Abstract
Coronavirus disease-19 (COVID-19) was first identified in Wuhan, China, and spread gradually throughout the world. There are multiple reports of prolonged viral shedding in people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); however, such findings have not been documented in Bangladesh. Herein, we present a case of metabolic syndrome that remained positive for SARS-CoV-2 RNA over a prolonged period. On clinical and laboratory examination, the patient was diagnosed with obesity, raised blood pressure, dyslipidemia, and uncontrolled glycemia. However, upon taking appropriate measures and controlling the plasma sugar level, he tested negative for SARS-CoV-2 RNA on the 72nd day since illness onset. We observed that COVID-19 patients with several comorbidities, such as metabolic syndrome, may shed the virus over a prolonged period. Therefore, strict public health measures and isolation rules should be followed by a high-risk population.
Keywords: SARS-CoV-2, COVID-19, virus shedding, metabolic syndrome
Introduction
In late 2019, coronavirus disease-2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged from Wuhan, China, and became a new worldwide public health concern.1 In Bangladesh, COVID-19 was first reported on 8 March 2020. Since then, 336,044 COVID-19 cases and 4702 deaths have been documented.2 Clinical manifestations vary from asymptomatic infection to mild upper respiratory tract infection and even acute respiratory distress syndrome. Transmission of SARS-CoV-2 occurs primarily through contact with respiratory droplets from COVID-19 patients.3 The duration of viral RNA shedding is defined as the number of days from the onset of symptoms to negative detection of SARS-CoV-2 RNA in respiratory tract specimens. This duration often determines the appropriate isolation period, as it is often used as a marker of infectivity and transmissibility. Asymptomatic carriers and people positive for SARS-CoV-2 RNA may continue to shed the virus, which is a potential indicator of viral infectivity and transmissibility.4
We report a case of prolonged viral shedding of SARS-CoV-2 RNA (over 72 days) in a COVID-19 patient with metabolic syndrome and elucidated the clinical comorbidity that might be considered as a future reference.
Case
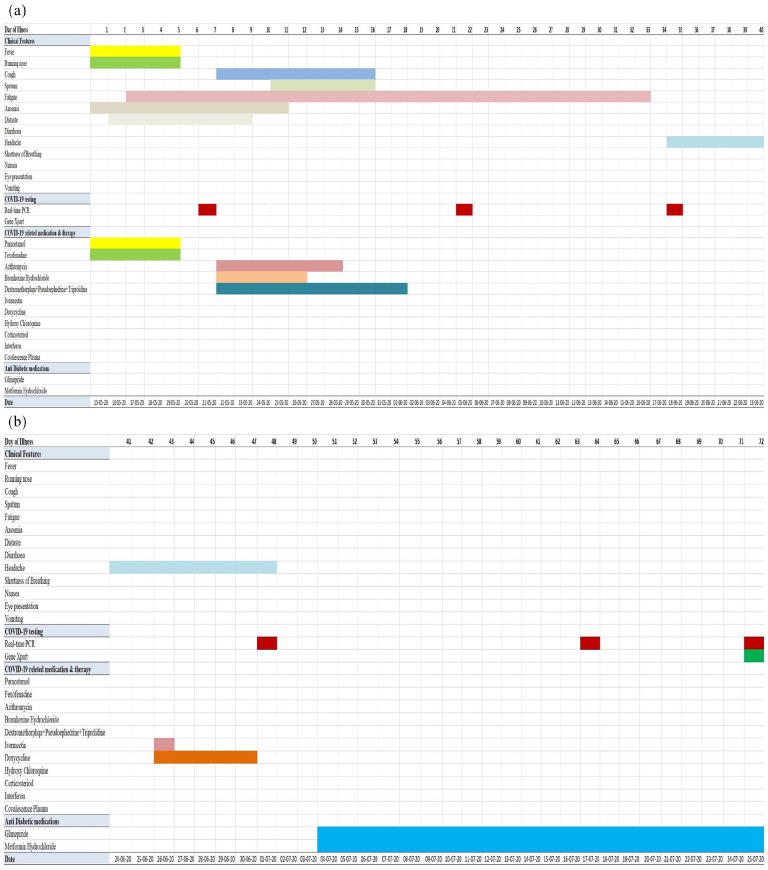
A male 42-year-old professional medium-sized vehicle driver complained of low-grade fever with a runny nose on 15 May 2020 (day 1 of illness) that lasted for 5 days and subsided upon taking antipyretic and anti-allergic medications. Subsequently, he gradually developed symptoms suggestive of COVID-19, such as anosmia (15–25 May 2020), loss of taste (16–23 May 2020), generalized fatigue (17 May–16 June 2020), and dry cough (22–30 May 2020). He had no history of breathing problems, diarrhea, or eye presentations such as redeye, tearing, foreign bodily sensations, or any other illness (Figure 1). On 21 May 2020 (day 7 of illness), his nasopharyngeal swab (NPS) specimen tested positive for SARS-CoV-2 RNA. He then underwent strict home isolation and continued it until his SARS-CoV-2 RNA test results were negative. Routine physical examination revealed that all parameters were within normal limits, except for slightly increased systolic blood pressure (130 mmHg) and obesity (body mass index (BMI): 32.8 kg/m2). Previous laboratory reports showed dyslipidemia and fatty changes in the liver upon ultrasonography of the hepatobiliary system (with occasional lipid-lowering drugs). Other parameters were within normal limits (Table 1).
Figure 1.
(a) Assessment of clinical symptoms, SARS-CoV-2 RNA testing, and medication history over the period of illness (days 1–40). (b) Assessment of clinical symptoms, SARS-CoV-2 RNA testing, and medication history over the period of illness (days 41–72).
Table 1.
Laboratory results of the case.
| Parameter | Reference value | 8 February 2019 | 21 August 2019 | 20 May 2020 | 4 July 2020 | 5 August 2020 |
|---|---|---|---|---|---|---|
| Biochemistry | ||||||
| Plasma glucose (Fasting) | 3.6–5.5 mmol/dL | 4.8 | 7.4 | 13.0 | 6.8 | |
| Plasma glucose (2-h ABF) | <7.8 mmol/dL | 23.3 | 9.7 | |||
| Serum cholesterol | <150 mg/dL | 167 | 192 | 195 | ||
| Triglycerides (TG) | 50–150 mg/dL | 261 | 240 | 307 | ||
| High-density lipoprotein (HDL) cholesterol | Male > 40 mg/dL | 30 | 33 | 39 | ||
| Low-density lipoprotein (LDL) cholesterol | <150 mg/dL | 85 | 111 | 95 | ||
| Total cholesterol: HDL ratio | <4.5 | 5.56 | 5.82 | 5 | ||
| Alanine aminotransferase (ALT) | Up to 40 U/L | 27 | 23 | 46 | ||
| Aspartate aminotransferase (AST) | <37 U/L | — | ||||
| Serum creatinine | 0.55–1.3 mg/dL | — | 1.04 | 1.0 | ||
| Thyroid stimulating hormone (TSH) | Adult 0.47–5.01 mIU/mL | — | 2.29 | |||
| Hematological profile | ||||||
| Red blood cells (RBC) | 4.5–5.5 × 1012/L | 5.20 | 5.2 | |||
| Hemoglobin | Male 13–17 g/dL | 15.1 | 15.0 | |||
| Erythrocyte sedimentation rate (ESR) | Male 00–10 mm in 1st hour | 06 | 08 | |||
| Packed cell volume (PCV)/hematocrit (HCT) | 0.40–0.64 L/L | 0.46 | 0.43 | |||
| Mean corpuscular volume (MCV) | 76–100 fL | 89 | 83.1 | |||
| Mean corpuscular hemoglobin (MCH) | 27–32 pg | 29 | 28.4 | |||
| Mean corpuscular hemoglobin concentration (MCHC) | 21–36 g/dL | 33 | 34.2 | |||
| RBC distribution width (RDW) | 11.60–14.80 | 14% | 10.04% | |||
| Total white blood cells (WBC) | 4.0–11.0 × 108/L | 5.5 | 6.5 | |||
| Circulating eosinophils | 56 | – | ||||
| Neutrophils | 40%–75% | 69% | 46% | |||
| Lymphocytes | 20%–40% | 27% | 42% | |||
| Monocytes | 02%–10% | 03% | 09% | |||
| Eosinophils | 0.1%–0.5% | 01% | 03% | |||
| Basophils | <0.1%–0.2% | 00% | 00% | |||
| Total platelet count | 150–400 × 106/L | 132 | 222 | |||
| Immunology &viral markers | ||||||
| Dengue IgG | Negative | |||||
| Dengue IgM | Negative | |||||
| Dengue NS1 Ag (ICT) | Negative | |||||
| Hepatitis B surface antigen (HBsAg) | Negative | |||||
| Anti-HCV | Negative | |||||
| Urine analysis | ||||||
| Physical examination | ||||||
| Specific gravity | 1.010–1.022 | — | 1.018 | |||
| Color | Pale yellow/straw | Straw | Yellow | |||
| Chemical examination | ||||||
| PH | 4.5–8.0 | — | 5.5 | |||
| Bilirubin | 0.02 mg/dL | — | Absent | |||
| Urobilinogen | 01.–1.8 mg/dL | — | Normal | |||
| Nitrite | Negative | — | Absent | |||
| Microscopic examination | ||||||
| Epithelial cells | 1–5/HPF | 1–2 | 1–2 | |||
| Pus cells | 0.5/HPF | 0–2 | 0–2 | |||
| Hyaline cast | 0.2/LPF | Nil | Nil | |||
| Stool analysis | ||||||
| Physical examination | ||||||
| Color | Brown/dark brown | Brown | ||||
| Consistency | Soft & formed | Loose | ||||
| Blood | Absent | Absent | ||||
| Mucus | Trace | Trace | ||||
| Worms | Absent | Absent | ||||
| pH | 6.5–7.5 | Acidic | ||||
| Pus cells | 1–5 | 1–5 | ||||
| Macrophage | Absent | Absent | ||||
| X-ray of chest P/A view | No significant abnormality is detected | |||||
| Full abdominal ultrasonography | Fatty change in the liver | Moderate fatty infiltration in the liver | ||||
His symptoms were managed both at home and with medications. Under home management, he followed hot steam inhalation, gargling with warm water, took herbal tea and lemonade, drank warm water with food, and occasionally performed freehand exercises. During this time, he took azithromycin, bromhexine hydrochloride, and cough-suppressant combinations (dextromethorphan + pseudoephedrine + triprolidine), ivermectin, and doxycycline. His COVID-19-related symptoms had resolved by day 17 (31 May 2020), except for mild fatigue that persisted for another 16 days. On day 22, his second COVID-19 test was positive for SARS-CoV-2 RNA and he continued home isolation accordingly. After clinical recovery, he was symptom-free but had an occasional nonspecific headache. He was non-diabetic based on a previous medical report, but routine checkup on 4 July 2020 revealed raised plasma sugar levels in fasting and 2-h postprandial glucose challenges. He started anti-diabetic medications (glimepiride and metformin hydrochloride) under an endocrinologist. On 25 July 2020 (day 72 of illness), 22 days after initiating anti-diabetic medication, he finally tested negative for SARS-CoV-2 RNA. Upon further follow-up (5 August 2020), plasma glucose monitoring revealed controlled glycemic status, but he had dyslipidemia with moderate fatty infiltration in the liver, for which he took appropriate measures. Throughout, he had no breathing problems and did not require anti-viral drugs or hospital admission. The entire illness period, PCR (polymerase chain reaction) testing, and medication profile are described in Figure 1 and Table 1.
His wife had also been detected with COVID-19 on 23 May 2020 and tested negative for SARS-CoV-2 on 4 June 2020 and was asymptomatic throughout her illness. Fortunately, none of the children were infected with COVID-19.
Discussion
To the best of our knowledge, this is the first report of prolonged shedding of SARS-CoV-2 in a COVID-19 patient with metabolic syndrome from Bangladesh.5 However, the period of viral infectivity and transmissibility among prolonged SARS-CoV-2 virus shedders often creates a dilemma regarding when patients should discontinue isolation or return to work following Bangladesh’s National Guidelines on Clinical Management of Coronavirus disease 2019.6 During the COVID-19 pandemic, several studies on prolonged viral shedding in COVID-19 patients with or without comorbidities have been published. A Taiwanese study reported a prolonged, 63-day shedding period in a healthy 50-year-old female COVID-19 patient.7 Elsewhere, a positive SARS-CoV-2 test on the 74th day of infection was documented in a 61-year-old male COVID-19 patient with mixed cellularity classical Hodgkin lymphoma and peripheral T-cell lymphoma, who had received convalescent plasma, and this study also highlighted that the presence of SARS-CoV-2 antibodies in the patient’s plasma was not effective in terms of viral clearance.8 Zhu et al.9 investigated clinical outcomes in 10 COVID-19 pneumonia cases among renal transplant recipients and documented prolonged clinical recovery and viral shedding periods compared to immunocompetent controls. In view of those studies on prolonged SARS-CoV-2 RNA shedders, a recent study observed that most of the RNA positives might already have crossed their infectious period and 50% of their PCR results had cycle threshold values in the mid-to-upper 30s signifying low viral load.10 Moreover, the Centers for Disease Control and Prevention (CDC) also described with several scientific notes that patients generally are not infectious despite remaining SARS-CoV-2 positive for a prolonged period.11 Thus, clinicians should cautiously interpret these prolonged SARS-CoV-2 shedders with multiple comorbidity.
Upon in-depth screening of our case, we incidentally found that he had metabolic syndrome, with diabetes mellitus (DM), hypertension, dyslipidemia, and fatty changes in the liver. An earlier study found that clinical conditions such as DM are associated with prolonged persistence of RNA in NPS and sputum specimens.12 A literature review revealed that the presence of obesity with DM and dyslipidemia was associated with a 6-fold increased risk of severe COVID-19 illness.13 Another study revealed that COVID-19 patients with higher BMI and non-alcoholic fatty liver disease suffered from a severe form of the disease.14
In this case, we observed that after receiving anti-diabetic medication that consisted of metformin hydrochloride and glimepiride, he started to feel better and subsequently tested negative for SARS-CoV-2 RNA. It might be coincidental to think that the patient might have cleared the virus spontaneously by innate immunity or the addition of metformin that controlled the glycemic status might have some role in this PCR negativity. A recent study suggested that uncontrolled DM may compromise the innate immunity system that works as a first line of defense against SARS-CoV-215 and that the addition of metformin can enhance angiotensin-converting enzyme 2 (ACE2), which has a potent anti-inflammatory effect on the pulmonary system and prevents the entry of SARS-CoV-2.16 Zhu et al. reported that COVID-19 cases with type 2 DM who had well-controlled blood glucose had better outcomes than those with poorly controlled blood sugar.17 Even then, these observations need to be proved by further studies. Our patient also received several experimental COVID-19 therapeutic drugs,18 such as azithromycin, ivermectin, and doxycycline, without apparent beneficial effects. Our findings emphasize that people with DM and other comorbidities should take all possible precautions and strictly control their glycemic status.
This case report has limitations. First, serum IgM/IgG-neutralizing antibody titers could not be evaluated at our facility. Second, the SARS-CoV-2 RNA viral load was not determined because a qualitative real-time PCR kit was used. Third, viral infectiveness could not be determined through cell culture since it was beyond the scope of the facility. Finally, genomic characterization could not be performed to evaluate virulence due to any changed genomic structure or mutated SARS-CoV-2 RNA genome.
Conclusion
Our results suggest that metabolic syndrome could be a risk factor for prolonged SARS-CoV-2 RNA viral shedding. Studies based on larger cohorts could help to characterize viral infectivity and transmissibility among prolonged SARS-CoV-2 shedders.
Acknowledgments
We thank Md. Abdullah Omar Nasif, Avirup Saha, Farzana Ansari, and Md. Shafayet Jamil for their technical support. In addition, we gratefully acknowledge the kind cooperation and assistance from the patient during the case study preparation.
Footnotes
Declaration of conflict of interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from *INSTITUTIONAL REVIEW BOARD (BSMMU/2020/6235)*.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: SM Rashed Ul Islam  https://orcid.org/0000-0002-8164-5905
https://orcid.org/0000-0002-8164-5905
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Directorate General of Health Services. Coronavirus COVID-19 dashboard, http://103.247.238.92/webportal/pages/covid19.php (2020, accessed 13 September 2020).
- 3. Chan JF, Yuan S, Kok K, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qi L, Yang Y, Jiang D, et al. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis 2020; 96: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome. J Cardiovasc Pharmacol Ther 2017; 22: 365–367. [DOI] [PubMed] [Google Scholar]
- 6. Directorate General of Health Services Ministry of Health Family Welfare. National Guidelines on clinical management of coronavirus disease 2019 (COVID-19), Version 7.0, p. 39, https://www.dghs.gov.bd/images/docs/Guideline/COVID_Guideline_v7.pdf (2020, accessed 30 June 2020).
- 7. Liu WD, Chang SY, Wang JT, et al. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect 2020; 81(2): 318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karataş A, İnkaya AÇ, Demiroğlu H, et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci 2020; 59(5): 102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol 2020; 77: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity — a strategy for containment. New Engl J Med 2020; 383: e120. [DOI] [PubMed] [Google Scholar]
- 11. CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention, https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcommunity%2Fstrategy-discontinue-isolation.html (2020, accessed 10 November 2020).
- 12. Wang K, Zhang X, Sun J, et al. Differences of severe acute respiratory syndrome coronavirus 2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with coronavirus disease 2019. Chest 2020; 158(5): 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng KI, Gao F, Wang XB, et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism 2020; 108: 154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji D, Qin E, Xu J, et al. Nonalcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol 2020; 73: 451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr 2020; 14(4): 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malhotra A, Hepokoski M, McCowen KC, et al. ACE2, Metformin, and COVID-19. Iscience 2020; 23: 101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and preexisting type 2 diabetes. Cell Metab 2020; 31: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US National Library of Medicine ClinicalTrials.gov. A comparative study on ivermectin and hydroxychloroquine on the COVID19 patients in Bangladesh, https://clinicaltrials.gov/ct2/show/NCT04434144 (accessed 2 September 2020).