Abstract
Background
A bidirectional relationship exists between the brain and the gastrointestinal tract. Foods containing bacteria that positively influence the gastrointestinal microbiome are termed, probiotics; compounds that promote the flourishing of these bacteria are termed, prebiotics. Whether microbiome influencing therapies could treat psychiatric conditions, including depression and anxiety, is an area of interest. Presently, no established consensus for such treatment exists.
Methods
This systematic review analyses databases and grey literature sites to investigate pre and/or probiotics as treatments for depression and/or anxiety disorders. Articles included are from within 15 years. Pre-determined inclusion exclusion criteria were applied, and articles were appraised for their quality using a modified-CASP checklist. This review focuses specifically on quantitative measures from patients with clinical diagnoses of depression and/or anxiety disorders.
Results
7 studies were identified. All demonstrated significant improvements in one or more of the outcomes measuring the of effect taking pre/probiotics compared with no treatment/placebo, or when compared to baseline measurements.
Discussion
Our review suggests utilising pre/probiotic may be a potentially useful adjunctive treatment. Furthermore, patients with certain co-morbidities, such as IBS, might experience greater benefits from such treatments, given that pre/probiotic are useful treatments for other conditions that were not the primary focus of this discourse. Our results are limited by several factors: sample sizes (adequate, though not robust); short study durations, long-term effects and propensity for remission undetermined.
Conclusion
Our results affirm that pre/probiotic therapy warrants further investigation. Efforts should aim to elucidate whether the perceived efficacy of pre/probiotic therapy in depression and/or anxiety disorders can be replicated in larger test populations, and whether such effects are maintained through continued treatment, or post cessation. Interventions should also be investigated in isolation, not combination, to ascertain where the observed effects are attributable to. Efforts to produce mechanistic explanations for such effect should be a priority.
Keywords: microbiome, mental health, nutritional treatment
Introduction
Humans are an amalgamation of both ‘self’ material and a plethora of ‘non-self’ microorganisms, termed microbiota. These two entities are interactive, and their symbiotic co-existence is crucial for maintaining health.
An ecosystem of microbiota is collectively known as a microbiome. One such example exists within the human gastrointestinal tract (GIT). A highly developed bidirectional relationship exists between the central nervous system (CNS) and the GIT (including the microbiota colonising the GIT), with each area seemingly capable of influencing the condition and functionality of the other. This relationship is termed the gut–brain axis (GBA).1–4 The potential effects of the GBA on physiology and pathophysiology are too vast to be covered in one review. Therefore, in light of the current mental health crisis, we have chosen to focus on the GBA’s relationship with ‘common mental disorder’ (CMD).
It is theorised that the behaviours, cognitive capacities and even the emotions of an individual may not be determined entirely by their nervous system, but also by the colonising GIT microbiota. The mechanistic details underpinning the GBA are complex and yet to be fully elucidated. However, it has already been identified that crucial to facilitating this interaction are: immunomodulatory mechanisms; afferent and efferent neuronal signalling afforded by the vagal nerve; and bidirectional enteroendocrine signalling.2 5
Many factors influence the GIT microbiome, from the mode of delivery at birth to genetic and epigenetic variables. One of the most significant influences is diet.4 Foods containing microbiota thought to exert a positive influence on an individual’s GIT microbiome are termed probiotics, and food types that promote the growth or flourishing of these bacteria are termed prebiotics.4 5 The growing appreciation of the interconnectedness between the GIT microbiome and the nervous system, combined with the knowledge that pre/probiotics can alter the condition and constitution of the GIT microbiome, has highlighted pre/probiotics as potentially therapeutically valuable agents in the treatment of certain psychiatric conditions.6 Among the conditions being investigated are anxiety disorders and depression. The extent to which pre/probiotics might be therapeutically useful (or even viable) in treating anxiety and/or depression is presently unknown, which provides a reasonable rationale for exploring their potential value. To better understand this, we will contemplate some of the mechanisms already identified as key for GBA interactions alongside existing experimental findings.
GBA mechanisms
The vagus nerve
The immune system is crucial in mediating the GBA interactions.1 2 7 At least two mechanisms for this have been described. Experimental findings suggest the vagus nerve is integral in mediating GBA interactions.8 Components of microbiota recognised as non-self provoke cells of the innate immune system to release pro-inflammatory cytokines. The presence of pro-inflammatory cytokines influences neuronal physiology via vagal nerve stimulation.5 9 Numerous animal studies support this and indicate that microbiota-induced effects on neuronal physiology can affect behaviour. For example, mice that were administered Bifidobactium longum to induce gut dysbiosis exhibited anxiolytic effects compared with control subjects; subsequent vagotomy blocked this effect.10
Signalling molecules
Neuronal biochemistry can also be affected by direct action of cytokines that cross the blood–brain barrier (BBB).9 Cytokines are produced when GIT microbiota or their metabolites gain access to the systemic circulation.11–13 This process is thought to be part of the pathophysiology of multiple medical conditions. For example, patients with depression show increased GIT permeability, which may enable luminal microbiota to enter systemic circulation and trigger pro-inflammatory states.12–14 Certain microorganisms are associated with such effects, while others appear to oppose these actions. Various lactobacilli and bifidobacteria strains—typically commensal within healthy human GIT microbiomes—have been shown to attenuate the presence of pro-inflammatory cytokines within he systematic circulation when administered as probiotic supplements.2 9 15 Contrastingly, other microbiota have been shown to exert the opposite effect. This may be significant, given that neuroinflammation has been identified as being key in the pathogenesis of multiple psychiatric conditions including both anxiety disorder and major depressive disorders (MDDs).9–14
Cross-talk
There is evidence indicating GIT microbiota affect the functioning of luminal GIT cells, which may affect host behaviour via endocrinal signalling. Microbial metabolites, such as short chain fatty acid (SCFA), are thought to have this effect.3 Certain commensals produce SCFAs by fermenting non-digestible dietary carbohydrates, like dietary fibre, as part of their natural physiology (fermentable compounds such as these are examples of prebiotics).2 5 SCFAs directly affect enteroendocrine cells within the GIT, triggering the release of various compounds including cholecystokinin and glucagon-like peptide 1. These compounds are endocrinologically active, and may induce changes in host physiology and behaviour—such as triggering satiety.3 Microbiota–host interactions of this nature are termed ‘cross-talk’.
Numerous compounds have been identified as potentially capable of influencing host behaviour by cross-talk. Tryptophan (TRP), a precursor of the neurotransmitter serotonin, is one such example.16 It is possible that cross-talk mechanisms may form part of the pathophysiology of anxiety disorders and/or depression, which highlights them as potentially utilisable therapeutic avenues for treating these conditions.11 16 Finally, it must be highlighted that the mechanisms by which GBA interactions are enacted suggest that the effects microbiota have on their host physiologically, or during pathophysiological states, may be detectable within the host’s biochemical profile. As such, it may prove useful to consider any effects that pre/probiotics have in humans through biochemical analysis, in addition to considering the global state of an individual subjectively and objectively.
Psychiatric disorders in society
The mechanisms and findings outlined provide a succinct tour of some of the information GBA studies have produced. Collectively, they indicate the necessity for further investigation; however, what they neglect to highlight is the importance that such research might hold within the context of clinical practice—particularly with regard to anxiety and depression. According to 2014 UK governmental statistics, 17% of people report experiencing the effects of a CMD weekly.17 CMD encompasses multiple conditions. Of these, anxiety disorders and depression are, respectively, the first and second largest contributors to this figure, comprising >9%. This review will focus on depression and anxiety as their symptoms are easier to quantify than other CMDs, yet still represent a significant proportion of CMDs as a whole. The prevalence of CMDs has consistently increased in adults, irrespective of age and gender, since 1993. In 2016/17, 1.4 million patients were referred with a clinical diagnosis of a CMD, 53% with a diagnosis of an anxiety or stress-related disorder and 33% with depression. The UK has a target for 50% of patients referred for anxiety disorder and depression—who complete treatment—to shift from a clinically recognised condition to a subclinical state. This target has not been met.17
These figures are included not to suggest that the treatment of anxiety and/or depression is failing, but to highlight the difficulty in treating such conditions. Our collective understanding of psychiatric disorders (and, broadly speaking, of mental health) is still developing, meaning it is vital to ensure our clinical approaches are multifaceted and evolving. The nascent field of ‘nutritional psychology’ could offer a way to enhance the efficacy of current treatments and/or provide novel treatments.
Recent reviews exploring pre/probiotic supplementation in other psychiatric disorders yielded null conclusions. However, this could be as a result of the heterogeneity of the studies included.18 19 Whether pre/probiotics are useful agents in the treatment of CMDs remains to be determined, though the pressing need for alternative treatment options, combined with the underlying scientific rationale supporting their potential efficacy, is reason enough to explore their worth further.
Objective
This review aims to ascertain whether individuals living with a clinically diagnosed anxiety disorder and/or depression can have a quantitatively measured reduction in the negative experience of their CMD by consuming a diet inclusive of prebiotics and/or probiotics, alongside any other treatment indicated in the National Institute for Health and Care Exellence (NICE) guidelines.
Population, intervention, comparison, outcome (PICO)
To inform the above objective, this review employs the population, intervention, comparison, outcome (PICO) framework detailed in table 1.
Table 1.
PICO information
| Population | Intervention(s) | Comparison | Outcome |
| Patients with a clinical diagnosis of an anxiety disorder, depression, or both conditions simultaneously, who are receiving treatment for their condition(s) in a way that is in concordance with NICE guidelines. | A diet inclusive of prebiotic foods. A diet inclusive of probiotic foods. A diet inclusive of both prebiotic and probiotic foods. |
Patients with a clinical diagnosis of an anxiety disorder, depression, or both conditions, who are receiving treatment that is in concordance with NICE guidelines, but are receiving no specific dietary interventions. | Analysis of quantitative measures that assess the impact of an anxiety disorder, depression, or both conditions simultaneously on an individual. |
NICE, National Institute for Health and Care Excellence; PICO, population, intervention, comparison, outcome.
Methods
A literature review was conducted using the PICO framework to structure the objective. Selected papers were appraised and scored using the Critical Appraisal Skills Programme (CASP) method. The bias of the selected papers was assessed using the Cochrane-risk-of-bias tool. The obtained data were then analysed against the objectives.
Eligibility criteria
While reviews exploring probiotics and anxiety and depression exist, there are no reviews that exclusively explore probiotics to supplement an existing treatment plan. In order to address this, specific criteria were outlined before conducting the literature search to identify suitable studies.
Inclusion criteria
Studies were identified for further consideration and potential inclusion if they met the following criteria: the study population must comprise human adults aged 18 and older; participants had a clinically recognised anxiety disorder and/or depression; participants received a dietary intervention of a prebiotic and/or probiotic; study findings must be expressed, at least in part, quantitatively; studies must have been published within the last 15 years (January 2003 to January 2019—the selected time period serves to differentiate between when information concerning microbiota was determined by bacterial culturing methods rather than the significantly more robust contemporary methods that utilise genomic sequencing to ascertain, with greater precision, which microbiota are present in an individual’s GIT); studies must present primary data—that is, they are not systematic reviews or review articles (however, articles of this nature were read to identify additional appropriate studies); studies must be available in the English language.
Exclusion criteria
Studies were excluded if any of the following conditions were applicable: participants were concurrently receiving any treatment that was in dispute of NICE guidelines (provided that a study participant had not been sectioned under the Mental Health Act (or equivalent), then there was no requirement that that individual should receive any form of treatment; as such, patients acting with informed consent who chose not to receive any aspect of the treatment protocol outlined in NICE guidelines, but chose to receive a dietary intervention in a study, were suitable for inclusion in this review—studies were only excluded when they appeared to be in dispute of NICE guidelines); the study investigated the effect of prebiotics and/or probiotics without regard for anxiety disorders and/or depression; the study’s findings were produced from animal models; the study’s findings were expressed only qualitatively; the study’s method or quality were deemed to be of a poor standard; the study was in disaccord with outlined inclusion criteria.
Sources in accord with the predetermined inclusion criteria and not exhibiting characteristics outlined in the exclusion criteria were deemed suitable for further investigation.
Information sources
With the eligibility criteria determined, a literature search was conducted.
Database searching
A systematic search was carried out of the following databases: MEDLINE, Embase, PsychINFO, AMED, Cochrane Library, PubMed and Web of Science. Limits applied were: publication date range 2003–2019, human studies, age >18 years, and English language.
Search strategy
A search strategy was devised and adapted to each database as required. The finalised search consisted of [Depress* OR “Low mood” OR Anxi* OR Stress* OR “Mental Health”] AND [prebiotic* OR probiotic* OR psychobiotic OR probiotic/tu OR “probiotic supplementation” OR symbiotic* OR bifidobac* OR bifidogenic OR “Gastrointestinal microbiome” OR “gut-brain axis” OR lactobac*].
Hand searching
To ensure relevant data were not missed, hand searching of ‘grey literature’ was also conducted. This consisted of advanced Google searching of specific domains likely to produce relevant findings. Specifically, the domains ‘nhs.net’ and ‘gov.uk’ were searched. Data were also yielded by searching relevant websites including the British Dietetic Association, NICE guidelines, NICE evidence search, MIND, and Web of Science conference archives.
Expert network consultations
Finally, to further minimise the possibility of missing any relevant findings, the scientific director of the American Gut Project and the lead of Dietary Intervention Studies at Cork University Ireland’s APC Microbiome project were contacted for their expert opinion regarding sources, unpublished conference findings or contemporary research relevant for this review. No previously unidentified sources were yielded.
Results
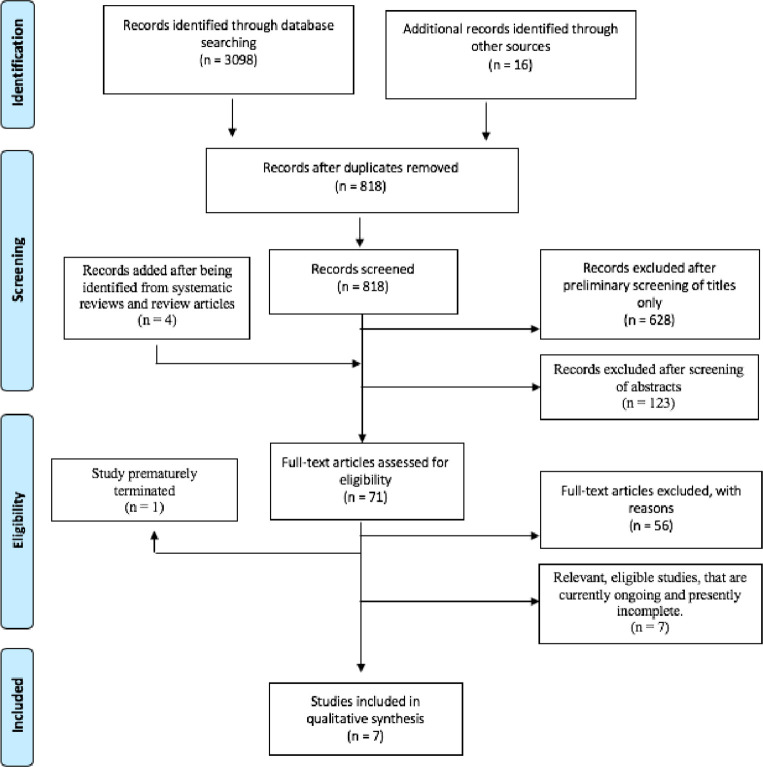
The outlined searching strategy was applied. The results of this are detailed below in Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flowchart format. (figure 1)
Figure 1.
An adapted PRISMA flowchart detailing search results acquired by actioning the strategy detailed in the Methods section. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses.
Study screening
Of the articles identified, 67 were deemed suitable for thorough evaluation. Non-primary sources were unsuitable for inclusion; however, they were included in a thorough evaluation to identify additional relevant articles, yielding a further four papers. Of this total of 71 articles, 56 were in breach of the pre-determined inclusion/exclusion criteria and were removed. Seven studies were ongoing, with results therefore unavailable—these were also excluded. One study was prematurely terminated.
Study selection
A total of seven articles were selected for inclusion. Key discriminators throughout this process included: studies conducted on non-human subjects; studies conducted on human subjects that lacked a clinical diagnosis; and studies that determined effect through non-quantitative measures. By adhering to the pre-determined conditions for this review, the studies identified investigated only populations with diagnosed depression (n=7); no suitable study investigated patients primarily for an anxiety disorder (n=0). However, given that certain included articles assess anxiety specifically within their test cohort, and that certain measured outcomes are highly relevant for assessing anxiety as well as depression (stress evaluators, or plasma cortisol, for example), both conditions will still be considered when evaluating the following results.
All seven studies investigated at least one probiotic strain; 3/7 studies investigated a single probiotic strain as an intervention, while 4/7 studies investigated the effect of administering multiple probiotic strains as a combined intervention. A total of 12 strains were investigated; most commonly tested were Lactobacillus acidophilus (tested in three articles), Lactobacillus casaei and Bifidobacterium bifidium (tested in two articles each). One study investigated combined prebiotic–probiotic therapy, and one article investigated isolated prebiotic therapy.
Article appraisal and data extraction
The salient details of the articles are presented in table 2, along with each study’s modified-CASP appraisal score.
Table 2.
Details of the articles, along with each study's modified CASP appraisal score
| Author, year, study type, location | Subjects | No. of subjects, F/M ratio |
Days of intervention, type of intervention | Method of administration, dose | Measured outcome | Study appraisal score |
| Rudzki et al (2019), RCT, Poland20 |
Patients with clinically diagnosed MDD, that are also on an SSRI treatment regimen in an outpatient setting | 79 43:17 (19 subjects lost to follow-up with gender unspecified in report) |
56 Probiotic: Lactobacilus plantarum 299 v |
Weeks 0–8:
2 probiotic containing capsules, 1 in the morning, 1 at night. Dose: 10× 109 CFU |
Primary: Psychiatric symptoms: Hamilton Depression rating scale (HAM-D-17) <=>; Symptom checklist-90 <=>; Perceived Stress Scale-10 <=>. Cognitive functions: Work speed in attention and perceptivity test \/; California Verbal Learning Test \/; Ruff-figural fluency test <=>; Stroop Test parts A & B <=>. Biochemical measures in systemic circulation: kynurenine (KYN) \/; 3-hydroxykynurenine:KYN ratio \/; KYN:TRP ratio <=>; TRP <=>; kynurenic acid <=>; anthranilic acid <=>; 3-hydroxy anthranilic acid <=>; TNFα <=>; IL-6 <=>; IL-1b <=>; cortisol plasma concentrations <=>. |
10 |
| Majeed et al (2018), RCT, India23 |
Patients with clinically diagnosed MDD and IBS | 40 17:3 |
90, further follow-up at day 105 Probiotic: Bacillus coagulans MTCC 5856 |
Weeks 0–11:
1 probiotic containing tablet daily Dose: 600 mg tablet containing 333.3 mg of B. coagulans – 2×109 CFU |
Primary measures: Depression symptoms: HAM-D-17 \/; Montgomery-Asberg Depression Rating \/. Secondary measures: Clinical Global Impression Improvement Rating Scale \/; Clinical Global Impression Severity Rating Scale \/; Gastrointestinal Discomfort Questionnaire (GI-DQ) \/; Modified Epworth Sleepiness Scale (mESS)\/; Montgomery-Asberg Depression Rating Scale \/; Centre for Epidemiological Studies Depression Scale \/; Irritable bowel syndrome quality of life questionnaire (IBS-QoL) \/ |
8 |
| Kazemi et al (2018), RCT, test, test, control, Iran21 |
Patients with clinically diagnosed MDD, also on SSRI treatment regimen in an outpatient setting | 110 enrolled, 81 completed trial, all participants accounted for 78:32 |
56 Probiotics: Lactobacillus helveticus Bifidobacterium longum OR Prebiotic: galacto-oligosaccharide |
Probiotic: 5 g sachet given once daily Dose: ≥10 × 109 CFU of bacterial strains (both strains cumulatively equal ≥10 × 109 CFU) Prebiotic: 5 g dose galacto-oligosaccharide +0.2% excipient. |
Primary outcomes: Depression symptoms: Beck Depression Inventory (BDI-II) \/ for probiotic, <=> prebiotic. Secondary outcomes: Biochemical measures: Serum KYN:TRP <=> for probiotic, <=> prebiotic; KYN:TRP once adjusted for serum isoleurine = \/ probiotic, <=> prebiotic; TRP: isoleurine ratio \/ for probiotic, <=> prebiotic; tryptophan:branched chain amino acid ratio <=> probiotic, <=> prebiotic. |
10 |
| Miyaoka et al (2018), open label case–control, Japan24 |
Patients with treatment resistant MDD, who are taking SSRIs | 40 24:16 |
56 Probiotic: Clostridium butyricum MIYAIRI 588 (CBM588) |
Weeks 0–4:
1 capsule daily Dose: 40 mg Weeks 2–8: 1 capsule daily Dose: 60 mg |
Primary outcomes: Depression symptoms: HAD-17 \/; Secondary outcomes: Depression symptoms: BDI-II \/. Anxiety symptoms: Beck Anxiety Inventory Score \/. |
5.5 |
| Ghorbani et al (2018), RCT, Iran25 |
Patients with clinically diagnosed moderate severity depression | 40 28:12 |
70 Probiotics: Lactobacilus casaei Lactobacillus acidophilus Lactobacillus bulgarigus Lactobacillus rhamnosus Bifidobacterium breve Bifidobacterium longum Streptococus thermophilus AND Prebiotic: Fructo-oligosaccharide |
Weeks 0–4:
1 tablet daily fluoxetine Dose: 20 mg Weeks 4–10: 1 tablet fluoxetine 2 synbiotic capsules daily Dose: 20 mg fluoxetine; 2 synbiotic capsules Capsule content: 500 mg of probiotics: (Lactobacillus casaei 3×108 CFU/g; Lactobacillus acidophilus 2×108 CFU/g; Lactobacillus bulgarigus 2×109 CFU/g; Lactobacillus rhamnosus 3×108 CFU/g; Bifidobacterium breve 2×108 CFU/g; Bifidobacterium longum 1×109 CFU/g; Streptococus thermophilus 3×108 CFU/g + 100 mg prebiotics: mg fructo-oligosaccharide |
Primary outcome: HAD-17 \/. |
9.5 |
| Bambling et al (2017), case series, Australia26 |
Patients with resistant depression and multiple, poor response to treatment in the past | 12 8:4 |
56, further follow-up at day 112 Probiotics: Lactobacillus acidophilus Bifidobacterium bifidum Streptoccocus thermophiles |
Weeks 0–8:
2 capsules daily Dose: 2×1010 CFU of all microorganisms with 1600 mg magnesium oratate |
Primary outcomes: Psychiatric symptoms: Mini International Neuropsychiatric Interview (MINI,5) \/; BDI-II \/; Outcome Questionnaire 45 (OQ45) \/; Quality of Life Measure \/. |
6 |
| Akkasheh et al (2016), RCT, Iran22 |
Patients with clinically diagnosed MDD | 40 34:6 |
56 Lactobacillus acidophilus Lactobacillus casei Bifidobacterium bifidum |
Weeks 0–8:
1 capsule daily Dose: Lactobacillus acidophilus (2×109 CFU/g) Lactobacillus casei (2×109 CFU/g) Bifidobacterium bifidum (2×109 CFU/g) |
Primary outcomes: Depression symptoms: BDI-II \/; Secondary outcomes: Biochemical measures: Serum insulin \/; homeostasis model assessment of insulin resistance \/; serum hs-CRP concentration \/; fasting plasma glucose <=>; homeostatic model assessment of β cell function <=>; quantitative insulin sensitivity check index <=>; lipid profiles <=>; total antioxidant capacity levels <=>. |
9.5 |
Studies are identified with the patient group, participant number, intervention type and regimen detailed. The measured outcomes from which any effect could be observed, along with the findings of the studies, are detailed.
/\ represents a statistically significant increase in a measured outcome parameter for the test versus control group; \/ indicates a statistically significant decrease in a measured outcome parameter; <=> indicates that no statistically significant change was detected between the groups.
A modified-CASP appraisal score is included. The modified-CASP appraisal tool is a novel evaluative method that allows a numerical value from 0 to 12 to be calculated for each article based on its performance with respect to the traditional CASP appraisal framework criteria. A higher score out of 12 indicates a better quality article. This information can be integrated with the findings in table 3, which detail the extent to which each study appears to be affected by various biases in order to augment a thorough critical analysis of the dataset.
BDI-II, Beck Depression Inventory II; CASP, Critical Appraisal Skills Programme; CFU, colony forming units; F/M, female/male; GI-DQ, Gastrointestinal Discomfort Questionnaire; HAM-D-17, 17 item Hamilton Depression Rating Scale; hsCRP, high sensitivity C-reactive protein; IBS, irritable bowel syndrome; IBS-QoL, Irritable Bowel Syndrome Quality of Life questionnaire; IL, interleukin; KYN, kynurenine; MDD, major depressive disorder; mESS, modified Epworth Sleepiness Scale; MINI,5, Mini International Neuropsychiatric Interview; OQ45, Outcome Questionnaire 45; SSRI, selective serotonin reuptake inhibitor; TNF, tumour necrosis factor; TRP, tryptophan.
Bias
Risk of bias in studies
The Cochrane risk-of-bias tool was employed to assess bias within the studies. Studies were reviewed with respect to the outlined sub-categories within this tool and graded for risk of bias into the categories high, low, unclear or N/A. Table 3 details this.
Table 3.
The risk of key biases present within each study
| Author, year | Random sequence generation | Allocation concealment | Selective reporting | Other biases | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete data outcome | Additional information |
| Rudzki et al (2019)20 | Low | Low | Unclear | Low | Low | Unclear | High | 19 lost to follow-up; information concerning these individuals not included in report |
| Majeed et al (2018)23 | Unclear | Unclear | Low | High | Unclear | Unclear | Low | Authors employed by manufacturer of the intervention—Sabinsa, USA. Sabinsa also funded the trial |
| Kazemi et al (2018)21 | Low | Low | Low | Low | Low | Unclear | High | Attrition bias present; however, all subjects accounted for and details of loss to follow-up well explained |
| Miyaoka et al (2018)24 | Unclear | High | Low | Unclear | High | High | Low | Random group allocation insufficiently detailed; open label design (no blinding). |
| Ghorbani et al (2018)25 | Low | Low | Low | Low | Low | Unclear | Low | Independent company employed for randomisation protocols, no loss to follow-up. Blinding of results unspecified |
| Bambling et al (2017)26 | N/A | N/A | Low | High | High | N/A | High | No randomisation or control group. Patients and investigators knew what intervention was. Patients lost to follow-up (accounted for). Potential conflict of interest—researchers work with companies that seed fund, produce and research probiotics |
| Akkasheh et al (2016)22 | Low | Unclear | Low | Unclear | Unclear | Unclear | Low | Randomisation of groups and allocation claimed to be done; claimed outcomes were blinded, though no explicit details provided (therefore unclear). Attrition bias, participants included in analysis with intention-to-treat approach. Conflict of interest not stated |
Discussion
Results impression
There is significant clinical and methodological heterogeneity within this review which prevents meta-analysis. The nuances of the investigated populations, the variety of measured outcomes employed to evaluate interventions, and the variety of pre/probiotics that exist (and the multitude of experimental approaches/combinations of these) are examples of some of the factors contributing to this heterogeneity. Despite this, the results indicate a common finding—a link may exist between probiotic/prebiotic–probiotic supplementation and reductions in quantitative measures of depression.
Key findings
Three of seven studies showed insignificant changes for certain outcomes; however, these were generally biochemical measures. Prebiotics, when investigated as isolated therapies, incurred non-statistically significant results.20–22 However, in no study do the measured outcomes indicate pre/probiotic interventions increase, or negatively impact, measures of anxiety/depression. Furthermore, every study demonstrated a significant, quantitatively evident, decrease/improvement of symptoms and/or biochemically relevant measures of anxiety and/or depression for probiotic or combined prebiotic–probiotic use. This is observed regardless of the specific probiotic, method of administration or length of trial, across all test cohorts, irrespective of depression severity or additional comorbidities.20–26 The current evidence indicates: prebiotics are unlikely to be effective as an isolate therapy for depression within the timeframes investigated21 25; probiotics may be useful agents20–26; concomitant prebiotic–probiotic therapy may also be useful, though it is not obvious whether these agents function synergistically from this cohort of studies.21 25 The results offer some insights into pre/probiotic use for anxiety, though these are largely limited, with no test cohort being investigated primarily for an anxiety disorder (though some test populations have clinically significant comorbid anxiety disorders).26
Interpreting questionnaire results
The 17 item Hamilton Depression Rating Scale (HAM-D-17) and Beck Depression Inventory II (BDI-II) were the two most commonly employed methods for evaluating subjects’ psychiatric condition pre/during/post invention. Probiotic/combined prebiotic–probiotic therapy significantly reduced patient scores on these tests in 6/7 studies.21–26 The exception, Rudzki et al,20 found no link between probiotic use and changes in HAM-D-17 scores. There were no significant effects on depression and anxiety symptoms with the Symptom Checklist-90 and Perceived Stress Scale-10.20 The intervention in this study was Lactobacillus plantarum 299V; interestingly, no other study investigated this bacterial strain. Despite this, notable effects were seen following L. plantarum administration with respect to the cognitive functions of test subjects and for certain biochemical measures. Considering this, it is reasonable to suggest that L. plantarum may be functionally active via the GBA (hence its effect on cognition), but its influence pertains less to factors affecting depression and/or anxiety.
Furthermore, this finding indicates that, of the 12 different probiotics investigated, 11 were potentially useful agents. The effect measured by HAM-D-17 and BDI-II is perhaps best appreciated when considering Kazemi et al 21 and Ghorbani et al.25 These papers scored the joint highest and joint second highest during appraisal, respectively, and had the lowest risks of bias. Both articles demonstrated marked improvement in BDI-II or HAM-D-17 scores in patients supplemented with probiotics (Kazemi—mean BDI-II—probiotic group: 18.25 at baseline–>9.0 end of trial; placebo: 18.74–>15.55. Ghorbani—mean HAM-D-17—prebiotic-probiotic group: 22.90–>3.65 (1.71 SD); placebo: 22.55–>4.80 (2.05 SD). Interestingly, this effect is present in quite different cohorts. Ghorbani et al investigated subjects with moderate severity depression, while Kazemi et al studied patients with more severe MDD who were simultaneously using selective serotonin reuptake inhibitor (SSRIs). This may suggest that any positive effect derived from probiotic administration would be felt across a variety of depression severities, and that such an effect would be independent of—even additional to—the effects gained from conventional NICE guidelines treatment, such as SSRIs.21 25 This is further supported in Bambling et al,26 where improvements noted in participant’s mean quality of life (QoL) scores after 8 weeks of daily probiotic use (57.5 at week 1–>70.0 at week 8) were undone after 8 weeks post-treatment cessation (average scores returned to baseline levels: 70.0 at week 8–>56.7 at week 16). Participants of this study had highly treatment-resistant MDD, though still appeared to experience some benefit from probiotic use. It is also noteworthy that, within this cohort, the most common comorbidity to treatment-resistant MDD was an anxiety disorder, of which 60% of subjects also met the necessary criteria.26 Under these circumstances, QoL scoring can serve, at least in part, as a measure of the effect of probiotics on both anxiety and depression as comorbidities.
Potential mechanisms underlying the observed results
It should be noted that while disruption to the GBA is common across a wide range of disorders, our understanding of the GBA is limited. Much of the current research into its mechanisms has been conducted on animal models; with a limited number of human studies, we cannot say with certainty whether increased serotonin levels in the gut are responsible for a cascade of molecular events that influence neuronal activity. However, the findings of this review lead us to question what mechanisms might be driving such effects.
Pro-inflammatory cytokines and immune responses
As outlined above, immunomodulatory mechanisms are thought to be key in mediating GBA interactions.3 9 13 14 However, studies that assessed immune function through serum measurements of pro-inflammatory cytokines or by directly assessing immune cell functions all found insignificant changes between test and control groups (Rudzki—TNFα, IL-6 and IL-1b: 1.70, 2.05 and 0.16, respectively, at baseline; 1.78, 2.19 and 0.36, respectively, post-intervention. Akkasheh—assessment of B cell function: 29.8 at baseline–>22.7 end of trial, all changes statistically insignificant). However, it should be appreciated that sampling cerebrospinal fluid might have offered more useful information regarding CMDs than circulating blood samples.20 22 Nonetheless, this finding is surprising as it is suggested that in many conditions where probiotics are seen to be useful therapeutic agents, their usefulness is related to their capacity to reduce immune production of endogenous, pro-inflammatory compounds—such as cytokines—as is the case in ulcerative colitis.27 28 Given the strength of evidence supporting the claim that there may be an underlying inflammatory basis in the pathogenesis of certain CMDs—including depression—such a mechanism would have been fitting.2 13 14 The absence of such a finding certainly does not indicate such mechanisms are insubstantial in GBA actions or in the pathogenesis of psychiatric conditions. However, it does suggest that other mechanisms may be mediating the observed effects. Another mechanism that may potentially be significant in explaining the aforementioned findings relates to the compound TRP and its metabolic pathways.
Tryptophan
TRP has been identified as potentially important in GBA interactions in psychiatric disorders as its metabolic pathways hypothetically could enable microbiota to have cross-talk interactions with their host.2 3 12 TRP is a metabolic precursor for serotonin and is present in the systemic circulation; it can cross the BBB, with the potential to raise serotonin levels in the CNS.29 However, TRP may instead progress down the kynurenine (KYN) pathway, from which serotonin cannot be synthesised.29 30 Microbiota have a modulatory effect over which metabolic pathway TRP progresses down.30–32 Considering the role that serotonin levels within the CNS have on the pathogenesis of CMDs, the effect probiotics have on TRP and KYN levels is of interest and might partially explain the global effects observed in test subjects.
Both Rudzki et al and Kazemi et al considered TRP, KYN and KYN:TRP ratios. Kazemi et al also considered KYN levels adjusted for isoleucine concentration, as isoleucine can reduce KYN concentration.33 Kazemi et al found a significant decrease in KYN and adjusted-KYN:TRP ratio in placebo versus probiotic (KYN (nmol/L)—placebo: 772.89–>798.49; probiotic: 757.59–>722.47. KYN:TRP (nmol/μg)—placebo: 11.0–>12.4; probiotic: 11.23–>9.58). Rudzki et al found decreased KYN (placebo: 2.17–>2.32; probiotic: 2.05–>1.82), but insignificant differences in KYN:TRP ratio between placebo and probiotic groups (KYN:TRP (both measured in μmol/L)—placebo: 0.04–>0.04; probiotic 0.04–>0.03). Rudzki et al’s KYN:TRP ratio, however, was non-adjusted for amino acids and may therefore be less insightful. Decreased KYN may in fact be a more significant finding, given that available TRP may have been converted into serotonin in the probiotic group, which could offer an explanation for the improvements observed in their performance in tasks assessing cognitive function.31 Furthermore, probiotics, including Lactobacillus plantarum 299 v specifically, have been shown to increase serotonin biosynthesis, which further strengthens this point.34 In either case, probiotic supplementation seemingly initiated biochemical changes in depressed patients for compounds considered to be significant in the pathogenesis of CMDs. This indicates further investigation is required into the effects of probiotics on TRP, KYN and other metabolic compounds, as they may offer insights into why the articles in this review resoundingly indicate that probiotics can improve symptoms of anxiety and depression in a quantitative sense.
Implications of findings
It is crucial that these findings are appreciated with regard to the clinical contexts in which they would be applied. For practical purposes, we try to observe the effect of an intervention on a condition in an isolated manner; however, this can overemphasise the clinical significance of the data. The reality is that anxiety disorders and depression affect individuals in a multitude of ways, manifest in multiple bodily systems, and arise in patients who may have other comorbidities. While one person might experience gastrointestinal symptoms as a result of their primary illness, another may find their cardiovascular health more affected. In essence, the effects of psychiatric morbidity are diffuse, variable and require treatment approaches that adequately appreciate their complexity—at the risk of cliché, they require a holistic approach. In this way, with a better understanding of its mechanisms, probiotics may prove to be a useful tool across a wide range of conditions.
Evidence exists supporting the benefits of probiotics in the treatment of numerous morbidities—from infectious/antibiotic-induced diarrhoea and irritable bowel syndrome (IBS) to autoimmune conditions such as ulcerative colitis.32 34 35 Microbiome studies are novel, and it is likely more information will surface as research continues—for example, preliminary data already suggest probiotics may be useful for the prevention of obesity and type 1 and type 2 diabetes. In fact, in this review, Akkasheh et al 22 demonstrate that probiotics significantly lower serum insulin concentration and resistance, while Majeed et al 23 showed significance decreases in intervention group scores for the Gastrointestinal Discomfort Questionnaire (GI-DQ), Modified Epworth Sleepiness Scale (mESS) and the Irritable Bowel Syndrome Quality of Life questionnaire (IBS-QoL).36 This information is of particular significance as patients with anxiety disorders and depression are predisposed to numerous comorbidities, including impaired insulin functioning and IBS.37 As such, the effect that probiotics have on patients with CMDs may be twofold: they may directly improve depression in line with the observed findings of this review, and/or they might beneficially impact a patient’s experience of their CMD by alleviating additional comorbidities. The overlap of conditions more commonly arising in patients with CMDs and conditions that probiotics may be useful for treating highlights the exciting potential that the clinical application of probiotic agents may hold for certain patient groups.
Limitations
There are some limitations affecting this review. First, it is acknowledged that while the review shows no negative effects of pre/probiotics, this could be a reflection of a positive reporting bias; this is supported by evidence that highlights a lack of systematic reporting of adverse events in connection with probiotic interventions.38 Further to this, a number of the interventions administered contained a combination of compounds—be this multiple probiotics, or other ingredients. Therefore, it cannot be ascertained with certainty which product is responsible for the observed results or whether a specific combination is required for such an effect. There is also anecdotal evidence that prebiotics may worsen outcomes under specific treatment conditions.39 The findings thus point more towards products that warrant further investigation in isolation or in combinations whereby effects can be better understood, rather than indicating the value of a specific product. An existing study highlights that a more appropriate study methodology would be to assess disease-specific and strain-specific efficacy.40 In the selected papers, study durations were short and populations small, meaning they were unable to provide full clarity on the effect the interventions might have. There was also a notable difference in sample sizes between studies with significantly fewer data for probiotic interventions. Without balanced data, a larger scale or longer trials, one cannot gain accurate information regarding: the overall effect of an intervention; whether the effects seen are lasting, long-acting or temporary; or whether there are any long-term dangers or complications associated with the prolonged use of the intervention. Thirdly, it should be noted that, while the studies are generally uniform in indicating the usefulness of probiotic-containing products for patients with clinically diagnosed depression, there is little scope to posit their use for patients affected by an anxiety disorder in the absence of depression. No studies were identified investigating this population without having additional confounding factors that made their results less relevant/useful; additional work is required to clarify whether such products might benefit this patient group. It should also be noted that even though the studies are widely in accordance in their findings, the included studies are of variable quality and subject to different degrees of bias. Finally, this review does not include papers published within the last year and therefore the conclusions may not necessarily reflect the most recently published data.
Conclusion
Our results affirm that pre/probiotic therapy warrants further investigation. Efforts should aim to elucidate whether the perceived potential efficacy of pre/probiotic therapy in depression and/or anxiety disorders can be replicated in larger test populations, and whether any effects are maintained through continued treatment, or post-cessation. Specific interventions should be investigated in isolation, not combination, to ascertain from where the observed effects arise. Detailing mechanistic explanations for any effects should be a priority. Consideration and research effort should also be directed towards identifying patient groups that may experience greater benefits of pre/probiotic therapy due to their own individual idiosyncrasies; as previously discussed, patients with comorbid depression and IBS may be such a group. Finally, it is imperative that biochemical analysis is included in our interpretation of the effects of microbiome studies. A biochemical focus may highlight processes that precede or follow notable changes in an individual, which could offer new insights or affirm present theories regarding why particular outcomes occur. This would provide a useful contribution to the growing literature for microbiome studies and may even inform our understanding of physiology and pathophysiology generally.
These preliminary findings suggest continued investigation, on a larger scale and over a longer time period, would be appropriate. However, purely from the information gathered in this review, it is valid to suggest that, for patients with clinically recognised depression: isolate, or adjuvant, prebiotic therapy is unlikely to affect an individual’s experience of their condition in a quantitatively evident way; and that isolate, or adjuvant, probiotic/combined prebiotic–probiotic therapy may offer a quantitatively measurable improvement in parameters relating to depression. However, there are inadequate data to suggest anything meaningful to support or refute the use of either pre/probiotic agents (or a combination of both) in patients with clinically recognised anxiety disorders; this would be a useful area to investigate further.
Acknowledgments
Special thanks to Librarian, Lisa McLaren, for her patience and advice during the review process. We are grateful to the NNEdPro Global Centre for Nutrition and Health for assistance towards APC costs.
Footnotes
Twitter: @macaninch
Contributors: SN acted as the main author of this project. MZ provided review, editing and amendments. EM assisted with methods, editing and supervision. KM offered senior supervision and final editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not applicable as no datasets were generated and/or analysed for this study.
References
- 1. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 2017;7:124–36. 10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sherwin E, Rea K, Dinan TG, et al. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 2016;32:96–102. 10.1097/MOG.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 3. Sherwin E, Sandhu KV, Dinan TG, et al. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs 2016;30:1019–41. 10.1007/s40263-016-0370-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh RK, Chang H-W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics - a review. J Food Sci Technol 2015;52:7577–87. 10.1007/s13197-015-1921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ng QX, Peters C, Ho CYX, et al. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord 2018;228:13–19. 10.1016/j.jad.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 7. Oriach CS, Robertson RC, Stanton C, et al. Food for thought: the role of nutrition in the microbiota-gut-brain axis. Clin Nutr Exp 2016;6:25–38. 10.1016/j.yclnex.2016.01.003 [DOI] [Google Scholar]
- 8. Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018;12:49 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011;23:1132–9. 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016;167:1125–36. 10.1016/j.cell.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang S, Wu X, Hu X, et al. Recognizing depression from the microbiota. Int J Mol Sci 2018;19:1592 10.3390/ijms19061592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392 10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res 2014;39)::624–44. 10.1007/s11064-014-1266-6 [DOI] [PubMed] [Google Scholar]
- 14. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013;246:199–229. 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity 2014;40:824–32. 10.1016/j.immuni.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin CR, Osadchiy V, Kalani A, et al. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 2018;6:133–48. 10.1016/j.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker C. Mental health statistics for England: prevalence, services and funding. House of Commons Library 2018. [Google Scholar]
- 18. Ng QX, Loke W, Venkatanarayanan N, et al. A systematic review of the role of prebiotics and probiotics in autism spectrum disorders. Medicina 2019;55:e129 10.3390/medicina55050129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng QX, Soh AYS, Venkatanarayanan N, et al. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology 2019;78:1–6. 10.1159/000498862 [DOI] [PubMed] [Google Scholar]
- 20. Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019;100:213–22. 10.1016/j.psyneuen.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 21. Kazemi A, Noorbala AA, Azam K, et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr 2019;38:522–8. 10.1016/j.clnu.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 22. Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016;32:315–20. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 23. Majeed M, Nagabhushanam K, Arumugam S, et al. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res 2018;62:1218 10.29219/fnr.v62.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyaoka T, Kanayama M, Wake R, et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder. Clin Neuropharmacol 2018;41:151–5 https://www.ncbi.nlm.nih.gov/pubmed/30234616 10.1097/WNF.0000000000000299 [DOI] [PubMed] [Google Scholar]
- 25. Ghorbani Z, Nazari S, Etesam F, et al. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch Neurosci 2018;5:e60507 http://archneurosci.com/en/articles/60507.html 10.5812/archneurosci.60507 [DOI] [Google Scholar]
- 26. Bambling M, Edwards SC, Hall S, et al. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017;25:271–4. 10.1007/s10787-017-0311-x [DOI] [PubMed] [Google Scholar]
- 27. Ng QX, Soh AYS, Loke W, et al. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res 2018;11:345–9. 10.2147/JIR.S174982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fedorak RN. Probiotics in the management of ulcerative colitis. Gastroenterol Hepatol 2010;6:688–90. [PMC free article] [PubMed] [Google Scholar]
- 29. Yadav VK. Serotonin: the central link between bone mass and energy metabolism : Karsenty G, Translational endocrinology of bone. 1st edn Cambridge, Massachusettes: Academic Press, 2013: 51–62. [Google Scholar]
- 30. Richard DM, Dawes MA, Mathias CW, et al. L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res 2009;2:45–60. 10.4137/IJTR.S2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waclawiková B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals 2018;11 10.3390/ph11030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sekine A, Okamoto M, Kanatani Y, et al. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. Springerplus 2015;4:48 10.1186/s40064-015-0826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012;18:4012-8–8. 10.3748/wjg.v18.i30.4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Vrese M, Marteau PR. Probiotics and prebiotics: effects on diarrhea. J Nutr 2007;137:803S–11. 10.1093/jn/137.3.803S [DOI] [PubMed] [Google Scholar]
- 36. Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, et al. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci 2016;17 10.3390/ijms17060928. [Epub ahead of print: 13 Jun 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinac M, Pehar D, Karlović D, et al. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clin Croat 2014;53:55-71. [PubMed] [Google Scholar]
- 38. Gwee K-A, Lee WW-R, Ling KL, et al. Consensus and contentious statements on the use of probiotics in clinical practice: a South East Asian gastro-neuro motility association working team report. J Gastroenterol Hepatol 2018;33:1707–16. 10.1111/jgh.14268 [DOI] [PubMed] [Google Scholar]
- 39. Hempel S. Safety of probiotics used to reduce risk and prevent or treat disease.. Evid Rep Technol Assess 2011:1–645. [PMC free article] [PubMed] [Google Scholar]
- 40. McFarland LV, Evans CT, Goldstein EJC. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med 2018;5:124. 10.3389/fmed.2018.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]