Abstract
Objectives
To determine the prevalence of neuropathic pain among terminally ill patients with cancer admitted to a general ward, using the International Association for the Study of Pain algorithm.
Methods
This prospective observational study was conducted at a tertiary care center. We enrolled terminally ill patients with cancer admitted to the general ward between September 2018 and September 2019. On the day of consultation with our palliative care team, pain management clinicians examined and diagnosed neuropathic pain using the International Association for the Study of Pain diagnostic criteria.
Results
A total of 108 patients were enrolled during the study period. The median age was 69 years (interquartile range [IQR] 58.3–76.8 years), 72 patients (66.7%) were men, and the median survival time was 33 days (IQR 14.3–62 days). Of the 108 patients, 33 (30.6%) had neuropathic pain. Patients with neuropathic pain had more severe pain than those without neuropathic pain.
Conclusions
The prevalence of neuropathic pain in terminally ill patients with cancer admitted to a Japanese general ward was 30.6%. Further studies are warranted to elucidate whether the accurate diagnosis of neuropathic pain can improve pain control and/or patient conditions.
Keywords: Cancer pain, neuropathic pain, nociceptive pain, general ward, prevalence, terminally ill cancer patients
Introduction
The management of pain in patients with cancer is important because pain is associated with insomnia, anxiety, depression, and decreased quality of life.1,2 Pain is evident in 48% of patients with early-stage cancer and in 60% to 90% of patients with advanced-stage disease.3,4 In particular, neuropathic pain (NP) in patients with cancer increases the overall pain intensity and analgesic consumption, and further decreases the quality of life.5–7 In addition to opioids, coanalgesics such as anticonvulsants and tricyclic antidepressants are known to be effective in treating NP in oncological patients.8 NP in patients with cancer is a source of concern for both patients and their families, and imposes increased medical costs and a considerable social burden.9,10
Determination of NP as the primary cause of pain in patients with cancer is critical. Although some studies have evaluated the prevalence of NP in patients with cancer, their target populations have been highly variable in terms of the cancer stage and the diagnostic criteria for NP.11,12 The prevalence of NP among terminally ill patients with cancer admitted to palliative care units was determined as 18.6% when the NP diagnostic algorithm of the International Association for the Study of Pain (IASP) was applied.13,14
The prevalence of NP in terminally ill cancer patients admitted to general wards remains unknown. Because most terminally ill patients with cancer are admitted to general wards in Japan, investigating the prevalence of NP in this setting is relevant. Overlooking NP in patients with cancer may lead to insufficient pain management. NP in these patients is mostly intractable, and considerable amounts of opioid analgesics might be required. However, coanalgesics have also been determined to be effective in treating NP.8
The primary aim of this study was to determine the prevalence of NP among terminally ill patients with cancer who were admitted to a general ward. The secondary aim was to compare the survival time, pain therapy, equivalent oral morphine dose, history of chemotherapy and/or biologic treatment, numerical rating scale (NRS) score, and pain type between NP and non-NP patients.
Patients and methods
Study design, setting, and patients
This was a single-center prospective observational study performed among terminally ill patients with cancer who were admitted to a general ward. We included consecutive cancer patients ≥20 years old who were admitted to Yokohama City University Medical Center and who consulted the palliative care team (PCT) between September 2018 and September 2019. We excluded patients with disturbances in consciousness, cognitive dysfunction, and psychiatric disorders, as well as those who declined or withdrew consent. We also excluded patients who did not complete the follow-up, and those who had survival times of ≥181 days from the registration day because we defined patients with survival times of ≤180 days as terminally ill.
Yokohama City University Medical Center is a 726-bed academic general hospital with a tertiary emergency center located in the center of Yokohama City, which is the second largest city in Japan with a population of 3.7 million. This hospital has no wards or beds that are specifically assigned to palliative care patients. All terminally ill patients with cancer are allotted a primary physician/surgeon or an appropriate specialty cancer team. The involvement of the PCT is at the discretion of the primary doctor/team or upon patient request.
Diagnosis of neuropathic pain
Pain management clinicians in the PCT diagnosed NP and determined its severity using the NRS on the day of the consultation. A definitive diagnosis of NP or non-NP was made using the NP diagnostic algorithm of the IASP.14 This algorithm states that the patient has ‘possible NP’ if the pain distribution is neuroanatomically plausible and the patient’s history suggests a relevant lesion or disease. The pain is then further classified by the presence of the following criteria: (1) sensory signs confined to the innervation territory of the nervous structure within the lesion, and (2) diagnostic confirmation of a lesion or disease explaining NP. Satisfaction of either one of these criteria confirms the diagnosis of ‘probable NP,’ and satisfaction of both criteria confirms the diagnosis of ‘definite NP.’ In the present study, both ‘probable NP’ and ‘definite NP’ were considered as NP.13 We examined pain type (somatic pain, visceral pain, and pure NP) based on each patient’s clinical history, clinical findings, and imaging results.
Patient characteristics
For each patient, we also recorded the age, sex, primary tumor site, extent of the disease (metastatic or local), metastatic locations, history of anticancer treatment, Eastern Cooperative Oncology Group (ECOG) Performance Status score, survival time, NRS score, and pain therapy.
Statistical analysis
All statistical analyses were conducted using JMP version 12 (SAS Institute Inc., Cary, NC, USA). Categorical and numerical data were analyzed using the chi-squared and Mann–Whitney U tests, respectively. Data pertaining to the NRS score and equivalent oral morphine dose were evaluated on the day of the consultation. Statistical significance was set at P < 0.05. We did not perform a sample size calculation because our study was a descriptive epidemiological study.
Ethical considerations
This study was approved by the ethics committee of the Yokohama City University Medical Center (B190905210, 29 August 2018), Yokohama, Japan, and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. All patients provided written informed consent. Our observational study complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.15
Results
During the study period, 211 patients consulted the PCT after admission. Patients who met the following exclusion criteria were excluded: 26 patients with disturbances of consciousness, 5 with cognitive dysfunction, 2 with psychiatric disorders, 13 who declined or withdrew consent, 3 who did not complete the follow-up, and 54 with survival time ≥181 days. Thus, 108 patients were eligible for the study and were statistically analyzed (Figure 1).
Figure 1.
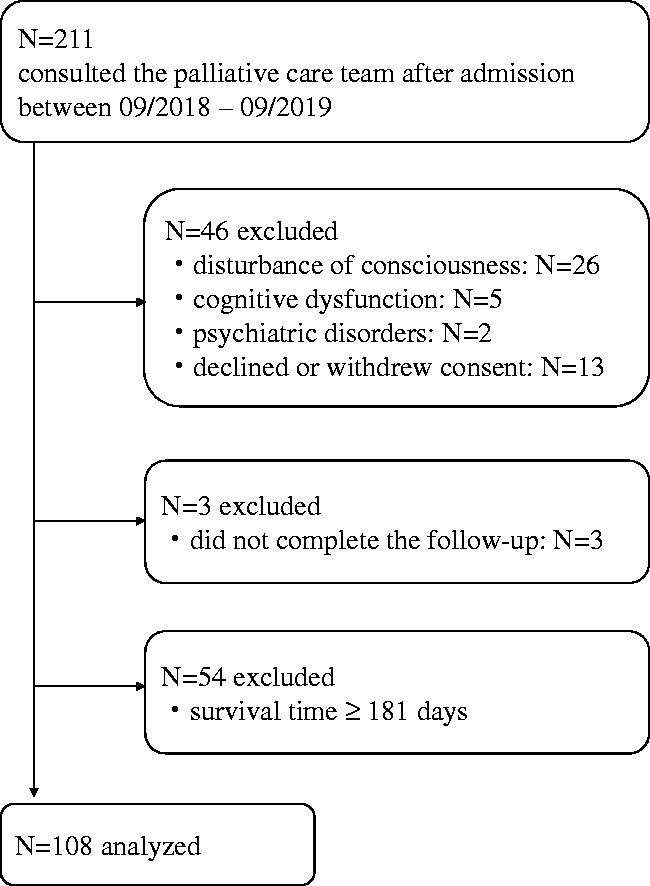
Flow chart of participant selection.
The baseline characteristics of all included patients are shown in Table 1. The median age was 69 years (interquartile range [IQR] 58.3–76.8 years), and 72 patients (66.7%) were men. The primary tumor sites were colon/rectum (13.0%), lung (12.0%), and stomach (12.0%). Metastatic disease was present in 107 patients (99.1%), and the most frequent metastatic locations were the lymph nodes (79.6%), liver (41.7%), bone (37.0%), and lung (29.6%). Regarding anticancer treatment, chemotherapy and/or biologic treatment were the most frequently used (80.6%), followed by surgery (53.7%) and radiation therapy (27.8%). No anticancer treatment was performed in 11.1% of subjects. The ECOG Performance Status scores were 3 and 2 in 48 (44.4%) and 35 patients (32.4%), respectively. The median survival time was 33 days (IQR 14.3–62 days).
Table 1.
Baseline characteristics of the 108 patients included in the study.
| Number | (%) | |
|---|---|---|
| Age, years | ||
| Median (IQR) | 69 (58.3–76.8) | |
| Sex | ||
| Male | 72 | 66.7 |
| Primary tumor site | ||
| Colon–rectum | 14 | 13.0 |
| Lung | 13 | 12.0 |
| Stomach | 13 | 12.0 |
| Pancreas | 11 | 10.2 |
| Pharynx | 5 | 4.6 |
| Breast | 5 | 4.6 |
| Kidney | 5 | 4.6 |
| Uterus | 5 | 4.6 |
| Prostate | 4 | 3.7 |
| Other | 33 | 30.6 |
| Extent of disease | ||
| Metastatic | 107 | 99.1 |
| Local | 1 | 0.9 |
| Metastatic locations | ||
| Lymph node | 86 | 79.6 |
| Liver | 45 | 41.7 |
| Bone | 40 | 37.0 |
| Lung | 32 | 29.6 |
| Peritoneal | 27 | 25.0 |
| History of anticancer treatment | ||
| Chemotherapy and/or biologic treatment | 87 | 80.6 |
| Surgery | 58 | 53.7 |
| Radiotherapy | 30 | 27.8 |
| None | 12 | 11.1 |
| ECOG Performance Status Score | ||
| 1 | 9 | 8.3 |
| 2 | 35 | 32.4 |
| 3 | 48 | 44.4 |
| 4 | 16 | 14.8 |
| Survival time, days | ||
| Median (IQR) | 33 (14.3–62) | |
| Mean (SD) | 41.9 (33.4) |
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; SD, standard deviation.
The pain characteristics of the 108 patients are shown in Table 2. The median NRS score was 6 (IQR 4–8). Seven patients (6.5%) had no pain, 14 (12.9%) had mild pain (NRS score 1–3), and 87 (80.6%) had moderate or severe pain (NRS score ≥4). At the time of consultation with the PCT, 70 patients (61.2%) were prescribed opioids (oxycodone, 32.4%), 101 patients (93.5%) were prescribed non-opioid medications (acetaminophen, 50.9%), and 10 patients (9.3%) were prescribed coanalgesics (pregabalin, 9.3%). Gabapentin was not used because gabapentin had not been approved for the treatment of NP in Japan in the study period. Twelve patients (11.1%) were unable to take oral medicines (data not shown).
Table 2.
Pain characteristics of the 108 patients included in the study.
| Number | (%) | |
|---|---|---|
| NRS score | ||
| Median (IQR) | 6 (4–8) | |
| 0 | 7 | 6.5 |
| 1–3 | 14 | 12.9 |
| ≥4 | 87 | 80.6 |
| Pain therapy | ||
| Opioids | 70 | 61.2 |
| Oxycodone | 35 | 32.4 |
| Hydromorphone | 8 | 7.4 |
| Fentanyl | 4 | 3.7 |
| Morphine | 4 | 3.7 |
| Other | 19 | 17.6 |
| Non-opioids | 101 | 93.5 |
| Acetaminophen | 55 | 50.9 |
| NSAIDs | 45 | 41.7 |
| Steroids | 1 | 0.9 |
| Coanalgesics | 10 | 9.3 |
| Pregabalin | 10 | 9.3 |
| TCA | 2 | 1.9 |
IQR, interquartile range; NRS, numerical rating scale; NSAIDs, nonsteroidal anti-inflammatory drugs; TCA, tricyclic antidepressants.
The prevalence of NP among the 108 eligible patients is shown in Table 3. Thirty-three patients (30.6%) were diagnosed with NP, including 27 with definite NP and six with probable NP, based on the NP diagnostic algorithm of the IASP. In contrast, 68 patients (62.9%) were diagnosed with non-NP, and 7 patients had no pain.
Table 3.
Prevalence of NP in the 108 patients included in the study.
| Diagnosis | Number | (%) |
|---|---|---|
| NP | 33 | 30.6 |
| Definite NP | 27 | |
| Probable NP | 6 | |
| Non-NP | 68 | 62.9 |
| No pain | 7 | 6.5 |
NP, neuropathic pain.
The characteristics of the 33 NP patients with cancer are shown in Table 4. The primary cause of NP was tumor-related in 26 patients (78.8%), related to chemotherapy and/or biologic treatment in 6 patients (18.2%), and non-oncologic in 1 patient (3.0%). Tumor infiltration or compression and lumbosacral plexopathy were the primary causes of tumor-related NP. The primary tumor sites were the lung (15.2%) and stomach (15.2%). Among the cancer types, patients with prostate cancer had the highest prevalence of NP (4/4, 100.0%),
Table 4.
Characteristics of the 33 patients with cancer and NP.
| Number | (%) | ||
|---|---|---|---|
| Cause of pain | |||
| Tumor-related NP | 26 | 78.8 | |
| Tumor infiltration or compression | 9 | 27.3 | |
| Lumbosacral plexopathy | 7 | 21.2 | |
| Cervical plexopathy | 3 | 9.1 | |
| Brachial plexopathy | 3 | 9.1 | |
| Other | 4 | 12.1 | |
| Treatment-related NP | 6 | 18.2 | |
| Chemotherapy and/or biologic treatment | 6 | 18.2 | |
| Radiotherapy | 0 | 0 | |
| Post-surgery neuralgia | 0 | 0 | |
| Non-oncologic NP | 1 | 3.0 | |
| Lumbar spinal stenosis | 1 | 3.0 | |
| Postherpetic neuralgia | 0 | 0 | |
| Primary tumor site | NP Pt/Cancer Pt (%) | ||
| Lung | 5 | 15.2 | 5/13 (38.5) |
| Stomach | 5 | 15.2 | 5/13 (38.5) |
| Prostate | 4 | 12.1 | 4/4 (100.0) |
| Breast | 2 | 6.1 | 2/5 (40.0) |
| Colon–rectum | 2 | 6.1 | 2/14 (14.3) |
| Uterus | 2 | 6.1 | 2/5 (40.0) |
| Other | 13 | 39.4 |
NP, neuropathic pain; NP Pt/Cancer Pt, number of NP patients/number of cancer patients (for each cancer type).
A comparison of NP and non-NP patient characteristics is shown in Table 5. Age (69 vs. 68.5 years), sex (22 vs. 44 men), ECOG Performance Status Score (3 vs. 3), survival time (40 vs. 32.5 days), and history of chemotherapy and/or biologic treatment (26 vs. 57 patients) were not significantly different between the two groups. In contrast, pain intensity was significantly higher in NP patients than in non-NP patients (median NRS score 7 vs. 5; P = 0.025). However, the daily equivalent oral morphine dose (23 vs. 15 mg) and the use of opioids (21 vs. 44 patients) were not significantly different between NP and non-NP patients. The use of coanalgesics was significantly more prevalent among NP patients (9 vs. 1 patient; P < 0.001). Regarding pain type, the coexistence of somatic pain was significantly more common in NP patients than in non-NP patients (66.7% vs. 22.1%; P < 0.001). NP caused by spinal cord compression, accompanied by somatic pain for vertebral bone metastases, was the most common (in 8 patients, data not shown). Conversely, the coexistence of visceral pain was significantly less common in NP patients than in non-NP patients (9.1% vs. 42.6%; P < 0.001). Most non-NP patients (21 patients, data not shown) had visceral pain caused by lymph node metastases in gastrointestinal, hepatobiliary, or pancreatic cancer. There was no significant difference between NP and non-NP patients in the prevalence of coexisting somatic and visceral pain (24.2% vs. 35.3%). No patients had pure NP.
Table 5.
Comparison between NP and non-NP patients.
| NP | Non-NP | ||
|---|---|---|---|
| n = 33 | n = 68 | P- value | |
| Age, years | |||
| Median (IQR) | 69 (60.5–74.5) | 68.5 (58–75.8) | 0.622 |
| Sex, number | |||
| Male | 22 | 44 | 0.846 |
| ECOG Performance Status Scale | |||
| Median | 3 | 3 | 0.882 |
| Survival time, days | |||
| Median (IQR) | 40 (17–69.5) | 32.5 (15.3–56.5) | 0.377 |
| Mean (SD) | 47.3 (34.6) | 41.4 (32.6) | |
| Pain therapy | |||
| Opioids, number (%) | 21 (63.6) | 44 (64.7) | 0.916 |
| Coanalgesics, number (%) | 9 (27.3) | 1 (1.5) | <.001 |
| Equivalent oral morphine dose, mg | |||
| Median (IQR) | 23 (0–40) | 15 (0–50) | 0.897 |
| History of chemotherapy and/or biologic treatment, number (%) | |||
| 26 (78.8) | 57 (83.8) | 0.535 | |
| NRS score | |||
| Median (IQR) | 7 (5.5–8) | 5 (4–8) | 0.025 |
| Pain type, number (%) | |||
| Somatic pain | 22 (66.7) | 15 (22.1) | <.001 |
| Visceral pain | 3 (9.1) | 29 (42.6) | <.001 |
| Somatic and visceral pain | 8 (24.2) | 24 (35.3) | 0.256 |
| Pure NP | 0 (0) | ||
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; NP, neuropathic pain; NRS, numerical rating scale; SD, standard deviation.
Discussion
This single-center observational study revealed that the prevalence of NP in terminally ill patients with cancer admitted to the general ward was 30.6%. To the best of our knowledge, this is the first study to investigate the prevalence of NP using the NP diagnostic algorithm of the IASP in terminally ill patients with cancer who were admitted to the general ward of a hospital in Japan.
The strengths of our study include the use of the IASP criteria to diagnose NP by pain specialists, because they are the standard criteria for the diagnosis of NP.14 Although other screening tools for NP have been applied in non-cancer patients, past studies have reported that their diagnostic accuracy is insufficient in patients with cancer because—as we also demonstrated—such patients rarely have pure NP and often show characteristics of mixed pain, with both neuropathic and nociceptive components.16,17 The nociceptive component of mixed pain in cancer patients may complicate the identification of the neuropathic component when screening tools such as painDETECT18 and S-LANSS16,19 are used. Thus, a thorough clinical assessment by pain specialists is fundamental for the diagnosis of NP in patients with cancer.17
Using the same IASP diagnostic criteria for NP, other investigators have reported a prevalence of NP of 18.6% in terminally ill patients with cancer admitted to the palliative care unit of a Japanese cancer center,13 and 41.4% among outpatients with cancer in a pain clinic with life expectancy ≥3 months.17 Our results are in accordance with these previous results, and collectively suggest that a certain fraction of patients with terminal cancer experience NP in a wide range of clinical settings (i.e., as inpatients/outpatients or in a general ward/palliative care unit).
The primary cause of NP in our patients was tumor-related, while a smaller fraction of patients experienced treatment-related NP. Tumor-related NP is caused by various mechanisms that depend on the cancer type. For example, spinal cord compression caused by vertebral bone metastasis20 has been observed in patients with lung, prostate, stomach, and other cancers. Lumbosacral plexopathy is mainly caused by lumbar sacral bone metastasis, cervical plexopathy by head and neck cancer, and brachial plexopathy by supraclavicular lymph node metastases. Interestingly, treatment-related NP in the present study was caused exclusively by chemotherapy or biologic treatment, and not by radiation or other therapies. The generalizability of this finding is unclear, however, because the number of patients included in this study was relatively small.
In the current study, there were no patients with pure NP; that is, all patients with NP also had nociceptive components, such as somatic and/or visceral pain (Table 5). A typical example of pure NP in patients with cancer is chemotherapy-induced peripheral neuropathic pain. It is caused by injury to the peripheral nerves as a result of the neurotoxicity of chemotherapy, and it usually manifests as pure NP, rather than mixed pain.13 The absence of pure NP in our patients may be because we only studied end-stage patients; the prevalence of nociceptive pain increases with the progression of cancer.3,4
Our results demonstrated that patients with NP had more intense pain than those without NP, although both groups of patients were receiving comparable equivalent oral morphine doses. Overall, our patients appeared to have been receiving insufficient amounts of narcotics because even non-NP patients experienced considerable levels of pain (median NRS score 5/10, IQR 4–8). However, it is unclear whether the observed higher pain intensity in NP patients could have been mitigated simply by increasing the dose of narcotics because NP is resistant to the effects of these drugs.16 Nevertheless, our results suggest the importance of NP diagnosis in cancer patients because NP leads to more intense pain, and effective treatment often requires multimodal therapies, including coanalgesics and radiation. Some of these therapies may be beyond the expertise of non-specialists in cancer pain control.
A significantly larger fraction of patients with NP were receiving coanalgesics compared with those without NP. This finding suggests that the primary doctors/teams had recognized the difficulties of treating pain using narcotics only in patients with NP, even before consulting the PCT, although it is unknown whether the primary doctors/teams had diagnosed the presence of NP. The accurate diagnosis of NP in patients with cancer can be difficult: it is characteristically different from typical NP without cancer because of the coexistence of nociceptive pain.16,17 Moreover, there are no guidelines for the use of coanalgesics in treating NP in cancer patients. For these reasons, it is necessary for pain management specialists to quantify the NP component involved in each patient's pain, and to evaluate the indications of coanalgesics in patients with cancer.
Our study has several limitations. First, it was a single-center study, and the sample size was relatively small. Moreover, the prevalence of NP is likely to differ among hospitals because cancer types and stages are varied. Second, the time of diagnosis of NP was limited to the first consultation. NP might have emerged later in some patients, as the cancer progressed. Third, it remains unknown whether a diagnosis of NP leads to meaningful changes in clinical outcomes, even when the PCT is consulted. Fourth, the limited number of samples in the present study might have affected the statistical significance of our results.
In conclusion, the prevalence of NP among terminally ill patients with cancer admitted to a Japanese general ward was 30.6% in our study. Cancer-related NP represented 78.8% of all NP cases. NP patients had significantly higher NRS scores and were more often prescribed coanalgesics by their primary doctor/team compared with their non-NP patients. Further studies are warranted to investigate whether the accurate diagnosis of NP can lead to better pain control and/or patient conditions.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yusuke Nagamine https://orcid.org/0000-0002-0443-5007
References
- 1.Mystakidou K, Parpa E, Tsilika E, et al. Comparison of pain quality descriptors in cancer patients with nociceptive and neuropathic pain. In Vivo 2007; 21: 93–97. [PubMed] [Google Scholar]
- 2.Portenoy RK, Miransky J, Thaler HT, et al. Pain in ambulatory patients with lung or colon cancer. Prevalence, characteristics, and effect. Cancer 1992; 70: 1616–1624. DOI: 10.1002/1097-0142(19920915)70:6<1616::aid-cncr2820700630>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MI, Rayment C, Hjermstad M, et al. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 2012; 153: 359–365. DOI: 10.1016/j.pain.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Christo PJ, Mazloomdoost D. Cancer pain and analgesia. Ann N Y Acad Sci 2008; 1138: 278–298. DOI: 10.1196/annals.1414.033. [DOI] [PubMed] [Google Scholar]
- 5.Mulvey MR, Boland EG, Bouhassira D, et al. Neuropathic pain in cancer: systematic review, performance of screening tools and analysis of symptom profiles. Br J Anaesth 2017; 119: 765–774. DOI: 10.1093/bja/aex175. [DOI] [PubMed] [Google Scholar]
- 6.Kerba M, Wu JS, Duan Q, et al. Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol 2010; 28: 4892–4897. DOI: 10.1200/JCO.2010.28.6559. [DOI] [PubMed] [Google Scholar]
- 7.Pina P, Sabri E, Lawlor PG. Characteristics and associations of pain intensity in patients referred to a specialist cancer pain clinic. Pain Res Manag 2015; 20: 249–254. DOI: 10.1155/2015/807432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urch CE, Dickenson AH. Neuropathic pain in cancer. Eur J Cancer 2008; 44: 1091–1096. DOI: 10.1016/j.ejca.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009; 7: 102. DOI: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BH, Torrance N, Bennett MI, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain 2007; 23: 143–149. DOI: 10.1097/01.ajp.0000210956.31997.89. [DOI] [PubMed] [Google Scholar]
- 11.Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat Med 2013; 27: 714–721. DOI: 10.1177/0269216312464408. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Gordo AM, Feliu J, Martinez B, et al. Descriptive analysis of clinical factors affecting terminally ill cancer patients. Support Care Cancer 2009; 17: 261–269. DOI: 10.1007/s00520-008-0460-y. [DOI] [PubMed] [Google Scholar]
- 13.Harada S, Tamura F, Ota S. The prevalence of neuropathic pain in terminally ill patients with cancer admitted to a palliative care unit: a prospective observational study. Am J Hosp Palliat Care 2016; 33: 594–598. DOI: 10.1177/1049909115577353. [DOI] [PubMed] [Google Scholar]
- 14.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008; 70: 1630–1635. DOI: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. DOI: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashibata T, Tagami K, Miura T, et al. Usefulness of painDETECT and S-LANSS in identifying the neuropathic component of mixed pain among patients with tumor-related cancer pain. Support Care Cancer 2020; 28: 279–285. DOI: 10.1007/s00520-019-04819-9. [DOI] [PubMed] [Google Scholar]
- 17.Tzamakou E, Petrou A, Tefa L, et al. Detection of neuropathic pain in end-stage cancer patients: diagnostic accuracy of two questionnaires. Pain Pract 2018; 18: 768–776. DOI: 10.1111/papr.12671. [DOI] [PubMed] [Google Scholar]
- 18.Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006; 22: 1911–1920. DOI: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 19.Hardy J, Quinn S, Fazekas B, et al. Can the LANSS scale be used to classify pain in chronic cancer pain trials? Support Care Cancer 2013; 21: 3387–3391. DOI: 10.1007/s00520-013-1921-5. [DOI] [PubMed] [Google Scholar]
- 20.Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003; 15: 211–217. DOI: 10.1016/s0936-6555(02)00400-4. [DOI] [PubMed] [Google Scholar]


