Abstract
Introduction:
Anemia is a condition in which the hemoglobin concentration falls below 11 g/dL. It impairs health and well-being in women and increases the risk of maternal and neonatal adverse outcomes. The availability of local information on the magnitude and associated factors has a major role in the management and control of anemia in women contributing to reduction in maternal morbidity and mortality.
Methods:
The purpose of this study was to see regional impacts and influencing factors related to the anemia among women in Ethiopia using the 2016 Ethiopia Demographic and Health Survey data. The anemia level among women aged 15–49 years (n = 5133) using the multilevel ordinal logistic regression model was analyzed.
Results:
In the study, 37.4% of women in Ethiopia had no anemia, above one-third percent (34.4%) of women had moderate anemia, and approximately 4% women’s anemia level was severe. The 27.1% of variation of anemia was due to between-region variations. The odds of being normal weight in the greater category of anemia level were 0.59 times less likely as compared to underweight women. The higher levels of anemia were increased by 2.31 for pregnant women as compared to non-pregnant women. Having work being in greater anemia levels was 0.88 times less likely as compared to had no work. Older women (35–49 years) in higher anemia levels were 2.1 times more likely as compared to younger women. The women living in the rural area being in the greater category anemia levels were 1.53 times more likely as compared to women living in the urban area. The higher level of anemia in the rich quintile was 0.72 times less likely to the higher level of anemia as compared in the poor quintile women.
Conclusion:
Pregnant women in Ethiopia are more exposed for the higher anemia level and women live in rural area have the greater anemia level.
Keywords: Multilevel model, anemia levels, women, region
Introduction
Anemia is considered as a condition in which the number and size of red blood cells, or the hemoglobin concentration falls below 11 g/dL. As a result, it leads to impairment of the capacity of the blood to transport oxygen around the body.1 It is observed as an indicator of both poor nutrition and poor health. Anemia also impairs health and well-being in women and increases the risk of maternal and neonatal adverse outcomes. Globally, anemia affected 1.62 billion people, of these, 56 million anemia cases were found in pregnant women.2
In low-income and middle-income countries, pregnant women experience high rates of anemia; Africa and South Asia were reported highest prevalence rates.3 Anemia can have negative effects on a woman’s health including maternal mortality and severe morbidity.4
Both the risk and consequences of anemia were more important in low-income and middle-income countries.5 Africa was ranked the second highest in terms of anemia (38.6%) and severe anemia (1.8%).6 It is a moderate and severe public health problem in all reproductive age groups of women (38.6%) and pregnant women (54%) of the Sub-Saharan Africa (SSA), respectively.7
Factors like age, sex, residential elevation (altitude), smoking behavior, and pregnancy status influence hemoglobin concentration.8 Previous studies have also documented several potential causes of anemia among women including rural residency,5 younger age,9 and pregnancy status.10,11 The study in Nepal showed that a high prevalence of anemia among underweight women. Similarly, overweight women were less likely to be anemic as compared with women with normal body mass index (BMI).12
The study in 2019 indicates that considerable geographic disparities in anemia prevalence rate occur within Ethiopia. Similarly, the study showed that anemia prevention strategies need to be targeted on rural residents, women with limited to no education, women who are breastfeeding, areas with poor latrine facilities, and women who are HIV positive.13 Different studies also showed that the prevalence of anemia is varied in different regions of the country. For instance, prevalence rates of 31.6% in Sidama14 and 39.94% in Woliyata Sodo15 were recorded in recent years. A higher rate which was 61.6% has also been reported in pregnant women in the Boditii heath center.16
In Ethiopia, varied prevalence rates of anemia among women have been observed with different factors across different parts of the country.10,17 For instance, large family size, low education status, rural residence, hookworm infestation, and HIV infection were identified as factors contributing to anemia in northern Ethiopia.17,18
The availability of local information on the magnitude and associated factors has a major role in the management and control of anemia levels in women contributing to reduction in maternal morbidity and mortality. Many studies focused on anemia in women have been limited to subnational assessments and subgroups of the women, with most studies being carried out with pregnant women. Despite the wider scope of the problem, little research data have been explored about coverage, regional variation, severity, and the extent of associate factors of anemia levels among women in Ethiopia. Although factors affecting anemia are considered to operate in different levels, existing national studies used single-level analysis techniques which may lead to incorrect estimation of parameters, false conclusion of the association and also cannot measure the contribution of the individual, household, and region to the total variation of anemia levels. Therefore, accounting for the nested structure of data may reduce bias in parameter estimation, increase precision and power, and consider as an efficient use of information available. Multilevel ordinal logistic regression (OLR) examines the effects of explanatory variables at different levels simultaneously. It produces more accurate estimates of parameter and significance tests compared with single-level logistic regression. Furthermore, it quantifies within- and between-groups variation on outcome which might be due to unobserved factors at different levels.19
Therefore, the aim of this study was to identify individual and regional level factors of anemia in reproductive age groups of women simultaneously using multilevel OLR model. In addition, the study aimed to quantify the amount of variation in anemia levels which was due to regional variation in Ethiopia.
Methods
Data sources
This study was national-based cross-sectional study design conducted as a part of the 2016 Ethiopia Demographic and Health Survey (2016 EDHS). Based on a nationally representative sample, it provides estimates at the national and regional levels and for urban and rural areas. The survey target groups were women age 15–49 years and men age 15–59 years in randomly selected households across Ethiopia. 2016 EDHS collected detailed information on background characteristics from 16,650 households, 15,683 female respondents, and 12,688 male respondents. This report presents comprehensive detailed final outcomes of the survey at the national level, for the nine regional states and two city administrations (Addis Ababa city and Dire Dawa city) of Ethiopia.20
The 2016 EDHS data are available to the general public by request in different formats from the Measure DHS website (http://idhsdata.org). The author applied the Measure DHS by briefly stating the objectives of the study and got the permission to download the women anemia level data set in SPSS format.
Nested structure of the data
The survey data set used for this study was based on multistage stratified cluster sampling. The structure of data in the population was hierarchical, and a sample from such a population can be viewed as a multistage sample. For multistage-clustered samples, the dependence among observations often comes from several levels of the hierarchy. In this study, the clustering of the data points within geographical regions offers a natural two-level hierarchical structure of the data, that is, women were nested within regions.
Sampling techniques and study participants
Stratified two-stage sampling technique was used to select enumeration areas (EAs) and households. An EA is a geographic area covering on average 181 households. The 2007 Ethiopia Population and Housing Census (PHC) were used as a sampling frame to select EAs. In the first stage, 645 EAs (202 in urban and 443 in rural) were selected with probability proportional to EA size. The EA size was the number of residential households in the EA as determined in the 2007 PHC. And those EAs with more households had higher probability of being selected. In the second stage, 16,650 households were selected by systematic sampling technique, with average of 28 households per EAs. All women in the selected household were eligible for anemia testing. Details on sampling technique, sample size, data collection tools, data quality control, and ethical concerns are available in 2016 EDHS report.20
All women who had data on anemia status were included in this study. A total of 5133 women who were tested for anemia were included in the analysis.
Data collection
The 2016 EDHS sample was stratified into urban and rural areas, yielding 21 sampling strata. Samples of EAs were selected independently in each stratum in two stages. Implicit stratification and proportional allocation were achieved at each of the lower administrative levels by sorting the sampling frame within each sampling stratum before sample selection, according to administrative units in different levels, and using a probability proportional to size selection at the first stage of sampling.
Measurement of study variables
The response variable in the study was women of reproductive age group of anemia level (normal, mild, moderate, or severe). Hemoglobin levels of the women were measured using HemoCue, which is the standard test used in the EDHS 2016, and all hemoglobin values were adjusted for both altitude and smoking status.20 Anemia is categorized as normal (>12 g/dL), mild (11–11.9 g/dL), moderate (8–10.9 g/dL), and severe (<8 g/dL) based on the World Health Organization (WHO) classification.8
Detailed description of factors that were expected to be associated with the level of anemia considered in this study was: sociodemographic (education level (illiterate, primary, secondary, or higher); work status (no or yes); age (15–19 years, 20–34 years, or 35–49 years); residence (urban or rural); and wealth of women (poor, middle, or rich)) and health-related characteristics (BMI (underweight, normal, overweight, or obese); status of current pregnancy (no or yes); and number of children (no child, one or two children, or more than two children)) of study participants.
Operational definitions
Anemia is a condition marked by low levels of hemoglobin in the blood. Iron is a key component of hemoglobin, and iron deficiency is estimated to be responsible for half of all anemias globally. Other causes of anemia include malaria, hookworm and other helminthes, other nutritional deficiencies, chronic infections, and genetic conditions.20
Eligibility and exclusion criteria
Inclusion criteria
This study provides a comprehensive overview of anemia level among reproductive age group of women in Ethiopia. Therefore, detailed reproductive history and maternal health issues were much information elicited from the woman’s age group of 15–49 years.20 All reproductive age groups of women (15–49 years) who were voluntarily consenting hemoglobin test during the survey period (18 January 2016 to 27 June 2016) were included in the study. In the exclusion criteria, the women of reproductive age group (15–49 years) who have not taken anemia test during the survey period were excluded from the study.
Method of data analysis
Many models for the ordinal dependent variable have been studied in the literature; in this specific study, cumulative logit model was used. The basic objective of ordered logit models is calculation of a cumulative probability for dependent variable being greater than the category called this model as the proportional odds model (POM).21–23 This model assumes that the effect of independent variable is same across all the categories of dependent variable; this is also called the proportional odds assumption or parallel lines assumption.24 The proportional odds assumption was tested using the likelihood-ratio test. It tests the null hypothesis that there is no difference in the effects of explanatory variables across the levels of anemia. The p-value ⩾ 0.05 is desirable to retain null hypothesis.25 Furthermore, each variable in the model was tested to identify the variables for which the proportional odds assumption was violated.
The POM model for the categorical variable order categories and a collection of explanatory variables for the subject is given by:
where:
For a response variable with categories and a set of predictors having the effect parameters the probability of response variable being less than or equal to category can be modeled by the logistic distribution as:
Under the assumption of proportional odds, the remains same and only intercepts vary for different categories of response variable. As the sign of is negative (subtracted), they show how the one unit increases in predictor increase in log-odds of being in the category greater than
are the intercepts and are different for each comparison ordinal categorical variable, and the relation between to ensure that
The slope coefficients are the same for all the categories of dependent variable; for continuous variables, the slope coefficients change in log-odds for one unit change in predictor; and for nominal predictors, the slope coefficients represent the effect of each category of nominal variable as compared to reference category.26
Multilevel OLR model
Many types of data, including observational study collected from the human and biological sciences, are nested in clustered. Multilevel models are for hierarchical nested data structure by allowing error components at different levels of hierarchy. The multilevel analysis is used to fit the model and to estimate the variation in response occurred within cluster and between clusters, the units of higher level. The variation between clusters represents random effect due to the inclusion of cluster level in the model. Multilevel OLR model is used for the analysis of hierarchical and ordinal dependent variable. It is used to model the ordinal categorical dependent by one or more independent variables. The ordinal categorical dependent follows the logistic distribution and nested with higher levels.27,28
Let the order categorical response of individual in the cluster with order categories coded as Then, the cumulative probability for ordered response up to category c is The multilevel random intercept (base model) cumulative log-odds model for ordinal response is written as:
where the measure of odds of being in the category less than or equal to C as compared to greater than the category C, and is the random effect of level 2 units and is assumed to follow normal distribution . The above random intercept model is mentioned when there are no explanatory variables. When model also has some fixed explanatory variables, then the above model can be written as:
where is the data matrix of fixed predictors; hence, the fixed effect is the same as for simple POM.
Austin et al.29 used a random intercept logistic regression model, whereas Moineddin et al.30 used both the random intercept and the random slopes logit models in which both the intercept and the slope randomly vary across clusters. Intraclass correlation coefficient (ICC) is used as assessment of how much variation in the response categories lies at the level 2 (group level). The ICC of within-groups variation for dichotomous and ordinal outcomes31 is defined as:
where is the variance level 2 error term, and 3.29 is the variance of standard logistic distribution.
The most commonly implemented estimation procedure for hierarchical generalized linear models is penalized quasi-likelihood, although this method has been shown to yield biased estimates of the variance components as well as the fixed effects when data are dichotomous.32 Data used in this study were reported and edited in SPSS (version 20), and Stata (version 13) was used for data analysis.
Results
Prevalence of anemia in Ethiopia
Table 1 shows the descriptive statistics for distribution of anemia level according to region of Ethiopia. Among 5133 women considered in the study, only 37.40% of women in Ethiopia had no anemia while above one-third percent (34.40%) of women had moderate anemia level, and approximately 4% (3.80%) women’s anemia level was severe. The result also revealed that more than half percent of women in both Amhara Region and Benishangul Region (54.70% and 54.40%, respectively) had no anemia while the highest percent of women in Somali Region, Afar Region, and Harari Region had moderate anemia level (70.40%, 65.00%, and 54.10%, respectively), and 14.40% of women in Somali Region and 10.8% of women in Dire Dawa had severe anemia (Table 1).
Table 1.
Prevalence of anemia across region in Ethiopia.
| Region | Anemia level (within region) | Total | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|
| Not anemic | Mild | Moderate | Severe | Value (df) | p-value | |||
| Tigray | Count (%) | 287 (48.30) | 176 (29.60) | 118 (19.90) | 13 (2.20) | 594 (100) | 1092.27 (30) | 0.000 |
| Afar | Count (%) | 60 (16.00) | 53 (14.20) | 243 (65.00) | 18 (4.80) | 374 (100) | ||
| Amhara | Count (%) | 389 (54.70) | 189 (26.60) | 119 (16.70) | 14 (2.00) | 711 (100) | ||
| Oromia | Count (%) | 245 (30.80) | 240 (30.20) | 285 (35.80) | 25 (3.10) | 795 (100) | ||
| Somali | Count (%) | 25 (5.60) | 43 (9.60) | 317 (70.40) | 65 (14.40) | 450 (100) | ||
| Benishangul | Count (%) | 234 (54.40) | 108 (25.10) | 84 (19.50) | 4 (0.90) | 430 (100) | ||
| SNNPR | Count (%) | 316 (46.20) | 180 (26.30) | 174 (25.40) | 14 (2.00) | 684 (100) | ||
| Gambela | Count (%) | 157 (40.90) | 86 (22.40) | 137 (35.70) | 4 (1.00) | 384 (100) | ||
| Harari | Count (%) | 32 (14.40) | 59 (26.60) | 120 (54.10) | 11 (5.00) | 222 (100) | ||
| Addis Ababa | Count (%) | 123 (49.40) | 60 (24.10) | 63 (25.30) | 3 (1.20) | 249 (100) | ||
| Dire Dawa | Count (%) | 53 (22.10) | 57 (23.80) | 104 (43.30) | 26 (10.80) | 240 (100) | ||
| Total | Count (%) | 1921 (37.40) | 1251 (24.40) | 1764 (34.40) | 197 (3.80) | 5133 (100) | ||
df: degree of freedom; SNNPR: Southern Nations, Nationalities, and People’s Region.
The study in Table 1 also showed that, using the chi-square test at 5% level of significant the prevalence of anemia level according to region had significantly associated.
Distribution of anemia levels according to influencing factors
Table 2 presents the percentage distribution of sociodemographic and health-related characteristics of anemia levels of women in Ethiopia. The result showed that among non-anemic women, more than 71% of women had normal BMI while less than 2% women were obese and 28.40% and 24.90% underweight and overweight women had severe anemia, respectively. The result also revealed that 88.60% of non-anemic women were non-pregnant while more than 56% of pregnant women had severe anemia. In this study, the result indicated that, among severe anemic level of women, 76.60% of women had no formal education, similarly 18.80%, 4.10%, and 0.50% of women with primary, secondary, and higher educational levels had severe anemic, respectively. The higher proportion of anemia level of women for non-working women was severe (83.80%) followed by moderate, mild, and non-anemic (73.10%, 70.30%, and 63.20%, respectively). Similarly, the proportion of anemia level differs by age of women. Accordingly, 69.50% of severe anemic age groups of women were 20–34 years while 6.60% of severe anemic women were in the age group of 34–49 years. More than 90% severe anemic level and 83.60% moderate anemia level of women were rural residence. In the study, women wealth index had also another influencing factor on their anemia level. The result showed that 77.20% severe anemia levels of women were poor whereas only 15.20% of severe anemic women were rich.
Table 2.
Percentage distribution of anemia levels according to influencing factors.
| Variables | Category | Anemia level | Total | Chi-square value (significance) | ||||
|---|---|---|---|---|---|---|---|---|
| Not anemic | Mild | Moderate | Severe | |||||
| Body mass index | Underweight | Count | 343 | 324 | 509 | 56 | 4199 | 300.38 (0.000)* |
| % | 17.90% | 25.90% | 28.90% | 28.40% | 100% | |||
| Normal | Count | 1379 | 807 | 897 | 72 | 934 | ||
| % | 71.80% | 64.50% | 50.90% | 36.50% | 100% | |||
| Overweight | Count | 162 | 92 | 230 | 49 | 3189 | ||
| % | 8.40% | 7.40% | 13.00% | 24.90% | 100% | |||
| Obese | Count | 37 | 28 | 128 | 20 | 1387 | ||
| % | 1.90% | 2.20% | 7.30% | 10.20% | 100% | |||
| Currently pregnant | No or unsure | Count | 1702 | 1081 | 1330 | 86 | 366 | 318.70 (0.000)* |
| % | 88.60% | 86.40% | 75.40% | 43.70% | 100% | |||
| Yes | Count | 219 | 170 | 434 | 111 | 191 | ||
| % | 11.40% | 13.60% | 24.60% | 56.30% | 100% | |||
| Highest educational level | No education | Count | 1113 | 754 | 1171 | 151 | 3548 | 66.02 (0.000)* |
| % | 57.90% | 60.30% | 66.40% | 76.60% | 100% | |||
| Primary | Count | 537 | 375 | 438 | 37 | 1585 | ||
| % | 28.00% | 300.0% | 24.80% | 18.80% | 100% | |||
| Secondary | Count | 176 | 79 | 103 | 8 | 1351 | ||
| % | 9.20% | 6.30% | 5.80% | 4.10% | 100% | |||
| Higher | Count | 95 | 43 | 52 | 1 | 3591 | ||
| % | 4.90% | 3.40% | 2.90% | .5% | 100% | |||
| Respondent currently working | No | Count | 1214 | 879 | 1290 | 165 | 191 | 65.41 (0.000)* |
| % | 63.2% | 70.3% | 73.1% | 83.80% | 100% | |||
| Yes | Count | 707 | 372 | 474 | 32 | 946 | ||
| % | 36.80% | 29.70% | 26.90% | 16.20% | 100% | |||
| Respondent’s current age | 15–19 years | Count | 598 | 301 | 405 | 47 | 2237 | 47.78 (0.000)* |
| % | 31.10% | 24.10% | 23.00% | 23.90% | 100% | |||
| 20–34 years | Count | 1271 | 906 | 1277 | 137 | 1950 | ||
| % | 66.20% | 72.40% | 72.40% | 69.50% | 100% | |||
| 35–49 years | Count | 52 | 44 | 82 | 13 | 978 | ||
| % | 2.70% | 3.50% | 4.60% | 6.60% | 100% | |||
| Total children ever born | No children | Count | 365 | 236 | 312 | 33 | 4155 | 6.55 (0.364) |
| % | 19.00% | 18.90% | 17.70% | 16.80% | 100% | |||
| One or two children | Count | 854 | 554 | 747 | 82 | 5133 | ||
| % | 44.50% | 44.30% | 42.30% | 41.60% | 100% | |||
| More than two children | Count | 702 | 461 | 705 | 82 | 2582 | ||
| % | 36.50% | 36.90% | 40.00% | 41.60% | 100% | |||
| Type of place of residence | Urban | Count | 437 | 233 | 289 | 19 | 793 | 36.62 (0.000)* |
| % | 22.70% | 18.60% | 16.40% | 9.60% | 100% | |||
| Rural | Count | 1484 | 1018 | 1475 | 178 | 1758 | ||
| % | 77.30% | 81.40% | 83.60% | 90.40% | 100% | |||
| Wealth index combined | Poor | Count | 782 | 620 | 1028 | 152 | 4199 | 174.41 (0.000)* |
| % | 40.70% | 49.60% | 58.30% | 77.20% | 100% | |||
| Middle | Count | 340 | 205 | 233 | 15 | 934 | ||
| % | 17.70% | 16.40% | 13.20% | 7.60% | 100% | |||
| Rich | Count | 799 | 426 | 503 | 30 | 3189 | ||
| % | 41.60% | 34.10% | 28.50% | 15.20% | 100% | |||
Significant variable at 5% level of significant.
In this study, bivariate chi-square analysis has been used and seven influencing variables considered were found to be statistically significant at 5% (since p < 0.05) significance level. Thus, the anemia levels had significantly associated with BMI, pregnant status, educational level, working status, age, place of residence, and wealth index of the women in Ethiopia (Table 2).
Fitted multilevel OLR models
Goodness-of-fit of the model
As women are nested in the high level of hierarchy such as women were nested in region (level 2), so we need to fit a two-level multilevel ordinal logit model to estimate women anemia level. In the two-level random intercept model, we allowed the cut points to vary at different levels of hierarchy. We take these random variations as a part of total random variation, that is, we distribute the whole random variation into parts associated with different levels of hierarchies. In the present problem, for the estimation of level of anemia, we used two-level multilevel random intercept ordinal logit models to find variation due to region level of women (Table 3).
Table 3.
Test for goodness-of-fit of the model.
| Criteria | Ordinal logistic regression model | Multilevel ordinal logistic regression model |
|---|---|---|
| Value | ||
| Number of observations | 5133 | 5133 |
| Number of groups (region) | 11 | |
| Observation per group | ||
| Minimum | 222 | |
| Maximum | 795 | |
| Log likelihood | −5945.01 | −5541.83 |
| LR chi-square (8) | 471.08 (0.000) | |
| Proportional odd assumptions test (LR chi-square (30)) | 3407.46 (0.201) | |
| Wald chi-square (15) | 439.61 (0.000) | |
| LR test vs ologit model:chi-bar-square (01) | 533.10 | |
| AIC | 11,912.02 | 11,121.67 |
| BIC | 11,983.99 | 11,245.99 |
| Region var(_cons) | 1.22 (0.000) | |
| Intraclass correlation | 0.27 | |
AIC: Akaike information criterion; BIC: Bayesian information criterion; LR: likelihood-ratio.
p-values of the model fitted are presented within parenthesis.
In the study at 5% level of significance, both OLR and multilevel OLR models were significant. The likelihood-ratio test statistic was 3407.46 which we compared with a chi-square distribution on 30 degrees of freedom and the associated p-value was 0.201, so we cannot reject the null hypothesis that the location parameters (slope coefficients) were the same across response categories, and we concluded that the proportional odds assumption holds. This study was chosen between OLR and multilevel OLR models; it depends on the between-region variation on the level of anemia. The estimated between-region variance was 1.22, which implies an ICC was 0.27, so approximately 27% of the variation in level of anemia was due to between-region variation. As shown in the summary table (Table 3), the likelihood-ratio chi-square values for between-region variance were found to be 533.10 with p < 0.05. Thus, the between-region variation on anemia level was significant. As shown in the result, both Akaike information criterion (AIC) and Bayesian information criterion (BIC) also supported the conclusion that the multilevel OLR models were a better fit to the data (Table 3).
Examining of region effects on anemia levels
Caterpillar plot
Caterpillar plot showed that the estimated residuals for the 11 regions of Ethiopia. Except one region, for a substantial number of regions, the 95% confidence interval did not overlap zero, indicated that the level of anemia was significantly above average (above the zero line) or below average (below the zero line) at the 5% level for these regions (Figure 1).
Figure 1.
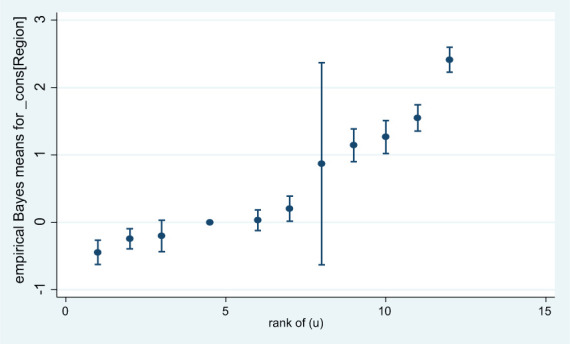
Caterpillar plot using predicted value and standard error for each region in rank order.
Predicted regional effect
From the result (Table 4), the effect of each region on prevalence of anemia levels from the smallest predicted region to the largest is determined. We can see that Somali Region and Harari Region had the highest rank, respectively. Therefore, the largest level of anemia prevalence had Somali Region and Harari Region, respectively, while Benishangul Region and Tigray Region had the smallest predicted anemia level prevalence, respectively.
Table 4.
Rank, and predicted effect of regions.
| uRank | Region | U | |
|---|---|---|---|
| 1 | 1 | Benishangul | −0.43 |
| 2 | 2 | Tigray | −0.17 |
| 3 | 3.5 | Amhara | 0.00 |
| 4 | 3.5 | Oromia | 0.00 |
| 5 | 5 | SNNPR | 0.15 |
| 6 | 6 | Addis Ababa | 0.21 |
| 7 | 7 | Gambela | 0.23 |
| 8 | 8 | Afar | 1.27 |
| 9 | 9 | Dire Dawa | 1.27 |
| 10 | 10 | Harari | 1.40 |
| 11 | 11 | Somali | 2.13 |
SNNPR: Southern Nations, Nationalities, and People’s Region.
Interpretation of fitted multilevel mixed-effects OLR models
According to Table 5, the three intercepts were used to differentiate the category of anemia level 1 for each comparison. The first cut point was estimated to be −0.62 and told us that the log-odds of having mild, moderate, or severe level of anemia relative to had not anemia were 0.62. This was corresponding to a probability of having mild, moderate, or severe anemia level of It follows that the probability of having instead had no anemia was The second cut point was estimated to be 0.60 and so the log-odds of having moderate or severe anemia level relative to had not anemia or mild were −0.60, which corresponds to a probability of 0.35. The probability of having instead had no anemia was 0.65. Finally, the third cut point was estimated to be 3.94 and told us the log-odds of having severe anemia level relative to had not anemia, mild or moderate were −3.94, which corresponds to a probability of 0.02. The probability of having instead had severe level of anemia was 0.98. Each cut point’s cumulative response probabilities vary across region. As in any multilevel model, one way to assess regional variation is to use the value of ICC, and each cut point’s cumulative response probability was computed by adding estimate of regional variation in each cut point.
Table 5.
Multilevel ordinal model fitted of anemia with the related factors.
| Anemia | Coefficient | Odds ratio | Standard error | Z | p > |z| | 95% confidence interval |
|---|---|---|---|---|---|---|
| Cut points | ||||||
| −0.62 | 0.19 | −3.22 | 0.001 | −0.99, −0.24 | ||
| 0.60 | 0.19 | 3.14 | 0.002 | 0.23, 0.98 | ||
| 3.94 | 0.21 | 18.89 | 0.000 | 3.53, 4.34 | ||
| BMI (underweight—reference) | ||||||
| Normal | −0.53 | 0.59 | 0.04 | −8.03 | 0.000* | 0.52, 0.67 |
| Overweight | 0.34 | 1.40 | 0.15 | 3.18 | 0.001* | 1.14, 1.72 |
| Obese | 0.94 | 2.56 | 0.39 | 6.27 | 0.000* | 1.91, 3.44 |
| Curprg (no—reference) | ||||||
| Yes | 0.84 | 2.31 | 0.17 | 11.43 | 0.000* | 2.00, 2.67 |
| Edulevel (illiterate—reference) | ||||||
| Primary | 0.065 | 1.07 | 0.07 | 0.96 | 0.338 | 0.93, 1.22 |
| Secondary | −0.11 | 0.89 | 0.11 | −0.92 | 0.358 | 0.70, 1.14 |
| Higher | −0.15 | 0.86 | 0.14 | −0.88 | 0.378 | 0.62, 1.20 |
| Work status (no—reference) | ||||||
| Yes | −0.13 | 0.88 | 0.05 | −2.09 | 0.037* | 0.78, 0.99 |
| Age (15–19 years—reference) | ||||||
| 20–34 years | 0.36 | 1.43 | .10 | 4.94 | 0.000* | 1.24, 1.644 |
| 35–49 years | 0.74 | 2.10 | .36 | 4.37 | 0.000* | 1.504, 2.92 |
| NChildren (no child—reference) | ||||||
| One or two children | 0.09 | 1.09 | 0.09 | 1.08 | 0.280 | 0.93, 1.28 |
| More than two children | 0.25 | 1.28 | 0.12 | 2.60 | 0.009* | 1.06, 1.55 |
| Residence (urban—reference) | ||||||
| Rural | 0.42 | 1.53 | 0.15 | 4.28 | 0.000* | 1.256, 1.85 |
| Wealth (poor—reference) | ||||||
| Middle | −0.25 | 0.78 | 0.06 | −3.17 | 0.002* | 0.66, 0.91 |
| Rich | −0.33 | 0.72 | 0.06 | −4.26 | 0.000* | 0.62, 0.84 |
| Region var(_cons) | 1.22 | 0.62 | 0.45, 3.30 | |||
BMI: body mass index.
Significant variable at 5% level of significant.
As shown in the fitted multilevel OLR model, BMI, current pregnancy, work status, age, number of children, residence, and wealth of women had significant effect on level of anemia in Ethiopia. Starting with the effect of BMI, women with normal weight had lower effect in the greater anemia level than underweight women. The odds of being normal weight in the greater category of anemia level were 0.59 times less likely as compared to underweight women. The odds in the greater category of anemia level were 1.40 times more likely for overweight as compared to underweight women. In regarding to obese women having greater levels of anemia were 2.56 times more likely as compared to underweight women while holding all other variables in the model constant. So, overweight and obese had incremental effect for higher levels of anemia in Ethiopia.
With regarding to current pregnancy of women, the odds of higher levels of anemia were increased by 2.31 for pregnant women as compared to non-pregnant women while holding all other variables in the model constant. Therefore, pregnant women in Ethiopia were more exposed for higher level of anemia.
Women having work being in greater anemia levels were 0.88 times less likely as compared to had no work. Adult women aged (20–34 years) being greater anemia levels were 1.43 times more likely as compared to younger women aged (15–19 years), and older women (35–49 years) higher anemia levels were 2.10 times more likely as compared to younger women. More number of children being in higher level of anemia were increased by 1.28 as compared to women have no child. The women living in the rural area being in the greater category anemia levels were 1.53 times more likely as compared to women living in urban area.
In addition, higher level of wealth index of women had negative influence for greater category of anemia level. The higher level of anemia in the middle quintile was reduced by 0.78 as compared to the higher level of anemia in the poor quintile, and the higher level of anemia in the rich quintile was 0.72 times less likely as compared to the higher level of anemia in the poor quintile women while holding all other variables in the model constant.
Discussion
This study was chosen between OLR and multilevel mixed-effects OLR models; it depends on the between-region variation effect on the level of anemia. Thus, the result showed that 27% was between-region variation on anemia level and significant impact on variability of anemia level. This finding was in line with other study findings in 2019 which indicated that considerable geographic disparities in anemia prevalence rate occur within Ethiopia.13 Also, a study in Sidama14 and Woliyata Sodo15 revealed that the prevalence of anemia was varied in different regions of the Ethiopia.
According to this study, women compared to normal weight, underweight women, overweight, and obese women had more likely for higher anemia levels in Ethiopia. This finding was contradicted to the finding of Nepal;9 the study in Nepal revealed that overweight women were less likely to be anemic as compared to women with normal BMI while the same evidence was reported in Nepal, in which high prevalence of anemia among underweight women.
This study also indicated that pregnant women in Ethiopia were more exposed for higher anemia levels. This result was in line with other study finding conducted in Ethiopia and other country studies,2,8,10,11 where the pregnancy status influences the hemoglobin concentration. This finding was in agreement with the study reported in Africa and South Asia,3,7 in which pregnant women experience high rates of anemia.
In this study, the result revealed that, aged women had positive effect for higher anemia levels. Our findings were also consistent with the study that indicated that women’s background characteristics were significant,8,9 in which age of women was potential causes of anemia in Ethiopia.
In this study, women who had more number of children showed a significant positive impact on the higher anemia levels for women in Ethiopia. This finding was in agreement with studies conducted in different parts of the country, Ethiopia;17, 18 in the studies, large family size varies in the prevalence rate of anemia in different parts of the country. This could be due to the possibility that more children might cause food insecurity in the household and women access unbalanced diet.
The women living in the rural area were more likely in the greater category of anemia level compared to women living in the urban area. This is consistent with a recent study conducted inside the country and outside the country,5,13,17,18 in which due to poor health-seeking behavior and inadequate infrastructure of health centers, women living in the rural area might not get care for maternal services and disease which may contribute to anemia.
Higher level of wealth index of women has negative influence for greater category of anemia levels. This is a similar to the finding conducted in the different studies,3–5 in which anemia was the highest prevalence rate in low-income and middle-income countries. Due to increasing price of foods and health services, women might not be afford the food and not treated for disease on time that may result anemia in Ethiopia.
Limitations
The data were adjusted for multistage sampling design used in the survey to make the findings of this study nationally reproducible. Nonetheless, the findings should be handled with cautions: first, the study did not consider the assessment of dietary factors and all important socioeconomic factors that might potentially have an impact on the development of anemia. Second, due to the cross-sectional design of the study, it was not possible to determine the cause–effect relationship between the predictor variables and anemia. Finally, sample size calculation was not performed for this study because we have used already collected data.
Conclusion
In this study, multilevel OLR model was fitted to measure the anemia level using fixed effect and random effect. Prevalence of anemia level according to region had significantly associated and more than 27% variation of anemia level was due to between-region variations in Ethiopia. The largest level of anemia prevalence was seen in Somali Region and Harari Region, respectively, while Benishangul Region and Tigray Region had the smallest predicted anemia level prevalence, respectively. From the fitted multilevel OLR model: women with normal weight had lower effect in the greater anemia level; overweight and obese had incremental effect for higher levels of anemia; pregnant women were more exposed for higher level of anemia; a women having work had negative effect on the higher level anemia; as women age increases, the level of anemia was also increased; women having more number of children had positive impact on the higher level of anemia; the households live in the rural area had greater level of anemia; and higher level of wealth index of women had negative influence for greater category of anemia level in Ethiopia.
Acknowledgments
The author acknowledges the Ethiopia Central Statistical Agency in Demographic and Health Survey program for accessing online the use of Ethiopia Demographic and Health Survey (EDHS) data for this study.
Footnotes
Author contributions: The author formulated the research question(s), designed the study, analyzed the data, interpreted the results, and wrote, reviewed and approved the final manuscript.
Availability of data and materials: The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The 2016 EDHS data are available to the general public by request in different formats from the Measure DHS website (http://idhsdata.org). The author applied the Measure DHS by briefly stating the objectives of the study and got the permission to download the women anemia levels data set in SPSS format.
ORCID iD: Enyew Assefa  https://orcid.org/0000-0001-9659-2415
https://orcid.org/0000-0001-9659-2415
References
- 1. World Health Organization. Essential nutrition actions improving maternal, newborn, infant and young child health and nutrition. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 2. Akhtar M, Hassan I. Severe anemia during late pregnancy. Case Rep Obstet Gynecol 2012; 2012: 485452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013; 1(1): e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumbiganon P, Laopaiboon M, Intarut N, et al. Indirect causes of severe adverse maternal outcomes: a secondary analysis of the WHO Multicountry Survey on Maternal and Newborn Health. BJOG 2014; 121: 32–39. [DOI] [PubMed] [Google Scholar]
- 5. Balarajan Y, Ramakrishnan U, Özaltin E, et al. Anemia in low-income and middle-income countries. Lancet 2011; 378: 2123–2135. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. The Global Prevalence of Anemia in 2011. Geneva: World Health Organization, 2015. [Google Scholar]
- 7. The World Bank. Prevalence of anemia among women of reproductive age. World Health Organization, Global health observatory data repository, World Health Statistics, 2018, https://data.worldbank.org/indicator/SH.ANM.ALLW.ZS
- 8. World Health Organization. Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. Geneva: WHO, 2011, https://www.who.int/vmnis/indicators/haemoglobin/en/ (accessed 1 March 2019). [Google Scholar]
- 9. Harding KL, Aguayo VM, Namirembe G, et al. Determinants of anemia among women and children in Nepal and Pakistan: an analysis of recent national survey data. Matern Child Nutr 2018; 14(Suppl. 4): e12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamu AL, Crampin A, Kayuni N, et al. Prevalence and risk factors for anemia severity and type in Malawian men and women: urban and rural differences. Popul Health Metr 2017; 15: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J-O, Lee JH, Ahn S, et al. Prevalence and risk factors for iron deficiency anemia in the Korean population: results of the fifth Korea national health and nutrition examination survey. J Korean Med Sci 2014; 29: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gautam S, Min H, Kim H, et al. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: evidence from recent national survey data. PLoS ONE 2019; 14(6): e0218288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kibret KT, Chojenta C, D’Arcy E, et al. Spatial distribution and determinant factors of anaemia among women of reproductive age in Ethiopia: a multilevel and spatial analysis. BMJ Open 2019; 9: e027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gebremedhin S, Enquselassie F, Umeta M. Prevalence and correlates of maternal anemia in rural Sidama, Southern Ethiopia. Afr J Reprod Health 2014; 18(1): 44–53. [PubMed] [Google Scholar]
- 15. Gedefaw L, Ayele A, Asres Y, et al. Anemia and associated factors among pregnant women attending antenatal care clinic in Wolayitasodo town, southern Ethiopia. Ethiop J Health Sci 2015; 25(2): 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lelissa D, Yilma M, Shewalem W, et al. Prevalence of anemia among women receiving antenatal care at Boditii health center, Southern Ethiopia. Clin Med Res 2015; 4: 79–86. [Google Scholar]
- 17. Melku M, Addis Z, Alem M, et al. Prevalence and predictors of maternal anemia during pregnancy in Gondar, northwest Ethiopia: an institutional based cross-sectional study. Anemia 2014; 2014: 108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebre A, Mulugeta A. Prevalence of anemia and associated factors among pregnant women in north western zone of Tigray, northern Ethiopia: a cross-sectional study. J Nutr Metab 2015; 2015: 165430–165437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health 2000; 21: 171–192. [DOI] [PubMed] [Google Scholar]
- 20. Ethiopia Demographic Health Survey. Addis Ababa; Calverton, MD: Central Statistical Agency and ICF International, 2016. [Google Scholar]
- 21. Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics 1990; 46(4): 1171–1178. [PubMed] [Google Scholar]
- 22. Agresti A. An introduction to categorical data analysis. New York: John Wiley & Sons, 1996. [Google Scholar]
- 23. Liu I, Agresti A. The analysis of ordered categorical data: an overview and a survey of recent developments. Test 2005; 14(1): 1–73. [Google Scholar]
- 24. McCullagh P. Regression models for ordinal data. J Royal Stat Soc Ser B 1980; 42: 109–142. [Google Scholar]
- 25. Bauer DJ, Sterba SK. Fitting multilevel models with ordinal outcomes: performance of alternative specifications and methods of estimation. Psychol Method 2011; 16(4): 373–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arfan M, Sherwani RAK. Ordinal logit and multilevel ordinal logit models: an application on wealth index MICS-survey data. Peer Rev Article 2017; 13: 211–226. [Google Scholar]
- 27. Khiari M, Rejeb JB. Determination of the regional impact on innovation with an ordinal logit and a multilevel analysis. Proced Soc Behav Sci 2015; 195: 592–602. [Google Scholar]
- 28. O’Connell AA. An illustration of multilevel models for ordinal response data. In: Proceedings of the eighth international conference on teaching statistics (ICOTS8) (Data and context in statistics education: towards an evidence-based society) Ljubljana, 11–16 July 2010. [Google Scholar]
- 29. Austin JT, Yaffee RA, Hinkle DE. Logistic regression for research in higher education. In: Paulsen M. (ed.) Higher education: handbook of theory and research. New York: Springer, 1992, p. 379–410. [Google Scholar]
- 30. Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol 2007; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snijders TAB, Berkhof J. Diagnostic checks for multilevel models. In: de Leeuw J, Meijer E. (eds) Handbook of Multilevel Analysis. New York: Springer, 2008, pp. 141–175. [Google Scholar]
- 32. Breslow N. Whither PQL? University of Washington Biostatistics Working Paper Series, No. 192, 2003, https://biostats.bepress.com/uwbiostat/paper192


