Abstract
Aims:
The therapeutic use of psychedelics is regaining scientific momentum, but similarly psychoactive ethnobotanical substances have a long history of medical (and other) uses in indigenous contexts. Here we aimed to evaluate patient outcomes in a residential addiction treatment center that employs a novel combination of Western and traditional Amazonian methods.
Methods:
The study was observational, with repeated measures applied throughout treatment. All tests were administered in the center, which is located in Tarapoto, Peru. Data were collected between 2014 and 2015, and the study sample consisted of 36 male inpatients who were motivated to seek treatment and who entered into treatment voluntarily. Around 58% of the sample was from South America, 28% from Europe, and the remaining 14% from North America. We primarily employed repeated measures on a psychological test battery administered throughout treatment, measuring perceived stress, craving frequency, mental illness symptoms, spiritual well-being, and physical and emotional health. Addiction severity was measured on intake, and neuropsychological performance was assessed in a subsample from intake to at least 2 months into treatment.
Results:
Statistically significant and clinically positive changes were found across all repeated measures. These changes appeared early in the treatment and were maintained over time. Significant improvements were also found for neuropsychological functioning.
Conclusion:
These results provide evidence for treatment safety in a highly novel addiction treatment setting, while also suggesting positive therapeutic effects.
Keywords: addiction, ayahuasca, safety, Takiwasi, traditional Amazonian medicine
Introduction
Addiction treatment was an exciting line of inquiry during the first wave of psychedelic research in the mid-20th century, but the field was marred by inadequate research methodology and growing controversies that led to a near total investigatory suspension.1–4 After a decades-long hiatus, a revitalized second wave is now well underway.5–7 Once again, addictions are a target of psychedelic research with some impressive early results,8–14 and there is hope for a breakthrough treatment similar to the recent success of MDMA-assisted psychotherapy for post-traumatic stress disorder.15 Yet while the psychiatric discovery and embrace of psychedelics is relatively recent, the use of similar substances in shamanic and ethnomedical contexts is much older and is likely to have ancient roots.16–18
Therapeutic use of ayahuasca
One such ethnobotanical substance is ayahuasca—the common name of the vine Banisteriopsis caapi, which contains monoamine oxidase-inhibiting β-carbolines and is traditionally used alone or with various admixtures19,20—but also the name of the decoction prepared from B. caapi and the leaves of a plant containing the psychedelic N,N-DMT,21 such as Psychotria viridis or Diplopterys cabrerana.22 Ayahuasca (i.e. the DMT-containing decoction) is powerfully psychoactive but appears to be safe when used appropriately.23–30 In recent times its therapeutic potential has been increasingly documented, particularly for the alleviation of substance abuse, depression, and anxiety-related disorders.31–37
Ayahuasca is not considered to carry a high intrinsic addiction potential,38,39 and indeed suggestions of anti-addictive outcomes have been reported since the earliest biomedical study on the sacramental use of ayahuasca,40 with evidence slowly accumulating since then.41–47 Various potential mechanisms have been proposed,48–52 yet quantitative studies of ayahuasca for addiction have only rarely been conducted in explicitly therapeutic settings,53,54 in part due to regulatory challenges.55
The Takiwasi Center
Running parallel to these developments is a well-established and nationally accredited therapeutic community in Peru, the Takiwasi Center, which has been employing ayahuasca in the treatment of addictions since 199256—around the same time that human psychedelic research resumed.57,58 However, Takiwasi is rather poorly characterized as an “ayahuasca-assisted” treatment, since a variety of other traditional techniques are used; for example, the traditional dieta (diet) is particularly important, during which a patient enters social seclusion while receiving restricted alimentation, along with the intake of prescribed medicinal plants.59,60 The ceremonial use of ayahuasca, diets, and other plant-based techniques proceed from traditional Amazonian medicine,61–63 which Takiwasi combine with Western psychotherapeutic and biomedical approaches.
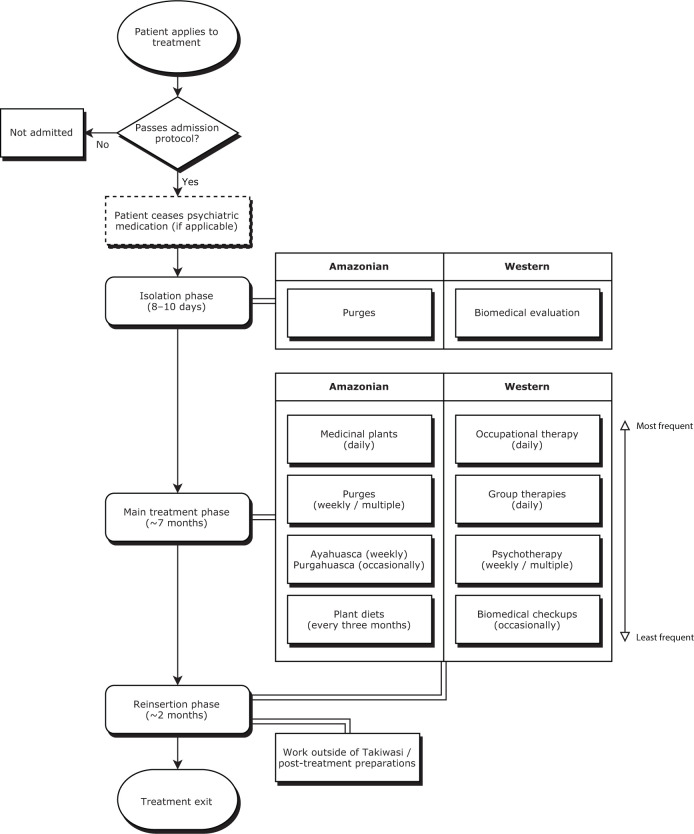
Being founded by a French medical doctor, the combination of Amazonian and Western medicine in Takiwasi is operated from within a biopsychosocial-spiritual framework.64 For example, clinical staff at the center may be healers with training in traditional Amazonian medicine, but there are also professional doctors, psychologists, and nurses. Due to potential interactions between pharmaceuticals and plant medicines,25 patients cease taking psychiatric medications prior to entry, although this does not apply to those with a history of psychosis, as they would not be accepted for treatment.56 Certain physical health conditions are also part of the center’s exclusion criteria, including diabetes, gastric ulcers, and epilepsy, but also renal, cardiac, respiratory or hepatic insufficiency.56,65 Once admitted, the ideal treatment process lasts around 9 months (although this is flexible), and progresses through stages of: (a) initial isolation (around 8–10 days); (b) main treatment (around 7 months); and finally (c) a reinsertion phase (around 2 months) where patients continue with the main treatment, but are preparing for post-treatment life and thus are able to leave the center and engage in work in the community (see Figure 1). Across the phases, treatment primarily revolves around physical detoxification (focusing on the use of emetic and psychoactive plant preparations), psychotherapy (group and individual), occupational therapy, community living, psychological and spiritual development (through psychoactive plant sessions and diets), and also biomedical evaluation. The application of medicinal plants in Takiwasi has parallels to psychedelic-assisted psychotherapy, although the Takiwasi framework differs in that its roots are in traditional Amazonian medicine. More detailed descriptions of these treatment procedures have been provided by Berlowitz et al.,66 Mabit et al.,67 Bustos,68 and O’Shaughnessy.69
Figure 1.
Outline of Takiwasi’s treatment regime (average timeline).
Study rationale
Takiwasi offers the potential for generating unique insights into the use of traditional medicines in addiction treatment, including, but not limited to the use of ayahuasca. Yet while the center’s own publications have reported positive patient outcomes,70 scientific evaluation has been lacking.71 It is of significance then that results from the first preliminary observational study of Takiwasi’s treatment have recently been published,66 with analyses showing improvements for treatment completers in terms of addiction severity, craving, emotional distress, and quality of life. Comparable results were recently reported in a second observational study,65 which showed post-treatment improvements in anxiety and depression scores, as well as improved scores on quality of life and spirituality. Although the effect sizes in these studies were large, the end-point analyses used in both studies opened questions about the timing of within-treatment changes, particularly with regard to treatment dropouts that were excluded from analysis. Here we build on these results by reporting on patient changes at multiple points within treatment.
Given the clinically and theoretically complex nature of addictions,72 we attempted to characterize within-treatment change as broadly as possible by following the multi-dimensional addiction recovery model proposed by Dodge et al.73 We thus measured patients on the following domains: (a) physical (somatic health); (b) bio-marker (cortisol, with results to be reported elsewhere); (c) dependency (craving); (d) psychological (stress, mental/emotional well-being, and neuropsychological functioning); (d) psychiatric (mental illness); (e) social (social functioning); and (f) spiritual (spiritual well-being).
Methods
The study was approved by the James Cook University Human Research Ethics Committee (H5267), and all participants gave written informed consent prior to participation. We used the STROBE cohort checklist when writing this report.74
Participants
Participation in the study was open to all patients who were: (a) seeking treatment for addiction, and (b) had passed Takiwasi’s admission protocol and been admitted as inpatients. Although we did not measure treatment motivation, all patients entered into treatment voluntarily, and previous studies suggest that the majority of Takiwasi patients arrive motivated to seek change.75 No patients declined to participate in the study. Data were collected from April 2014 to August 2015, and while not all patients completed the full course of treatment, no patients dropped out of the study while in treatment (although study participation ended once a patient had left treatment).
As only male inpatients are admitted to Takiwasi, the final sample consisted of 36 male inpatients with ages on treatment admission ranging from 20 to 50 years (M = 29, SD = 7), and total time in treatment (from entry to exit) ranging from 3 to 367 days (M = 183, SD = 118). South Americans made up 58% of the sample, with 28% European, and the remaining 14% North American. Prior to treatment, the most commonly consumed drugs were alcohol (83%), cannabis (71%), and cocaine (51%), with poly-drug use being common (66%). Of the sample, 61% completed the treatment, 22% exited voluntarily (i.e. against staff recommendation), 14% were suspended from treatment, and one patient (3%) abandoned the treatment without advising staff. Further demographics can be found in Table 1.
Table 1.
Patient demographics.
| Percentage of the study samplea | |
|---|---|
| Country of residence | |
| Peru | 33 |
| Rest of South America | 25 |
| France | 17 |
| Rest of Europe | 11 |
| North/Central America | 14 |
| Age (on admission) | |
| 20–29 | 58 |
| 30–39 | 31 |
| 40–49 | 8 |
| >49 | 3 |
| Religion (on admission) | |
| None | 50 |
| Christian | 39 |
| Buddhist | 5 |
| Islamic | 3 |
| Other | 3 |
| Drug use 30 days prior to treatmentb | |
| Alcohol | 83 |
| Cannabis | 71 |
| Cocaine and derivatives | 51 |
| Sedatives | 26 |
| Opiates | 23 |
| Amphetamines | 20 |
| Barbiturates | 13 |
| Hallucinogens | 9 |
| Poly-drug use | 66 |
| Total time spent in treatment | |
| <1 month | 17 |
| 1–3 months | 11 |
| 4–6 months | 17 |
| 7–9 months | 17 |
| >9 months | 38 |
| Treatment exit | |
| Completed | 61 |
| Voluntary | 22 |
| Suspendedc | 14 |
| Abandoned | 3 |
N = 36.
Drug use subcategories are not mutually exclusive.
Three patients were suspended for leaving the center and consuming alcohol and cocaine.
Design
Similar to Berlowitz et al.66 and in accordance with World Health Organization recommendations for the evaluation of traditional medicines,76 the overall study design consisted of an observational “black box” view of patient change throughout treatment, which was not intended to isolate specific aspects of the treatment for analysis. We therefore obtained repeated measures on psychological variables in order to assess clinical change in a global sense.
Measures
Addiction severity
The fifth edition of the Addiction Severity Index (ASI)77,78 is a widely used structured clinical interview that attempts to quantify a patient’s addiction severity across seven life problem areas: medical, alcohol, drug, employment, legal, family, and psychiatric. Higher scores indicate greater problem severity for each dimension.
Clinical battery
The clinical battery tests were selected for their relevance in the addiction literature, but also for the suitability of individual test items within a residential treatment context. The measures used were as follows.
Perceived Stress Scale (PSS)
The PSS79,80 was used to measure psychological stress over the previous month. Analyses were made on the 10-item subset (PSS-10), due to its improved psychometric properties.81 Higher scores indicate greater perceived stress.
Craving Experience Questionnaire–frequency (CEQ-F)
The CEQ-F82 was used to measure the frequency of craving experienced over the past 30 days. Craving is conceptualized in terms of frequency of desire, craving-related imagery, and intrusive thoughts. Higher scores indicate greater frequency of craving over the month prior.
Brief Symptom Inventory (BSI)
The BSI83 was used to assess the prevalence of psychiatric disorder symptoms over the previous 7 days. The test is a shorter 53-item version of the Symptom Checklist-90 Revised,84 and both instruments measure psychiatric symptoms across nine dimensions. Only results for the Global Severity Index (GSI) are reported here, where higher scores indicate greater overall problem severity.
Spiritual Well-Being Scale (SWBS)
The SWBS85 was used to assess two dimensions at the time of testing: Religious Well-Being (RWB) and Existential Well-Being (EWB). RWB items explicitly address religious and spiritual notions of God, whereas EWB items are secular and probe life satisfaction and meaning. On both dimensions, higher scores indicate greater well-being.
Short Form Health Survey 36 version 2 (SF-36v2)
The SF-36v286 was used to capture perceived changes in health over the past 4 weeks. While the SF-36v2 measures eight health domains, we only report here the global measures of: (a) physical health (Physical Component Summary; PCS), and (b) mental/emotional health (Mental Component Summary; MCS). For both domains, higher scores indicate better health.
Self-Evaluated Transition (SET)
The SET is a single 5-choice item from the SF-36v2 that captures perceived change in general health over the past year. The patient rates their current “health in general” compared with 1 year prior as either: 1 (much better), 2 (somewhat better), 3 (about the same), 4 (somewhat worse), or 5 (much worse).
Neuropsychological functioning
The Repeated Battery for the Assessment of Neuropsychological Functioning Update (RBANS)87 was used to test for abnormal cognitive functioning. With two equivalent testing forms, the Spanish version allows for a single retest only. The instrument assesses performance through 12 subtests that comprise five indexes: immediate memory, visuospatial, language, attention, and delayed memory. For all indexes, higher scores indicate better performance (with low scores relative to age bracketed norms suggesting cognitive impairment).
Procedures
Addiction severity
The ASI was administered to patients on intake only, most often by a co-author of this work (I.B.), but at times by Takiwasi staff instead. It was administered on average 2 days into treatment (SD = 3 days).
Clinical battery
Measurements on the clinical battery were made at important treatment points that we termed milestones. In consultation with Takiwasi staff, the selected milestones were: (a) treatment admission, (b) approximately 1 month after each diet, and (c) exit from treatment (although it should be noted that in practice treatment exit could occur at any point for a variety of reasons). There were five milestones in total, herein designated M1–M5.
Dieting provided a natural measurement point in treatment, since it occurs with some regularity (around every 2–3 months) and marks a consolidation point for patients within the treatment. Moreover, the diets are followed by a reflective phase where patient plant intake is negligible, which allowed us to minimize interference from the acute effects of psychoactive plants when taking repeated measures. From M1 to M5, the sample sizes were 22, 19, 18, 13, and 9, respectively (N = 36). The average number of days in treatment (with standard deviations) for patients at M1–M5 were: 3 (3), 110 (31), 169 (31), 245 (30), and 309 (27).
Neuropsychological functioning
The RBANS was administered on treatment intake, and at a follow-up point either at the end of treatment, or at least 2 months into treatment (n = 8). The average number of days in treatment before the second administration was 153 (SD = 70). Given the language-dependent nature of some test sections, the RBANS was only administered to fluent Spanish speakers.
Analyses
All analyses were conducted using R.88 Effect sizes for t-tests were calculated with effsize.89 Mixed-effects models were generated using lme4,90 with significance values from lmerTest using Satterthwaite’s method.91,92
Intake comparisons
For group analysis we compared the sample’s intake ASI scores with normative values from mainstream inpatient centers. Due to the cultural diversity of Takiwasi’s patients and the lack of global ASI normative data, these comparisons were unavoidably cross-cultural. One-sample t-tests were used to check for significant differences. We also compared Takiwasi patients’ intake scores (M1) on the clinical battery against available normative samples using one-sample t-tests.
Within-treatment changes
We analyzed the clinical battery in terms of predicted change over time from M1 versus M2–M5 (plotted against normative values for comparative purposes). Mixed-effects models were used to maximize the data available for analysis (i.e. we included all data points in the analyses, irrespective of a patient’s time in treatment at study enrollment, or reason for treatment exit), and also to account for the lack of statistical independence due to repeated measures.93 In all models the fixed effect was treatment milestone (categorical), with patient as the random effect. All models were random intercept only, and thus implied the modeling assumption of an invariant effect of time spent in treatment across patients. Since treatment milestone was a proxy for time spent in treatment, we also tested the models with the addition of total treatment time (in days) as a predictor, but its addition was not warranted based on Akaike information criterion (AIC) values.
For neuropsychological functioning, we compared intake and within-treatment performance using paired-samples t-tests, using Hedges’94 correction for Cohen’s d as the effect size.
Dropout analysis
We compared early treatment dropouts (i.e. those who spent less than 30 days in treatment) against the rest of the sample via a logistic regression, using demographics and ASI intake scores as predictors, and a binary “dropout” variable as the outcome.
Results
Intake profile
We first characterize the Takiwasi sample on intake, making comparisons against available normative values.
Addiction severity
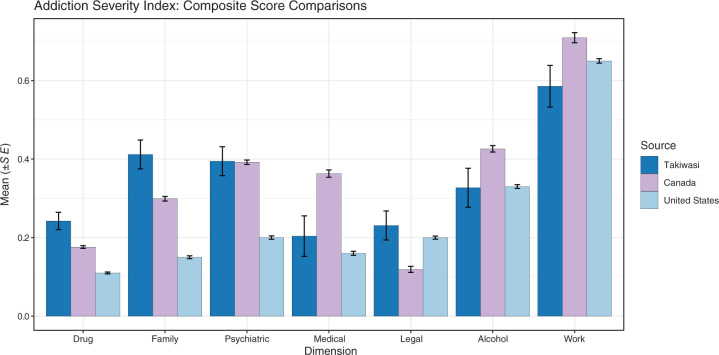
Figure 2 shows ASI composite score means and standard errors for the Takiwasi sample (n = 34; ASI data were unavailable for two patients), Canadian addiction patients being readmitted to treatment with at least three previous attempts (sample sizes range from 517 to 1474),95 and USA addiction inpatients (N = 3133).77
Figure 2.
ASI samples and normative comparisons.
Compared with the Canadian sample, Takiwasi patients had significantly higher scores on (a) drug, t(33) = 3.00, p < 0.01, Cohen’s d = 0.51, 95% CI for d (−0.19, 1.22); (b) family, t(33) = 3.06, p < 0.01, d = 0.53, CI (−0.18, 1.24); and (c) legal, t(33) = 3.03, p < 0.01, d = 0.52, CI (−0.19, 1.23). Significantly lower scores for Takiwasi patients were found for (a) medical, t(33) = −3.08, p < 0.01, d = −0.53, CI (−1.24, 0.18); and (b) work t(33) = −2.33, p = 0.026, d = −0.40, CI (−1.10, 0.31).
When compared with the US sample, Takiwasi patients had significantly higher scores for (a) drug, t(33) = 5.99, p < 0.001, d = 1.03, 95% CI for d (0.28, 1.77); (b) family, t(33) = 7.11, p < 0.001, d = 1.22, CI (0.46, 1.98); and (c) psychiatric, t(33) = 5.28, p < 0.001, d = 0.91, CI (0.17, 1.64).
Clinical battery
For the intake sample (n = 22; except for CEQ, n = 27), we first made comparisons where non-clinical norms were available. We found that (a) PCS scores were not significantly different from the US average (N = 4024)96: t(21) = −1.23, p = 0.233, Cohen’s d = −0.26, 95% CI for d (−1.15, 0.63); (b) MCS scores were significantly lower than the US average (N = 4024)96: t(21) = −9.47, p < 0.001, d = −2.02, CI (−3.11, −0.93); (c) PSS-10 scores were significantly higher than the US male average (N = 968)97: t(21) = 8.59, p < 0.001, d = 1.83, CI (0.78, 2.89); and finally (d) GSI scores were significantly higher than non-clinical US males (N = 361)98: t(21) = 8.13, p < 0.001, d = 1.73, CI (0.69, 2.77).
For those measures where only clinical comparisons were available, we found that (a) RWB scores were possibly lower than US mental health patients (N = 182)99: t(21) = −2.21, p = 0.038, d = −0.47, 95% CI for d (−1.37, 0.43); (b) EWB scores were not significantly different from US mental health patients (N = 182)99: t(21) = −0.41, p = 0.686, d = −0.09, CI (−0.97, 0.80); and (c) CEQ-F scores were significantly higher than an Australian sample of alcohol abuse outpatients (N = 276)82: t(26) = 4.01, p < 0.001, d = 0.77, CI (−0.05, 1.59).
Within-treatment changes
Clinical battery
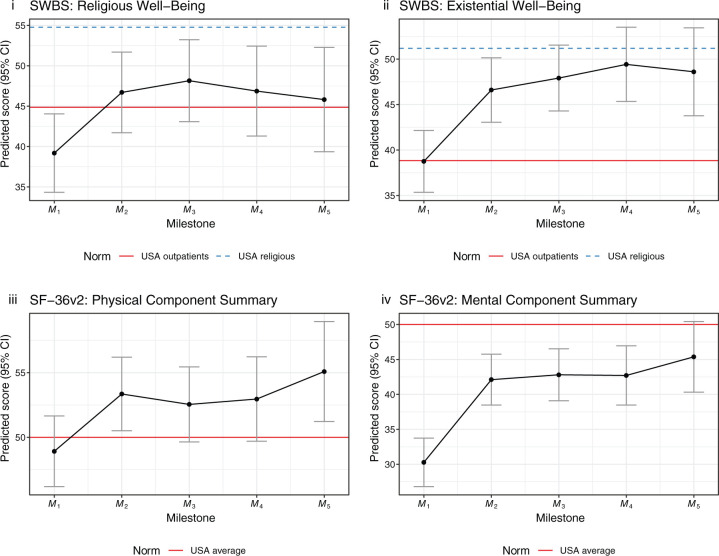
Here we present predictive mixed-effects models for patient change throughout treatment on the clinical battery, where each estimate is relative to M1, plotted alongside norms from the sources previously mentioned in the intake analyses. Figure 3 shows model estimates for those tests where higher scores indicate positive clinical outcomes. Estimates are significant at p < 0.001, except for RWB: M2 (p < 0.01), M4 (p < 0.01), M5 (p = 0.05); and PCS: M2 (p < 0.01), M3 (p = 0.023), M4 (p = 0.026), M5 (p < 0.01).
Figure 3.
Mixed-effects model estimates (positive grouping).
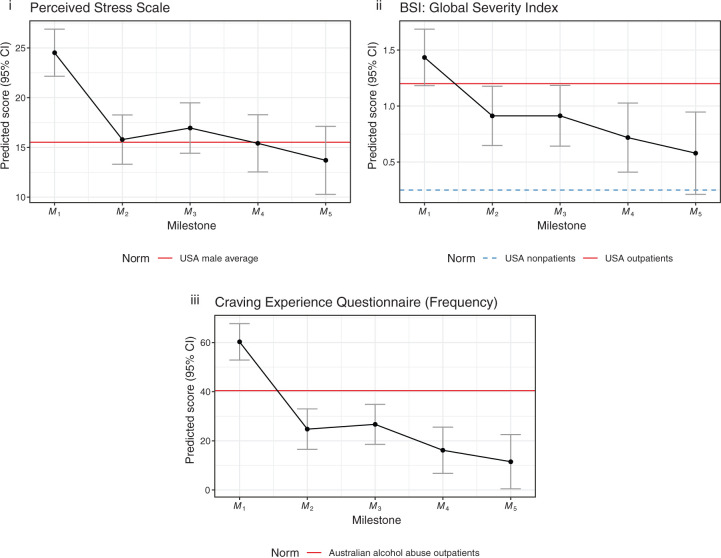
Figure 4 shows model estimates for those tests where higher scores indicate negative clinical outcomes. All estimates are significant at p < 0.001, except for GSI M3 (p < 0.01). Estimate confidence intervals for both positive and negatively grouped tests can be found in Tables 2 and 3.
Figure 4.
Mixed-effects model estimates (negative grouping).
Table 2.
Confidence intervals for model estimates (positive grouping).
| Scale | 95% CIs for model estimates (by milestone) | ||||
|---|---|---|---|---|---|
| Intake | Change versus intake | ||||
| M 1 | M 2 | M 3 | M 4 | M 5 | |
| SWBS | |||||
| RWB | [34.5, 43.9] | [3.3, 11.7] | [4.4, 13.5] | [2.5, 12.9] | [0.3, 13.0] |
| EWB | [35.5, 42.1] | [4.4, 11.3] | [5.4, 12.9] | [6.4, 15.0] | [4.7, 15.0] |
| SF-36v2 | |||||
| PCS | [46.3, 51.6] | [1.7, 7.2] | [0.6, 6.7] | [0.6, 7.5] | [2.0, 10.3] |
| MCS | [26.9, 33.6] | [8.0, 15.6] | [8.5, 16.6] | [7.8, 17.0] | [9.5, 20.7] |
CI, confidence interval; EWB, Existential Well-Being; MCS, Mental Component Summary; Mn, treatment milestone; PCS, Physical Component Summary; RWB, Religious Well-Being; SF-36v2, Short Form Health Survey 36 version 2; SWBS, Spiritual Well-Being Scale.
Significance values. p < 0.001 for all estimates except as noted. RWB: M2 (p < 0.01), M4 (p < 0.01), M5 (p = 0.05). PCS: M2 (p < 0.01), M3 (p = 0.023), M4 (p = 0.026), M5 (p < 0.01).
Table 3.
Confidence intervals for model estimates (negative grouping).
| Scale | 95% CIs for model estimates (by milestone) | ||||
|---|---|---|---|---|---|
| Intake | Change versus intake | ||||
| M 1 | M 2 | M 3 | M 4 | M 5 | |
| PSS-10 | [22.2, 26.8] | [−11.2, −6.2] | [−10.3, −4.9] | [−12.2, −6.0] | [−14.5, −7.1] |
| GSI | [1.2, 1.7] | [−0.8, −0.2] | [−0.8, −0.2] | [−1.1, −0.4] | [−1.3, −0.5] |
| CEQ-F | [53.0, 67.5] | [−43.0, −28.1] | [−41.5, −25.8] | [−53.4, −34.9] | [−59.8, −37.7] |
CEQ-F, Craving Experience Questionnaire (frequency); CI, confidence interval; GSI, Global Severity Index; Mn, treatment milestone; PSS-10, Perceived Stress Scale (10-item version).
Significance values. p < 0.001 for all estimates except for GSI M3 (p < 0.01).
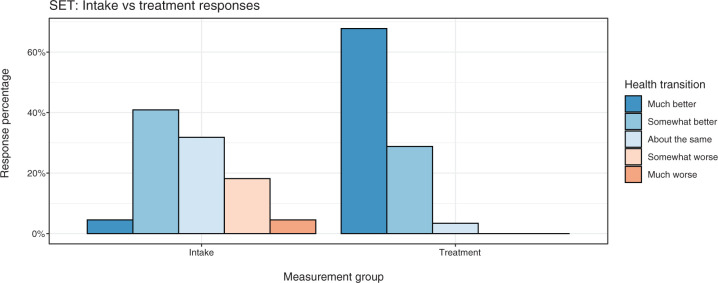
Self-evaluated transition
Figure 5 shows response percentages for the SET item, comparing health transition on intake (M1; n = 22) versus repeated measures during treatment (M2–M5; n = 28, with 59 administrations total). Excluding intake, no patients in treatment ever rated their health as “somewhat worse” or “much worse,” and there were only two that ever rated their health as “about the same.”
Figure 5.
SF-36v2: Self-evaluated transition.
Neuropsychological functioning
Mean score increases from intake to treatment for the group (n = 8) were found for all indexes. However, paired-samples t-tests for treatment versus intake scores were only significant for Total Scale, t(7) = 3.37, p = 0.012, g = 0.55, 95% CI for g (0.17, 0.92); and Delayed Memory, t(7) = 2.73, p = 0.029, g = 0.74, CI (0.08, 1.40). Complete index comparisons can be found in Table 4.
Table 4.
RBANS: intake versus treatment.
| Index | Intakea | Treatmenta | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | Change | 95% CIb | g | |
| Total Scale | 82.9 | 12.4 | 90.2 | 10.9 | 7.4* | [2.2, 12.5] | 0.55 |
| I. Memory | 84.6 | 13.8 | 89.9 | 16.1 | 5.2 | [−0.5, 11.0] | 0.29 |
| Visuospatial | 83.8 | 11.4 | 86.1 | 11.9 | 2.4 | [−4.9, 9.7] | 0.18 |
| Language | 96.1 | 7.3 | 101.8 | 7.7 | 5.6 | [−3.8, 15.0] | 0.67 |
| Attention | 88.0 | 18.5 | 92.8 | 13.4 | 4.8 | [−1.6, 11.1] | 0.20 |
| D. Memory | 82.9 | 15.4 | 94.5 | 8.6 | 11.6* | [1.6, 21.7] | 0.74 |
n = 8.
Confidence interval for mean change from intake to treatment.
Significance values. Total Scale: p = 0.012. Delayed Memory: p = 0.029. All others: p > 0.05.
Dropout analysis
Logistic regression analyses showed no significant relationships between early treatment dropouts (i.e. those who spent less than 30 days in treatment; n = 6) and the rest of the sample (n = 30) for either nationality, religion, or ASI intake scores (for all dimensions). However, patient age at treatment admission was significant, and for simplicity we report t-test results for age between the two samples: Early treatment dropouts were younger (M = 22 years, SD = 2) than the rest of the sample (M = 30 years, SD = 7), t(26) = −5.24, p < 0.001, Cohen’s d = −1.24, 95% CI for d (−2.17, −0.30).
Discussion
Takiwasi patients on admission
Our results indicate that Takiwasi patients can be expected to have a high severity of addiction on intake, with ASI elevations at least on drug and family problems in comparison to mainstream centers. Based on ASI psychiatric cutoff scores,100 Takiwasi patients are also likely to have psychiatric co-morbidity on admission, a finding supported by intake GSI elevations, and also recent results from Berlowitz et al.75 where affective and anxiety disorders were found to be prevalent. Overall, the Takiwasi patients’ addiction severity profile was comparable to the most severe Canadian readmission sample reported by Simoneau and Brochu,95 where patients with higher problem severity were re-seeking treatment after at least three prior admissions. However, medical problems in the Takiwasi sample were less severe than the Canadian sample, being more comparable in that domain to the US inpatient average.
The intake profile on the clinical battery supported the ASI findings, and portrayed patients that are likely to be highly stressed, suffering from mental illness symptoms, and experiencing frequent craving. Mental and emotional health is likely to be especially low, although we did not find evidence that physical health is low on average. Religious and existential well-being were comparable to US clinical populations, and cognitive functioning may be in the “low average” range.87
Within-treatment changes
Over the course of treatment, we find that Takiwasi patients are likely to make clinically significant improvements on a variety of measures relevant to addiction. Specifically, patients are predicted to see strong increases in mental and emotional health, in addition to increased meaning and purpose in life. Large reductions in perceived stress, mental illness symptoms, and craving can also be expected. The most dramatic shifts appeared earlier in the treatment, and these changes seem to be at least maintained over time (if not further improved), although we had limited statistical power to assess later stage changes. Although Berlowitz et al.66 found that Takiwasi treatment completers had large and clinically significant improvements on nearly all measures, no significant changes in physical health were found. However, as the authors note, ASI medical composite scores may not be sufficiently sensitive for this purpose. Our application of the SF-36v2 supported this interpretation, as the physical health models suggested a general improvement in health that was maintained over the course of treatment. Additionally, self-reported health transitions were nearly universally positive for all measurement points beyond intake. Cognitive functioning provided further evidence for improvement, which was most notable in the domain of delayed memory.23
While our results accord with the end-point analyses of Berlowitz et al.66 and Giovannetti et al.,65 here we increased the temporal resolution of within-treatment measurement, finding that clinical improvements occur relatively quickly (compared with the length of the full treatment), suggesting that dropouts roughly beyond the third month are unlikely to be caused by lack of clinical change. However, our results did suggest that younger patients may be more likely to drop out of treatment early on. Further investigations of patient change within the opening months of treatment appear to be warranted.
Finally, we found some divergence in the modeling of spiritual well-being throughout treatment. Takiwasi’s treatment regime is complex, and spirituality and religion play multifaceted roles within it. Psychedelics are known to induce altered states of consciousness of a “mystical” type101–103 with potentially profound implications,104,105 and ayahuasca can be similarly potent.106,107 It is interesting then to note the differences that we observed for Takiwasi patients, with strong predicted increases in existential well-being (perhaps even toward levels seen in US religious samples), whereas spiritual well-being formulated in explicitly religious terms was more uncertain. This contrast possibly reflects pre-existing differences in religiosity (e.g. 50% of the sample were non-religious on admission), but also suggests differences for patients in coming to terms with the apparent spiritual content of ayahuasca and other plant ceremonies.108
Limitations and significance
Our observational study design precludes the causal attribution of patient change to particular aspects of the treatment, although the use of ayahuasca gives an obvious and empirically supported target for treatment effects.34,46 Importantly however, the design does not distinguish between specific treatment interventions and the unusual environment of a residential center: Other variables such as the passage of time, the potential development of community and friendships within treatment, the lack of access to drugs of abuse, and the removal of patients from their daily life circumstances would all be probable contributing factors to changes seen within treatment. On the other hand, it is worth noting that many of the treatment processes are demanding (e.g. many of the plants utilized in Takiwasi induce emesis), and they are unlikely to be considered at all pleasant by patients. In this sense it is impressive that patients with serious addiction problems continue with the treatment for as long as they often do.
Despite the above limitations, the uniformity of the present results would be surprising if the effects of the treatment were broadly deleterious. Thus at a minimum, we provide evidence of treatment safety in a highly novel setting. However, while ayahuasca is known to have an acceptable safety profile from a pharmacological perspective,26,34,109 many of the Amazonian techniques used in Takiwasi are not yet well studied scientifically, despite indigenous and community usage of the same plants in medical and other contexts.59,63,110,111 Thus although Takiwasi’s exclusion criteria,56,65 biomedical evaluation, and practitioner expertise likely increase the safety margin—particularly for international and urban patients where interactions not seen in traditional settings increase in likelihood (e.g. pharmaceutical interactions)—further study is needed on the basic effects of Amazonian medicinal plants and practices.
Our results also strongly suggest a therapeutic effect, but we cannot yet determine if these patient changes translate into addiction treatment success. Linking our findings to treatment effectiveness would require longitudinal studies that follow-up on patients once they return to their communities, where there are likely to be more opportunities for relapse. Moreover, it is difficult to assess how well these results would generalize to other patients seeking treatment, given that the Takiwasi admission protocol filters out those who are not yet motivated to commit to treatment,66 implying that the treatment may be suited to certain patient profiles.75 Yet irrespective of the admission protocol, it seems unlikely that patients who are not ready to change would stand to benefit from this approach, especially considering the difficult nature of the treatment. However, given the cultural diversity and relatively severe addiction profile of the sample, our results do suggest that this mode of treatment may hold promise in those areas where conventional treatment approaches are failing.
Overall, our findings are consistent with the contemporary literature on the use of indigenous psychoactive plant sacraments,34,37,39,40,47,54,112,117 and the use of classical hallucinogens more generally.9,12,36,41,118–126 However, the present results should not be associated with the use of ayahuasca alone, especially considering that it forms one element in a complicated treatment protocol (see Figure 1). For example, when asked to select the most important aspects of the treatment, Takiwasi healers in fact most frequently chose dietary retreats, even though ayahuasca ceremonies were also considered to be important.64,127–130 Moreover, the focus on medicinal plants and emesis may be connected to effects in the gut–brain axis,131 which has recently been linked with opioid dependence.
Conclusion
The resurgence of psychedelic research holds promise for the addiction treatment field, and Takiwasi provides a unique parallel to these developments, by now carrying decades of clinical experience. Takiwasi’s treatment protocol is more deeply connected with traditional medical practices, and while such an approach may have its own benefits and be more (or less) appropriate for specific populations, further clinical work is called for in order to investigate treatment effectiveness. Qualitative work may also be helpful not only in providing converging lines of evidence for treatment effects and potential mechanisms, but for further delineating the nature of the treatment itself, particularly in relation to concepts of health, illness, and safety, and how they relate to the re-emerging field of psychedelic medicine.
Footnotes
Author contributions: David M. O’Shaughnessy: Conceptualization, Methodology, Investigation, Formal analysis, Funding acquisition, Writing—Original draft.
Ilana Berlowitz: Investigation, Writing—Review & editing.
Robin Rodd: Supervision, Writing—Review & editing.
Zoltán Sarnyai: Supervision, Writing—Review & editing.
Frances Quirk: Supervision, Funding acquisition, Writing—Review & editing.
Author’s Note: Ilana Berlowitz is also affiliated with Faculty of Medicine, Institute of Anatomy, University of Zurich, Zurich, Switzerland.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Publication made possible in part by support from the Berkeley Research Impact Initiative (BRII) sponsored by the UC Berkeley Library.
ORCID iD: David M. O’Shaughnessy  https://orcid.org/0000-0002-0698-099X
https://orcid.org/0000-0002-0698-099X
Contributor Information
David M. O’Shaughnessy, College of Medicine and Dentistry, James Cook University, Townsville, QLD 4811, Australia.
Ilana Berlowitz, Department of Psychology, University of Fribourg, Fribourg, Switzerland.
Robin Rodd, Division of Social Sciences, Duke Kunshan University, Kunshan, Jiangsu, China.
Zoltán Sarnyai, Laboratory of Psychiatric Neuroscience, Centre for Molecular Discovery, Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, QLD, Australia.
Frances Quirk, New England Institute of Healthcare Research, Faculty of Medicine and Health, University of New England, Armidale, NSW, Australia.
References
- 1. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry 2020; 10: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Novak SJ. LSD before Leary: Sidney Cohen’s critique of 1950s psychedelic drug research. Isis 1997; 88: 87–110. [DOI] [PubMed] [Google Scholar]
- 3. Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs 1998; 30: 381–418. [DOI] [PubMed] [Google Scholar]
- 4. Krebs TS, Johansen PO. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol 2012; 26: 994–1002. [DOI] [PubMed] [Google Scholar]
- 5. Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 2017; 42: 2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi T, Gold JA. A review of emerging therapeutic potential of psychedelic drugs in the treatment of psychiatric illnesses. J Neurol Sci 2020; 411: 116715. [DOI] [PubMed] [Google Scholar]
- 7. Strassman RJ. Hallucinogenic drugs in psychiatric research and treatment. Perspectives and prospects. J Nerv Ment Dis 1995; 183: 127–138. [DOI] [PubMed] [Google Scholar]
- 8. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015; 29: 289–299. [DOI] [PubMed] [Google Scholar]
- 9. Bogenschutz MP, Johnson MW. Classic hallucinogens in the treatment of addictions. Prog Neuro-Psychopharmacol Biol Psychiatry 2016; 64: 250–258. [DOI] [PubMed] [Google Scholar]
- 10. Bogenschutz MP. It’s time to take psilocybin seriously as a possible treatment for substance use disorders. Am J Drug Alcohol Abuse 2016; 43: 4–6. [DOI] [PubMed] [Google Scholar]
- 11. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 2014; 28: 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. Epub ahead of print 21 July 2016. DOI: 10.3109/00952990.2016.1170135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noorani T, Garcia-Romeu A, Swift TC, et al. Psychedelic therapy for smoking cessation: qualitative analysis of participant accounts. J Psychopharmacol 2018; 32: 756–769. [DOI] [PubMed] [Google Scholar]
- 14. Krupitsky EM, Burakov A, Romanova T, et al. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abuse Treat 2002; 23: 273–283. [DOI] [PubMed] [Google Scholar]
- 15. Multidisciplinary Association for Psychedelic Studies. FDA grants breakthrough therapy designation for MDMA-assisted psychotherapy for PTSD, agrees on special protocol assessment for phase 3 trials, www.maps.org/news/media/6786 (accessed 26 August 2017).
- 16. Grob CS. Psychiatric research with hallucinogens: what have we learned? Yearb Ethnomed Study Conscious 1994; 3: 91–112. [Google Scholar]
- 17. Ogalde JP, Arriaza BT, Soto EC. Identification of psychoactive alkaloids in ancient Andean human hair by gas chromatography/mass spectrometry. J Archaeol Sci 2009; 36: 467–472. [Google Scholar]
- 18. Miller MJ, Albarracin-Jordan J, Moore C, et al. Chemical evidence for the use of multiple psychotropic plants in a 1,000-year-old ritual bundle from South America. Proc Natl Acad Sci 2019; 116: 11207–11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luna LE. Indigenous and mestizo use of ayahuasca: an overview. In: Santos RG. (ed.) The ethnopharmacology of ayahuasca. Kerala: Transworld Research Network, 2011, pp.1–21. [Google Scholar]
- 20. Schultes RE. Ethnopharmacology of the northwest Amazon: unexpected chemical discoveries. Rev Acad Colomb Cienc Exactas Fís Nat 1987; 16: 85–92. [Google Scholar]
- 21. Timmermann C, Roseman L, Schartner M, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep 2019; 9: 16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Domínguez-Clavé E, Soler J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull 2016; 126: 89–101. [DOI] [PubMed] [Google Scholar]
- 23. Bouso JC, González D, Fondevila S, et al. Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: a longitudinal study. PLoS One 2012; 7: e42421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bouso JC, Fábregas JM, Antonijoan RM, et al. Acute effects of ayahuasca on neuropsychological performance: differences in executive function between experienced and occasional users. Psychopharmacology 2013; 230: 415–424. [DOI] [PubMed] [Google Scholar]
- 25. Callaway JC, Grob CS. Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for severe adverse interactions. J Psychoactive Drugs 1998; 30: 367–369. [DOI] [PubMed] [Google Scholar]
- 26. Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007; 102: 24–34. [DOI] [PubMed] [Google Scholar]
- 27. Lanaro R, Bressanim de Aquino Calemi DB, Togni LR, et al. Ritualistic use of ayahuasca versus street use of similar substances seized by the police: a key factor involved in the potential for intoxications and overdose? J Psychoactive Drugs 2015; 47: 132–139. [DOI] [PubMed] [Google Scholar]
- 28. Riba J, Rodriguez-Fornells A, Urbano G, et al. Subjective effects and tolerability of the South American psychoactive beverage ayahuasca in healthy volunteers. Psychopharmacology 2001; 154: 85–95. [DOI] [PubMed] [Google Scholar]
- 29. dos Santos RG, Balthazar FM, Bouso JC, et al. The current state of research on ayahuasca: a systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J Psychopharmacol 2016; 30: 1230–1247. [DOI] [PubMed] [Google Scholar]
- 30. dos Santos RG, Bouso JC, Hallak JEC. Ayahuasca, dimethyltryptamine, and psychosis: a systematic review of human studies. Ther Adv Psychopharmacol 2017; 7: 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coe MA, McKenna DJ. The therapeutic potential of ayahuasca. In: Camfield D, McIntyre E, Sarris J. (eds) Evidence-based herbal and nutritional treatments for anxiety in psychiatric disorders. Switzerland: Springer, 2017, pp.123–137. [Google Scholar]
- 32. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol 2016; 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González D, Cantillo J, Pérez I, et al. Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacology (Berl) 2020; 237: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamill J, Hallak J, Dursun SM, et al. Ayahuasca: psychological and physiologic effects, pharmacology and potential uses in addiction and mental illness. Curr Neuropharmacol 2019; 17: 108–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Labate BC, Cavnar C. (eds). The therapeutic use of ayahuasca. Berlin: Springer-Verlag, 2014. [Google Scholar]
- 36. dos Santos RG, Osório FL, Crippa JAS, et al. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol 2016; 6: 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soler J, Elices M, Dominguez-Clavé E, et al. Four weekly ayahuasca sessions lead to increases in “acceptance” capacities: a comparison study with a standard 8-week mindfulness training program. Front Pharmacol 2018; 9: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fábregas JM, González D, Fondevila S, et al. Assessment of addiction severity among ritual users of ayahuasca. Drug Alcohol Depend 2010; 111: 257–261. [DOI] [PubMed] [Google Scholar]
- 39. Lawn W, Hallak JE, Crippa JA, et al. Well-being, problematic alcohol consumption and acute subjective drug effects in past-year ayahuasca users: a large, international, self-selecting online survey. Sci Rep 2017; 7: 15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grob CS, McKenna DJ, Callaway JC, et al. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nerv Ment Dis 1996; 184: 86–94. [DOI] [PubMed] [Google Scholar]
- 41. dos Santos RG, Hallak JEC. Therapeutic use of serotoninergic hallucinogens: a review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev 2020; 108: 423–434. [DOI] [PubMed] [Google Scholar]
- 42. Oliveira-Lima AJ, Santos R, Hollais AW, et al. Effects of ayahuasca on the development of ethanol-induced behavioral sensitization and on a post-sensitization treatment in mice. Physiol Behav 2015; 142: 28–36. [DOI] [PubMed] [Google Scholar]
- 43. Cruz JI, Nappo SA. Is ayahuasca an option for the treatment of crack cocaine dependence? J Psychoactive Drugs 2018; 50: 247–255. [DOI] [PubMed] [Google Scholar]
- 44. Labate BC, dos Santos RG, Strassman R, et al. Effect of Santo Daime membership on substance dependence. In: Labate BC, Cavnar C. (eds) The therapeutic use of ayahuasca. Berlin: Springer-Verlag, 2014, pp.153–159. [Google Scholar]
- 45. Loizaga-Velder A. A psychotherapeutic view on the therapeutic effects of ritual ayahuasca use in the treatment of addiction. MAPS Bull Spec Ed 2013; 23: 36–40. [Google Scholar]
- 46. Nunes AA, dos Santos RG, Osório FL, et al. Effects of ayahuasca and its alkaloids on drug dependence: a systematic literature review of quantitative studies in animals and humans. J Psychoactive Drugs 2016; 48: 195–205. [DOI] [PubMed] [Google Scholar]
- 47. Talin P, Sanabria E. Ayahuasca’s entwined efficacy: an ethnographic study of ritual healing from ‘addiction’. Int J Drug Policy 2017; 44: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Rios MD, Grob CS, Baker JR. Hallucinogens and redemption. J Psychoactive Drugs 2002; 34: 239–248. [DOI] [PubMed] [Google Scholar]
- 49. Kuypers KPC, Riba J, De la Fuente Revenga M, et al. Ayahuasca enhances creative divergent thinking while decreasing conventional convergent thinking. Psychopharmacology (Berl) 2016; 233: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liester MB, Prickett JI. Hypotheses regarding the mechanisms of ayahuasca in the treatment of addictions. J Psychoactive Drugs 2012; 44: 200–208. [DOI] [PubMed] [Google Scholar]
- 51. Tupper KW. Entheogenic healing: the spiritual effects and therapeutic potential of ceremonial ayahuasca use. In: Ellens JH. (ed.) The healing power of spirituality: how faith helps humans thrive. Westport: Praeger, 2009, pp.269–282. [Google Scholar]
- 52. Winkelman M. Alternative and traditional medicine approaches for substance abuse programs: a shamanic perspective. Int J Drug Policy 2001; 12: 337–351. [Google Scholar]
- 53. Fernández X, dos Santos RG, Cutchet M, et al. Assessment of the psychotherapeutic effects of ritual ayahuasca use on drug dependency: a pilot study. In: Labate BC, Cavnar C. (eds) The therapeutic use of ayahuasca. Berlin: Springer-Verlag, 2014, pp.183–196. [Google Scholar]
- 54. Thomas G, Lucas P, Capler NR, et al. Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev 2013; 6: 30–42. [DOI] [PubMed] [Google Scholar]
- 55. McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther 2004; 102: 111–129. [DOI] [PubMed] [Google Scholar]
- 56. Mabit J. Ayahuasca in the treatment of addictions. In: Roberts TB, Winkelman M. (eds) Psychedelic medicine: new evidence for hallucinogenic substances as treatments. Vol. 2 Westport: Praeger, 2007, pp.87–105. [Google Scholar]
- 57. Strassman RJ. Human hallucinogenic drug research in the United States: a present-day case history and review of the process. J Psychoactive Drugs 1991; 23: 29–38. [DOI] [PubMed] [Google Scholar]
- 58. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res 1996; 73: 121–124. [DOI] [PubMed] [Google Scholar]
- 59. Sanz-Biset J, Cañigueral S. Plant use in the medicinal practices known as “strict diets” in Chazuta valley (Peruvian Amazon). J Ethnopharmacol 2011; 137: 271–288. [DOI] [PubMed] [Google Scholar]
- 60. Sanz-Biset J, Cañigueral S. Plants as medicinal stressors, the case of depurative practices in Chazuta valley (Peruvian Amazonia). J Ethnopharmacol 2013; 145: 67–76. [DOI] [PubMed] [Google Scholar]
- 61. Luna LE. The healing practices of a Peruvian shaman. J Ethnopharmacol 1984; 11: 123–133. [DOI] [PubMed] [Google Scholar]
- 62. Jauregui X, Clavo Z, Jovel E, et al. “Plantas con madre”: plants that teach and guide in the shamanic initiation process in the east-central Peruvian Amazon. J Ethnopharmacol 2011; 134: 739–752. [DOI] [PubMed] [Google Scholar]
- 63. Sanz-Biset J, Campos de la Cruz J, Epiquién-Rivera MA, et al. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J Ethnopharmacol 2008; 122: 333–362. [DOI] [PubMed] [Google Scholar]
- 64. Berlowitz I, Ghasarian C, Walt H, et al. Conceptions and practices of an integrative treatment for substance use disorders involving Amazonian medicine: traditional healers’ perspectives. Rev Bras Psiquiatr 2017; 40: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giovannetti C, Arce SG, Rush B, et al. Pilot evaluation of a residential drug addiction treatment combining traditional Amazonian medicine, ayahuasca and psychotherapy on depression and anxiety. J Psychoactive Drugs 2020; 52: 472–481. [DOI] [PubMed] [Google Scholar]
- 66. Berlowitz I, Walt H, Ghasarian C, et al. Short-term treatment effects of a substance use disorder therapy involving traditional Amazonian medicine. J Psychoactive Drugs 2019; 51: 323–334. [DOI] [PubMed] [Google Scholar]
- 67. Mabit J, Giove R, Vega J. Takiwasi: the use of Amazonian shamanism to rehabilitate drug addicts, www.takiwasi.com (1996, accessed 26 July 2020).
- 68. Bustos S. The house that sings: the therapeutic use of icaros at Takiwasi. Shaman’s Drum 2006; 73: 33–39. [Google Scholar]
- 69. O’Shaughnessy DM. Takiwasi: addiction treatment in the “Singing House”. PhD Thesis, James Cook University, Australia, 2017. [Google Scholar]
- 70. Mabit J. Blending traditions—using indigenous medicinal knowledge to treat drug addiction, www.maps.org/news-letters/v12n2/12225mab.pdf (2002, accessed 14 June 2020).
- 71. Labate BC, Santos RG, Anderson B, et al. The treatment and handling of substance dependence with ayahuasca: reflections on current and future research. In: Labate BC, MacRae E. (eds) Ayahuasca, ritual and religion in Brazil. London: Equinox, 2010, pp.205–227. [Google Scholar]
- 72. Skinner MD, Aubin HJ. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev 2010; 34: 606–623. [DOI] [PubMed] [Google Scholar]
- 73. Dodge K, Krantz B, Kenny PJ. How can we begin to measure recovery? Subst Abuse Treat Prev Policy 2010; 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Berlowitz I, Walt H, Ghasarian C, et al. Who turns to Amazonian medicine for treatment of substance use disorder? Patient characteristics at the Takiwasi addiction treatment center. J Stud Alcohol Drugs 2020; 81: 416–425. [PubMed] [Google Scholar]
- 76. World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicine. Report no. WHO/EDM/TRM/2000.1, www.who.int/publications-detail/general-guidelines-formethodologies-on-research-and-evaluation-of-traditional-medicine (2000, accessed 2 May 2020).
- 77. McLellan AT, Cacciola JC, Alterman AI, et al. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict 2006; 15: 113–124. [DOI] [PubMed] [Google Scholar]
- 78. Urschel HC, III, Blair J, McLellan TA. Indice De Severidad De Adicción 5ta Edición. Measurement instrument, www.phmcresearch.org/products/addiction-severity-index (accessed 22 January 2017).
- 79. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- 80. Remor E. Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). Span J Psychol 2006; 9: 86–93. [DOI] [PubMed] [Google Scholar]
- 81. Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskmap S. (eds) The social psychology of health. Newbury Park: SAGE, 1988, pp.31–67. [Google Scholar]
- 82. May J, Andrade J, Kavanagh DJ, et al. The craving experience questionnaire: a brief, theory-based measure of consummatory desire and craving. Addiction 2014; 109: 728–735. [DOI] [PubMed] [Google Scholar]
- 83. Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983; 13: 595–605. [PubMed] [Google Scholar]
- 84. Derogatis LR, Unger R. Symptom Checklist-90-Revised. In: Weiner IB and Craighead WE (Eds) The Corsini encyclopedia of psychology. 4th ed. Hoboken: John Wiley & Sons, 2010. [Google Scholar]
- 85. Paloutzian RF, Bufford RK, Wildman AJ. Spiritual Well-Being Scale: mental and physical health relationships. In: Cobb M, Puchalski CM, Rumbold B. (eds) Oxford textbook of spirituality in healthcare. Oxford: Oxford University Press, 2012. [Google Scholar]
- 86. Ware JE., Jr. SF-36 health survey update. Spine 2000; 25: 3130–3139. [DOI] [PubMed] [Google Scholar]
- 87. Randolph C. RBANS Update: manual. Bloomington: NCS Pearson, PsychCorp, 2012. [Google Scholar]
- 88. R Core Team. R. A language and environment for statistical computing. R Foundation for Statistical Computing, www.r-project.org (2020, accessed 19 October 2018).
- 89. Torchiano M. Effsize—a package for efficient effect size computation. R Package Version 0.7.9. Zenodo, 2020. [Google Scholar]
- 90. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 91. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J Stat Softw 2017; 82: 1–26. [Google Scholar]
- 92. Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods 2016; 49: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 93. Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2000; 61: 310–317. [DOI] [PubMed] [Google Scholar]
- 94. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat 1981; 6: 107–128. [Google Scholar]
- 95. Simoneau H, Brochu S. Addiction severity index profile of persons who reenter treatment for substance use disorders. Subst Abus 2017; 38: 432–437. [DOI] [PubMed] [Google Scholar]
- 96. Maruish ME. User’s manual for the SF-36v2 health survey. 3rd ed. Lincoln: QualityMetric Incorporated, 2011. [Google Scholar]
- 97. Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol 2012; 42: 1320–1334. [Google Scholar]
- 98. Derogatis LR. Brief symptom inventory: administration, scoring, and procedures manual. Bloomington: Pearson, 1993. [Google Scholar]
- 99. Bufford RK, Paloutzian RF, Ellison CW. Norms for the Spiritual Well-Being Scale. J Psychol Theol 1991; 19: 56–70. [Google Scholar]
- 100. Cacciola JS, Pecoraro A, Alterman AI. Development of ASI psychiatric severity cut-off scores to identify co-occurring psychiatric disorders. Int J Ment Health Addict 2008; 6: 77–92. [Google Scholar]
- 101. Doblin R. Pahnke’s ‘Good Friday experiment’: a long-term follow-up and methodological critique. J Transpers Psychol 1991; 23: 1–28. [Google Scholar]
- 102. Griffiths RR, Richards WA, McCann U, et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 2006; 187: 268–283. [DOI] [PubMed] [Google Scholar]
- 103. Pahnke WN. The psychedelic mystical experience in the human encounter with death. Harv Theol Rev 1969; 62: 1–21. [Google Scholar]
- 104. MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 2011; 25: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Roseman L, Nutt DJ, Carhart-Harris RL. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 2018; 8: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kjellgren A, Eriksson A, Norlander T. Experiences of encounters with ayahuasca—“the vine of the soul”. J Psychoactive Drugs 2009; 41: 309–315. [DOI] [PubMed] [Google Scholar]
- 107. Luna LE, White SF. Ayahuasca reader: encounters with the Amazon’s sacred vine. Santa Fe: Synergetic Press, 2000. [Google Scholar]
- 108. Trichter S, Klimo J, Krippner S. Changes in spirituality among ayahuasca ceremony novice participants. J Psychoactive Drugs 2009; 41: 121–134. [DOI] [PubMed] [Google Scholar]
- 109. Riba J. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther 2003; 306: 73–83. [DOI] [PubMed] [Google Scholar]
- 110. Berlowitz I, Torres EG, Walt H, et al. “Tobacco is the chief medicinal plant in my work”: therapeutic uses of tobacco in Peruvian Amazonian medicine exemplified by the work of a maestro tabaquero. Front Pharmacol 2020; 11: 594591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Politi M, Friso F, Saucedo G, et al. Traditional use of Banisteriopsis caapi alone and its application in a context of drug addiction therapy. J Psychoactive Drugs. Epub ahead of print 27 September 2020. DOI: 10.1080/02791072.2020.1820641. [DOI] [PubMed] [Google Scholar]
- 112. Argento E, Capler R, Thomas G, et al. Exploring ayahuasca-assisted therapy for addiction: a qualitative analysis of preliminary findings among an Indigenous community in Canada. Drug Alcohol Rev 2019; 38: 781–789. [DOI] [PubMed] [Google Scholar]
- 113. Bergman RL. Navajo peyote use: its apparent safety. Am J Psychiatry 1971; 128: 695–699. [DOI] [PubMed] [Google Scholar]
- 114. Pascarosa P, Futterman S, Halsweig M. Observations of alcoholics in the peyote ritual: a pilot study. Ann NY Acad Sci 1976; 273: 518–524. [DOI] [PubMed] [Google Scholar]
- 115. Albaugh BJ, Anderson PO. Peyote in the treatment of alcoholism among American Indians. Am J Psychiatry 1974; 131: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 116. Halpern JH, Sherwood AR, Hudson JI, et al. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol Psychiatry 2005; 58: 624–631. [DOI] [PubMed] [Google Scholar]
- 117. Uthaug MV, van Oorsouw K, Kuypers KPC, et al. Sub-acute and long-term effects of ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacology 2018; 235: 2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Johnson MW, Garcia-Romeu A, Johnson PS, et al. An online survey of tobacco smoking cessation associated with naturalistic psychedelic use. J Psychopharmacol 2017; 31: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol 2014; 29: 57–68. [DOI] [PubMed] [Google Scholar]
- 120. Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 2011; 68: 71–78. [DOI] [PubMed] [Google Scholar]
- 121. Hendricks PS, Thorne CB, Clark CB, et al. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol 2015; 29: 280–288. [DOI] [PubMed] [Google Scholar]
- 122. Hendricks PS, Clark CB, Johnson MW, et al. Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. J Psychopharmacol 2014; 28: 62–66. [DOI] [PubMed] [Google Scholar]
- 123. Johansen PO, Krebs TS. Psychedelics not linked to mental health problems or suicidal behavior: a population study. J Psychopharmacol 2015; 29: 270–279. [DOI] [PubMed] [Google Scholar]
- 124. Carhart-Harris RL, Roseman L, Bolstridge M, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep 2017; 7: 13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Halpern JH. The use of hallucinogens in the treatment of addiction. Addict Res 1996; 4: 177–189. [Google Scholar]
- 126. Baumeister D, Barnes G, Giaroli G, et al. Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Ther Adv Psychopharmacol 2014; 4: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013; 36: 305–312. [DOI] [PubMed] [Google Scholar]
- 128. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clinical Investigation 2015; 125: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression – A systematic review. Clinical Psychology Rev 2021; 83: 101943. [DOI] [PubMed] [Google Scholar]
- 130. Fotiou E, Gearin AK. Purging and the body in the therapeutic use of ayahuasca. Soc Sci Med 2019; 239: 112532. [DOI] [PubMed] [Google Scholar]
- 131. Simpson S, Kimbrough A, Boomhower B, et al. Depletion of the microbiome alters the recruitment of neuronal ensembles of oxycodone intoxication and withdrawal. eNeuro 2020; 7: ENEURO.0312–192020. [DOI] [PMC free article] [PubMed] [Google Scholar]