Abstract
Background:
There are limited data about the racial difference in the characteristics of chronic obstructive pulmonary disease (COPD) patients who are treated at clinics. We aimed to compare sociodemographic and clinical characteristics between US and Korean COPD patients using large-scale nationwide COPD cohorts.
Methods:
We used the baseline demographic and clinical data of COPD patients aged 45 years or older with at least a 10 pack-per year smoking history from the Korean COPD Subtype Study (KOCOSS, n = 1686) cohort (2012–2018) and phase I (2008–2011) of the US Genetic Epidemiology of COPD (COPDGene) study (n = 4477, 3461 were non-Hispanic whites [NHW], and 1016 were African Americans [AA]).
Results:
Compared to NHW, AA had a significantly lower adjusted prevalence ratio (aPR) of cough >3 months (aPR: 0.67; 95% CI [confidence interval]: 0.60–0.75) and phlegm >3 months (aPR: 0.78, 95% CI: 0.70–0.86), but higher aPR of dyspnea (modified Medical Round Council scale ⩾2) (aPR: 1.22; 95% CI: 1.15–1.29), short six-minute walk distance (<350 m) (aPR: 1.98; 95% CI: 1.81–2.14), and poor quality of life (aPR: 1.10; 95% CI: 1.05–1.15). Compared to NHW, Koreans had a significantly lower aPR of cough >3 months (aPR: 0.53; 95% CI: 0.47–0.59), phlegm >3 months (aPR: 0.75; 95% CI: 0.67–0.82), dyspnea (aPR: 0.72; 95% CI: 0.66–0.79), and moderate-to-severe acute exacerbation in the previous year (aPR: 0.73; 95% CI: 0.65–0.82). NHW had the highest burden related to chronic bronchitis symptoms and cardiovascular diseases related to comorbidities.
Conclusion:
There are substantial differences in sociodemographic characteristics, clinical presentation, and comorbidities between COPD patients from the KOCOSS and COPDGene, which might be caused by interactions between various intrapersonal, interpersonal, and environmental factors of the ecological model. Thus, a broader and more comprehensive approach would be necessary to understand the racial differences of COPD patients.
Keywords: chronic obstructive pulmonary disease, cohort, ecological model, racial difference
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common non-communicable diseases worldwide, and its global burden is increasing,1 especially in Asian countries.2 COPD is characterized by persistent respiratory symptoms and airflow limitation,3 but the clinical presentations of COPD appear to be substantially different depending on the race of the patient.4
Several post hoc analyses of therapeutic interventions showed different clinical characteristics between Asian and overall populations.5,6 Asian patients were more likely to be men, have a lower body mass index (BMI), and experience more severe airflow limitation than overall populations.4–6 However, most of the studies which compared racial differences were based on the results of therapeutic interventional trials or population-based observational studies. These studies may not reflect the characteristics of COPD patients who are treated at clinics.
We hypothesized that sociodemographic and clinical characteristics in COPD patients are different by race. In this study, we aimed to compare sociodemographic and clinical characteristics between US and Korean COPD patients using large-scale nationwide COPD cohorts in each country.
Methods
Study population
We used the baseline demographic and clinical data from the Korean COPD Subgroup Study (KOCOSS) cohort (2012–2018) and phase I (2008–2011) of the US Genetic Epidemiology of COPD (COPDGene) study. The KOCOSS is a multicenter cohort study of COPD patients of tertiary hospitals in South Korea.7 The COPDGene study is a multicenter cohort study of non-hispanic whites (NHW) and African Americans (AA) with at least a 10 pack-year smoking history in the US.8,9
For our analysis, we included patient data of adults aged 45 years or older with at least a 10 pack per-year smoking history and whose post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity was <0.7. The KOCOSS included 1686 Korean participants, and the COPDGene study included 4477 participants, of which 3461 were NHW, and 1016 were AA.
Data collection
Baseline sociodemographic information includes age, sex, smoking history, BMI, and education level. Overweight was defined as BMI ⩾25.0 kg/m2 and BMI ⩾23.0 kg/m2 for the NHW and AA and Koreans, respectively.10 In terms of clinical characteristics, symptoms [cough >3 months, phlegm >3 months, and modified medical round council (mMRC) scale], acute exacerbations in the previous year, six-minute walk distance, and severity of airflow limitation were included. The severity of airflow limitation was defined based on post-bronchodilator forced expiratory volume in 1 s (FEV1) as mild (FEV1 ⩾80% predicted), moderate (50% ⩽FEV1 <80% predicted), or severe-to-very severe (FEV1 <50% predicted).3 Comorbidities included cardiovascular disease, diabetes, gastroesophageal reflux, and osteoporosis. Quality of life was also assessed using the St George’s Respiratory Questionnaire (SGQR) in the COPDGene or the COPD-specific version of SGRQ (SGRQ-C) in the KOCOSS. It is well known that the SGRQ-C score is equivalent to the original SGRQ.11
Ethics and consent
Study protocols of the KOCOSS and COPDGene were approved by the institutional review board (IRB) of each participating hospital. Written informed consent was obtained from all patients of both studies. Ethical approval for this study was obtained from the institutional review board of the Samsung Medical Center (no. 2014-06-132).
Statistical analysis
Continuous and categorical variables were compared among the three groups using analysis of variance and χ2 tests, respectively. To compare the clinical characteristics by race, we generated all the variables as bivariate outcomes and conducted multivariable logistic regression models. Then we calculated prevalence ratios (PRs) using the predicted proportion from logistic regression. In this analysis, we adjusted for age, sex, BMI category (underweight, normal, overweight, obese, and unknown), smoking status (former and current), and post-bronchodilator FEV1 <50% predicted. In addition, we conducted a subgroup analysis by the severity of airflow limitations (mild-to-moderate versus severe-to-very severe) in all the characteristics. p values <0.05 were considered significant, and two-sided tests were used in all calculations. Statistical analyses were performed using STATA 14.0 (StataCorp LP, College Station, TX, USA).
Results
Sociodemographic and clinical characteristics
As shown in Table 1, Koreans in the KOCOSS were older and were more likely to be men than NHW and AA in the COPDGene study (p <0.01 for both). The ratio of men to women among current smokers was highest in Koreans (24.8) and lowest in NHW (1.3) (p <0.01). The mean BMI of NHW and AA was similar, while the BMI of Koreans was significantly lower (NHW versus AA versus Koreans, 27.9 versus 27.9 versus 22.3 kg/m2, p <0.01). Koreans had the lowest educational status, and NHW had the highest (NHW versus AA versus Koreans, 34.4 versus 59.0 versus 86.5 %, p <0.01). The KOCOSS data showed a pulmonary tuberculosis prevalence of 24% (these data were not available in the COPDGene data).
Table 1.
Characteristics of COPD patients, with smoking history in the COPDGene and KOCOSS.
| COPDGene cohort | KOCOSS cohort | p Value | ||
|---|---|---|---|---|
| NHW (n = 3461) | AA (n = 1016) | Koreans (n = 1686) | ||
| Age, years | 64.3 (8.3) | 58.6 (8.2) | 69.5 (7.6) | <0.01 |
| Sex, male | 1948 (56.3) | 555 (54.6) | 1641 (97.3) | <0.01 |
| Smoking status | <0.01 | |||
| Former | 2185 (63.1) | 351 (34.5) | 1221 (72.4) | |
| Current | 1276 (36.9) | 665 (65.5) | 465 (27.6) | |
| BMI, kg/m2 (n = 6154) | 27.9 (5.9) | 27.9 (6.7) | 22.3 (3.7) | <0.01 |
| Education, high school or less (n = 6148) | 1191 (34.4) | 599 (59.0) | 1447 (86.5) | <0.01 |
| Symptoms | ||||
| Cough >3 months (n = 6025) | 1486 (43.9) | 368 (37.7) | 357 (21.5) | <0.01 |
| Phlegm >3 months (n = 5996) | 1364 (40.5) | 366 (37.7) | 502 (30.3) | <0.01 |
| mMRC (n = 6055) | 1.9 (1.5) | 2.1 (1.5) | 1.4 (0.9) | <0.01 |
| AE in the previous year | ||||
| ⩾1 or more moderate-to-severe AE | 1295 (37.4) | 384 (37.8) | 377 (22.4) | <0.01 |
| Six-minute walk distance, m (n = 5670) | 387.4 (121.6) | 336.7 (125.2) | 389.7 (225.9) | <0.01 |
| COPD group | <0.01 | |||
| Mild | 610 (17.6) | 177 (17.4) | 273 (16.2) | |
| Moderate | 1442 (41.7) | 482 (47.4) | 931 (55.2) | |
| Severe | 918 (26.5) | 244 (24.0) | 412 (24.4) | |
| Very severe | 491 (14.2) | 113 (11.1) | 70 (4.2) | |
| Cardiovascular disease | ||||
| Hypertension (n = 6155) | 1617 (46.7) | 544 (53.5) | 665 (39.6) | <0.01 |
| Congestive heart failure (n = 6154) | 154 (4.5) | 51 (5.0) | 56 (3.3) | 0.07 |
| Dyslipidemia (n = 6147) | 1541 (44.5) | 301 (29.6) | 196 (11.7) | <0.01 |
| Myocardial infarction (n = 6155) | 286 (8.3) | 59 (5.8) | 81 (4.8) | <0.01 |
| Peripheral vascular disease (n = 6153) | 122 (3.5) | 20 (2.0) | 29 (1.7) | <0.01 |
| Diabetes mellitus (n = 6156) | 394 (11.4) | 155 (15.3) | 302 (18.0) | <0.01 |
| Gastro-esophageal reflux (n = 6150) | 1107 (32.0) | 198 (19.5) | 154 (9.2) | <0.01 |
| Osteoporosis (n = 6153) | 515 (14.9) | 62 (6.1) | 67 (4.0) | <0.01 |
| Quality of life (n = 5495) | ||||
| Total score | 35.9 (22.4) | 40.1 (24.2) | 36.0 (21.0) | <0.01 |
| Symptoms | 42.1 (25.7) | 44.0 (26.3) | 45.5 (20.7) | <0.01 |
| Impacts | 25.8 (21.1) | 30.1 (24.0) | 25.3 (19.6) | <0.01 |
| Activity | 50.6 (29.0) | 55.5 (29.9) | 48.0 (23.1) | <0.01 |
Variables were expressed as mean (SD) or number (%).
AA, African American; AE, acute exacerbation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; COPDGene, US Genetic Epidemiology of COPD study; KOCOSS, Korean COPD Subtype Study; mMRC, modified Medical Round Council; NHW, non-Hispanic white.
Compared to NHW, AA had a significantly lower aPR of cough >3 months (aPR: 0.67; 95% CI [confidence interval]: 0.60–0.75) and phlegm >3 months (aPR: 0.78, 95% CI: 0.70–0.86), but higher aPR of dyspnea (mMRC ⩾ 2) (aPR: 1.22; 95% CI: 1.15–1.29), short six-minute walk distance (<350 m) (aPR: 1.98; 95% CI: 1.81–2.14), and poor quality of life (SGQR total score ⩾ 25) (aPR: 1.10; 95% CI: 1.05–1.15). Compared to NHW, Koreans had a significantly lower aPR of cough >3 months (aPR: 0.53; 95% CI: 0.47–0.59), phlegm >3 months (aPR: 0.75; 95% CI: 0.67–0.82), dyspnea (aPR: 0.72; 95% CI: 0.66–0.79), moderate-to-severe AE in the previous year (aPR: 0.73; 95% CI: 0.65–0.82), and severe-to-very severe COPD (aPR: 0.50; 95% CI: 0.44–0.55). Korean COPD patients had poorer quality of life (SGQR-C total score ⩾25) (aPR: 1.07; 95% CI: 1.01–1.12) compared to NHW (Table 2).
Table 2.
Adjusted prevalence ratios and 95% confidence intervals of clinical characteristics in all patients.
| COPDGene cohort | KOCOSS cohort | ||
|---|---|---|---|
| NHW | AA | Koreans | |
| PR (95% CI) | PR (95% CI) | ||
| Age ⩾65 years | Reference | 0.61 (0.54, 0.68) | 1.42 (1.34, 1.50) |
| Sex, male | Reference | 0.98 (0.91, 1.04) | 1.77 (1.71, 1.83) |
| Smoking status, current | Reference | 1.52 (1.39, 1.65) | 0.85 (0.76, 0.94) |
| Overweight or obese (n = 6154) | Reference | 0.95 (0.90, 1.00) | 0.55 (0.50, 0.59) |
| Education, high school or less (n = 6148) | Reference | 1.64 (1.52, 1.77) | 2.57 (2.43, 2.71) |
| Symptoms | |||
| Cough >3 months (n = 6111) | Reference | 0.67 (0.60, 0.75) | 0.53 (0.47, 0.59) |
| Phlegm >3 months (n = 6082) | Reference | 0.78 (0.70, 0.86) | 0.75 (0.67, 0.82) |
| mMRC ⩾2 (n = 6131) | Reference | 1.22 (1.15, 1.29) | 0.72 (0.66, 0.79) |
| Moderate-to-severe AE in the previous year | Reference | 1.05 (0.94, 1.16) | 0.73 (0.65, 0.82) |
| Six-minute walk distance <350 m (n = 5670) | Reference | 1.98 (1.81, 2.14) | 1.07 (0.94, 1.19) |
| Severe-to-very severe COPD | Reference | 0.98 (0.89, 1.07) | 0.50 (0.44, 0.55) |
| Cardiovascular disease | |||
| Hypertension (n = 6155) | Reference | 1.34 (1.25, 1.42) | 0.81 (0.74, 0.88) |
| Congestive heart failure (n = 6145) | Reference | 1.33 (0.89, 1.77) | 0.77 (0.50, 1.03) |
| Dyslipidemia (n = 6147) | Reference | 0.80 (0.72, 0.88) | 0.24 (0.20, 0.27) |
| Myocardial infarction (n = 6155) | Reference | 0.88 (0.63, 1.12) | 0.45 (0.33, 0.57) |
| Peripheral vascular disease (n = 6144) | Reference | 0.70 (0.36, 1.04) | 0.34 (0.19, 0.50) |
| Diabetes mellitus (n = 6156) | Reference | 1.63 (1.33, 1.94) | 1.61 (1.34, 1.88) |
| Gastro-esophageal reflux (n = 6150) | Reference | 0.64 (0.55, 0.73) | 0.35 (0.29, 0.41) |
| Osteoporosis (n = 6153) | Reference | 0.47 (0.34, 0.60) | 0.42 (0.29, 0.54) |
| Poor quality of life* (n = 5571) | Reference | 1.10 (1.05, 1.15) | 1.07 (1.01, 1.12) |
Defined as the St George’s Respiratory Questionnaire (SGRQ) or COPD-specific version of SGRQ total score ⩾25.
Adjusted for age, sex, BMI categories (underweight, normal, overweight, obese, and unknown), smoking (former, and current), post-bronchodilator FEV1 <50% predicted.
AA, African American; AE, acute exacerbation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COPDGene, US Genetic Epidemiology of COPD; KOCOSS, Korean COPD Subtype Study; mMRC, modified Medical Round Council; NHW, non-Hispanic white; PR, prevalence ratio.
Regarding comoribidities, AA had a significantly lower aPR of dyslipidemia (aPR: 0.80; 95% CI: 0.72–0.88), gastro-esophageal reflux (aPR: 0.64; 95% CI: 0.55–0.73), and osteoporosis (aPR: 0.47; 95% CI: 0.34–0.60), but had a higher aPR of hypertension (aPR: 1.34; 95% CI: 1.25–1.42) and diabetes mellitus (aPR: 1.63; 95% CI: 1.33–1.94) compared to NHW. Koreans had a significantly lower aPR of hypertension (aPR: 0.81; 95% CI: 0.74–0.88), dyslipidemia (aPR: 0.24; 95% CI: 0.20–0.27), myocardial infarction (aPR: 0.45; 95% CI: 0.33–0.57), peripheral vascular disease (aPR: 0.34; 95% CI: 0.19–0.50), gastro-esophageal reflux (aPR: 0.35; 95% CI: 0.29–0.41), and osteoporosis (aPR: 0.42; 95% CI: 0.29–0.54) and had a higher aPR of diabetes mellitus (aPR: 1.61; 95% CI: 1.34–1.88), compared to NHW (Table 2). When we conducted additional analysis using COPD severity (mild-to-moderate or severe-to-very severe), the results were similar (Supplemental Table 1).
Discussion
The analysis of the COPDGene registry from the US and the KOCOSS registry from Korea showed that sociodemographic and clinical characteristics were significantly different between COPD patients from two cohorts. Among the three racial groups, NHW had the highest-burden related to chronic bronchitis symptoms and cardiovascular diseases related to comorbidities. In comparison, AA were most likely to be current smokers, reported more dyspnea, and had worse exercise capacity. Korean COPD patients were more likely to be men, older, and underweight compared to the NHW and AA COPD patients. They were also least likely to have symptoms and comorbidities except for diabetes. These findings were consistent irrespective of COPD severity.
Age, sex, smoking history, and education were significantly different among NHW, AA, and Korean COPD patients. This might be due to the rapid entry into an aging society and the predominantly high prevalence of male smokers in Korea.12,13 While women smoke at nearly the same rate as men in western countries, the 2019 Organization for Economic Cooperation and Development (OECD) data indicated that less than 5% of Korean women smoke.13 This high smoking rate is associated with low educational attainment, which is a well-known risk factor for COPD.14 In addition, Korean COPD patients had significantly lower BMI, which would be associated with nutrition and diet habit. These sociodemographic characteristics might interact with different prevalences of comorbidities as a COPD risk factor; a relatively high incidence of tuberculosis and a low prevalence of asthma in Korea, resulting in racial differences in clinical phenotype.4,15 Compared to NHW, AA are known to have a higher prevalence of childhood asthma.16 This might account for the relatively young age in AA in this study.
Regarding symptoms, NHW had more symptoms including cough and sputum than AA, suggesting the chronic bronchitis type of COPD would be predominant among the NHW COPD patients; whereas AA were younger and current smokers with lower education levels, more dyspnea, and poor exercise capacity, which was similar to the previous study.17 Compared to NHW and AA of the COPDGene, Koreans of the KOCOSS had significantly milder symptoms including cough, sputum, dyspnea, fewer previous exacerbations, and had fewer cardiovascular comorbidities, irrespective of COPD severity (Supplemental Table 1). Less symptom burden and lower exacerbation rate in patients from the KOCOSS might be attributed to relatively easy access to medical care.
We found that the comorbidity profile is different among groups. NHW had the highest burden of cardiovascular comorbidities and osteoporosis, while AA had comorbidity patterns driven by hypertension and diabetes mellitus, and Koreans had the lowest burden of comorbidities, except for diabetes mellitus. Given that COPD is often regarded as part of multimorbidity particularly in the elderly and these comorbidities are associated with COPD poor outcomes,18,19 different strategies among races may be required for optimal management of COPD-related comorbidities.
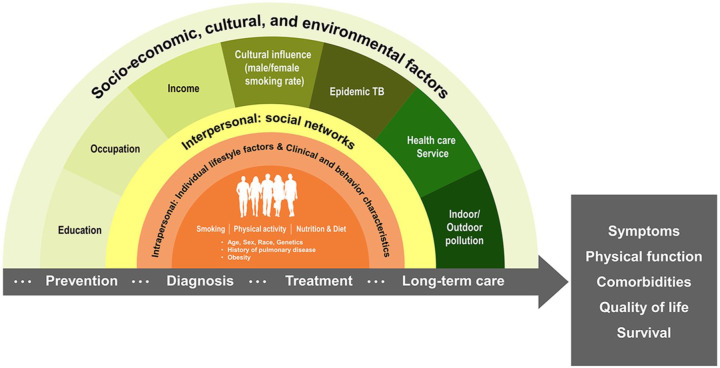
A simple model is hardly applicable to explain racial differences in sociodemographic and clinical characteristics of COPD patients from the two cohorts. Considering individual, interpersonal, social, and cultural factors are interactively linked in a complex manner, an ecological model comprehensively addressing these factors is necessary (Figure 1). The specific composition of individual, interpersonal, social, and cultural risk factors of COPD might be linked to the healthcare system in each country, further compounding differences by race in disease burden and presentation such as symptoms, physical function, and comorbidities (Figure 1). Or some of the differences in clinical features might be due to inter-racial genetic differences.20 Finally, environmental factors such as indoor/outdoor pollution levels, which continue to have a significant effect on the rates of COPD in Asia,21 might contribute to racial differences in our study.
Figure 1.
The ecological model of chronic obstructive pulmonary disease development and outcome adapted from Dahlgren and Whitehead (1991).22
There are several limitations to this study. First, both cohorts were hospital based, so participants would not represent COPD patients in each country studied. However, both cohorts were nationwide studies recruiting patients from multiple hospitals across each respective country. However, further study with a representative sample would be necessary to confirm the study findings. Second, the analysis was retrospective in nature, so all factors in ecological models such as indoor/outdoor pollution or genetics were not available. Third, because the two cohorts were conducted a few years apart, racial differences of some symptoms, quality of life, and outcomes might be due to different therapeutic approaches over time.
In conclusion, there are substantial differences in sociodemographic characteristics, clinical presentation, and comorbidities between COPD patients from the COPDGene and KOCOSS, which might be caused by interactions between various intrapersonal, interpersonal, and environmental factors of the ecological model. Thus, a broader and more comprehensive approach linking research-oriented clinical centers to national health registries or air pollution surveillance data would be necessary to understand the racial differences of COPD patients and to develop clinical guidelines for COPD prevention, diagnosis, treatment, and long-term care in the precision medicine era. Furthermore, it would be necessary to develop local guidelines reflecting racial differences of COPD patients and provide more personalized care for COPD.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_2040622320982455 for Understanding racial differences of COPD patients with an ecological model: two large cohort studies in the US and Korea by Hye Yun Park, Hyun Lee, Danbee Kang, Hye Sook Choi, Yeong Ha Ryu, Ki-Suck Jung, Don D. Sin, Juhee Cho and Kwang Ha Yoo in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors would like to thank Sang Eun Lee (Samsung Medical Information and Media Services at Samsung Medical Center) for her support.
Footnotes
Author contributions: HYP, HL, JC, and KHY were responsible for the conception and design of the study. HYP, HL, DK, JC, and KHY undertook the analysis and interpretation of the data. HYP, HL, DK, DDS, JC, and KHY drafted the manuscript. HYP, HL, HSC, YHR, KSJ, DDS, JC, and KHY made a critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Research of Korea Centers for Disease Control and Prevention (2016ER670100, 2016ER670101, 2016ER670102, 2018ER670100, 2018ER670101, and 2018ER670102) for the KOCOSS cohort and was supported by the National Heart, Lung, and Blood Institute (grants U01 HL089897 and U01 HL089856) for the COPDGene cohort. The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an industry advisory committee composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion.
Ethics approval and consent to participate: Consent to participate in this study was obtained from each subject in the KOCOSS and COPDGene. Ethics approval for this study was obtained from the institutional review board of Samsung Medical Center (no. 2014-06-132).
ORCID iD: Hye Yun Park  https://orcid.org/0000-0002-5937-9671
https://orcid.org/0000-0002-5937-9671
Availability of data and material: All data extracted in this study are included in this article.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hye Yun Park, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Hyun Lee, Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, South Korea.
Danbee Kang, Center for Clinical Epidemiology, Samsung Medical Center, Seoul, Republic of Korea Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, South Korea.
Hye Sook Choi, Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, South Korea.
Yeong Ha Ryu, Division of Pulmonary, Department of Internal Medicine, Dongkang Hospital, Ulsan, South Korea.
Ki-Suck Jung, Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, South Korea.
Don D. Sin, Respiratory Division, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
Juhee Cho, Department of Clinical Research Design & Evaluation, SAIHST, Sungkyunkwan University, 81 Irwon-ro, Gangnam-gu, Seoul 06351, South Korea Center for Clinical Epidemiology, Samsung Medical Center, Seoul, Republic of Korea Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Kwang Ha Yoo, Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 05030, South Korea.
References
- 1. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med 2018; 6: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GOLD. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.org (2020; accessed 1 October 2020).
- 4. Lee H, Shin SH, Gu S, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med 2018; 16: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology 2011; 16: 825–835. [DOI] [PubMed] [Google Scholar]
- 6. Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis 2017; 12: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JY, Chon GR, Rhee CK, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the KOrea COpd subgroup study team cohort. J Korean Med Sci 2016; 31: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maselli DJ, Bhatt SP, Anzueto A, et al. Clinical epidemiology of COPD: insights from 10 years of the COPDGene study. Chest 2019; 156: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Physical status: the use and interpretation of anthropometry: report of a World Health Organization (WHO) expert committee. Geneva: World Health Organization, 1995. [PubMed] [Google Scholar]
- 11. Meguro M, Barley EA, Spencer S, et al. Development and validation of an improved, COPD-specific version of the St George respiratory questionnaire. Chest 2007; 132: 456–463. [DOI] [PubMed] [Google Scholar]
- 12. Um J, Zaidi A, Choi S-J. Active ageing index in Korea – comparison with China and EU countries. Asian Soc Work Policy Rev 2019; 13: 87–99. [Google Scholar]
- 13. OECD. Health at a glance 2019. Available at: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2019_4dd50c09-en (2019; accessed 9 December 2020).
- 14. Pleasants RA, Riley IL, Mannino DM. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2016; 11: 2475–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SW, Kim YS, Kim D-S, et al. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci 2011; 26: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayden LP, Cho MH, Raby BA, et al. Childhood asthma is associated with COPD and known asthma variants in COPDGene: a genome-wide association study. Respir Res 2018; 19: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han MK, Curran-Everett D, Dransfield MT, et al. Racial differences in quality of life in patients with COPD. Chest 2011; 140: 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013; 22: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J 2006; 28: 1245–1257. [DOI] [PubMed] [Google Scholar]
- 20. Gim J, An J, Sung J, et al. A between ethnicities comparison of chronic obstructive pulmonary disease genetic risk. Front Genet 2020; 11: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh YM, Bhome AB, Boonsawat W, et al. Characteristics of stable chronic obstructive pulmonary disease patients in the pulmonology clinics of seven Asian cities. Int J Chron Obstruct Pulmon Dis 2013; 8: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dahlgren G, Whitehead M. Policies and strategies to promote social equity in health. Stockholm: Institute for Futures Studies, 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_2040622320982455 for Understanding racial differences of COPD patients with an ecological model: two large cohort studies in the US and Korea by Hye Yun Park, Hyun Lee, Danbee Kang, Hye Sook Choi, Yeong Ha Ryu, Ki-Suck Jung, Don D. Sin, Juhee Cho and Kwang Ha Yoo in Therapeutic Advances in Chronic Disease