Abstract
Normal eating behavior is coordinated by the tightly regulated balance between intestinal and extra-intestinal homeostatic and hedonic mechanisms. By contrast, food addiction represents a complex, maladaptive eating behavior that reflects alterations in brain–gut–microbiome (BGM) interactions and a shift of this balance towards hedonic mechanisms. Each component of the BGM axis has been implicated in the development of food addiction, with both brain to gut and gut to brain signaling playing a role. Early life influences can prime the infant gut microbiome and brain for food addiction, which might be further reinforced by increased antibiotic usage and dietary patterns throughout adulthood. The ubiquitous availability and marketing of inexpensive, highly palatable and calorie dense food can further shift this balance towards hedonic eating through both central (disruptions in dopaminergic signaling) and intestinal (vagal afferent function, metabolic toxaemia, systemic immune activation, changes to gut microbiome and metabolome) mechanisms. In this Review, we propose a systems biological model of BGM interactions, which incorporates published reports on food addiction, and provides novel insights into treatment targets aimed at each level of the BGM axis.
Introduction
The obesity epidemic continues to be a major public health problem both in the USA and globally.1,2 Obesity is defined as a body mass index (BMI) ≥30 kg/m2 and a BMI ≥40 kg/m2 is considered extreme obesity, with overweight classified as 25–29.9 kg/m.1,2 The prevalence of obesity worldwide has tripled since 1975, with ~39% of the world’s adult population being overweight and 13% being obese in 2016.2 In the USA alone, the number of individuals with obesity continues to dramatically increase, with >35% of individuals being overweight, >37% obese and 8% morbidly obese.2,3 Obesity is the biggest driver of preventable chronic diseases and healthcare costs in the USA, with current cost estimates ranging from $147–210 billion per year.4 Despite the magnitude of the problem and the associated healthcare costs, drug development efforts have largely failed and proposed treatments have had disappointing outcomes, with only modest reductions and frequent weight regain after successful weight loss.4,5
Obesity has a complex and multifactorial aetiology and the limited progress in obesity treatments can in large part be attributed to the failure to apply a systems biology-based approach to understand its pathophysiology and to develop individualized strategies to achieve sustained weight loss and prevention6,7 A growing body of largely preclinical studies support the concept of bidirectional signaling within the brain–gut–microbiome (BGM) axis in the pathophysiology of obesity, mediated by metabolic, endocrine, neural and immune system mediated mechanisms.8 Signaling from the brain through the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis influences many gastrointestinal processes, including motility and transit,9 fluid and mucus secretion,9 immune activation, intestinal permeability,10 relative gut microbial abundances,11 as well gene expression patterns in certain pathogenic gut microorganisms.12 Changes in the gut luminal environment can affect gut microbial community composition and function.13,14 Conversely, the gut microbiota can communicate with the brain via hundreds of metabolites,15-17 which are sensed by specialized cells in the gut, including enteroendocrine cells, enterochromaffin cells and primary or secondary afferent nerve endings. Sensing of bacterial metabolites by these cells results in neural signaling to the brain, interactions with gut-based immune cells leading to local and systemic immune activation, or the metabolite might achieve sufficient concentrations in the circulation to directly access brain circuits by crossing the blood–brain barrier.18 Short-chain fatty acids (SCFAs), the main byproduct of microbial fermentation of dietary fiber, have emerged as key mediators of BGM signaling.19 These saturated fatty acids can influence the central nervous system (CNS) through immune-,20 endocrine-,21 and vagal- pathways.22
The gut microbiome and bidirectional BGM interactions are programmed through influences during pregnancy and the first 1,000 days of life,23 and are subject to multiple perturbations from within the body (including from metabolism, gut microbiota interactions and energy expenditure) and from the environment (for example, via food, stress and medications) throughout life (Figure. 1). Perturbations at any level of the BGM system, resulting in compromised inhibitory mechanisms that normally regulate food intake, can bias ingestive behaviors towards predominantly hedonic-driven eating beahviours, cravings and overeating24-27. An extensive literature exists on the homeostatic regulation of food intake and maintenance of body weight via interactions between hypothalamic nuclei and orexogenic and anorexogenic gut hormones, in addition to chemical signals derived from adipose tissue, in particular leptin.28-30 However, it is ultimately the complex balance between gut-derived orexogenic (ghrelin, insulin) and anorexogenic signals (including cholecystokinin, neuropeptide Y (NPY) and glucagon-like peptide-1 (GLP1)), gut microbial metabolites (SCFAs and amino acid metabolites), stress mediators (corticotropin releasing factor (CRF)), and the motivational drive generated by the central reward system (dopaminergic reward system) and prefrontal cortical inhibitory mechanisms that determine how much we eat.31-33
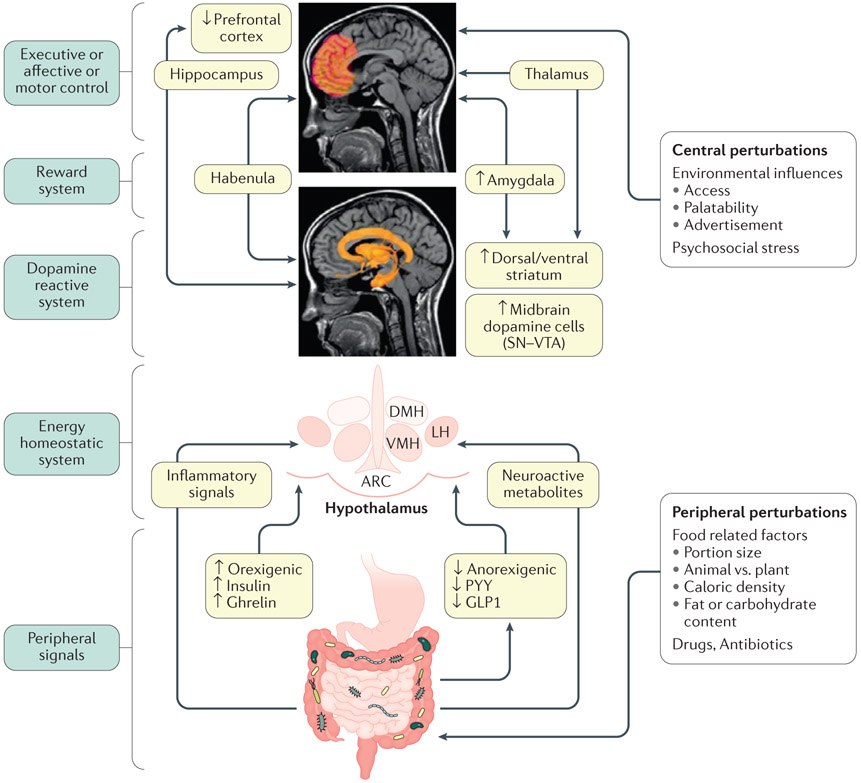
Figure 1. Model of brain–gut–microbiome interactions in ingestive behavior.
In the periphery, gut-generated and vagally transmitted orexogenic and anorexogenic signals interact with specific nuclei in the hypothalamus in the homeostatic regulation of food intake. Food-related factors interact with gut microorganisms and gut microbial metabolites modulate the release of orexogenic and anorexogenic peptides from enteroendocrine cells in the distal small intestine, shifting the balance between anorexogenic and orexogenic signaling in the hypothalamus. In addition, gut microorganisms can signal to the brain via inflammatory mediators (such as lipopolysaccharides) and neuroactive metabolites (such as tryptophan metabolites). Centrally, interactions between several brain networks, including the prefrontal cortex, the dopaminergic reward system and the sensorimotor system underlie the hedonic regulation of food intake. Several environmental influences such as food advertisements, food cues engage the extended reward system which can override the homeostatic control mechanisms. Exposure to visual and sensory cues, as well as psychosocial stress play important role in this process. Blue boxes on the left represent different parts of the BGM axis. Light green boxes in the center show mechanisms involved in altered BGM interactions in food addiction. Upward arrows show upregulation, downward arrows show downregulation. Modified with permission from Volkow et al. Biological Psychiatry 2013 EM: DONE
A particular type of eating behavior which has been referred to as food addiction, plays an important part in the pathophysiology of obesity.34,35 Food addiction is the continued consumption of highly palatable foods even after energy requirements have been met and despite known negative physical and psychological consequences in response to uncontrolled food ingestion.34,36 Similar to other forms of substance abuse, food addiction represents an addiction-like response to food (especially foods rich in sugar and fat) or the process of eating itself in susceptible individuals.37,38
Since it was first proposed by T. Randolph in 1956,39 there has been an ongoing controversy over the term of food addiction,40,41 despite strong arguments supporting shared underlying pathophysiology between drug and food addiction.33,42 On a behavioral level, individuals with food addiction as identified by the Yale Food Addiction Scale (YFAS)43 meet the diagnostic criteria for substance abuse disorder found in the Diagnostic and Statistical Manual of Mental Disorders44, which involves loss of control over eating, excessive time or focus on food, neglect of other activities and continuation of the behavior despite known negative consequences.45-47 An increasing number of research reports on the biological alterations in the extended reward network in both humans and rodents also point towards strong similarities in the mechanisms underlying substance use disorders and food addiction, including substantial commonalities between the neural substrates underlying the substance abuse and at least some forms of obesity that involve food addiction.31-33 For example, the biological similarities between individuals with obesity with food addition and individuals with drug addiction include, but are not limited to, changes in the dopaminergic pathways within the reward system, and in cortical performance monitoring, both of which are involved in processes associated with reward sensitivity, motivation, interoceptive awareness, stress reactivity and self-control.48-50 Despite these similarities, there are also clear differences. Although the development of predominantly hedonic driven eating behaviors involves food-induced alterations in multiple peripheral and central mechanisms of the BGM axis, drug addiction results from a direct effect of the drug on the brain.51,52 In addition, in contrast to the nearly universal development of addiction upon exposure to a drug, food addiction as assessed by the YFAS score is present in only a subgroup of individuals with obesity.46,53 On the basis of questionnaire-based surveys and by other methods of assessment, food addiction is present in 25–37% of individuals with obesity, reaching rates of up to 60% in those who are morbidly obese or in patients who undergo bariatric surgery.36,38,54-60 Food addiction is also highly associated with eating disorders such as bulimia nervosa and binge eating disorder.61,62
Previous work on obesity and food addiction crosses over multiple fields of research: neuroscience, gastroenterology, microbiology, endocrinology, immunology and many others. For example, the gut microbiome, intestinal signaling, extraintestinal signaling (visual, olfactory, food memories), early life programming of food preferences and many other factors can contribute to food addiction. Here, we review and build on past work to create a systems-based BGM model of obesity and food addiction. Systems biology is an interdisciplinary field of study that focuses on complex interactions within multiple biological systems, rather than focusing on individual mechanisms. One of the aims of systems biology is to model and discover emergent properties of cells, tissues and organisms functioning as a system rather than as individual parts. We believe that such an interdisciplinary, system-based approach is able to create a more nuanced understanding of food addiction, as shown in Figure 1. In this Review, we summarize the physiology of food addiction in obesity as it relates to alterations within the brain and the gut microbiome. We will introduce key factors that influence the BGM axis such as diet, antibiotics, early life adversity, food cues and psychological stress, during the prenatal and postnatal period, and during adulthood. This Review also discusses several therapies aimed at food addiction in individuals with obesity, including those targeted at the gut, the microbiome and the brain, and highlights limitations and areas for future research in the field.
Ingestive behaviour physiology
The role of the gut microbiome
Ingestive behavior represents a delicate balance between homeostatic and hedonic regulatory mechanisms in the CNS, orchestrated by a number of gut peptides, neuronal impulses, endocrine signals and countless other influences, including signals generated by the gut microbiota (Figure 1).
BGM interactions involving gut peptides that regulate ingestive behavior have been the most extensively studied. The gastric hormone ghrelin has an important role in producing hunger and craving,63,64 perhaps through amplification of dopaminergic signaling mechanisms,65 whereas the intestinal hormones GLP166 and peptide YY67 trigger satiety and associated behavioral changes. Increased production of microbiota-derived SCFAs can stimulate enteroendocrine cells to release GLP168 and peptide YY69, while decreasing the secretion of ghrelin.70
Insulin is another orexogenic hormone, as hyperinsulinaemia, regardless of plasma glucose levels, contributes to increased sensations of hunger and results in a heightened palatability of sucrose.71 Animal evidence suggests that disruptions of microbial SCFA metabolism can promote insulin resistance and hyperinsulinaemia,72 thereby potentially shifting the balance towards hedonic behaviors. For example, studies in mice have shown that increased production of acetate by an altered gut microbiota can lead to activation of the parasympathetic nervous system which in turn promotes increased glucose-stimulated insulin secretion and increased ghrelin secretion resulting in hyperphagia.73
Additionally, gut microbiota-derived secondary bile acids can regulate insulin sensitivity through signaling involving the nuclear farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5 (also known as G-protein coupled bile acid receptor 1 GBAR1).74 Activation of intestinal FXR in a mouse model induces microbial production of the secondary bile acid lithocholic acid, driving TGR5 signaling and triggering GLP1 secretion from enteroendocrine L-cells.75 Exposure to oral broad-spectrum antibiotics (combination of ampicillin, vancomycin, neomycin sulfate, and metronidazole) successfully inhibited microbial lithocholic acid production and completely reversed improvements in insulin sensitivity.75 In a single blind randomized controlled trial of 20 individuals with obesity, administration of an oral antibiotic (vancomycin) for just 1 week resulted in reduced microbial diversity (mainly affecting Firmicutes), with an associated drop in secondary bile acids as well as in insulin sensitivity.76
An example of microbial regulation of food preferences has been shown in a study demonstrating that fruit flies fed a diet deficient in essential amino acids show preferential intake of amino acid-rich foods.77 These preferences are, however, blunted by the presence of both Acetobacter pomorum and lactobacilli.77 Of note, neither Acetobacter pomorum nor lactobacilli were capable of modulating food intake individually, suggesting that these microorganisms must work together to influence host behavior.77 Although the mechanisms driving food seeking behaviors in this model remain unclear, microbial modulation of neuronal TOR signaling has been previously proposed as an important mediator of nutrient balance and growth in Drosophila.78,79 In fruit flies exposed to a nutrient scarce environment, Lactobacillus plantarum can promote protein assimilation from the diet, resulting in increased production of branched-chain amino acids.79 These amino acids activate central nervous system (CNS) TOR kinase activity, resulting in the release of insulin-like peptides.79
The role of the brain
Neuroimaging studies have improved our understanding of the role of the brain in ingestive behavior in both animals and more recently in humans, especially the interplay of various brain networks involved in homeostatic mechanisms versus food addiction (non-homeostatic).50,80-82 The homeostatic component of food intake is comprised of hormonal regulators of hunger, satiety and adiposity levels.83,84 The hypothalamus is the primary brain area within the homeostatic system that regulates food ingestion and energy balance, and hence is often referred to as the ‘satiety centre’ or ‘feeding centre’ of the brain.85-87
Normal ingestive behavior is under the control of the extended reward network, which includes brain regions from the core reward network such as the nucleus accumbens, ventral tagmental area or substantia nigra, and are regulated by cognitive network regions such are the prefrontal cortex.88,89 The extended reward network is involved in the processing of rewarding stimuli and modulation of food-seeking behavior,90,91 inhibitory control,92 cognitive performance monitoring,93,94 interoceptive and sensory awareness,88,95,96 and integrating salient information to make decisions regarding food intake.97-100 This processing includes brain regions concerned with interconnecting brain networks such as reward, salience, emotional regulation, the somatosensory system and cortical inhibition (prefrontal control) networks (Figure 2).31,33,90,99 The salience network is responsible for monitoring the homeostatic state of the body to make adaptive adjustments to real or expected disturbances in homeostasis through autonomic nervous system, as well as behavioral responses.98,101 In food addiction (as in substance abuse), the saliency of a specific type of reward (food or drug) becomes greater at the expense of other rewards.32,48 The emotional regulation network is activated by stimuli threatening the homeostasis of the organism, and provides a rapid feedback inhibition of such activation via its connections with the salience network.89,102,103 Advanced analytical techniques such as brain network metrics based on graph theory, which measure the underlying architecture and flow of communication between brain regions and networks, and multivariate machine learning methods that predict obesity have been applied to phenotype hedonic ingestion-related brain signatures, with a focus on alterations in the extended reward network.88,89,104-107
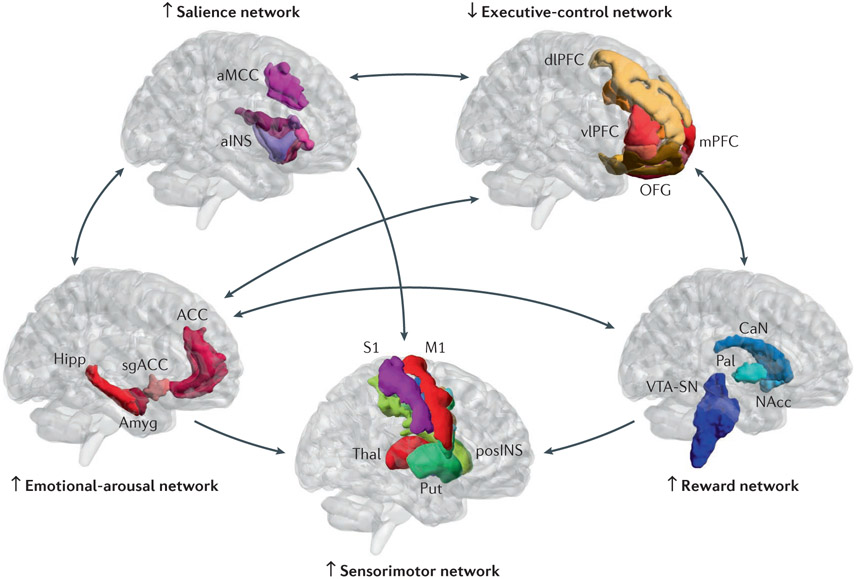
Figure 2. Model of altered brain network interactions in food addiction.
Several brain networks interact in the regulation of ingestive behavior. In food addiction, increased engagement of the salience network by food cues engages the executive control network leading to increased attention to food, and the emotional arousal network. Insufficient inhibitory control of the reward and of the emotional arousal networks by the executive control network plays a key part in shifting the balance from predominantly homeostatic to hedonic and regulation of food intake. The salience network (aMCC, anterior mid cingulate cortex; aINS, anterior insula) responds according to the subjective salience of any interoceptive or exteroceptive stimulus reaching the brain, or to the expectation of such stimuli, and coordinates appropriate attentional, behavioral, affective and visceral autonomic responses to such stimuli. The executive control network (dlPFC, dorsolateral prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; mPFC, medial prefrontal cortex; OFG, orbitofrontal gyrus) is activated during tasks involving executive functions such as attention, working memory, planning and response selection. Under normal circumstances, it exerts an inhibitory influence on the emotional arousal and the reward networks. The reward network (Nacc, nucleus accumbens; VTA-SN, ventral tegmental area – substantia nigra; CaN, caudate nucleus; Pal, pallidum) is a group of neural structures responsible for motivation, ‘wanting’ desire or craving for a reward. It is under inhibitory control by the executive control network. Its main neurotransmitter is dopamine. The sensorimotor network (Thal, thalamus; Put, putamen; pINS, posterior insula; S1/S2, primary/secondary sensory cortex; M1/M2, primary/secondary motor cortex) receives sensory input from the body, is important for awareness of body sensations and the generation of appropriate motor responses and behaviors.
The Emotional arousal network (ACC, anterior cingulate cortex; Hipp, hippocampus; Amyg, amygdala; sgACC, subgenual anterior cingulate cortex) is activated by perceived or real perturbation of the organism’s homeostasis. Bidirectional arrows between brains depict reported bidirectional network interactions. Up and down arrows next to brains illustrate reported up and down regulation of the individual networks
Homeostatic versus hedonic systems
Homeostatic system.
The hypothalamus acts as a hub integrating information from the external environment, such as food availability and stress, and from the internal milieu of the host to meet real or perceived nutrient needs.108,109 Lesions in the hypothalamus, in both humans and animals can lead to increases in appetite, food ingestion and weight gain.110,111 Numerous studies have been directed at identifying the molecular mechanisms within the hypothalamus underlying these processes.85,108,112 For example, animal models have demonstrated that brief electrical stimulation of nuclei within the hypothalamus can cause the increased expression of genes related to Agouti-related protein (AGRP)–NPY–γ-aminobutyric acid (GABA) signaling, which in turn can cause voracious food ingestion.113,114 These targeted cells have, therefore, been referred to as ‘hunger neurons’.86,115 The hypothalamus has close interactions with corticolimbic and medullary pontine regions integrating sensory information mediated by vagal afferents, affective state and cognitive modulation to generate appropriate motor responses and adaptive eating behaviors.85
Hedonic system.
Individuals with obesity and with food addiction are more likely than individuals who are lean or obese without food addiction to display a heightened motivation to eat highly palatable foods, consume more calories from fat and protein, and have at least 20% prevalence rates with comorbid conditions, such as depression, binge eating, and decreased quality of life functioning, beyond that observed with obesity alone.116-119 The closely regulated balance between hedonic and homeostatic aspects of ingestive behavior can be altered when normal inhibitory regulation of the reward system is compromised via decreased modulation or connectivity, resulting in overconsumption of food. There are similarities between food addiction and other addictive behaviors, as both reflect an imbalance in responses within the brain’s extended reward system to stimuli from the environment.33,120 In food addiction, such uncoupling can be the result of central as well as peripheral disturbances in brain–gut interactions, including diet-induced neuroplastic changes in the sensitivity of vagal afferent nerve terminals and of hypothalamic nuclei to satiety hormones,121-123 emotional state, and easy access to highly processed and palatable foods that all modify the rewarding properties of food, thereby leading to overconsumption.32,48 Both human and animal studies have shown that increased cravings and food addiction behaviors result in increased conditioning and motivation to seek these highly palatable foods, and these behaviors are based on alterations of regions within the extended reward network.31,33,124,125
Evidence also exists suggesting that, similar to other addiction like behaviors, food addiction is associated with a reduced response of the reward network.126 For example, ingestion of rewarding foods or drugs leads to reduced dopamine signaling within the reward system in both individuals with obesity and those with drug addiction,127 suggesting that a reduction in dopamine signaling (by both the reduced release of dopamine and the downregulation of dopamine receptors) might contribute to the overconsumption and increased cravings of the drug of choice.128-130 According to the dopamine deficiency hypothesis, reduced dopamine release in the striatum alters corticostriatal communication between the basal ganglia (core reward) and the extended reward system, resulting in compromised inhibition of connectivity in the reward regions.131 Reduced cortical inhibition of reward regions is also associated with greater cravings and reduced disinhibition scores.132-134According to this theory, hypo-dopaminergic function also leads to reduced levels of subjective well-being, as it is linked to dysregulation of other neurotransmitters such as 5-HT, enkephalins and GABA.131 To compensate for this dopamine deficiency, it has been suggested that affected individuals will engage in behaviors that stimulate the brain’s production and utilization of dopamine, such as by the overconsumption of highly palatable and rewarding foods,131,135,136 increasing the risk of developing food addiction and obesity.137,138 Thus, a stronger stimulus (for example, increased food intake) is required to overcome the reduced responsiveness of the dopamine system, similar to mechanisms identified in disorders of addiction, and failure to achieve this goal is associated with food cravings and the engagement of the stress response.31,32,48,139,140 Stress induced eating usually depends on a number of factors such as the length of the stress, type of stressor, type of foods available, especially if calorie dense and highly palatable, length of time exposed to the food, and satiety and hunger levels.124 Studies have shown that during stress, increased cortisol levels could contribute to increased gluconeogenesis, an upregulation of corticotrophin releasing factor (CRF) in the amygdala and other limbic regions, and consequently the blunting of HPA axis function, which in turn leads to low dopamine and reward functioning commonly associated with food addiction.125 Stress has also been associated with increases in ghrelin and cortisol and related increases in craving and intake of highly palatable foods, which were higher in those with obesity and overweight than lean individuals.141 A study performed in obese rats demonstrates downregulation of striatal dopamine D2 receptors compared to lean rats, similar to what has been shown in previous studies of humans addicted to drugs.142,143 Furthermore, D2 receptor knock down mice rapidly develop compulsive-like food seeking behaviors when high-fat food is readily available.142
Even though dopamine has been the most thoroughly investigated signaling system in addictive behaviors, several neurotransmitters other than dopamine and neuropeptides are involved both in the homeostatic regulation of food intake (including orexin, leptin and ghrelin, corticotropin releasing factor) and have been implicated in the rewarding effects of food, cannabinoids, opioids and serotonin.48 Moreover, neurons in ventral tegmental area and/or nucleus accumbens express GLP1, ghrelin, leptin, insulin, orexin and melanocortin receptors, suggesting that these hormones or peptides can influence the rewarding responses to food.48 Rats that are fed a diet high in saturated fats and refined sugars for two months demonstrate reduced hippocampal brain-derived neurotrophic factor, with a concomitant reduction in synaptic plasticity.144
Food addiction pathological mechanisms
The first 1,000 days of life represent a crucial developmental period for the gut-associated immune system and the BGM axis.23,145,146 Preclinical models of myelination and brain development suggest that the early life microbiome regulates myelination of the prefrontal cortex147 and facilitates proper striatal synapse function.148 Additionally, commensal microorganisms might also have a role in programming the HPA axis for stress responses,149 a system implicated in obesity and food addiction behavior,150-153 whereby excess cortisol and related steroids, such as those from a disrupted of HPA axis, can drive adipogenesis154 and increase food cravings.152 Early life exposure to different microorganisms, antibiotics, dietary factors and stress shape the relative abundances and richness of the gut microbiota, influence immune system and brain development, modulate microbial communication with the CNS and program maladaptive BGM interactions.8,155,156 Although these interactions and their roles in the development of obesity have been well described (Figure 3),8,155,156 their links with maladaptive eating behaviors is incompletely understood.
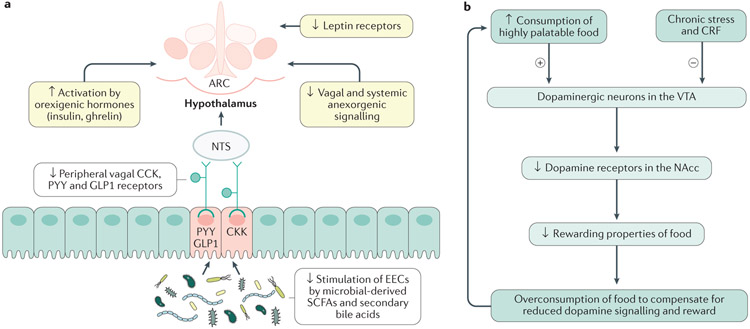
Figure 3. Mechanisms in the homeostatic and hedonic systems leading to food addiction.
a ∣ Diet-induced disinhibition of vagal and hypothalamic satiety mechanisms. A high fat, low fibre diet reduces the release of satiety hormones (GLP1, PYY, CCK) from enteroendocrine cells (EECs) in the gut by dietary fibre-derived short-chain fatty acids (SCFAs), leads to downregulation of receptors for satiety hormones molecules on vagal afferents innervating the EECs, and to a downregulation of the vagally-mediated satiety signaling to the arcuate nucleus of the hypothalamus (ARC). Hypothalamic receptors mediating the effect of other anorexigenic signals (leptin) reaching the ARC via the systemic circulation are also downregulated, resulting in an unrestrained effect of orexogenic signals (ghrelin, insulin, cortisol). Thus, chronic exposure to a high fat, low fibre diet downregulates the inhibitory mechanisms of homeostatic regulation of ingestive behaviors. b ∣ Diet-induced changes in the extended reward system. According to the dopamine deficiency hypothesis, a reduction of dopaminergic stimulation of neurons in the NAc as a result of reduced dopamine relase from the VTA, and a downregulation of dopamine receptors on NAc neurons, reduces the rewarding effects of ingested foods, and leads to craving and overconsumption of unhealthy food in an attempt to compensate for the reduced dopamine signaling. Chronic stress-induced CRF release and glucocorticoid levels also have an inhibitory effect on dopamine signaling. Up and downward arrows inside boxes illustrate reported up and downregulation of respective mechanisms.
Prenatal developmental influences
Maternal prenatal factors have been shown to influence development of the infant BGM axis, with evidence suggesting an important role of the prenatal maternal diet in influencing the neonate gut microbiome. For example, greater maternal consumption of dairy during pregnancy was positively associated with a greater relative abundance of members of the genus Clostridium in the faecal microbiome of 145 infants, adjusted for maternal BMI, feeding method and parity.157 Similarly, a maternal high-fat diet was associated with depletion of the genus Bacteroides in the neonatal gut microbiome, which persisted through 4–6 weeks of age.158 These changes might be mediated indirectly by maternal dietary influences on the composition of breast milk, or directly from effects of the maternal diet on the fetal gut microbiome.157,158,159 Maternal psychosocial stress has been implicated in the development of an obese phenotype. For example, severe maternal stress due to bereavement in the prenatal period was associated with increased BMI in the male adult offspring in a study of 120,000 men, regardless of trimester that the bereavement occurred in.160 In mice, moderate maternal stress during pregnancy was found to influence postnatal brain development and gene expression in the paraventricular nucleus of the hypothalamus, the hypothalamic regulator of the HPA axis, ultimately resulting in deficits of neuroplasticity and central stress responsiveness. This effect was partially mediated by stress-induced alterations in the maternal vaginal microbiome.161 As maternal antibiotic use during the second or third trimester is associated with an increased offspring risk of obesity regardless of pre-gravid BMI in a study of 436 mother–child dyads,162 and the vaginal microbiota play an important part in shaping the infant microbiome, as demonstrated in a very well-characterized group of 9 mothers and their 10 newborns,163 it is possible that changes in maternal vaginal microbial abundance associated with antibiotic exposure or diet during pregnancy might also increase the risk of obesity in the offspring.
Postnatal influences
Early diet.
The infant gut microbiota is sensitive to early life nutrition. Human breast milk contains >200 different human milk oligosaccharides (HMOs), a type of prebiotic that cannot be degraded by gut glycoside hydrolases or absorbed via intestinal membrane transporters, suggesting that their primary target is the infant’s gut microbiota.164 The limited small intestinal bioavailability of HMOs enables efficient delivery to the developing infant gut microbiota, most notably Bifidobacterium, which can degrade these sugars.164 Exclusively breastfed infants show a more diverse Bifidobacterium microbiota (175 faecal samples from 7 infants) compared with exclusively formula-fed infants (154 faecal samples from 7 infants), which might set the stage for future, beneficial BGM interactions165 given that a robust Bifidobacterium community has been shown to be protective against intestinal infections166 and associated with appropriate development of the infant immune system.167 Limited data exist on the effect of combination feeding (breastmilk and formula) on the gut microbiome, despite how common it is.168 Bogen et al.,169 in an observational study of over 70,000 infants, suggest that a longer duration of partial breastfeeding is necessary to yield a similar protective effect against obesity compared with exclusive breastfeeding; combination feeding for >26 weeks yielded a similar protective effect (odds ratio (OR) of developing obesity: 0.70; 95% CI: 0.61–0.81) as exclusive breastfeeding for 16–26 weeks (OR: 0.71; 95% CI: 0.56–0.92).
The relative abundances of the infant gut microbiota and its associated microbial transcriptome change substantially once solid food is incorporated at around 9 months of age, including increased abundances of Bacteroidetes and elevated SCFA levels, as suggested by a high-quality, 2.5-year case study of 60 faecal samples from a single infant170. However, evidence has shown that the faecal microbiota profile assessed at 3 months of age (composed of primarily Bacteroidaceae, Bifidobacteriaceae, Enterobacteriaceae, Lachnospiraceae, Ruminococcaceae, and Veillonellaceae) is a more reliable predictor of future risk of being overweight compared to querying the microbiota profile at 12 months, as suggested by a study of 1,087 infants171 These findings are supported by a meta-analysis of >200,000 participants that found that breastfeeding was associated with a statistically significant reduced risk of obesity in children (pooled adjusted OR: 0.78; 95% CI: 0.74–0.81), with a subset of studies even revealing a dose–response relationship between breastfeeding duration and a reduction in obesity risk.172
Highly processed foods, which are filled with large amounts of salt, sugar, fat and additives have become increasingly available in the developed world.173,174 A pattern of increased exposure and ingestion of such foods in childhood might program food preferences and increase the risk of the development of food addiction into adulthood.175 Additionally, the relentless marketing of such foods, starting in childhood, has contributed to the increased uncontrollable consumption and cravings of unhealthy foods, especially in children.176,177
Antibiotics.
An analysis of outpatient antibiotic prescription rates in 2010 found that >70% of prescriptions in the USA were written for antibiotics, with the highest prescribing rates for children under 10 years of age and with an average of three doses of antibiotics by the age of 2 years.178 In a USAcohort study of 333,353 children, antibiotic prescriptions were significantly associated with a diagnosis of childhood obesity (HR: 1.26; 95%CI: 1.23–1.28).179 In a longitudinal study of 39 healthy children, the gut microbiota of antibiotic-treated children was found to be less diverse at multiple phylogenetic levels, with some species even dominated by a single strain. However, the gut microbiome, largely appeared to return to baseline within 1 month of antibiotic exposure.180 In a Danish study examining over 28,000 mother–child dyads, early administration of antibiotics – within the first 6 months of life – was associated with an increased risk of being overweight at a 7-year follow-up in children from normal weight, but not overweight, mothers; the gut microbiota of study participants were not examined in this study.181 Though it is difficult to draw definitive conclusions from natural history and cross sectional epidemiological studies, numerous preclinical studies182-185 also support a negative effect of early life antibiotics on energy metabolism, the immune system and obesity. Mice that received a single dose of low-dose penicillin at birth showed enhanced high-fat diet-induced obesity as adults; this phenotype could be successfully transferred to germ-free mice by the penicillin-selected microbiota.182 Another mouse study showed that a single early-life macrolide antibiotic course resulted in persistent perturbations to the gut microbiome (increased Akkermansia muciniphila attributed to reductions in competitor mucin-degrading bacteria) and the immune system (reduction in small intestinal CD4+ IL-17A+ lymphocytes, decreased intestinal IgA secretion).186 Collectively, these studies suggest that the antibiotic-altered microbiota, and not the antibiotic itself, has a causal role in driving obesogenic metabolic and immunological changes in mice. It remains to be determined if and how the antibiotic induced microbiome alterations can influence the brain and alter ingestive behaviors.
Early adversity.
A history of early adverse life events (EALs) such as natural disaster, parents divorcing, emotional or physical abuse, sickness or death of a family member, predisposes individuals to develop obesity and food addiction in adulthood, mainly through mechanisms associated with stress, inflammation, emotional perturbations, maladaptive coping responses and metabolic disturbances.187,188 Studies including a meta-analysis have demonstrated that trauma and abuse during the developmental period is significantly associated with greater odds of adulthood obesity and substance abuse (OR: 1.34, 95% CI: 1.24–1.45, P<.001).189-191 Preclinical models of early adversity (maternal separation model) have observed that addictive behaviors can develop later in life, but these animal models require further investigation in humans, especially as the underlying mechansims are unknown and these animal studies are poor models of human behaviour.192 For example, rats exposed to limited nesting stress in the post-natal period had an immature HPA axis, which was associated with reduced gut microbiota diversity, with an especially notable reduction in bacteria capable of degrading fiber.193 Although the causal relationship between adversity during childhood and adult obesity is incompletely understood, it has been suggested that overconsumption of highly palatable foods even when hunger and satiation have been met are a possible coping mechanisms to deal with the increased stress responsiveness seen in individuals with a history of EALs.194-197 In a study of 186 men and women comparing healthy individuals with those with obesity, a history of EALs was associated with alterations in resting state functional connectivity, shown using MRI of brain regions in the extended reward network.198 These EAL-related alterations probably contributed to an increased probability of developing food addiction and obesity later in life. In a network analysis, sex-differences were also noted in the interactions between early life adversity, brain connectivity and food addiction, suggesting that the development of food addiction might be driven by different factors in men versus women.198 For example, compared to men, women show increased post-prandial activations in the brain’s reward regions, which might increase susceptibility to cravings for highly palatable foods and result in hyperphagia.199,200 Although the exact molecular mechanisms by which women, compared to men, experience this increased susceptibility remain unclear, results from a pilot study of 63 individuals with varying BMI levels suggest that EALs might contribute to the development of food addiction by interfering with interactions between the brain and gut microbiome, specifically microbial tryptophan metabolism and reward brain regions such as the amygdala, anterior insula and nucleus accumbens.201
Adult environmental influences.
Several environmental factors might contribute to the pathophysiology of obesity by influencing BGM interactions in the adult. Below we discuss some of these environmental factors in detail.
Diet.
Cheap, highly processed and easily accessible high caloric and palatable foods are abundant in the developed world.202 Studies have shown that foods that are enhanced for taste and salience not only increase cravings and ingestion of these foods, but contextual factors such as stress can serve as conditional cues for future food intake and long-term weight gain.125,203,204 In fact, overconsumption of highly palatable foods, in particular those containing high levels of fat and sugar, reduces the rewarding thresholds of such foods when ingested, in relationship to reduced levels of dopamine and dopamine receptors in the brain, therefore requiring an increased intake of such foods to generate the same satisfaction.205,206 Although long-term ingestion of such highly palatable foods has been shown to alter gut microbial diversity and relative abundances in humans, it is important to note that the adult microbiome is relatively resistant to short-term changes in diet, as suggested by a high-quality study of 98 individuals.207,208
Food cues.
Studies have shown that portion sizes are directly related to a compromised ability to control food intake, an important feature of food addiction.209 Food labels and plate and utensil sizes can moderate the portion control effect by increasing food intake.210 Although the exact mechanisms are unknown, these development and marketing driven food-related cues, which are ubiquitous in Western media and marketing, influence individuals with obesity to consume a greater number of calories than lean individuals.211,212,213,214 Individuals with obesity have also reported an increased preference and craving for foods rich in fat and sugar, in a study of over 46,000 adults.215
Psychosocial stress.
Psychosocial stress can also stimulate ingestive behavior in the adult by increasing appetite, cravings and motivation to consume highly palatable foods, thereby contributing to stress-related weight gain in obesity.125,216,217 In individuals with obesity, strong associations have been shown between perceived stress and food addiction, snacking, cravings and abnormal eating patterns.124,218 For example, a study of 339 adults (mean BMI = 26.7 ± 5.4 kg/m2) demonstrated that chronic stress can influence levels of the orexogenic hormone insulin, as well as glucose and cortisol responses, which in turn can lead to increased food intake and weight gain.219 Paradoxically, although the ingestion of ‘comfort foods’ high in fat and sugar can reduce subjectively perceived stress, various studies have shown that ingestion of such foods can also lead to increased autonomic responses, disrupt the HPA axis and increase cortisol and ghrelin levels, which have been associated with increased cravings and ingestive behaviors.132,220-222
In a mouse model, chronic psychosocial stress resulted in a global reduction in gut microbiota richness and diversity, including a reduction of the relative abundance of Akkermansia223, which has been suggested to have beneficial effects within the context of human obesity and metabolic syndrome by previous investigations.224 These stress-induced perturbations were also associated with changes in the functional profile of the gut microbiome, with decreased synthesis and metabolism of SCFAs, tryptophan and tyrosine.223 These changes might have been mediated, at least in part, by alterations in immunoregulatory signals, as these mice showed transient elevations in number of IL-10+ T regulatory (Treg) cells, which were suppressed over time.223
Amino acid metabolites.
Tryptophan and its metabolites – serotonin (5-HT), kynurenine and indole – have been implicated as important mediators of BGM interactions within the context of obesity and food addiction.225,226 5-HT, due in part to its diverse roles as a neurotransmitter in both the gastrointestinal tract (that is, in processes such as peristalsis, secretion and absorption) and the CNS (that is, in regulation of pain modulation, sleep and mood), is the most extensively studied of these metabolites.227 95% of the body’s 5-HT is stored in gastrointestinal enterochromaffin cells (ECCs) and the gut microbiota, through the production of SCFAs and secondary bile acids, can regulate 5-HT synthesis and its release from ECCs.228-230 Human studies incorporating acute tryptophan depletion (typically involving providing individuals with a large protein load containing non-tryptophan large neutral amino acids to saturate amino acid blood-brain barrier transporters, thereby limiting the transport of endogenous tryptophan), a validated method to transiently reduce peripheral and central 5-HT synthesis, underscore the effect of changes in 5-HT release on food preference.231 In a study of 55 women following acute tryptophan depletion, participants with overweight showed a statistically significant increase in sweet calorie intake and preference for sweet foods compared with a placebo intervention.232 By contrast, the lean group showed no differences, suggesting that individuals with overweight might be more susceptible to changes in tryptophan metabolism and 5-HT availability.232 Host and microorganisms participate in different aspects of tryptophan metabolism: although host cells have the major role in the kynurenine pathway, microbial cells are primarily involved in the indole pathway.233
Although 5-HT has been the most extensively studied tryptophan metabolite, the majority of tryptophan is converted by host cells to kynurenine.234 In the gastrointestinal tract, kynurenine is synthesized from tryptophan by the rate limiting enzyme indoleamine-2,3-dioxygenase (IDO), which can be upregulated by inflammatory cytokines or downregulated by reactive oxygen species, such as hydrogen peroxide produced by intestinal Lactobacillus.235,236 As both kynurenine and tryptophan compete to cross the blood–brain barrier through the same, easily saturated transporter, inflammation-associated or microbiota-associated changes in peripheral kynurenine concentrations might also influence central 5-HT levels.237 Alternatively, increased flux through the kynurenine pathway can influence the brain through neuroactive downstream metabolites such as kynurenate and quinolinate, which function as an N-methyl-D-aspartate (NMDA) antagonist and an NMDA excitotoxin or neurotoxin, respectivly.235 The balance of tryptophan metabolism might be preferentially shunted towards the kynurenine pathway in individuals with obesity, as serum kynurenine, kynurenate and quinolinate levels show positive associations with BMI.238
Another important group of tryptophan metabolites are the indoles. Most undigested dietary tryptophan in the gut lumen is converted exclusively by gut microorganisms to indole.239 In animal and human studies, indole has been shown to play an important part in modulating kynurenine synthesis7, strengthening the mucosal intestinal barrier,14 attenuating CNS inflammation,15 and modulating GLP1 secretion,240 all of which have been shown to be disrupted in states of obesity.238,241-243 One study investigating the role of stool indole metabolites in 63 healthy individuals found positive associations between indole, skatole and indoleacetic acid and food addiction behaviors, with regions of the extended reward network (nucleus accumbens, amygdala, and anterior insula) playing an important part in this interaction.201
Metabolic toxaemia.
In mouse models of obesity, a diet high in fat (60% lard) and low in dietary fibre has been implicated in the longterm disruptions in gut microbiota diversity,244 while diets high in fibre result in positive alterations in ingestive behavior (decreased food intake, increased satiety)245. When dietary fiber is reduced or unavailable, certain gut microorganisms such as Akkermansia mucinophilia consume the glycans making up mucins in the mucus layer of the gut, thereby compromising intestinal barrier function.246 This phenomenon is referred to as increased ‘leakiness’ of the gut (Figure 4). Sonnenburg et al.244 showed in mice that a low fibre diet results in a substantial loss of microbiota diversity and abundance, which was magnified in each successive generation, up to the fourth and final generation studied.244 Remarkably, supplementation with a high fibre diet alone was insufficient to normalize microbial diversity.244 Reduced intestinal barrier function results in increased access of membrane bound lipopolysaccharide (LPS) from Gram-positive microorganisms to TLR4 receptors on host epithelial and immune cells, contributing to inappropriate immune activation.247-250
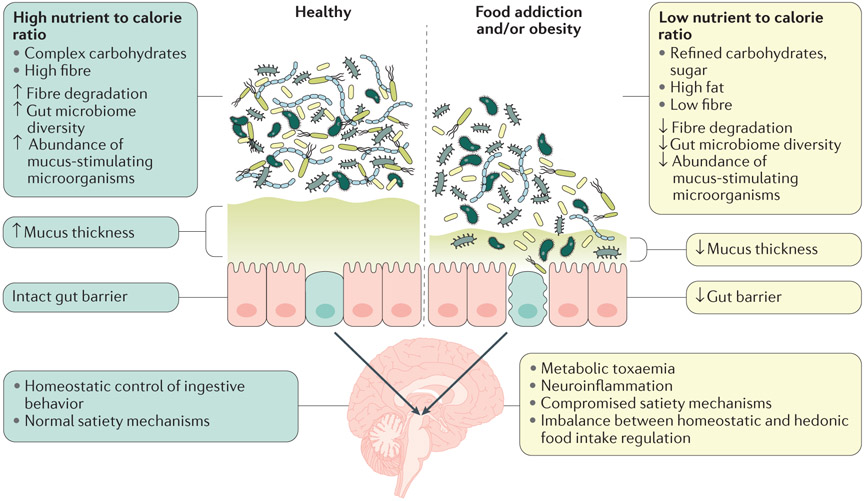
Figure 4. Interactions between food, gut microbiota and intestinal permeability in the regulation of ingestive behavior.
Left panel: A healthy diet (high in fibre, low in fat and sugar) is associated with a high diversity of the gut microbiota, including an abundance of taxa involved in stimulating mucus production in humans and animal models.323,324 The combination of an intact mucus layer and tight intestinal epithelium results in an intact gut barrier. Right panel: An unhealthy diet (high in fat, sugar and low in fiber) is associated with a reduced microbial diversity, reduction of mucus stimulating microorganisms, reduction in mucus layer thickness and an increase in epithelial leakiness. This process results in reduced intestinal barrier function (leaky gut) and activation of the gut associated immune system by microbial products such as lipopolysaccharide (LPS). The combination of a leaky gut and an overabundance of inflammatory bacterial products is thought to result in elevated plasma levels of LPS and proinflammatory cytokines. This state of metabolic endotoxaemia has been shown to reduce central satiety mechanisms by influencing enteroendocrine secretion of the satiety hormones PYY, cholecystokinin and serotonin (5-HT), and by reducing the expression of anorexigenic peptide receptors and leptin receptors on vagal afferents and in the hypothalamus, respectively, leading to a disinhibition of satiety mechanisms. Up and downward arrows inside boxes represent reported up and downregulation of mechanisms and of microbiome measures. Modified with permission from Cani & Everard. Mol. Nutr Food Res 60:58-66; 2016 OK, but would replace the last one with reduction of net mucus production
The combination of a leaky gut and an overabundance of inflammatory bacterial products is thought to result in elevated plasma levels of LPS and proinflammatory cytokines, including IL-1β, IL-6 and TNF.251 Increased systemic immune activation can shift tryptophan metabolism towards the kynurenine pathway and away from 5-HT or indole synthesis, as described previously.252 This state of metabolic endotoxaemia has been shown to reduce central satiety mechanisms by influencing enteroendocrine secretion of the satiety hormones PYY, cholecystokinin and 5-HT253-255 and by reducing the expression of anorexigenic peptide receptors and leptin receptors on vagal afferents256 and in the hypothalamus, respectively.257 In this way, vagal afferent neurons in the presence of a high-fat diet remain in an orexigenic state, regardless of whether food was consumed, driving hyperphagia and obesity.258 In addition to changes in ingestive behavior, there are likely numerous other mechanisms contributing to high-fat diet-induced obesity, such as gut microbiota-driven remodeling of the intestinal transcriptome to favor an obesogenic signaling cascade.259
Although the gut microbiota has an important role in the generation of inflammatory mediators, it might also be protective against the development of metabolic endotoxaemia. For example, mice fed a high-fat diet that was supplemented with oligofructose, a prebiotic fiber that preferentially increases gut Bifidobacterium abundance, showed reduced endotoxaemia, decreased levels of plasma and adipose tissue proinflammatory cytokines, and improved glucose tolerance.260 In this study, no relationship was seen between endotoxaemia and any other bacterial group except for Bifidobacterium.260 The translatability of these preclinical findings to human metabolic disease remains to be determined.
In summary, disruptions during both early life (prenatal influences, including maternal diet, antibiotic exposure and early adversity) and adulthood (diet and/or psychosocial stressors) can have a profound effect on the gut microbiome and the brain, setting the stage for food addiction. The associated changes in amino acid metabolism and metabolic toxaemia perpetuate these maladaptive changes at all levels of the BGM axis (Figure 1).
Clinical implications of food addiction
The proposed system biological model of altered BGM interactions resulting in maladaptive changes in ingestive behavior provides not only a plausible explanation for the refractory nature of obesity to many traditional therapeutic strategies, but also presents a rationale for several new therapeutic strategies (Figure 5, Box 1).
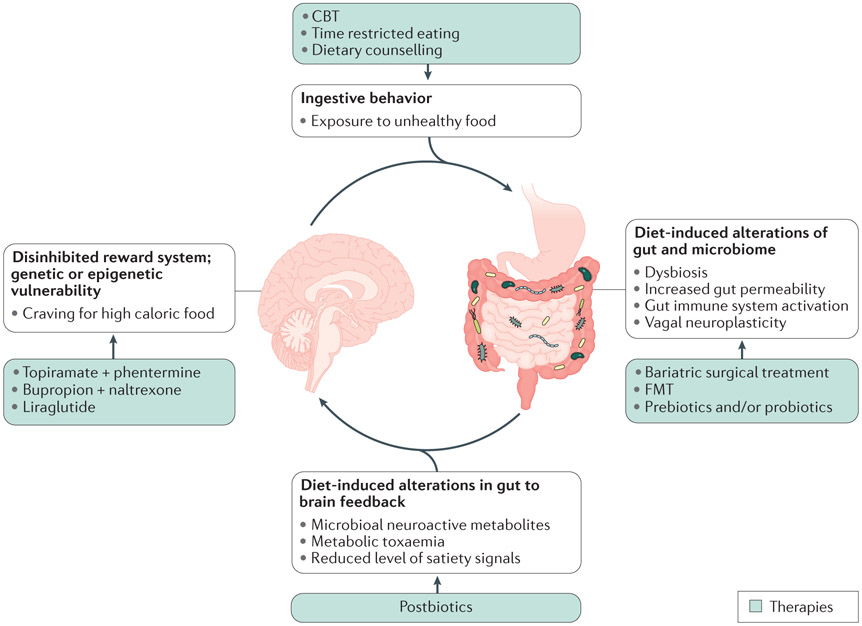
Figure 5. Circular model of brain gut microbiome interactions in obesity and targets for intervention.
The interaction of genetic and epigenetic factors influences the balance between hedonic and homeostatic control of ingestive behavior, and the risk for the development of hedonic dominance. When exposed to ubiquitous food of high caloric density (fat, sugar) and low in fiber, predisposed individual will overconsume such foods, resulting in changes in the gut and the microbiome as shown in Fig. 3. The resulting change in gut to brain signaling can further compromises homeostatic regulation of food intake and reinforces the disinhibition of the reward system. Targets for intervention and therapeutic modalities include altered ingestive behavior (cognitive behavioral therapy, CBT; time restricted eating; dietary counseling), alterations of gut and microbiome (bariatric endoscopic and surgical treatment; faecal microbota transplantation (FMT); prebiotics and probiotics); altered gut to brain feedback (postbiotics such as butyrate, tryptophan derived compounds, including indoles, and other amino acid metabolites.) and altered reward system (centrally acting medications).
Box 1 ∣. Therapeutic targets within the brain gut microbiome axis for obesity.
| Brain Targets | Approaches for Obesity Treatment |
|---|---|
| Cognitive Control |
|
| Reward Processes |
|
| Homeostatic Control |
|
| Gut Remodeling |
|
| Gut Absorption |
|
| Gut Microbiota |
|
Gut-directed therapies
As the gut is the primary source of hunger and satiety signals that regulate homeostatic feeding behaviors, it is not surprising that several obesity treatments, including bariatric surgery, have aimed to modify these gut mechanisms. Most of the bariatric procedures result in satisfactory and sustained weight loss and prompt resolution of the metabolic syndrome, a substantial improvement over the transient and more modest effects seen with medical therapies.261,262 Bariatric surgery-related weight loss is multifactorial, with the most common procedures, Roux-en-Y gastric bypass (RYGB) and laparoscopic sleeve gastrectomy (LSG), resulting in hypophagia. This hypophagia is not only a consequence of a reduced gastric capacity but also of marked reductions in appetite, food preferences and food addiction,263-265 which predicted long-term weight loss outcomes.266-268 Evidence also exists for the role of bariatric surgery-induced weight loss driving remission of food addiction (as assessed by YFAS); one study of 14 patients with obesity and with food addiction prior to surgery demonstrated remission of food addiction in 93% following surgery-induced weight loss (P<0.001).269 The mechanisms behind the post-bariatric surgery reductions in food addiction scores and brain responses to highly palatable food cues, as suggested by the aforementioned small, pilot studies, are incompletely understood.263,265 Both, RYGB and LSG result in increases in blood levels of anorexigenic gut peptides (GLP1, PYY) that, in part, mediate changes in appetite and food addiction after bariatric surgery.263,270-272 However, several other BGM pathways have also been implicated from both preclinical (mouse models) and clinical studies as possible explanations for these changes, including enhanced microbial production of polyamines and GABA, changes in bile acid profiles and FXR pathway signaling, and increased production of SCFAs.273-277 Preliminary work in individuals with obesity undergoing bariatric surgery suggest that changes in gut microbiome composition and microbial metabolism of aromatic amino acids and glutamate were associated with reductions in appetite, food addiction and changes in food preferences, suggesting a possible causal role of these metabolites in behavioral responses.278-283 However, future studies are needed to confirm causality between gut microbial metabolites and food addiction in humans. Another important consideration is the well-known reduction in systemic low-grade inflammation and endotoxaemia seen after bariatric surgery.284 As discussed earlier, this anti-inflammatory effect could increase hypothalamic sensitivity to satiety signals and to insulin, resulting in a shift towards more homeostatic regulation of ingestive behaviors.285-290 Some studies have suggested that because of the involvement of the brain’s reward system in both food addiction and addiction of other substances, bariatric surgery might increase alcohol use.291
Microbiome-directed therapies
Microbiome-directed therapies, including faecal microbiota transplantation (FMT), represent a novel therapeutic option for obesity and metabolic syndrome. Small clinical studies (one study had 18 individuals, 9 receiving autologous FMT and 9 receiving allogenic FMT from a lean donor, and a second study had 38 individuals, 12 receiving autologous FMT, 26 receiving allogenic FMT from a lean donor) have shown that FMT from a lean donor resulted in an increase of butyrate-producing bacteria and improved insulin sensitivity in recipients with metabolic syndrome.292,293 The improved insulin sensitivity and associated changes in the faecal microbiome were not sustained at 18-week follow-up, suggesting a resilient faecal core microbiome.293 It remains unclear if FMT, when combined with lifestyle modification and brain-directed therapies, might result in longer-term success. Notably, lower recipient baseline faecal microbiota diversity was predictive of success of FMT.293 Ingestive behaviors were not assessed in those studies. It is well known that microbial products such as SCFAs modulate feeding behavior via central mechanisms.19 For example, the intake of a type of fiber that selectively increases gut microbial propionate production was associated with lowering the subjective appeal and the brain reward activation (as measured by brain MRI) to highly-palatable-food pictures in a study of 20 healthy men without obesity.21 It is important to note, however, that FMT is not without risks, including the rare but well-documented risks of bacteraemia or sepsis, ileus, perforation and aspiration, in addition to the more common, transient gastrointestinal complaints such as abdominal pain or changes in bowel habit.294
Time restricted eating
Increased interest exists in the potential health benefits of different types of time-restricted feeding (TRF) on obesity, cardiovascular health and ageing, including intermittent feeding and the fasting mimicking diet.295-297 In mice, ad libitum exposure to a high-fat diet resulted in changes in circadian rhythms and feeding behaviors that led to increased energy intake and weight gain.298 These changes could be reversed by TRF.299 One study showed that individuals with overweight, but otherwise healthy, adhering to TRF with the assistance of a smartphone application significantly reduced their daily-energy intake, in part by reducing late-night intake of alcohol and snacks. This behavioral change resulted in sustained weight loss up to 1 year after the intervention.300 The role that the microbiome plays in mediating the effects of TRF in humans is not known. However, in animal and in humans, gut microbiota composition and function display oscillations during the day that are associated with the circadian rhythm of the host and are dictated by the host’s food intake patterns.301 These oscillations in microbial metabolite production might have a major effect on the circadian epigenetic and transcriptional landscape of the gut and the liver, and eating behaviors leading to compromised oscillations have been associated with obesity and metabolic syndrome.302 There are other potential factors that might contribute to the benefits of TRF, including reduction of intake of snacks and calories, and changes in the gut microbial environment due to an increase in fasting associated patterns of motility and secretion.
Brain-directed therapies
Based on the premise that obesity is the result of an imbalance between energy intake and expenditure, for many years the dominant pharmacological approach to obesity was based on molecules that decrease appetite and/or stimulate energy expenditure.303-305 Of the medications now available for weight loss in the USA, some are aimed to decrease appetite by directly affecting the hypothalamus (phentermine, bupropion, naltrexone, or locarserin). Others are aimed to modulate reward circuits in the brain (naltrexone, bupropion, or topiramate), reducing the subjective pleasantness of palatable foods and compulsive food cravings, as well as decreasing the response to food cues at reward regions in the brain.306-309 A dual effect, reducing appetite and reward-based eating, is achieved through the use of hormonal satiety signals, like GLP1 agonists (liraglutide or exenatide).278,310 Very little is known about the effect of these anti-obesity medications on the gut microbiota. A small study in 19 individuals with type 2 diabetes mellitus have shown that treatment with liraglutide for 42 days resulted in a statistically significant increase in relative abundance of the genus Akkermansia.311
Cognitive behavioral therapy (CBT) in individuals with obesity with food addiction aims to change specific thoughts, beliefs, and cognition directly related to the feelings and behaviors attributed to uncontrollable ingestive behaviors and cravings.312,313 Since CBT strengthens prefrontal control mechanisms,314-319 cognitive reappraisal and attention strategies through CBT are thought to strengthen the inhibitory control of the prefrontal regions on the extended reward networks by influencing appetitive motivation and reducing food addiction in individuals with obesity.320-323
Key open research questions and future directions
Considerable progress has been made in our understanding of changes in BGM interactions in food addiction and obesity; yet, the majority of studies have been performed in rodents and there are few longitudinal, mechanistic studies in humans that support the translational relevance of these findings, which would guide more effective therapies. There is currently no evidence in humans that food addiction is the result of an altered gut microbiome, or that food addiction is driven by particular gut microbial metabolites. Furthermore, given the complexity and bidirectional signaling within the BGM axis, it is unlikely that a single microbial metabolite could causally explain the behavioral changes. Considering the influence of early life experiences, environmental factors, stress, emotions, genetic factors, and dietary influences in humans, microbial influences might only explain a small component of the variance in the development of food addiction. Several approaches are necessary to move this field beyond the current reliance on largely associative studies in small populations. Microbiome characterization by shotgun metagenomics, metatranscriptomics and proteomics studies in well phenotyped human populations combined with big data analysis will be required to identify a microbial signature of food addiction. At the same time, mechanistic studies using targeted interventions, which have been shown to be effective in reducing predominantly hedonic driven eating behaviors in humans, such as bariatric surgery, TRF or cognitive behavioral therapy are needed to probe for a causal role of the gut microbiome. Such human studies should be combined with reverse translational studies, evaluating the effect of faecal microbial transfer on rodent feeding behaviors and body weight.
Conclusions
Altered BGM interactions manifesting as dysregulated eating behavior and resulting in obesity, can best be understood as a complex, circular system that is stable and highly resistant to change (Figure 4). The close interactions between diet and gut microbial signals, the effect of these signals on satiety and inflammatory mediators from the gut, and their disruptive effect on homeostatic mechanisms in the brain, leads to a shift towards a greater influence of hedonic reward mechanisms and a reduction in inhibitory control. These changes in turn drive the preferred intake of high caloric foods reinforcing the gut dysbiosis (Figure 4). As traditional therapies aimed at individual aspects of this system, including most traditional dieting strategies, have failed, novel therapies must be based on a new understanding of the systems properties of BGM interactions (Figure 1). A combination of therapeutic approaches targeting different nodes of this system, and individualization of treatments based on differences in gut microbial composition and function are required to provide greater clinical benefits.
Key Points.
Food addiction refers to maladaptive ingestive behaviors resulting from a shift from primarily homeostatic to hedonic regulatory mechanisms of food intake; this shift reflects alterations at all levels of the brain–gut–microbiome (BGM) axis.
Normal ingestive behavior is the result of the tightly regulated interplay between orexogenic and anorexogenic gut hormones, leptin signaling from adipose tissue, hypothalamic nuclei, the dopaminergic reward system and prefrontal inhibitory influences.
In food addiction, a disinhibition of reward and anorexogenic mechanisms at all levels of the BGM axis results in unrestrained craving for food.
Several adverse early life events, including nutrition, stress and antibiotic intake can influence the development of BGM interactions and of ingestive behavior.
Lifelong dietary choices can modulate BGM Interactions and eating behaviors; for example, chronic ingestion of a typical Western diet can result in systemic low-grade immune system activation that can reduce feedback inhibitory mechanisms restraining food intake.
Current pharmacological treatment options for food addition are limited, bariatric surgery is the only therapy providing long-term benefits, but novel treatment approaches (including time restricted eating and cognitive behavioral interventions) are being evaluated.
Acknowledgements
We would like to acknowledge Claudia P. Sanmiguel for her contributions in making editorial suggestions to the gut-directed therapies section of this review to Cathy Liu for invaluable editorial servives.
Acknowledgements/Funding Support:
EA Mayer has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK048351, DK064539 and DK096606). AG has been supported by the following grants: DK106528 (NIDDK), ULTR001881/DK041301 (CURE/CTSI)
Glossary
- Systems biology:
An interdisciplinary field of study that focuses on complex interactions within multiple biological systems, rather than focusing on individual mechanisms.
- Hedonic-driven eating behaviors:
Also known as ‘food addiction’, these behaviours correspond to the continued consumption of highly palatable foods even after energy requirements have been met.
- Dopaminergic reward system:
Refers to the extensive network of neurons in the extended reward network that depend on dopamine as the primary neurotransmitter for reward-related processing.
- Extended reward network:
Used interchangeablely with ‘greater reward system’, this term describes brain regions concerned with interconnecting brain networks such as reward and salience networks, and is associated with processing of rewarding stimuli and modulation of food-seeking behaviors.
- Neural substrates:
A brain region or network that is associated with a specific behavior.
- Cortical performance monitoring:
Refers to processes associated with reward sensitivity, motivation, interoceptive awareness, stress reactivity and self-control.
- Salience:
The salience brain network is responsible for monitoring the homeostatic state of the body to make adaptive adjustments to real or expected disturbances in homeostasis through the autonomic nervous system and behavioral responses.
- Corticostriatal communication:
Refers to the extensive communication network between the brain’s cortex, which houses the extended reward network (including the frontal cortex and insula), and the striatum, which houses the core reword network (nucleus accumbens, basal ganglia).
- Ventral tegmental area:
Key region of the midbrain that houses the dopaminergic cell bodies that project to all regions of the core and extended reward network.
- Nucleus accumbens:
Region of the basal ganglia and a key hub for the core reward system, responsible for many dopaminergic processes, especially those related to pleasure, motivation, and aversion.
- Prebiotic:
Refers to dietary fibre or other substrates that can only be digested by commensal gut microorganisms, thereby promoting gut microbiota diversity and health.
- Maladaptive coping:
Behaviors that are used to cope with situations to alleviate stress or symptoms, but are not necessarily healthy and do not address the core cause of the stress.
- Psychological stress:
Sufficient levels of stress orginating from the environment that can cause dyregulation of homeostatic responses to cause physical or psychological symptoms.
- Perceived stress:
A measure of the degree to which events in an individual’s life are assessed as stressful. The most widely used scale for perceived stress is the Perceived Stress Scale.
Footnotes
Competing interests
E.A.M. serves on the scientific advisory boards of Amare, Axial Biotherapeutics, Bloom Science, Danone, Viome, UBiome, Pendulum, Mahana Therapeutics and APC Microbiome Ireland. A.G. and V.O. declare no competing interests.
References
- 1.CDC, C. f. D. C. a. P. Overweight and Obesity, <http://www.cdc.gov/obesity/data/adult.html> (2014).
- 2.http://www.who.int/mediacentre/factsheets/fs311/en/. Obesity and overweight. Fact sheet, 2016).
- 3.http://stateofobesity.org/rates/. Obesity Rates & Trends, 2016).
- 4.Biener A, Cawley J & Meyerhoefer C The High and Rising Costs of Obesity to the US Health Care System. Journal of General Internal Medicine 32, S6–S8, doi: 10.1007/s11606-016-3968-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancini MC & de Melo ME The burden of obesity in the current world and the new treatments available: focus on liraglutide 3.0 mg. Diabetol Metab Syndr 9, 44, doi: 10.1186/s13098-017-0242-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y et al. Obesity: pathophysiology and intervention. Nutrients 6, 5153–5183, doi: 10.3390/nu6115153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymsfield SB & Wadden TA Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 376, 254–266, doi: 10.1056/NEJMra1514009 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Osadchiy V, Martin CR & Mayer EA The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol 17, 322–332, doi: 10.1016/j.cgh.2018.10.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EA et al. Functional GI disorders: from animal models to drug development. Gut 57, 384–404, doi:gut.2006.101675 [pii] 10.1136/gut.2006.101675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keita AV & Soderholm JD The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 22, 718–733, doi: 10.1111/j.1365-2982.2010.01498.x (2010). [DOI] [PubMed] [Google Scholar]
- 11.Yu M et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 138, 231–239, doi: 10.1016/j.jpba.2017.02.008 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Moreira CG et al. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. MBio 7, doi: 10.1128/mBio.00826-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houlden A et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun 57, 10–20, doi: 10.1016/j.bbi.2016.04.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sovran B et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci Rep 9, 1437, doi: 10.1038/s41598-018-35228-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF & Stanton C gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113, 411–417, doi: 10.1111/j.1365-2672.2012.05344.x (2012). [DOI] [PubMed] [Google Scholar]
- 16.Shishov VA, Kirovskaia TA, Kudrin VS & Oleskin AV [Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12]. Prikl Biokhim Mikrobiol 45, 550–554 (2009). [PubMed] [Google Scholar]
- 17.Asano Y et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol 303, G1288–1295, doi: 10.1152/ajpgi.00341.2012 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Maslowski KM et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286, doi: 10.1038/nature08530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalile B, Van Oudenhove L, Vervliet B & Verbeke K The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16, 461–478, doi: 10.1038/s41575-019-0157-3 (2019). [DOI] [PubMed] [Google Scholar]
- 20.McLoughlin RF, Berthon BS, Jensen ME, Baines KJ & Wood LG Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr 106, 930–945, doi: 10.3945/ajcn.117.156265 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Byrne CS et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr 104, 5–14, doi: 10.3945/ajcn.115.126706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lal S, Kirkup AJ, Brunsden AM, Thompson DG & Grundy D Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281, G907–915, doi: 10.1152/ajpgi.2001.281.4.G907 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Diaz Heijtz R Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med 21, 410–417, doi: 10.1016/j.siny.2016.04.012 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Bliss ES & Whiteside E The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Front Physiol 9, 900, doi: 10.3389/fphys.2018.00900 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torres-Fuentes C, Schellekens H, Dinan TG & Cryan JF The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol 2, 747–756, doi: 10.1016/S2468-1253(17)30147-4 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Ochoa-Reparaz J & Kasper LH The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr Obes Rep 5, 51–64, doi: 10.1007/s13679-016-0191-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buhmann H, le Roux CW & Bueter M The gut-brain axis in obesity. Best Pract Res Clin Gastroenterol 28, 559–571, doi: 10.1016/j.bpg.2014.07.003 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Myers MG Jr., Leibel RL, Seeley RJ & Schwartz MW Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21, 643–651, doi: 10.1016/j.tem.2010.08.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyenet SJ & Schwartz MW Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 97, 745–755, doi: 10.1210/jc.2011-2525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi MA & Stuber GD Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab 27, 42–56, doi: 10.1016/j.cmet.2017.09.021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkow ND, Wang GJ, Fowler JS, Tomasi D & Baler R Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci 11, 1–24, doi: 10.1007/7854_2011_169 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Wang GJ, Tomasi D & Baler RD Obesity and addiction: neurobiological overlaps. Obesity reviews : an official journal of the International Association for the Study of Obesity 14, 2–18, doi: 10.1111/j.1467-789X.2012.01031.x (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindgren E et al. Food addiction: A common neurobiological mechanism with drug abuse. Front Biosci (Landmark Ed) 23, 811–836 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Gearhardt AN, Corbin WR & Brownell KD Food addiction: an examination of the diagnostic criteria for dependence. J Addict Med 3, 1–7, doi: 10.1097/ADM.0b013e318193c993 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD & Potenza MN Can food be addictive? Public health and policy implications. Addiction 106, 1208–1212, doi: 10.1111/j.1360-0443.2010.03301.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte EM & Gearhardt AN Associations of Food Addiction in a Sample Recruited to Be Nationally Representative of the United States. Eur Eat Disord Rev 26, 112–119, doi: 10.1002/erv.2575 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Schulte EM, Potenza MN & Gearhardt AN A commentary on the “eating addiction” versus “food addiction” perspectives on addictive-like food consumption. Appetite 115, 9–15, doi: 10.1016/j.appet.2016.10.033 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Gearhardt AN, Davis C, Kuschner R & Brownell KD The addiction potential of hyperpalatable foods. Curr Drug Abuse Rev 4, 140–145 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Randolph TG The descriptive features of food addiction; addictive eating and drinking. Q J Stud Alcohol 17, 198–224 (1956). [PubMed] [Google Scholar]
- 40.Meule A & Gearhardt AN Food addiction in the light of DSM-5. Nutrients 6, 3653–3671, doi: 10.3390/nu6093653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corsica JA & Pelchat ML Food addiction: true or false? Curr Opin Gastroenterol 26, 165–169, doi: 10.1097/MOG.0b013e328336528d (2010). [DOI] [PubMed] [Google Scholar]
- 42.Hone-Blanchet A & Fecteau S Overlap of food addiction and substance use disorders definitions: analysis of animal and human studies. Neuropharmacology 85, 81–90, doi: 10.1016/j.neuropharm.2014.05.019 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Gearhardt AN, Corbin WR & Brownell KD Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav 30, 113–121, doi: 10.1037/adb0000136 (2016). [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force Diagnostic and statistical manual of mental disorders : DSM-5. 5th edn, (American Psychiatric Association, 2013). [Google Scholar]
- 45.Sengor G & Gezer C Food addiction and its relationship with disordered eating behaviours and obesity. Eat Weight Disord, doi: 10.1007/s40519-019-00662-3 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Penzenstadler L, Soares C, Karila L & Khazaal Y Systematic Review of Food Addiction as Measured with the Yale Food Addiction Scale: Implications for the Food Addiction Construct. Curr Neuropharmacol 17, 526–538, doi: 10.2174/1570159X16666181108093520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burrows T, Kay-Lambkin F, Pursey K, Skinner J & Dayas C Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J Hum Nutr Diet 31, 544–572, doi: 10.1111/jhn.12532 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang GJ, Tomasi D & Baler RD The addictive dimensionality of obesity. Biol Psychiatry 73, 811–818, doi: 10.1016/j.biopsych.2012.12.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Sun Y, Lu L & Shi J Similarities and Differences in Neurobiology. Adv Exp Med Biol 1010, 45–58, doi: 10.1007/978-981-10-5562-1_3 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Kalon E, Hong JY, Tobin C & Schulte T Psychological and Neurobiological Correlates of Food Addiction. International review of neurobiology 129, 85–110, doi: 10.1016/bs.irn.2016.06.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiLeone RJ, Taylor JR & Picciotto MR The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 15, 1330–1335, doi: 10.1038/nn.3202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers PJ Food and drug addictions: Similarities and differences. Pharmacol Biochem Behav 153, 182–190, doi: 10.1016/j.pbb.2017.01.001 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ouellette AS et al. Establishing a food addiction diagnosis using the Yale Food Addiction Scale: A closer look at the clinically significant distress/functional impairment criterion. Appetite 129, 55–61, doi: 10.1016/j.appet.2018.06.031 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Davis C et al. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 57, 711–717, doi: 10.1016/j.appet.2011.08.017 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Meule A How Prevalent is “Food Addiction”? Front Psychiatry 2, 61, doi: 10.3389/fpsyt.2011.00061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avena NM, Gearhardt AN, Gold MS, Wang GJ & Potenza MN Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci 13, 514; author reply 514, doi: 10.1038/nrn3212-c1 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Ziauddeen H & Fletcher PC Is food addiction a valid and useful concept? Obesity reviews : an official journal of the International Association for the Study of Obesity 14, 19–28, doi: 10.1111/j.1467-789X.2012.01046.x (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziauddeen H, Farooqi IS & Fletcher PC Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 13, 279–286, doi: 10.1038/nrn3212 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Muller A et al. Food addiction and other addictive behaviours in bariatric surgery candidates. Eur Eat Disord Rev 26, 585–596, doi: 10.1002/erv.2629 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Sevincer GM, Konuk N, Bozkurt S & Coskun H Food addiction and the outcome of bariatric surgery at 1-year: Prospective observational study. Psychiatry Res 244, 159–164, doi: 10.1016/j.psychres.2016.07.022 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Gearhardt AN, Boswell RG & White MA The association of “food addiction” with disordered eating and body mass index. Eat Behav 15, 427–433, doi: 10.1016/j.eatbeh.2014.05.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gearhardt AN, White MA & Potenza MN Binge eating disorder and food addiction. Curr Drug Abuse Rev 4, 201–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakazato M et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198, doi: 10.1038/35051587 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Wren AM et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50, 2540–2547, doi: 10.2337/diabetes.50.11.2540 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Betancourt L & Smith RG Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 20, 1772–1785, doi: 10.1210/me.2005-0084 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Shah M & Vella A Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord 15, 181–187, doi: 10.1007/s11154-014-9289-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karra E, Chandarana K & Batterham RL The role of peptide YY in appetite regulation and obesity. J Physiol 587, 19–25, doi: 10.1113/jphysiol.2008.164269 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolhurst G et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371, doi: 10.2337/db11-1019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cani PD et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 90, 1236–1243, doi: 10.3945/ajcn.2009.28095 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Parnell JA & Reimer RA Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr 89, 1751–1759, doi: 10.3945/ajcn.2009.27465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodin J, Wack J, Ferrannini E & DeFronzo RA Effect of insulin and glucose on feeding behavior. Metabolism 34, 826–831 (1985). [DOI] [PubMed] [Google Scholar]
- 72.Jiao N et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics 50, 244–254, doi: 10.1152/physiolgenomics.00114.2017 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Perry RJ et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 534, 213–217, doi: 10.1038/nature18309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang S et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21, 159–165, doi: 10.1038/nm.3760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pathak P et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588, doi: 10.1002/hep.29857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vrieze A et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol 60, 824–831, doi: 10.1016/j.jhep.2013.11.034 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Leitao-Goncalves R et al. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol 15, e2000862, doi: 10.1371/journal.pbio.2000862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribeiro C & Dickson BJ Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol 20, 1000–1005, doi: 10.1016/j.cub.2010.03.061 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Storelli G et al. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14, 403–414, doi: 10.1016/j.cmet.2011.07.012 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Kahathuduwa CN, Boyd LA, Davis T, O’Boyle M & Binks M Brain regions involved in ingestive behavior and related psychological constructs in people undergoing calorie restriction. Appetite 107, 348–361, doi: 10.1016/j.appet.2016.08.112 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Berthoud HR Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 21, 888–896, doi: 10.1016/j.conb.2011.09.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simon JJ et al. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight 2, doi: 10.1172/jci.insight.92970 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abizaid A, Gao Q & Horvath TL Thoughts for food: brain mechanisms and peripheral energy balance. Neuron 51, 691–702, doi: 10.1016/j.neuron.2006.08.025 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Gao Q & Horvath TL Neurobiology of feeding and energy expenditure. Annual review of neuroscience 30, 367–398, doi: 10.1146/annurev.neuro.30.051606.094324 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Berthoud HR, Munzberg H & Morrison CD Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 152, 1728–1738, doi: 10.1053/j.gastro.2016.12.050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berthoud HR & Morrison C The brain, appetite, and obesity. Annu Rev Psychol 59, 55–92, doi: 10.1146/annurev.psych.59.103006.093551 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Harding IH et al. Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. International journal of obesity 42, 448–454, doi: 10.1038/ijo.2017.237 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Gupta A et al. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage. Clinical 7, 506–517, doi: 10.1016/j.nicl.2015.01.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta A et al. Sex differences in the influence of body mass index on anatomical architecture of brain networks. International journal of obesity (2005) 41, 1185–1195, doi: 10.1038/ijo.2017.86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kenny PJ Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679, doi: 10.1016/j.neuron.2011.02.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkow ND, Wang GJ & Baler RD Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15, 37–46, doi: 10.1016/j.tics.2010.11.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartholdy S, Dalton B, O’Daly OG, Campbell IC & Schmidt U A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev 64, 35–62, doi: 10.1016/j.neubiorev.2016.02.010 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Gearhardt AN, Yokum S, Stice E, Harris JL & Brownell KD Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci 9, 932–938, doi: 10.1093/scan/nst059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steward T et al. Food addiction and impaired executive functions in women with obesity. Eur Eat Disord Rev 26, 574–584, doi: 10.1002/erv.2636 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Stice E, Yokum S, Burger KS, Epstein LH & Small DM Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 4360–4366, doi: 10.1523/JNEUROSCI.6604-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olivo G et al. Resting-State Brain and the FTO Obesity Risk Allele: Default Mode, Sensorimotor, and Salience Network Connectivity Underlying Different Somatosensory Integration and Reward Processing between Genotypes. Front Hum Neurosci 10, 52, doi: 10.3389/fnhum.2016.00052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrow JD, Maren S & Robinson TE Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behavioural brain research 220, 238–243, doi:Doi 10.1016/J.Bbr.2011.02.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia-Garcia I et al. Alterations of the salience network in obesity: a resting-state fMRI study. Human brain mapping 34, 2786–2797, doi: 10.1002/hbm.22104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volkow ND & Baler RD NOW vs LATER brain circuits: implications for obesity and addiction. Trends in neurosciences 38, 345–352, doi: 10.1016/j.tins.2015.04.002 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Wijngaarden MA et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res 287, 127–134, doi: 10.1016/j.bbr.2015.03.016 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Seeley WW et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356, doi:Doi 10.1523/Jneurosci.5587-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menon V & Uddin LQ Saliency, switching, attention and control: a network model of insula function. Brain structure & function 214, 655–667, doi: 10.1007/s00429-010-0262-0 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zald DH The human amygdala and the emotional evaluation of sensory stimuli. Brain research. Brain research reviews 41, 88–123 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Kilpatrick LA et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 146, 1212–1221, doi: 10.1053/j.gastro.2014.01.023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park BY, Chung CS, Lee MJ & Park H Accurate neuroimaging biomarkers to predict body mass index in adolescents: a longitudinal study. Brain Imaging Behav, doi: 10.1007/s11682-019-00101-y (2019). [DOI] [PubMed] [Google Scholar]
- 106.Meng Q et al. Disrupted topological organization of the frontal-mesolimbic network in obese patients. Brain Imaging Behav 12, 1544–1555, doi: 10.1007/s11682-017-9802-z (2018). [DOI] [PubMed] [Google Scholar]
- 107.Baek K, Morris LS, Kundu P & Voon V Disrupted resting-state brain network properties in obesity: decreased global and putaminal cortico-striatal network efficiency. Psychol Med 47, 585–596, doi: 10.1017/S0033291716002646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harrold JA & Halford JC The hypothalamus and obesity. Recent Pat CNS Drug Discov 1, 305–314 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Munzberg H, Qualls-Creekmore E, Berthoud HR, Morrison CD & Yu S Neural Control of Energy Expenditure. Handb Exp Pharmacol 233, 173–194, doi: 10.1007/164_2015_33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.King BM The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87, 221–244, doi: 10.1016/j.physbeh.2005.10.007 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Purnell JQ, Lahna DL, Samuels MH, Rooney WD & Hoffman WF Loss of pons-to-hypothalamic white matter tracks in brainstem obesity. Int J Obes (Lond) 38, 1573–1577, doi: 10.1038/ijo.2014.57 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Carmo-Silva S & Cavadas C Hypothalamic Dysfunction in Obesity and Metabolic Disorders. Adv Neurobiol 19, 73–116, doi: 10.1007/978-3-319-63260-5_4 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Fu O et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat Commun 10, 4560, doi: 10.1038/s41467-019-12478-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zagmutt S, Mera P, Soler-Vazquez MC, Herrero L & Serra D Targeting AgRP neurons to maintain energy balance: Lessons from animal models. Biochem Pharmacol 155, 224–232, doi: 10.1016/j.bcp.2018.07.008 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Morrison CD & Berthoud HR Neurobiology of nutrition and obesity. Nutr Rev 65, 517–534 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Pedram P et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS One 8, e74832, doi: 10.1371/journal.pone.0074832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chao AM et al. Prevalence and psychosocial correlates of food addiction in persons with obesity seeking weight reduction. Compr Psychiatry 73, 97–104, doi: 10.1016/j.comppsych.2016.11.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pursey KM, Stanwell P, Gearhardt AN, Collins CE & Burrows TL The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients 6, 4552–4590, doi: 10.3390/nu6104552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eichen DM, Lent MR, Goldbacher E & Foster GD Exploration of “food addiction” in overweight and obese treatment-seeking adults. Appetite 67, 22–24, doi: 10.1016/j.appet.2013.03.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Michaelides M, Thanos PK, Volkow ND & Wang GJ Translational neuroimaging in drug addiction and obesity. ILAR J 53, 59–68 (2012). [DOI] [PubMed] [Google Scholar]
- 121.Hommel JD et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810, doi: 10.1016/j.neuron.2006.08.023 (2006). [DOI] [PubMed] [Google Scholar]
- 122.Lutter M & Nestler EJ Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139, 629–632, doi: 10.3945/jn.108.097618 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morton GJ, Cummings DE, Baskin DG, Barsh GS & Schwartz MW Central nervous system control of food intake and body weight. Nature 443, 289–295, doi: 10.1038/nature05026 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Sinha R & Jastreboff AM Stress as a common risk factor for obesity and addiction. Biol Psychiatry 73, 827–835, doi: 10.1016/j.biopsych.2013.01.032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinha R Role of addiction and stress neurobiology on food intake and obesity. Biological psychology 131, 5–13, doi: 10.1016/j.biopsycho.2017.05.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Onaolapo AY & Onaolapo OJ Food additives, food and the concept of ‘food addiction’: Is stimulation of the brain reward circuit by food sufficient to trigger addiction? Pathophysiology 25, 263–276, doi: 10.1016/j.pathophys.2018.04.002 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Stice E, Spoor S, Bohon C, Veldhuizen MG & Small DM Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of abnormal psychology 117, 924–935, doi: 10.1037/a0013600 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stoeckel LE et al. Effective connectivity of a reward network in obese women. Brain Res Bull 79, 388–395, doi: 10.1016/j.brainresbull.2009.05.016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stoeckel LE et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage 41, 636–647, doi: 10.1016/j.neuroimage.2008.02.031 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Volkow ND et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26, 6583–6588, doi: 10.1523/JNEUROSCI.1544-06.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blum K, Thanos PK & Gold MS Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol 5, 919, doi: 10.3389/fpsyg.2014.00919 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jastreboff AM et al. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes care 36, 394–402, doi: 10.2337/dc12-1112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Loeber S et al. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes (Lond) 36, 1334–1339, doi: 10.1038/ijo.2011.184 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Martin LE et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring, Md.) 18, 254–260, doi: 10.1038/oby.2009.220 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Blum K, Oscar-Berman M, Barh D, Giordano J & Gold M Dopamine Genetics and Function in Food and Substance Abuse. J Genet Syndr Gene Ther 4, doi: 10.4172/2157-7412.1000121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hardman CA, Herbert VM, Brunstrom JM, Munafo MR & Rogers PJ Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiol Behav 105, 1202–1207, doi: 10.1016/j.physbeh.2011.12.022 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Volkow ND et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage 42, 1537–1543, doi: 10.1016/j.neuroimage.2008.06.002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gaiser EC et al. Elevated Dopamine D2/3 Receptor Availability in Obese Individuals: A PET Imaging Study with [(11)C](+)PHNO. Neuropsychopharmacology 41, 3042–3050, doi: 10.1038/npp.2016.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volkow ND, Wang GJ, Fowler JS & Telang F Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos T R Soc B 363, 3191–3200, doi:Doi 10.1098/Rstb.2008.0107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Volkow ND, Wang GJ, Fowler JS & Telang F Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363, 3191–3200, doi: 10.1098/rstb.2008.0107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sinha R, Gu P, Hart R & Guarnaccia JB Food craving, cortisol and ghrelin responses in modeling highly palatable snack intake in the laboratory. Physiology & behavior 208, 112563, doi: 10.1016/j.physbeh.2019.112563 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnson PM & Kenny PJ Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13, 635–641, doi: 10.1038/nn.2519 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pepino MY et al. Sweet Dopamine: Sucrose Preferences Relate Differentially to Striatal D2 Receptor Binding and Age in Obesity. Diabetes 65, 2618–2623, doi: 10.2337/db16-0407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molteni R, Barnard RJ, Ying Z, Roberts CK & Gomez-Pinilla F A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112, 803–814, doi: 10.1016/s0306-4522(02)00123-9 (2002). [DOI] [PubMed] [Google Scholar]
- 145.Roessmann U & Gambetti P Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol 70, 308–313 (1986). [DOI] [PubMed] [Google Scholar]
- 146.Zhang SC Defining glial cells during CNS development. Nat Rev Neurosci 2, 840–843, doi: 10.1038/35097593 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Hoban AE et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6, e774, doi: 10.1038/tp.2016.42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Diaz Heijtz R et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108, 3047–3052, doi: 10.1073/pnas.1010529108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sudo N et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558, 263–275, doi: 10.1113/jphysiol.2004.063388 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zellner DA et al. Food selection changes under stress. Physiol Behav 87, 789–793, doi: 10.1016/j.physbeh.2006.01.014 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Oliver G, Wardle J & Gibson EL Stress and food choice: a laboratory study. Psychosom Med 62, 853–865 (2000). [DOI] [PubMed] [Google Scholar]
- 152.Epel E, Lapidus R, McEwen B & Brownell K Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26, 37–49, doi: 10.1016/s0306-4530(00)00035-4 (2001). [DOI] [PubMed] [Google Scholar]
- 153.Bose M, Olivan B & Laferrere B Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes 16, 340–346, doi: 10.1097/MED.0b013e32832fa137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee MJ, Pramyothin P, Karastergiou K & Fried SK Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta 1842, 473–481, doi: 10.1016/j.bbadis.2013.05.029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cong X, Henderson WA, Graf J & McGrath JM Early Life Experience and Gut Microbiome: The Brain-Gut-Microbiota Signaling System. Adv Neonatal Care 15, 314–323; quiz E311–312, doi: 10.1097/ANC.0000000000000191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neuman H, Forsythe P, Uzan A, Avni O & Koren O Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev 42, 489–499, doi: 10.1093/femsre/fuy018 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Lundgren SN et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 6, 109, doi: 10.1186/s40168-018-0490-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chu DM et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 8, 77, doi: 10.1186/s13073-016-0330-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bhagavata Srinivasan SP, Raipuria M, Bahari H, Kaakoush NO & Morris MJ Impacts of Diet and Exercise on Maternal Gut Microbiota Are Transferred to Offspring. Front Endocrinol (Lausanne) 9, 716, doi: 10.3389/fendo.2018.00716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hohwu L, Li J, Olsen J, Sorensen TI & Obel C Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish National Cohort Study. PLoS One 9, e97490, doi: 10.1371/journal.pone.0097490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jasarevic E et al. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci, doi: 10.1038/s41593-018-0182-5 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Mueller NT et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 39, 665–670, doi: 10.1038/ijo.2014.180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dominguez-Bello MG et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107, 11971–11975, doi: 10.1073/pnas.1002601107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marcobal A & Sonnenburg JL Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 18 Suppl 4, 12–15, doi: 10.1111/j.1469-0691.2012.03863.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roger LC, Costabile A, Holland DT, Hoyles L & McCartney AL Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 156, 3329–3341, doi: 10.1099/mic.0.043224-0 (2010). [DOI] [PubMed] [Google Scholar]
- 166.Tamburini S, Shen N, Wu HC & Clemente JC The microbiome in early life: implications for health outcomes. Nat Med 22, 713–722, doi: 10.1038/nm.4142 (2016). [DOI] [PubMed] [Google Scholar]
- 167.Hart AL et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 53, 1602–1609, doi: 10.1136/gut.2003.037325 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Sullivan A, Farver M & Smilowitz JT The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr Metab Insights 8, 1–9, doi: 10.4137/NMI.S29530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bogen DL, Hanusa BH & Whitaker RC The effect of breast-feeding with and without formula use on the risk of obesity at 4 years of age. Obes Res 12, 1527–1535, doi: 10.1038/oby.2004.190 (2004). [DOI] [PubMed] [Google Scholar]
- 170.Koenig JE et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108 Suppl 1, 4578–4585, doi: 10.1073/pnas.1000081107 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Forbes JD et al. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices With Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr 172, e181161, doi: 10.1001/jamapediatrics.2018.1161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yan J, Liu L, Zhu Y, Huang G & Wang PP The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14, 1267, doi: 10.1186/1471-2458-14-1267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Monteiro CA et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr 21, 18–26, doi: 10.1017/S1368980017001379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Martinez Steele E et al. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 6, e009892, doi: 10.1136/bmjopen-2015-009892 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hall KD Did the Food Environment Cause the Obesity Epidemic? Obesity 26, 11–13, doi: 10.1002/oby.22073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sadeghirad B, Duhaney T, Motaghipisheh S, Campbell NR & Johnston BC Influence of unhealthy food and beverage marketing on children’s dietary intake and preference: a systematic review and meta-analysis of randomized trials. Obesity reviews : an official journal of the International Association for the Study of Obesity 17, 945–959, doi: 10.1111/obr.12445 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Uribe R & Fuentes-Garcia A The effects of TV unhealthy food brand placement on children. Its separate and joint effect with advertising. Appetite 91, 165–172, doi: 10.1016/j.appet.2015.03.030 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Hicks LA, Taylor TH Jr. & Hunkler RJUS outpatient antibiotic prescribing, 2010. N Engl J Med 368, 1461–1462, doi: 10.1056/NEJMc1212055 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Stark CM, Susi A, Emerick J & Nylund CM Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut 68, 62–69, doi: 10.1136/gutjnl-2017-314971 (2019). [DOI] [PubMed] [Google Scholar]
- 180.Yassour M et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8, 343ra381, doi: 10.1126/scitranslmed.aad0917 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI & Jess T Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 35, 522–529, doi: 10.1038/ijo.2011.27 (2011). [DOI] [PubMed] [Google Scholar]
- 182.Cox LM et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721, doi: 10.1016/j.cell.2014.05.052 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Candon S et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One 10, e0125448, doi: 10.1371/journal.pone.0125448 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gaskins HR, Collier CT & Anderson DB Antibiotics as growth promotants: mode of action. Anim Biotechnol 13, 29–42, doi: 10.1081/ABIO-120005768 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Cho I et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626, doi: 10.1038/nature11400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ruiz VE et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 8, 518, doi: 10.1038/s41467-017-00531-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hemmingsson E Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr Obes Rep 7, 204–209, doi: 10.1007/s13679-018-0310-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Farr OM et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovasc Dis 25, 479–488, doi: 10.1016/j.numecd.2015.01.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hemmingsson E, Johansson K & Reynisdottir S Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity 15, 882–893, doi: 10.1111/obr.12216 (2014). [DOI] [PubMed] [Google Scholar]
- 190.Forster GL, Anderson EM, Scholl JL, Lukkes JL & Watt MJ Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiol Stress 9, 29–39, doi: 10.1016/j.ynstr.2018.08.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Whitesell NR et al. Childhood exposure to adversity and risk of substance-use disorder in two American Indian populations: the meditational role of early substance-use initiation. J Stud Alcohol Drugs 70, 971–981 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moffett MC et al. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73, 321–330, doi: 10.1016/j.bcp.2006.08.003 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moussaoui N et al. Chronic Early-life Stress in Rat Pups Alters Basal Corticosterone, Intestinal Permeability, and Fecal Microbiota at Weaning: Influence of Sex. J Neurogastroenterol Motil 23, 135–143, doi: 10.5056/jnm16105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Isohookana R, Marttunen M, Hakko H, Riipinen P & Riala K The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Compr Psychiatry 71, 17–24, doi: 10.1016/j.comppsych.2016.08.002 (2016). [DOI] [PubMed] [Google Scholar]
- 195.Windle M et al. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J Am Coll Health 66, 246–251, doi: 10.1080/07448481.2018.1431892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Campbell JA, Farmer GC, Nguyen-Rodriguez S, Walker RJ & Egede LE Using path analysis to examine the relationship between sexual abuse in childhood and diabetes in adulthood in a sample of US adults. Prev Med 108, 1–7, doi: 10.1016/j.ypmed.2017.12.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Van Niel C, Pachter LM, Wade R Jr., Felitti VJ & Stein MT Adverse events in children: predictors of adult physical and mental conditions. J Dev Behav Pediatr 35, 549–551, doi: 10.1097/DBP.0000000000000102 (2014). [DOI] [PubMed] [Google Scholar]
- 198.Osadchiy V et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes Sci Pract 5, 416–436, doi: 10.1002/osp4.362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martin B et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 148, 4318–4333, doi: 10.1210/en.2007-0161 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Inam QU, Ikram H, Shireen E & Haleem DJ Effects of sugar rich diet on brain serotonin, hyperphagia and anxiety in animal model of both genders. Pak J Pharm Sci 29, 757–763 (2016). [PubMed] [Google Scholar]
- 201.Osadchiy V et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PloS one 13, e0201772, doi: 10.1371/journal.pone.0201772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leigh SJ, Lee F & Morris MJ Hyperpalatability and the Generation of Obesity: Roles of Environment, Stress Exposure and Individual Difference. Curr Obes Rep 7, 6–18, doi: 10.1007/s13679-018-0292-0 (2018). [DOI] [PubMed] [Google Scholar]
- 203.Chao A, Grilo CM, White MA & Sinha R Food cravings, food intake, and weight status in a community-based sample. Eat Behav 15, 478–482, doi: 10.1016/j.eatbeh.2014.06.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mozaffarian D, Hao T, Rimm EB, Willett WC & Hu FB Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 364, 2392–2404, doi: 10.1056/NEJMoa1014296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schulte EM, Joyner MA, Potenza MN, Grilo CM & Gearhardt AN Current considerations regarding food addiction. Curr Psychiatry Rep 17, 563, doi: 10.1007/s11920-015-0563-3 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Nunes-Neto PR et al. Food addiction: Prevalence, psychopathological correlates and associations with quality of life in a large sample. J Psychiatr Res 96, 145–152, doi: 10.1016/j.jpsychires.2017.10.003 (2018). [DOI] [PubMed] [Google Scholar]
- 207.Wu GD et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108, doi: 10.1126/science.1208344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu Z & Knight R Dietary effects on human gut microbiome diversity. Br J Nutr 113 Suppl, S1–5, doi: 10.1017/S0007114514004127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.English L, Lasschuijt M & Keller KL Mechanisms of the portion size effect. What is known and where do we go from here? Appetite 88, 39–49, doi: 10.1016/j.appet.2014.11.004 (2015). [DOI] [PubMed] [Google Scholar]
- 210.Kerr MA, McCann MT & Livingstone MB Food and the consumer: could labelling be the answer? Proc Nutr Soc 74, 158–163, doi: 10.1017/S0029665115001676 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Lucan SC, Maroko AR, Sanon OC & Schechter CB Unhealthful Food-and-Beverage Advertising in Subway Stations: Targeted Marketing, Vulnerable Groups, Dietary Intake, and Poor Health. J Urban Health 94, 220–232, doi: 10.1007/s11524-016-0127-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Coates AE, Hardman CA, Halford JCG, Christiansen P & Boyland EJ Social Media Influencer Marketing and Children’s Food Intake: A Randomized Trial. Pediatrics 143, doi: 10.1542/peds.2018-2554 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Norman J et al. Sustained impact of energy-dense TV and online food advertising on children’s dietary intake: a within-subject, randomised, crossover, counter-balanced trial. Int J Behav Nutr Phys Act 15, 37, doi: 10.1186/s12966-018-0672-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Folkvord F, Anschutz DJ, Wiers RW & Buijzen M The role of attentional bias in the effect of food advertising on actual food intake among children. Appetite 84, 251–258, doi: 10.1016/j.appet.2014.10.016 (2015). [DOI] [PubMed] [Google Scholar]
- 215.Deglaire A et al. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Sante study). Eur J Clin Nutr 69, 40–46, doi: 10.1038/ejcn.2014.139 (2015). [DOI] [PubMed] [Google Scholar]
- 216.Geiker NRW et al. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obesity reviews : an official journal of the International Association for the Study of Obesity 19, 81–97, doi: 10.1111/obr.12603 (2018). [DOI] [PubMed] [Google Scholar]
- 217.Chao A, Grilo CM, White MA & Sinha R Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol 20, 721–729, doi: 10.1177/1359105315573448 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sinha R Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141, 105–130, doi: 10.1196/annals.1441.030 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chao AM, Jastreboff AM, White MA, Grilo CM & Sinha R Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity (Silver Spring, Md.) 25, 713–720, doi: 10.1002/oby.21790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dallman MF Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21, 159–165, doi: 10.1016/j.tem.2009.10.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Christiansen AM, Dekloet AD, Ulrich-Lai YM & Herman JP “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav 103, 111–116, doi: 10.1016/j.physbeh.2011.01.015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ulrich-Lai YM et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A 107, 20529–20534, doi: 10.1073/pnas.1007740107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bharwani A et al. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63, 217–227, doi: 10.1016/j.psyneuen.2015.10.001 (2016). [DOI] [PubMed] [Google Scholar]
- 224.Depommier C et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25, 1096–1103, doi: 10.1038/s41591-019-0495-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gheorghe CE et al. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr Opin Pharmacol 48, 137–145, doi: 10.1016/j.coph.2019.08.004 (2019). [DOI] [PubMed] [Google Scholar]
- 226.Osadchiy V, Martin CR & Mayer EA Gut Microbiome and Modulation of CNS Function. Compr Physiol 10, 57–72, doi: 10.1002/cphy.c180031 (2019). [DOI] [PubMed] [Google Scholar]
- 227.O’Mahony SM, Clarke G, Borre YE, Dinan TG & Cryan JF Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277, 32–48, doi: 10.1016/j.bbr.2014.07.027 (2015). [DOI] [PubMed] [Google Scholar]
- 228.Wikoff WR et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106, 3698–3703, doi: 10.1073/pnas.0812874106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276, doi: 10.1016/j.cell.2015.02.047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim DY & Camilleri M Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol 95, 2698–2709, doi: 10.1111/j.1572-0241.2000.03177.x (2000). [DOI] [PubMed] [Google Scholar]
- 231.Hood SD, Bell CJ & Nutt DJ Acute tryptophan depletion. Part I: rationale and methodology. Aust N Z J Psychiatry 39, 558–564, doi: 10.1080/j.1440-1614.2005.01627.x (2005). [DOI] [PubMed] [Google Scholar]
- 232.Pagoto SL et al. Acute tryptophan depletion and sweet food consumption by overweight adults. Eat Behav 10, 36–41, doi: 10.1016/j.eatbeh.2008.10.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roager HM & Licht TR Microbial tryptophan catabolites in health and disease. Nat Commun 9, 3294, doi: 10.1038/s41467-018-05470-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bender DA Biochemistry of tryptophan in health and disease. Mol Aspects Med 6, 101–197 (1983). [DOI] [PubMed] [Google Scholar]
- 235.Schwarcz R, Bruno JP, Muchowski PJ & Wu HQ Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13, 465–477, doi: 10.1038/nrn3257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Marin IA et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 7, 43859, doi: 10.1038/srep43859 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stavrum AK, Heiland I, Schuster S, Puntervoll P & Ziegler M Model of tryptophan metabolism, readily scalable using tissue-specific gene expression data. J Biol Chem 288, 34555–34566, doi: 10.1074/jbc.M113.474908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Favennec M et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 23, 2066–2074, doi: 10.1002/oby.21199 (2015). [DOI] [PubMed] [Google Scholar]
- 239.Kennedy PJ, Cryan JF, Dinan TG & Clarke G Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412, doi: 10.1016/j.neuropharm.2016.07.002 (2017). [DOI] [PubMed] [Google Scholar]
- 240.Chimerel C et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep 9, 1202–1208, doi: 10.1016/j.celrep.2014.10.032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mangge H et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity 22, 195–201, doi: 10.1002/oby.20491 (2014). [DOI] [PubMed] [Google Scholar]
- 242.Buckman LB et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun 35, 33–42, doi: 10.1016/j.bbi.2013.06.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Teixeira TF, Collado MC, Ferreira CL, Bressan J & Peluzio Mdo C Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res 32, 637–647, doi: 10.1016/j.nutres.2012.07.003 (2012). [DOI] [PubMed] [Google Scholar]
- 244.Sonnenburg ED et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215, doi: 10.1038/nature16504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rasoamanana R, Even PC, Darcel N, Tome D & Fromentin G Dietary fibers reduce food intake by satiation without conditioned taste aversion in mice. Physiol Behav 110–111, 13–19, doi: 10.1016/j.physbeh.2012.12.008 (2013). [DOI] [PubMed] [Google Scholar]
- 246.Tailford LE, Crost EH, Kavanaugh D & Juge N Mucin glycan foraging in the human gut microbiome. Front Genet 6, 81, doi: 10.3389/fgene.2015.00081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shen W et al. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male C57BL/6J mice. J Nutr 144, 1181–1187, doi: 10.3945/jn.114.194332 (2014). [DOI] [PubMed] [Google Scholar]
- 248.Ding S et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One 5, e12191, doi: 10.1371/journal.pone.0012191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cani PD et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772, doi: 10.2337/db06-1491 (2007). [DOI] [PubMed] [Google Scholar]
- 250.Chang M, Alsaigh T, Kistler EB & Schmid-Schonbein GW Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One 7, e40087, doi: 10.1371/journal.pone.0040087 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hotamisligil GS Inflammation and metabolic disorders. Nature 444, 860–867, doi: 10.1038/nature05485 (2006). [DOI] [PubMed] [Google Scholar]
- 252.Wang Q, Liu D, Song P & Zou MH Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 20, 1116–1143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Larraufie P, Dore J, Lapaque N & Blottiere HM TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol 19, doi: 10.1111/cmi.12648 (2017). [DOI] [PubMed] [Google Scholar]
- 254.Palazzo M et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol 178, 4296–4303 (2007). [DOI] [PubMed] [Google Scholar]
- 255.Kidd M, Gustafsson BI, Drozdov I & Modlin IM IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil 21, 439–450, doi: 10.1111/j.1365-2982.2008.01210.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.de Lartigue G, Ronveaux CC & Raybould HE Vagal plasticity the key to obesity. Mol Metab 3, 855–856, doi: 10.1016/j.molmet.2014.09.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.de Lartigue G, Barbier de la Serre C, Espero E, Lee J & Raybould HE Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab 301, E187–195, doi: 10.1152/ajpendo.00056.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.de Lartigue G, de La Serre CB & Raybould HE Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 105, 100–105, doi: 10.1016/j.physbeh.2011.02.040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Qin Y et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol 19, 7, doi: 10.1186/s13059-018-1389-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cani PD et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50, 2374–2383, doi: 10.1007/s00125-007-0791-0 (2007). [DOI] [PubMed] [Google Scholar]
- 261.Peterli R et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 319, 255–265, doi: 10.1001/jama.2017.20897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chang SH et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 149, 275–287, doi: 10.1001/jamasurg.2013.3654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scholtz S et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63, 891–902, doi: 10.1136/gutjnl-2013-305008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pepino MY et al. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 22, E13–20, doi: 10.1002/oby.20649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sanmiguel C et al. Bariatric surgery is associated with changes in the brain’s reward system architecture and eating behaviors. . Gastroenterology 150, S824 (2016). [Google Scholar]
- 266.Kanerva N, Larsson I, Peltonen M, Lindroos AK & Carlsson LM Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: findings from the Swedish Obese Subjects (SOS) study. Am J Clin Nutr 106, 136–145, doi: 10.3945/ajcn.116.149112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Konttinen H, Peltonen M, Sjostrom L, Carlsson L & Karlsson J Psychological aspects of eating behavior as predictors of 10-y weight changes after surgical and conventional treatment of severe obesity: results from the Swedish Obese Subjects intervention study. The American journal of clinical nutrition 101, 16–24, doi: 10.3945/ajcn.114.095182 (2015). [DOI] [PubMed] [Google Scholar]
- 268.Makaronidis JM et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 107, 93–105, doi: 10.1016/j.appet.2016.07.029 (2016). [DOI] [PubMed] [Google Scholar]
- 269.Pepino MY, Stein RI, Eagon JC & Klein S Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring) 22, 1792–1798, doi: 10.1002/oby.20797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Basso N et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc 25, 3540–3550, doi: 10.1007/s00464-011-1755-5 (2011). [DOI] [PubMed] [Google Scholar]
- 271.le Roux CW et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246, 780–785, doi: 10.1097/SLA.0b013e3180caa3e3 (2007). [DOI] [PubMed] [Google Scholar]
- 272.Faulconbridge LF et al. Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: An fMRI study. Obesity (Silver Spring) 24, 1054–1060, doi: 10.1002/oby.21464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li JV et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60, 1214–1223, doi: 10.1136/gut.2010.234708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li M et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 105, 2117–2122, doi: 10.1073/pnas.0712038105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Arora T et al. Roux-en-Y Gastric Bypass Surgery Induces Early Plasma Metabolomic and Lipidomic Alterations in Humans Associated with Diabetes Remission. PLoS One 10, e0126401, doi: 10.1371/journal.pone.0126401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gralka E et al. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am J Clin Nutr 102, 1313–1322, doi: 10.3945/ajcn.115.110536 (2015). [DOI] [PubMed] [Google Scholar]
- 277.Ryan KK et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188, doi: 10.1038/nature13135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Coveleskie K et al. The effect of the GLP-1 analogue Exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes Sci Pract 3, 434–445, doi: 10.1002/osp4.124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Braas D et al. Dynamic Changes in Gut Microbial Derived Indole and Phenol Products after Bariatric Surgery and its Relationship to Weight Loss. Gastroenterology 154, S158 (2018). [Google Scholar]
- 280.Jacobs J et al. Glutamate and Hedonic Eating: Role of the Brain-Gut-Microbiome Axis on Changes on Hedonic Eating after Bariatric Surgery. Gastroenterology 154, s201 (2018). [Google Scholar]
- 281.Lee CJ et al. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes Surg, doi: 10.1007/s11695-019-03976-4 (2019). [DOI] [PubMed] [Google Scholar]
- 282.Guo Y et al. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol 178, 43–56, doi: 10.1530/EJE-17-0403 (2018). [DOI] [PubMed] [Google Scholar]
- 283.Luijten J, Vugts G, Nieuwenhuijzen GAP & Luyer MDP The Importance of the Microbiome in Bariatric Surgery: a Systematic Review. Obes Surg 29, 2338–2349, doi: 10.1007/s11695-019-03863-y (2019). [DOI] [PubMed] [Google Scholar]
- 284.Monte SV et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 151, 587–593, doi: 10.1016/j.surg.2011.09.038 (2012). [DOI] [PubMed] [Google Scholar]
- 285.Iannelli A, Anty R, Schneck AS, Tran A & Gugenheim J Inflammation, insulin resistance, lipid disturbances, anthropometrics, and metabolic syndrome in morbidly obese patients: a case control study comparing laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Surgery 149, 364–370, doi: 10.1016/j.surg.2010.08.013 (2011). [DOI] [PubMed] [Google Scholar]
- 286.Iannelli A et al. Body composition, anthropometrics, energy expenditure, systemic inflammation, in premenopausal women 1 year after laparoscopic Roux-en-Y gastric bypass. Surg Endosc 28, 500–507, doi: 10.1007/s00464-013-3191-1 (2014). [DOI] [PubMed] [Google Scholar]
- 287.Yadav R et al. Effect of Roux-en-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Front Immunol 8, 1512, doi: 10.3389/fimmu.2017.01512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Peng Y, Li JZ, You M & Murr MM Roux-en-Y gastric bypass improves glucose homeostasis, reduces oxidative stress and inflammation in livers of obese rats and in Kupffer cells via an AMPK-dependent pathway. Surgery 162, 59–67, doi: 10.1016/j.surg.2017.01.012 (2017). [DOI] [PubMed] [Google Scholar]
- 289.Lindegaard KK, Jorgensen NB, Just R, Heegaard PM & Madsbad S Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr 7, 12, doi: 10.1186/s13098-015-0012-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.van de Sande-Lee S et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 60, 1699–1704, doi: 10.2337/db10-1614 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Blackburn AN, Hajnal A & Leggio L The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict Biol 22, 1540–1553, doi: 10.1111/adb.12436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vrieze A et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 e917, doi: 10.1053/j.gastro.2012.06.031 (2012). [DOI] [PubMed] [Google Scholar]
- 293.Kootte RS et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab 26, 611–619 e616, doi: 10.1016/j.cmet.2017.09.008 (2017). [DOI] [PubMed] [Google Scholar]
- 294.Dailey FE, Turse EP, Daglilar E & Tahan V The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol 49, 29–33, doi: 10.1016/j.coph.2019.04.008 (2019). [DOI] [PubMed] [Google Scholar]
- 295.Longo VD & Panda S Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab 23, 1048–1059, doi: 10.1016/j.cmet.2016.06.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Melkani GC & Panda S Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 595, 3691–3700, doi: 10.1113/JP273094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Di Francesco A, Di Germanio C, Bernier M & de Cabo R A time to fast. Science 362, 770–775, doi: 10.1126/science.aau2095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kohsaka A et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–421, doi: 10.1016/j.cmet.2007.09.006 (2007). [DOI] [PubMed] [Google Scholar]
- 299.Hatori M et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–860, doi: 10.1016/j.cmet.2012.04.019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gill S & Panda S A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798, doi: 10.1016/j.cmet.2015.09.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Thaiss CA et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529, doi: 10.1016/j.cell.2014.09.048 (2014). [DOI] [PubMed] [Google Scholar]
- 302.Racz B, Duskova M, Starka L, Hainer V & Kunesova M Links between the circadian rhythm, obesity and the microbiome. Physiol Res 67, S409–S420, doi: 10.33549/physiolres.934020 (2018). [DOI] [PubMed] [Google Scholar]
- 303.Ara R et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess 16, iii-xiv, 1–195, doi: 10.3310/hta16050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shin JH & Gadde KM Clinical utility of phentermine/topiramate (Qsymia) combination for the treatment of obesity. Diabetes Metab Syndr Obes 6, 131–139, doi: 10.2147/DMSO.S43403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hainer V & Aldhoon-Hainerova I Tolerability and safety of the new anti-obesity medications. Drug Saf 37, 693–702, doi: 10.1007/s40264-014-0206-3 (2014). [DOI] [PubMed] [Google Scholar]
- 306.Billes SK, Sinnayah P & Cowley MA Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res 84, 1–11, doi: 10.1016/j.phrs.2014.04.004 (2014). [DOI] [PubMed] [Google Scholar]
- 307.Wellman PJ & Maher TJ Synergistic interactions between fenfluramine and phentermine. Int J Obes Relat Metab Disord 23, 723–732 (1999). [DOI] [PubMed] [Google Scholar]
- 308.Lam DD et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149, 1323–1328, doi: 10.1210/en.2007-1321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.McElroy SL et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry 61, 1039–1048, doi: 10.1016/j.biopsych.2006.08.008 (2007). [DOI] [PubMed] [Google Scholar]
- 310.Anderberg RH et al. Glucagon-Like Peptide 1 and Its Analogs Act in the Dorsal Raphe and Modulate Central Serotonin to Reduce Appetite and Body Weight. Diabetes 66, 1062–1073, doi: 10.2337/db16-0755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wang Z et al. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol Diabetes Metab 1, e00009, doi: 10.1002/edm2.9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Foster D, Sanchez-Collins S & Cheskin LJ Multidisciplinary Team-Based Obesity Treatment in Patients With Diabetes: Current Practices and the State of the Science. Diabetes Spectr 30, 244–249, doi: 10.2337/ds17-0045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Cooper Z & Fairburn CG A new cognitive behavioural approach to the treatment of obesity. Behav Res Ther 39, 499–511 (2001). [DOI] [PubMed] [Google Scholar]
- 314.Klumpp H, Fitzgerald DA, Angstadt M, Post D & Phan KL Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychol Med 44, 3109–3121, doi: 10.1017/S0033291714000567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Jensen KB et al. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153, 1495–1503, doi: 10.1016/j.pain.2012.04.010 (2012). [DOI] [PubMed] [Google Scholar]
- 316.Seminowicz DA et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14, 1573–1584, doi: 10.1016/j.jpain.2013.07.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Brunoni AR et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord 162, 43–49, doi: 10.1016/j.jad.2014.03.026 (2014). [DOI] [PubMed] [Google Scholar]
- 318.Clay SW, Allen J & Parran T A review of addiction. Postgrad Med 120, E01–07, doi: 10.3810/pgm.2008.07.1802 (2008). [DOI] [PubMed] [Google Scholar]
- 319.Labus J et al. Randomised clinical trial: symptoms of the irritable bowel syndrome are improved by a psycho-education group intervention. Aliment Pharmacol Ther 37, 304–315, doi: 10.1111/apt.12171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.An H, He RH, Zheng YR & Tao R Cognitive-Behavioral Therapy. Adv Exp Med Biol 1010, 321–329, doi: 10.1007/978-981-10-5562-1_16 (2017). [DOI] [PubMed] [Google Scholar]
- 321.Sawamoto R et al. Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosoc Med 11, 14, doi: 10.1186/s13030-017-0099-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.O’reilly GA, Cook L, Spruijt-Metz D & Black DS Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obesity Reviews 15, 453–461, doi:Doi 10.1111/Obr.12156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lappalainen R et al. The effectiveness and applicability of different lifestyle interventions for enhancing wellbeing: the study design for a randomized controlled trial for persons with metabolic syndrome risk factors and psychological distress. Bmc Public Health 14, doi:Artn 310 Doi 10.1186/1471-2458-14-310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Makki K, Deehan EC, Walter J & Backhed F The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 23, 705–715, doi: 10.1016/j.chom.2018.05.012 (2018). [DOI] [PubMed] [Google Scholar]
- 325.Desai MS et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 167, 1339–1353 e1321, doi: 10.1016/j.cell.2016.10.043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]