-diabetics. Acta Diabetol. 2001;38:77–82. doi: 10.1007/s005920170017. [DOI] [PubMed] [Google Scholar]
- 30.Shang J, Wang Q, Zhang H, Wang X, Wan J, Yan Y, et al. The relationship between diabetes mellitus and COVID-19 prognosis:a retrospective cohort study in Wuhan, China. Am J Med. 2020:1–9. doi: 10.1016/j.amjmed.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Response:Acute Hyperglycemic Crises with Coronavirus Disease-19:Case Reports (Diabetes Metab J 2020 44 349-53) Diabetes Metab J. 2020;44:484–485. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]


