Abstract
Daratumumab, a human immunoglobulin G1 kappa monoclonal antibody that targets CD38, is currently approved as monotherapy and in varying combinations with approved anti-myeloma regimens in both newly diagnosed multiple myeloma and relapsed refractory multiple myeloma. Originally developed for intravenous administration, the subcutaneous formulation of daratumumab (daratumumab and hyaluronidase-fihj) was recently approved by the US Federal Drug Administration and European Commission in 2020. In clinical trials, compared with the intravenous formulation, subcutaneous daratumumab (Dara-SC) has significantly shorter administration time (median first dose 7 h versus 3–5 min, respectively), lower rates of infusion-related reactions (median first dose 50% versus less than 10%, respectively), and lower volume of infusion (median 500–1000 ml versus 15 ml, respectively). Otherwise, the pharmacokinetics, safety profile, and efficacy are comparable. This review summarizes the pivotal trials that led to the approval of Dara-SC, highlights important clinical considerations for the use of Dara-SC, and provides practical guidelines for the administration of Dara-SC in the clinic.
Keywords: CD38, daratumumab, monoclonal antibody, multiple myeloma, myeloma, newly diagnosed, relapsed refractory, subcutaneous
Introduction
Daratumumab is a human immunoglobulin G1 kappa monoclonal antibody that targets CD38, which is highly expressed on the surface of multiple myeloma (MM) cells, and induces MM cell death via several mechanisms, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and apoptosis.1 Daratumumab, originally developed as an intravenous formulation (Dara-IV), was the first monoclonal antibody approved for the treatment of MM, receiving its initial approval from the US Food and Drug Administration (FDA) as monotherapy for the treatment of relapsed/refractory MM (RRMM) in November of 2015 based on results of the early-phase clinical trials GEN501 and SIRIUS.2,3 Since that time, Dara-IV has been approved in combination with various standard backbone regimens in both RRMM and newly diagnosed MM (NDMM).4–9 Recently, the subcutaneous formulation of daratumumab (daratumumab and hyaluronidase-fihj) was approved by the FDA in May 2020, followed by European Medicines Agency (EMA) approval in June 2020, for the treatment of adult patients with MM. Table 1 summarizes the current US FDA- and EMA-approved indications for the use of both intravenous and subcutaneous daratumumab.
Table 1.
US FDA and EMA approved indications for daratumumab in multiple myeloma (MM).
| Dara-SC# | Dara IV‡ |
|---|---|
| Newly diagnosed MM (transplant ineligible) | Same in addition to |
| With bortezomib, melphalan, +prednisone (DVMP)* | With bortezomib, thalidomide, + dexamethasone (DVTd): newly diagnosed MM (transplant eligible)* |
| With lenalidomide + dexamethasone (DRd)* | With pomalidomide + dexamethasone (DPd): relapsed/refractory MM |
| Relapsed/refractory MM | |
| With lenalidomide + dexamethasone (DRd)* | |
| With bortezomib + dexamethasone (DVd)* | |
| As monotherapy: relapsed/refractory to three lines of therapy (including a PI + IMiD) or double refractory (to a PI + IMiD) | |
| In patients whose prior therapy included a PI and an IMiD and who have demonstrated disease progression on the last therapy* |
US FDA approved indications for Dara-SC.
US FDA approved indications for Dara-IV.
EMA approved indications for daratumumab via SC administration or IV infusion.
D, daratumumab; d, dexamethasone; EMA, European Medicines Agency; FDA, Federal Drug Administration; IMiD, immunomodulatory agent; IV, intravenous; M, melphalan; P, pomalidomide; P, prednisone; PI, proteasome inhibitor; R, lenalidomide; SC, subcutaneous; T, thalidomide; V, bortezomib.
Challenges in clinical practice that arise with the standard administration of Dara-IV (16 mg/kg typically given once weekly in cycles 1 and 2, every 2 weeks in cycles 3–6, and every 4 weeks thereafter in 28-day cycles) include infusion time, volume, and infusion-related reactions (IRRs). Of note, there are other dosing schemas of daratumumab-based regimens (i.e. daratumumab combined with bortezomib and dexamethasone in the CASTOR study).4 In clinical trials, the median duration of the first, second, and subsequent Dara-IV infusions were 7.0, 4.3, and 3.4 h, respectively, for a volume of 1000 ml in the first infusion, and 500 ml in subsequent infusions.10 The feasibility of splitting the first dose of daratumumab over 2 days has also been investigated and is shown to be well tolerated with reduced time required for the first infusion (median infusion time of 4.3 h on day 1, cycle 1, and 4.2 h on day 2, cycle 1).11 Recent data have also demonstrated that infusing daratumumab at an accelerated rate of 90 min is safe and effective.12,13 The rate of IRRs observed across clinical studies of Dara-IV is approximately 50%.10 These factors significantly impact healthcare resource utilization, as well as quality-of-life metrics. The approval of subcutaneous daratumumab (Dara-SC), based on the pivotal early phase trial PAVO [ClinicalTrials.gov identifier: NCT02519452], the phase III COLUMBA study [ClinicalTrials.gov identifier: NCT03277105] and preliminary results of the ongoing phase II PLEAIDES trial [ClinicalTrials.gov identifier: NCT03412565], is an important advancement in the treatment paradigm of MM. Dara-SC offers convenient dosing, as it is a fixed-dose injection of 15 ml that can be administered over 3–5 min, compared with several hours with the intravenous formulation. Moreover, Dara-SC in clinical trials has been shown to have comparable efficacy and a similar safety profile to the intravenous formulation, with significantly lower rates of IRRs, making Dara-SC a favorable treatment option.14–16
Here, we review the first in-human study of Dara-SC (PAVO) that assessed the safety and pharmacokinetics (PK) of subcutaneous daratumumab, and the phase III COLUMBA and phase II PLEAIDES trials that led to the approval of Dara-SC. We also highlight important clinical considerations for the use of Dara-SC and provide practical guidelines for the administration of Dara-SC in the clinic.
Clinical development of subcutaneous daratumumab
In its subcutaneous formulation, daratumumab is combined with recombinant human hyaluronidase PH20 (rHuPH20; ENHANZE drug delivery technology; Halozyme, Inc. San Diego, CA, USA), which is an endoglycosidase that degrades the glycosaminoglycan hyaluronan in the subcutaneous space and allows for increased dispersion and absorption of injected drugs at more rapid infusion rates.17,18 The pivotal trials of Dara-SC are summarized in Table 2. The phase Ib, three-part study PAVO (MMY1004) was the first in-human study to assess the PK profile, safety, and antitumor activity of Dara-SC in combination with rHuPH20 in patients with RRMM.15 The study included patients with RRMM who had received at least two previous lines of therapy [including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD)] and no prior anti-CD38 therapy. A sum of 53 patients were enrolled in part 1 (dose-escalation study) and 25 patients were enrolled in part 2 (dose-expansion study). In part 1 of the PAVO study, also known as the mix-and-deliver part of the study, daratumumab was mixed with rHuPH20 (DARA-MD) and administered subcutaneously via syringe pump at two dose levels, 1200 mg + rHuPH20 30,000 U in a total volume of 60 ml over ~20 min infusion time (n = 8) and 1800 mg + rHuPH20 45,000 U in a total volume of 90 ml over ~30 min infusion time (n = 45), in 28-day cycles, based on the standard schedule of administration of Dara-IV. Patients in both dosing cohorts were heavily pretreated populations with a median of four to five prior lines of therapy and the majority were refractory to both a PI and IMiD. The primary PK endpoint was trough concentration (Ctrough) at the end of weekly dosing before cycle 3, day 1 (C3D1), which has been correlated with the efficacy of daratumumab.19 The PK profile of the 1800 mg dosing cohort showed similar or greater mean Ctrough at C3D1 (744.20 µg/ml) as compared with the 16 mg/kg Dara-IV dosing cohorts in the GEN501 (617.17 µg/ml) and SIRIUS (573.49 µg/ml) studies. The most common treatment-emergent adverse events (TEAEs) in the 1200 mg cohort were thrombocytopenia, upper respiratory-tract infection, insomnia, and decreased appetite (37.5% each), whereas anemia (33.3%), upper respiratory-tract infection, pyrexia, and diarrhea (26.7% each) were the most common TEAEs in the 1800 mg cohort. IRRs occurred in 12.5% and 24.4% of the 1200 mg and 1800 mg dosing groups, respectively. The IRRs were generally grade 1 or 2 in severity, and the majority occurred during the first infusion, with most IRRs occurring during or within 6 h of the start of infusion (within the first 60 min from the start of infusion in 31%, within 60–120 min in 3%, within 120–180 min in 9%, within 180–240 min in 13%, within 240–300 min in 19% and within 300–360 min in 16%). The overall response rates (ORRs) of the 1200 mg and 1800 mg dosing cohorts were 25.0% and 42.2%, respectively. Overall, DARA-MD in part 1 of the PAVO study was well tolerated, with PK concentrations, safety profile, and responses in the 1800 mg dosing group consistent with that of Dara-IV in the GEN501 and SIRIUS studies.
Table 2.
Pivotal clinical trials of subcutaneous daratumumab (Dara-SC).
| Study | Trial name [ClinicalTrials.gov identifier] | Phase | n | Regimen (dose) | ORR (%) | IRR (%) |
|---|---|---|---|---|---|---|
| Clinical trials with Dara-SC in RRMM | ||||||
| Usmani et al.15 | PAVO [NCT02519452] | I/II | 8 | Daratumumab-MD (1200 mg)a | 25% | 13% |
| 45 | Daratumumab-MD (1800 mg)a | 42% | 24% | |||
| 25 | Dara-SC (1800 mg)b | 52% | 16% | |||
| Mateos et al.14 | COLUMBA [NCT03277105] | III | 522 | Dara-SC (1800 mg) versus IV | 41% versus 37% | 13% versus 35% |
| Chari et al.16 | PLEAIDES [NCT03412565] | II | 65 | Dara-SC (1800 mg) + Rd | 94% | 5% |
| Clinical trials with Dara-SC in NDMM | ||||||
| Chari et al.16 | PLEAIDES [NCT03412565] | II | 67 | Dara-SC (1800 mg) + VRd* | 97% | 9% |
| 67 | Dara-SC (1800 mg) + VMP** | 90% | 9% | |||
Transplant eligible.
Transplant ineligible.
Part 1 of PAVO using mix-and-deliver formulation.
Part 2 of PAVO using premixed formulation.
d, dexamethasone; Dara-SC, subcutaneous daratumumab; IRR, infusion-related reaction; IV, intravenous; M, melphalan; MD, mix and deliver; NCT, National Clinical Trial; NDMM, newly diagnosed multiple myeloma; ORR, overall response rate; P, prednisone; PFS, progression-free survival; R, lenalidomide; RRMM, relapsed/refractory multiple myeloma; SC, subcutaneous; V, bortezomib.
Based on these safety and efficacy results, part 2 of the PAVO study evaluated the safety and efficacy of a premixed and fixed dose of the subcutaneous formulation of daratumumab 1800 mg with rHuPH20 with a lower injection volume (15 ml), administered manually via handheld syringe over only 3–5 min (Dara-SC). Of the 25 patients enrolled in part 2, the median age was 68 (51–85) years, patients had received a median of three prior lines of therapy and 56% were refractory to both a PI and IMiD. Consistent with part 1 of the PAVO study, the mean Ctrough for end of weekly dosing at cycle 3, day 1, was similar or higher following Dara-SC 1800 mg compared with previous monotherapy studies of Dara-IV. The most common TEAEs were lymphopenia (32%), arthralgia (28%), back pain (28%), and thrombocytopenia, diarrhea, and nasopharyngitis (each occurring in 24% of patients). Only 16% of patients experienced IRRs, the majority of which occurred during the first administration of Dara-SC with a median time to onset of 70 (9–80) min. At a median follow up of 14.2 months, the ORR was 52% and median progression-free survival (PFS) was 12.0 (5.6–16.6) months.
Given the acceptable PK profile, safety, and efficacy results of the PAVO study, the phase III, randomized COLUMBA study evaluated the non-inferiority of Dara-SC to Dara-IV in patients with RRMM.14 Patients who had received at least three previous lines of therapy, including a PI and an IMiD, or were double refractory to both a PI and IMiD, and no prior anti-CD38 therapy, were included. Patients in the subcutaneous group received daratumumab at a dose of 1800 mg co-formulated with rHuPH20 2000 U/ml (based on dose determination in PAVO), and the intravenous group received daratumumab at the standard dose (16 mg/kg). The non-inferiority co-primary endpoints were ORR [at least more than partial response (⩾PR)] and the maximum Ctrough at the end of weekly dosing at cycle 3, day 1. A total of 522 patients were enrolled. This was a heavily pretreated patient population with a median of four prior lines of therapy. Baseline characteristics were similar between the two groups, albeit for a higher proportion of patients in the subcutaneous cohort having a European Cooperative Oncology Group score ⩾ 1 (76% versus 66%) and high-risk cytogenetics (26% versus 17%). At a median follow up of 7.5 months, the ORR was 41% in the subcutaneous group versus 37% in the intravenous group, meeting the predefined non-inferiority criteria. Moreover, the mean maximum Ctrough in the Dara-SC arm was consistently similar or slightly higher (593 µg/ml) than that intravenous arm (522 µg/ml), and the geometric means ratio of the maximum Ctrough for the subcutaneous versus intravenous group was 108% [90% confidence interval (CI): 95.74–121.67], with the lower limit of the 90% CI exceeding 80% and meeting the non-inferiority criterion. The most common grade 3 or 4 TEAEs occurring in the Dara-SC versus Dara-IV arm were similar in both groups: anemia (13% versus 14%), neutropenia (13% versus 8%), thrombocytopenia (14% versus 14%). IRRs were significantly lower in the Dara-SC group, occurring in 13% versus 34% of patients in the Dara-IV group [odds ratio (OR) 0.28; 95% CI: 0.18–0.44; p < 0.0001]. The majority of IRRs occurred following the first dose and were grade 1 or 2. Of note, the median time to onset for IRRs after the first dose was longer in the Dara-SC group (3.4 h) compared with the intravenous group (1.5 h). The median PFS was 5.6 months versus 6.1 months in the Dara-SC versus intravenous daratumumab groups, respectively, which is similar to that of the previous intravenous daratumumab monotherapy trials GEN501 and SIRIUS. Overall survival data at time of publication was not yet mature. In summary, the COLUMBA study found that Dara-SC was non-inferior in terms of efficacy and PK profile, with a similar safety profile but significantly lower rates of IRRs when compared with the intravenous formulation.
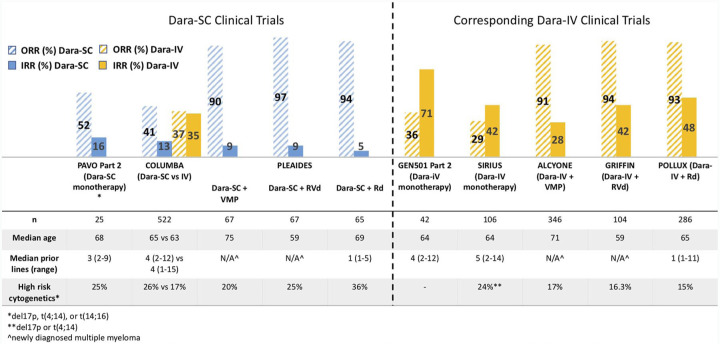
Dara-SC is also being studied in the ongoing phase II trial PLEIADES, which is a non-randomized, open-label study of Dara-SC combined with standard MM backbone regimens in both NDMM and RRMM. The primary endpoint analysis and updated efficacy and safety data were recently published.16 In the two NDMM arms of the study, Dara-SC 1800 mg was administered in combination with either bortezomib, lenalidomide, and dexamethasone (D-VRd; n = 67) in transplant-eligible patients, or bortezomib, melphalan, and prednisone (D-VMP; n = 67) in transplant-ineligible patients. In the RRMM arm, Dara-SC 1800 mg was administered in combination with lenalidomide and dexamethasone (D-Rd; n = 65). The primary endpoint was the ORR for the D-VMP and D-Rd cohorts and very good partial response (VGPR) or better rate in the D-VRd cohort. Baseline characteristics of the three cohorts are shown in Figure 1.
Figure 1.
Response and safety data in clinical trials of subcutaneous and intravenous daratumumab.
d, dexamethasone; Dara-IV, intravenous daratumumab; Dara-SC, subcutaneous daratumumab; IRR, infusion-related reaction; M, melphalan; n, sample size; N/A, not applicable; ORR, overall response rate; P, prednisone; R, lenalidomide; V, bortezomib.
The primary endpoints were met for all cohorts. At a median follow up of 14.3 and 14.7 months in the D-VMP and D-Rd cohorts, respectively, the ORR was 89.6% and 93.8%, with a VGPR rate of 77.6% and 78.5%, respectively. Moreover, minimal residual disease negativity was achieved in 16.4% of patients in the D-VMP, and 15.4% of patients in the D-Rd cohorts. At a median follow up of 3.9 months (primary analysis), the VGPR rate was 71.6% in the D-VRd cohort, and the ORR was 97%. IRRs were observed in 7.5% of patients (15/199) across all cohorts, and the majority were grade 1 or 2, with most patients experiencing IRRs during first treatment administration. No unexpected TEAEs were identified with the Dara-SC combination therapies, with the most common grade 3 or 4 hematologic TEAE being neutropenia in the D-VRd (28.4%) and D-Rd (49.2%) cohorts and thrombocytopenia in the D-VMP (43.3%) cohorts. Common non-hematologic TEAEs included pneumonia in 3%, 7.5%, and 12.3% of patients in the D-VRd, D-VMP, and D-Rd cohorts, respectively. A Dara-SC + Kd arm for patients with RRMM (who have received only one prior line of therapy for MM which included at least two consecutive cycles of lenalidomide therapy) has been added to the PLEIADES trial, although results have not yet been reported.
As shown in Figure 1, the pivotal clinical trials of Dara-SC have demonstrated similar efficacy with significantly reduced rates of IRRs as compared with Dara-IV in comparable patient populations and treatment combinations. Although it is important to be cautious when making direct comparisons between clinical trials, the phase III COLUMBA study showed Dara-SC monotherapy to be non-inferior to Dara-IV monotherapy. This finding is consistent with the Dara-SC combination therapies in PLEAIDES, demonstrating comparable outcomes to previous clinical studies of Dara-IV.
Subcutaneous daratumumab in systemic light-chain (AL) amyloidosis
Dara-SC is also being studied in systemic light-chain (AL) amyloidosis, a rare plasma-cell disorder characterized by the deposition of insoluble amyloid fibrils into tissues and organs which leads to progressive organ dysfunction. Although bortezomib-based therapies, such as CyBorD (cyclophosphamide, bortezomib, and dexamethasone), have improved outcomes in AL amyloidosis, better therapies are needed.20,21 Moreover, the 1000 ml volume of the first infusion of Dara-IV can be problematic in patients with both cardiac and renal amyloid who can be fluid overloaded at baseline. Primary results from the ongoing, randomized phase III ANDROMEDA study [ClinicalTrials.gov identifier: NCT03201965] evaluating Dara-SC in combination with CyBorD (DARA-CyBorD) in patients with newly diagnosed AL amyloidosis were recently presented.22,23 A total of 388 patients were enrolled, with a median age of 63, and the majority of patients having multiorgan involvement. The primary endpoint of the study was overall hematologic complete response (CR) rate. At a median duration of follow up of 11.4 months, the hematologic CR rate was 53% versus 18% in the DARA-CyBorD group compared with CyBorD alone (OR 5.1; 95% CI: 3.2–8.2; p < 0.0001). Additionally, the VGPR rate was 79% versus 49% in patients receiving DARA-CyBorD versus CyBorD, respectively (OR 3.8; p < 0.0001). Moreover, responses with DARA-CyBorD were achieved more rapidly as compared with CyBorD alone, with median time to CR being 85 days versus 60 days. DARA-CyBorD also delayed major organ deterioration PFS (defined as hematologic progression, end-stage cardiac or renal disease, or death; hazard ratio 0.58; 95% CI 0.37–0.93; p = 0.023). DARA-CyBorD had an acceptable safety profile consistent with that previously observed for Dara-SC and CyBorD. Systemic IRRs occurred in only 7% of patients, all were grade 1 or 2, and the majority occurred at first administration. Overall, initial results from the ANDROMEDA study suggest that DARA-CyBorD significantly improves outcomes for patients with AL amyloidosis compared with CyBorD alone.
Clinical considerations with subcutaneous daratumumab
Bodyweight
In contrast to the weight-based dosing of the intravenous formulation of daratumumab, Dara-SC is administered at a fixed dose. The relationship between PK and patient bodyweight was thus assessed in the COLUMBA and PLEAIDES studies. In the phase III COLUMBA study, the bodyweight-based subgroup analysis showed that Dara-SC achieved adequate exposure consistent with that of Dara-IV regardless of bodyweight.14 Patients in the lightest subgroup (⩽65 kg) did have about a 60% higher mean maximum Ctrough and those in the heaviest subgroup (>85 kg) had about a 12% lower mean maximum Ctrough with Dara-SC as compared with Dara-IV. The incidence of adverse events with Dara-SC versus Dara-IV was lower in the heaviest subgroup [51 (78%) of 65 patients versus 54 (89%) of 61 patients] and higher in the lightest subgroup [88 (95%) of 93 patients versus 82 (89%) of 92 patients]. However, the safety profile overall was similar among the bodyweight subgroups. The incidence of IRRs with Dara-SC was lower than with Dara-IV, regardless of bodyweight. The one adverse event which differed by bodyweight was the incidence of neutropenia. In the Dara-SC group, any-grade neutropenia occurred in 26%, 15%, and 17% for the ⩽65 kg, 65–85 kg, and >85 kg subgroups, respectively. The overall incidence of any-grade neutropenia in patients receiving Dara-SC was 19%. Of note, baseline grade 2 neutropenia in the Dara-SC ⩽50 kg group was higher with Dara-SC (46%) compared with Dara-IV (19%), which may account for the difference in the higher neutropenia rate observed at lower bodyweights with Dara-SC (Janssen internal data). Importantly, the ORRs in all bodyweight subgroups were consistent with the overall population. In the heaviest subgroup (>85 kg), the ORR was 44%, which was similar to the ORR of 41% for all patients.
As in the COLUMBA study, the bodyweight subgroup analysis in the PLEAIDES study found the lowest bodyweight subgroups (⩽65 kg) had higher Ctrough, and the heaviest bodyweight subgroups had lower Ctrough, with Dara-SC across the D-VRd, D-VMP, and D-Rd cohorts.16 However, the mean Ctrough at the end of weekly dosing was above the previously determined recommended target saturation for Dara-IV19 across all cohorts, and as discussed previously, the ORRs of the Dara-SC combination therapies in the PLEAIDES study were comparable with those with Dara-IV reported previously. Based on the results of the bodyweight subgroup analyses in COLUMBA and PLEAIDES, bodyweight does not appear to have a clinically significant effect on the safety or efficacy of fixed-dose Dara-SC.
Immunogenicity
Although rHuPH20 is a recombinant form of human protein, which is less likely to promote an immune response compared with non-human proteins, there remains a potential for antibody response.24 The clinical relevance of anti-drug antibodies can vary widely, however, and it is important to determine the effect of neutralizing antibodies on drug efficacy in clinical trials. The incidence of treatment-emergent rHuPH20 antibodies was 6–16% among patients in the PAVO, COLUMBA, and PLEAIDIES studies, which were all non-neutralizing. This is consistent with immunogenicity previously reported for the enzyme, and thus does not appear to have any clinically relevant effect.24
Practical considerations of subcutaneous daratumumab administration in the clinic
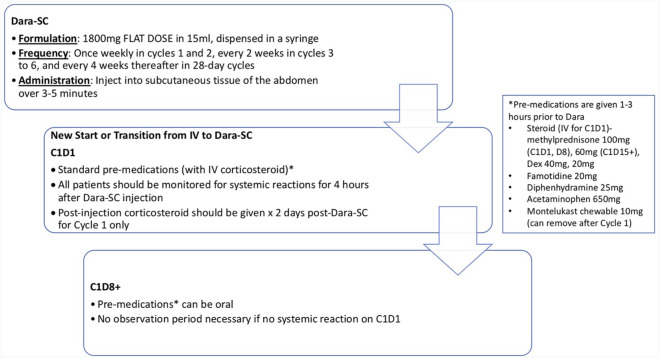
The dosing and administration schema for Dara-SC, as well as a suggested guideline for monitoring post-injection, is outlined in Figure 2. Dara-SC is given as an 1800 mg fixed-dose injection into the subcutaneous tissue of the abdomen over 3–5 min at the same schedule as Dara-IV. Pre-medications for Dara-SC remain the same as for Dara-IV, that is corticosteroid, famotidine, diphenhydramine, acetaminophen, and montelukast (in cycle 1). Given systemic reactions occurred in about 10% patients with the first injection of Dara-SC, falling to <1% with subsequent injections (mostly low grade), and the median time of reaction onset was 3.7 h (range: 9 min to 3.5 days) in clinical trials, we recommend monitoring for systemic reactions for 4 h after injection on cycle 1, day 1 for daratumumab-naïve patients. To date, there are no published studies on the transition of patients receiving Dara-IV to Dara-SC, although in our experience to date, there have been no IRRs with the institution of all standard pre-medications with the first Dara-SC administration. As we gain experience and understanding in clinical practice of IRRs with Dara-SC, observation time on cycle 1, day 1 of both daratumumab-naïve and patients transitioning Dara-IV to Dara-SC can be re-evaluated. If there were no IRRs in dose 1, subsequent cycle days do not likely require an observation period.
Figure 2.
Dosing and administration schema for subcutaneous daratumumab.
C1D1, cycle 1, day 1; C1D8+, cycle 1, day 8 and thereafter; Dara-SC, subcutaneous daratumumab; Dex, dexamethasone; IV, intravenous.
Given the very low rates of IRRs with Dara-SC, there is potentially a lesser need for pre- and post-dose corticosteroids with the subcutaneous formulation. Initial results of part 3 of the PAVO study, evaluating the safety of pre- and post-dose corticosteroid tapering during Dara-SC administration, were recently presented.25 In this study, patients received either a 3‑week or 2-week steroid tapering schedule. In the 3-week tapering schedule, pre-dose methylprednisolone was administered as 100 mg on cycle 1, day 1, 60 mg on cycle 1, day 8, and 30 mg on cycle 1, day 15, with the post‑dose methylprednisolone 20 mg for 2 days following cycle 1, day 1, and 1 day following cycle 1, day 8 and cycle 1, day 15. In the 2-week tapering schedule, pre-dose methylprednisolone was administered as 100 mg on cycle 1, day 1 and 60 mg on cycle 1, day 8, with the post‑dose methylprednisolone 20 mg for 2 days following cycle 1, day 1, and 1 day following cycle 1, day 8. The tapering schedules were assessed by a ‘3 + 3’ design followed by cohort expansion. A total of 30 patients were enrolled in the study (15 in each of the tapering cohorts). Overall, patients had received a median of two (two to seven) prior lines of therapy and 37% were refractory to both a PI and an IMiD. The duration of follow up was 7.7 months and 5.6 months for the 3‑week and 2‑week tapering cohorts, respectively. In each of the tapering cohorts, 53% of patients experienced a grade 3 or 4 TEAE, most commonly upper respiratory-tract infection, fatigue, and nausea. Importantly, no IRRs were reported in the 3-week group and in the 2-week group, three patients (20%) had an IRR which occurred on the first dose and were mostly grade 1 or 2. The ORR was 40%, consistent with the ORR in the COLUMBA trial. These results suggest that pre- and post-dose corticosteroids in RRMM patients receiving Dara-SC can be tapered rapidly without increased rates of IRRs while maintaining similar efficacy to Dara-IV. The ability to shorten the duration of steroid use will become particularly important when considering future combination studies with Dara-SC and novel therapies such as T-cell redirectors, chimeric antigen-receptor therapy, and checkpoint inhibitors, in which limiting steroids with these agents is preferable, given their potential impact on efficacy.
Conclusion
In summary, Dara-SC monotherapy demonstrated non-inferiority to Dara-IV monotherapy in the phase III COLUMBA study. Moreover, the addition of Dara-SC to VRd, VMP, and Rd in the PLEAIDES study has shown comparable response rates to previous clinical studies of Dara-IV in the same combinations. The PK and safety profiles of Dara-SC were also consistent across trials with that of Dara-IV and importantly, the incidence of IRRs was significantly lower with Dara-SC compared to Dara-IV.
Given the favorable benefit/risk profile of Dara-SC, the approval of Dara-SC in MM based on the results of the clinical trials discussed above will have a significant impact on clinical practice and healthcare resource utilization. There are currently two other anti-neoplastic agents approved for subcutaneous injection in combination with rHuPH20, trastuzumab and rituximab.26,27 Unlike subcutaneous rituximab which requires patients to have received at least one treatment of rituximab intravenously due to risk of hypersensitivity, Dara-SC can be administered as a subcutaneous injection with the first dose. Importantly, Dara-SC allows for much shorter administration times compared with its IV formulation, which is more convenient for both patients and healthcare providers and likely to improve patient compliance, as well as decrease cost. As seen in the COLUMBA study, administration of a modified Cancer Therapy Satisfaction Questionnaire showed that the subcutaneous group had consistently more positive perception and greater satisfaction with treatment than those in the intravenous group.14 Moreover, the lower incidence of IRRs seen with Dara-SC compared with Dara-IV will further reduce healthcare resource utilization given decreased need to manage infusion-related reactions. Other benefits of Dara-SC to consider may also be the potential for at-home administration beyond cycle 1 if no IRRs are observed. Such advantages will be very beneficial when considering needs such as minimizing risk of infection during transit or nosocomial acquisition, particularly in scenarios like the current COVID-19 global pandemic.
There are currently numerous ongoing studies of Dara-SC in both NDMM and RRMM (Table 3). The improved benefit/risk profile of the subcutaneous formulation of daratumumab will be particularly important in reducing treatment-related morbidity in vulnerable patient populations such as older adults with MM. Three phase II and III clinical trials are evaluating the use of Dara-SC in combination with IMiD and PI backbones in frail and older adult patients with NDMM [ClinicalTrials.gov identifiers: NCT03993912, NCT04052880, NCT04151667]. The phase III study PERSEUS [ClinicalTrials.gov identifier: NCT03710603] is evaluating the combination of Dara-SC with VRd versus VRd in patients with NDMM. As compared with the phase II study GRIFFIN (MMY2004) [ClinicalTrials.gov identifier: NCT02874742], which used the intravenous formulation, the use of Dara-SC in the phase III study PERSEUS may limit additional toxicity to patients treated with the quadruplet regimen. The phase III study AURIGA [ClinicalTrials.gov identifier: NCT03901963] is evaluating the use of Dara-SC + lenalidomide versus lenalidomide alone as maintenance therapy following frontline transplant. In the RRMM setting, Dara-SC in combination with pomalidomide and dexamethasone (Pd) versus Pd is being studied in the phase III APOLLO study [ClinicalTrials.gov identifier: NCT03180736]. Dara-SC is also being evaluated in combination with novel therapies such as bispecific T-cell redirection antibodies (TRIMM-2) [ClinicalTrials.gov identifier: NCT04108195]. TRIMM-2, a phase Ib study, will evaluate the safety and preliminary antitumor activity of Dara-SC in combination with talquetamab (which binds to GPRC5D) and teclistamab (which binds to BCMA). Although temporarily halted due to the global COVID-19 pandemic, LIGHTHOUSE is a phase III study which will aim confirm the preliminary efficacy and safety results of the phase I/II ANCHOR study [ClinicalTrials.gov identifier: NCT03481556] for the combination of melfulfen (a first-in-class anti-cancer peptide–drug conjugate that rapidly delivers an alkylating payload into tumor cells), Dara-SC, and dexamethasone in RRMM. Results of these and the other ongoing clinical trials with Dara-SC will undoubtedly open the door for the next wave of advancement in MM therapy.
Table 3.
Ongoing clinical trials of subcutaneous daratumumab in multiple myeloma.
| NCT number | Title | Phase | n | Recruitment |
|---|---|---|---|---|
| Newly diagnosed multiple myeloma | ||||
| NCT03993912 | A phase III study comparing lenalidomide and Dara-SC (R-Dara-SC) versus lenalidomide and dexamethasone (Rd) in frail subjects with previously untreated multiple myeloma who are ineligible for high dose therapy | III | 294 | Recruiting |
| NCT04052880 | A phase II study of Dara-SC in combination with dose-attenuated bortezomib, lenalidomide, and dexamethasone in elderly newly diagnosed multiple myeloma patients | II | 38 | Recruiting |
| NCT04151667 | Phase II study of daratumumab based response adapted therapy for older adults with newly diagnosed multiple myeloma | II | 32 | Recruiting |
| NCT03710603 | A phase III study comparing daratumumab, VELCADE (bortezomib), lenalidomide, and dexamethasone (D-VRd) versus VELCADE, lenalidomide, and dexamethasone (VRd) in subjects with previously untreated multiple myeloma who are eligible for high-dose therapy (PERSEUS) | III | 690 | Active, not recruiting |
| NCT03652064 | A phase III study comparing daratumumab, VELCADE (bortezomib), lenalidomide, and dexamethasone (D-VRd) with VELCADE, lenalidomide, and dexamethasone (VRd) in subjects with untreated multiple myeloma and for whom hematopoietic stem cell transplant is not planned as initial therapy | III | 395 | Active, not recruiting |
| NCT03901963 | A randomized study of daratumumab plus lenalidomide versus lenalidomide alone as maintenance treatment in patients with newly diagnosed multiple myeloma who are minimal residual disease positive after frontline autologous stem cell transplant (AURIGA) | III | 214 | Recruiting |
| NCT04497961 | Daratumumab versus lenalidomide maintenance therapy for multiple myeloma: a randomized pilot study comparing patient-reported health related quality of life measures | II | 100 | Not yet recruiting |
| Relapsed refractory multiple myeloma | ||||
| NCT03180736 | A phase III study comparing pomalidomide and dexamethasone with or without daratumumab in subjects with relapsed or refractory multiple myeloma who have received at least one prior line of therapy with both lenalidomide and a proteasome inhibitor (APOLLO) | III | 304 | Active, not recruiting |
| NCT03314181 | A phase I/II, multicenter, dose-escalation and expansion study of combination therapy with venetoclax, daratumumab and dexamethasone (with and without bortezomib) in subjects with relapsed or refractory multiple myeloma | II | 104 | Recruiting |
| NCT03871829 | A phase II study of Dara-SC administration in combination with carfilzomib and dexamethasone (DKd) compared with carfilzomib and dexamethasone (Kd) in participants with multiple myeloma who have been previously treated with Dara-IV to evaluate daratumumab retreatment | II | 230 | Recruiting |
| Novel combinations | ||||
| NCT04108195 | A phase Ib study of subcutaneous daratumumab regimens in combination with bispecific T-cell redirection antibodies for the treatment of subjects with multiple myeloma | II | 100 | Recruiting |
| NCT03837509 | A randomized open-label phase I/II study of INCB001158 combined with Dara-SC, compared with Dara-SC, in participants with relapsed or refractory multiple myeloma | I/II | 98 | Recruiting |
| Smoldering myeloma | ||||
| NCT03301220 | A phase III randomized, multicenter study of subcutaneous daratumumab versus active monitoring in subjects with high-risk smoldering multiple myeloma | III | 389 | Active, not recruiting |
Dara-IV, intravenous daratumumab; Dara-SC, subcutaneous daratumumab; NCT, National Clinical Trial.
Footnotes
Conflict of interest statement: LS: no relevant disclosures; JR: speaking fees from Celgene and Janssen, advisory board and consulting fees from Celgene, Janssen, Bristol Myers Squibb, Oncopeptides, Adaptive Biotechnologies, X4 Pharmaceuticals, Karyopharm, and Antegene; HJC: employed by the Multiple Myeloma Research Foundation, advisory board and consulting fees from Genetech, Celgene, Bristol Myers Squibb, GlaxoSmithKline and received research funding from Takeda, Celgene, and Genetech; SJ: advisory board and consulting fees from Celgene, Bristol Myers Squibb, Janssen Pharmaceuticals and Merck; DM: advisory board and consulting fees from Janssen, Celgene, Bristol Myers Squibb, Takeda, Legend, GlaxoSmithKline, Kinevant, and Foundation Medicine; SP: consulting fees from Foundation Medicine, research funding from Celgene and Karyopharm; SR: no relevant disclosures; LT: no relevant disclosures; DV: no relevant disclosures; AC: advisory board and consulting fees from Amgen, Antegene, Celgene, Janssen, Karyopharm, Millennium/Takeda, Novartis Pharmaceuticals, Oncopeptides, Sanofi; research funding from Amgen, Celgene, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Pharmacyclics.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Joshua Richter  https://orcid.org/0000-0002-0274-0585
https://orcid.org/0000-0002-0274-0585
Deepu Madduri  https://orcid.org/0000-0002-7305-9690
https://orcid.org/0000-0002-7305-9690
Ajai Chari  https://orcid.org/0000-0002-0405-7480
https://orcid.org/0000-0002-0405-7480
Contributor Information
Larysa Sanchez, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Joshua Richter, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Hearn Jay Cho, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Sundar Jagannath, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Deepu Madduri, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Samir Parekh, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Shambavi Richard, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Lowena Tam, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Daniel Verina, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Ajai Chari, Multiple Myeloma Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place, Box 1185, New York, NY 10029, USA.
References
- 1. Sanchez L, Wang Y, Siegel DS, et al. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol 2016; 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 3. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207–1219. [DOI] [PubMed] [Google Scholar]
- 4. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–766. [DOI] [PubMed] [Google Scholar]
- 5. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 6. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017; 130: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518–528. [DOI] [PubMed] [Google Scholar]
- 8. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019; 380: 2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019; 394: 29–38. [DOI] [PubMed] [Google Scholar]
- 10. DARZALEX®. (Daratumumab) injection for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc, 2019. [Google Scholar]
- 11. Chari A, Martinez-Lopez J, Mateos MV, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019; 134: 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamadeh IS, Reese ES, Arnall JR, et al. Safety and cost benefits of the rapid daratumumab infusion protocol. Clin Lymphoma Myeloma Leuk 2020; 20: 526–532.e1. [DOI] [PubMed] [Google Scholar]
- 13. Barr H, Dempsey J, Waller A, et al. Ninety-minute daratumumab infusion is safe in multiple myeloma. Leukemia 2018; 32: 2495–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol 2020; 7: e370–e380. [DOI] [PubMed] [Google Scholar]
- 15. Usmani SZ, Nahi H, Mateos MV, et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood 2019; 134: 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chari A, Rodriguez-Otero P, McCarthy H, et al. Subcutaneous daratumumab plus standard treatment regimensin patients with multiple myeloma across lines of therapy (PLEIADES): an open-label Phase II study. Br J Haematol. Epub ahead of print 30 July 2020. DOI: 101111/bjh16980. [DOI] [PubMed] [Google Scholar]
- 17. Bookbinder LH, Hofer A, Haller MF, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release 2006; 114: 230–241. [DOI] [PubMed] [Google Scholar]
- 18. Locke KW, Maneval DC, LaBarre MJ. ENHANZE® drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv 2019; 26: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu XS, Yan X, Puchalski T, et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin Pharmacol Ther 2017; 101: 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 2017; 129: 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015; 126: 612–615. [DOI] [PubMed] [Google Scholar]
- 22. Kastritis E, Palladini G, Minnema MC. Subcutaneous daratumumab+ cyclophosphamide, bortezomib, and dexamethasone (CyBorD) in patients with newly diagnosed light chain (AL) amyloidosis: primary results from the phase 3 ANDROMEDA study [LBA]. Presented at the 25th Annual European Hematology Congress: Virtual, 11–21 June 2020. Abstract #LB2604. [Google Scholar]
- 23. Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood 2020; 136: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosengren S, Dychter SS, Printz MA, et al. Clinical immunogenicity of rHuPH20, a hyaluronidase enabling subcutaneous drug administration. AAPS J 2015; 17: 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nahi H, Usmani SZ, Mateos MV, et al. Corticosteriod tapering in patients with relapsed or refractory multiple myeloma receiving subcutaneous daratumumab (DARA SC): part 3 of the open-label, multicenter, phase Ib PAVO Study. Presented at the 25th Annual European Hematology Congress: Virtual, 11–21 June 2020. Abstract #EP1038. [Google Scholar]
- 26. RITUXAN HYCELA® (Rituximab and hyaluronidase human) injection, for subcutaneous use. South San Francisco, CA: Biogen and Genentech USA, Inc, 2017. [Google Scholar]
- 27. HYLECTA HYLECTA® (Trastuzumab and hyaluronidase-oysk) injection, for subcutaneous use. South San Francisco, CA: Genentech USA, Inc, 2019. [Google Scholar]