Abstract
Aims.
To examine whether World Health Organization (WHO) risk level reductions in drinking were achievable, associated with improved functioning, and maintained over time among patients at varying initial alcohol dependence severity levels.
Design and setting.
Secondary data analysis of multisite randomized clinical trials: the US COMBINE Study and the UK Alcohol Treatment Trial (UKATT).
Participants.
Individuals with alcohol dependence enrolled in COMBINE (n=1383; 68.8% male) and seeking treatment for alcohol problems in UKATT (n=742; 74.1% male).
Interventions.
Naltrexone, acamprosate, or placebo, and combined behavioral intervention or medication management in COMBINE. Social behavior network therapy or motivational enhancement therapy in UKATT.
Measurements.
WHO risk level reductions were assessed via calendar method. Alcohol dependence was measured by the Alcohol Dependence Scale, the Leeds Dependence Questionnaire, and the Diagnostic and Statistical Manual of Mental Disorders. Measures of functioning included alcohol-related consequences (Drinker Inventory of Consequences and Alcohol Problems Questionnaire), mental health (Short Form Health Survey), and liver enzyme tests.
Findings.
One- and 2-level reductions in WHO risk levels in the last month of treatment were maintained at the 1-year follow-up [aOR(95% CI) 1-level reduction in COMBINE: 3.51 (2.73, 4.29) and UKATT: 2.65 (2.32, 2.98)] and associated with fewer alcohol-related consequences [e.g., B(95% CI) 1-level reduction COMBINE: −26.22 (−30.62, −21.82)], better mental health [e.g., B(95% CI) 1-level reduction UKATT: 9.53 (7.36, 11.73)], and improvements in γ-glutamyltransferase [e.g., B(95% CI) 1-level reduction UKATT: −89.77 (−122.50, −57.04)] at the end of treatment, even among patients with severe alcohol dependence. Results were similar when abstainers were excluded.
Conclusions.
Reductions in World Health Organization risk levels for alcohol consumption appear to be achievable, associated with better functioning, and maintained over time in both the United States and the United Kingdom.
Keywords: World Health Organization Drinking Risk Levels, Alcohol Use Disorder, Reduced Alcohol Consumption, Alcohol Treatment Outcomes, Low Risk Drinking, Alcohol Dependence
Introduction
The question of whether reductions in drinking (versus abstinence) are achievable and stable treatment outcomes for individuals with alcohol use disorder (AUD) has been debated extensively [1]. The European Medicines Agency (EMA) recommends abstinence as a primary endpoint and also accepts intermediate harm reduction endpoints, including reductions in total alcohol consumption, reduction in heavy drinking days, or reduction in World Health Organization (WHO) risk levels [2], which are defined by alcohol consumed per day (low risk: 1–40g for males/1–20g for females, medium risk: 41–60g for males/21–40g for females, high risk: 61–100g for males/41–60g for females, very high risk: 101+g for males/61+g for females)[3].
The inclusion of harm reduction endpoints is important because most individuals who receive treatment for AUD have difficulty sustaining abstinence [4–6] and many individuals with AUD prefer drinking reduction goals [7–11]. However, questions remain regarding whether non-abstinent drinking reductions can be achieved and maintained, particularly among individuals with more severe AUD[12,13].
WHO Risk Levels as a Drinking Reduction Target
Recent studies have shown that at least one- and two-level reductions in WHO risk levels are associated with significant improvements in how patients with AUD feel and function [14,15]. Furthermore, reductions in WHO risk levels are achieved by a majority of patients and are maintained for up to one-year following treatment [16]. The reductions in WHO risk levels are sensitive to medication effects [17–19] and associated with reduced risk of alcohol dependence, comorbid psychiatric/drug use disorders, and liver disease [20–23]. Yet, most recent studies of the WHO risk level reductions as endpoints have examined the endpoint in alcohol pharmacotherapy trials conducted in the US, leaving a knowledge gap about the utility of the WHO levels with nonpharmacological treatments and in other countries. Replicating the findings in non-US samples and in psychotherapy trials is important for understanding the generalizability of the WHO risk level reductions as treatment endpoints. Furthermore, previous work examining WHO risk level reductions [17–19] has not examined whether WHO risk level reductions are achieved, associated with functioning, and maintained over time among individuals with different levels of AUD severity.
To address these gaps in the literature, the current study examined whether the prevalence, functional outcomes, and maintenance of the WHO risk level reductions were replicated in a non-US psychotherapy trial, and extended previous research by examining WHO risk level reductions by initial levels of alcohol dependence severity in two samples: the US COMBINE pharmacotherapy trial study [24], and the United Kingdom (UK) Alcohol Treatment Trial (UKATT), a psychosocial treatment trial conducted in the UK [25]. The aims were to (1) estimate the prevalence of WHO risk level reductions, (2) test whether reductions in WHO risk levels in the last month of treatment were associated with improved functioning, (3) test whether reductions in WHO risk levels were maintained at one-year follow-up, and (4) evaluate whether initial alcohol dependence severity moderated the associations between reductions in WHO risk levels and functional outcomes and maintenance of WHO risk level reductions over time. We hypothesized that one- and two-level reductions in WHO risk levels would be achieved, associated with improved functional outcomes, and maintained over time in both the COMBINE and UKATT samples. Based on prior research showing that low risk drinking is more likely among individuals with lower alcohol dependence severity [12,26–28], we hypothesized reductions in WHO risk levels would be more likely to be achieved, associated with improved functional outcomes, and maintained over time among those with lower initial alcohol dependence severity.
Methods
Participants and procedures
The data for the current study were from the COMBINE and UKATT studies[24,25].
COMBINE.
Participants in COMBINE (n=1383) met the criteria for alcohol dependence based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) [29], and had at least 2 heavy drinking days in a consecutive 30-day period within the 90 days prior to the baseline assessment. Exclusion criteria included comorbid psychiatric diagnoses, illicit drug dependence, and any medical conditions in which naltrexone or acamprosate were contraindicated. Participants were recruited from 11 sites and randomized to one of nine treatment conditions based on receipt of: 1) active naltrexone (100 mg/day) or placebo naltrexone, 2) active acamprosate (3000 mg/day) or placebo acamprosate, and 3) medication management (MM) or combined behavioral intervention (CBI) with MM, or 4) CBI only. Treatment occurred over 16 weeks. Follow-up assessments were completed up to one-year post-treatment. Participants were male (68.8%) and non-Hispanic White (76.7%) [Black (7.9%), Asian (0.3%), Hispanic (11.2%), American-Indian/Alaskan Native (1.3%), multi-racial (1.3%), “other” race (1.2%)], with average age of 44.4 years (SD=10.2).
UKATT.
Inclusion criteria for UKATT were being over age 16, seeking help for alcohol problems, and being literate in English. Exclusion criteria included comorbid psychiatric diagnoses, severe cognitive impairment, and residential instability. Patients (n=742) were recruited from 7 sites and randomized to receive 4 sessions of motivational enhancement therapy or 12 sessions of social behavior and network therapy. Treatment occurred over 12 weeks. Follow-up assessments were completed at the end of treatment and one-year after trial entry. Participants were mostly male (74.1%), with average age of 41.6 (SD=10.1). Nearly all were non-Hispanic White (95.6%); other races included Asian (2.2%), Black (1.3%), “other” (0.9%).
Measures
Alcohol consumption.
WHO risk levels were measured using the timeline follow-back method [30] and calculated based on the average number of grams of alcohol consumed per day over one-month time periods. We calculated the WHO risk level at baseline (month prior to the screening), at end of treatment (month 4 in COMBINE and month 3 in UKATT), and at one-year follow-up (the month prior to the one year follow-up). Complete drinking data were available from 93.1% (end of treatment) and 79.5% (one-year follow-up) of participants in COMBINE and 92.9% (end of treatment) and 79.1% (one-year follow-up) of participants in UKATT.
We calculated two binary variables representing at least a one-level or at least a two-level reduction in the WHO risk levels from baseline to the last month of treatment and from baseline to the one-year follow-up. For the one-level reduction, the reference group included those with no change or an increase in the WHO risk level from baseline to the treatment/follow-up months. For the two-level reduction, the reference group included those with a one-level reduction, no change, or increase in the WHO risk level from baseline to the treatment/follow-up months.
Functioning at end of treatment.
Liver function was assessed in both COMBINE and UKATT by liver enzyme tests of alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT), with lower levels of ALT and GGT associated with better health outcomes [31].
Alcohol-related consequences were assessed by the Drinker Inventory of Consequences (DrInC) [32] in COMBINE and the Alcohol Problems Questionnaire (APQ) [33] in UKATT. Reliabilities of the DrInC and APQ exceeded Cronbach’s α=0.80. Mental health was assessed using the 12-item Short Form Health Survey (SF-12)[34] in COMBINE and the 36-item Short Form Health Survey (SF-36) in UKATT[35]. We used T-scores (with average functioning of 50 and standard deviation of 10) for the SF-36 and SF-12 mental health subscale, with higher scores indicating better mental health functioning. The reliability of the Short Form Health Survey items exceeded Cronbach’s α=0.80.
Alcohol dependence severity.
Initial alcohol dependence severity was measured at the baseline assessment using expected a posteriori scores derived from a moderated nonlinear factor analysis (MNLFA)[36]. MNLFA is a form of integrative data analysis that can be used to combine items from different measures that are theoretically measuring the same construct [37,38]. Items were included from the Alcohol Dependence Scale (ADS)[39] and DSM-IV[29] in COMBINE and from the Leeds Dependence Questionnaire (LDQ)[40] in UKATT. The alcohol dependence severity MNLFA scores, which are standardized across samples [36], align with alcohol dependence criteria counts and, despite higher average MNLFA scores in UKATT (t(df)=−7.29 (2110), p<0.001), they have comparable distributions across studies (Figure 1).
Figure 1.
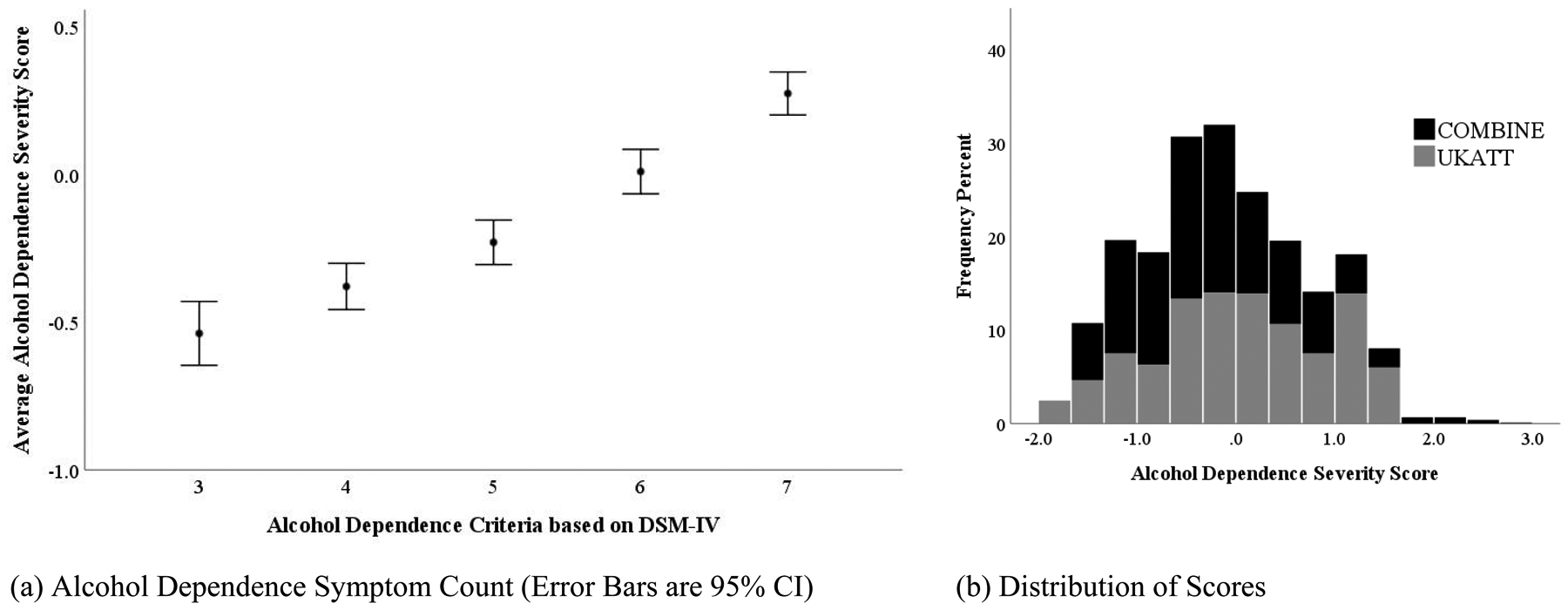
The Relationship of Alcohol Dependence Severity Expected A Posteriori Scores Derived from Moderated Nonlinear Factor Analysis [36] with DSM-IV Alcohol Dependence Criteria Count (panel a) and Distribution of Alcohol Dependence Severity Expected A Posteriori Scores in the COMBINE and UKATT Studies (panel b)
Note. Alcohol dependence severity was measured using expected a posteriori scores (similar to z-scores that are standardized across studies) derived from a moderated nonlinear factor analysis (MNLFA) of measures from COMBINE and UKATT [36].
We also examined cutoffs for alcohol dependence severity obtained from clinician ratings (COMBINE) and cutoff scores for the LDQ (UKATT). In COMBINE, clinician-rated severity of alcohol dependence on the Structured Clinical Interview for DSM-IV (SCID)[41] was used with ratings of “mild”, “moderate”, or “severe.” For UKATT, we used cutoffs derived from the LDQ[42], with LDQ scores 0–15=“mild”, 16–23=“moderate”, and 24–30=“severe”.
Statistical analysis
Frequency statistics were used to examine the prevalence within each study of achieving WHO risk level reductions and maintenance of WHO risk level reductions by dependence severity categories of “mild”, “moderate”, and “severe”. Linear regression models were used to examine the effects of end of treatment WHO risk level reductions in predicting end of treatment functioning (e.g., drinking consequences, ALT, GGT, mental health), with interaction terms used to determine if these results were moderated by initial alcohol dependence severity, as measured by continuous MNLFA factor scores. Logistic regression models were used to examine the probability of maintaining the WHO risk level reductions from the end of treatment to the one-year follow-up. Interaction terms in the logistic regression models were used to examine the odds of maintaining one- and two-level reductions at the one-year follow-up as a function of achieving one- and two-level reductions at the end of treatment, respectively, and whether dependence severity moderated these associations. All analyses were conducted using Mplus version 8.2 [43]. Missing outcome and drinking data were accommodated via multiple imputation with 50 imputed datasets and all studied variables were included in the imputation file [44–46]. Missing data ranged from 1% at baseline to 29% at the end of treatment. Sex, age, and initial WHO risk had no missing data and were included as covariates in all analyses. Clustering within sites was accommodated using the sandwich estimator in Mplus. We report exact p-values and, for consistency with prior analyses of the WHO risk levels [14–16], did not correct for multiple comparisons. The analyses and hypotheses were not pre-registered and that the results should be considered exploratory.
Sensitivity analyses.
We re-estimated all models excluding abstainers. These analyses provided a test of whether non-abstinent drinking reductions in the last month of treatment were associated with improvements in functioning and maintained over time.
Results
Descriptive Analyses
As shown in Table 1, at trial entry (baseline), the majority of individuals in both studies were in the very high risk level and none were abstinent. In the last month of treatment and at the one-year follow-up, most individuals were in the abstinent, low risk, or medium risk levels. Significantly more individuals in UKATT than COMBINE were high risk or very high risk in the last month of treatment and at one-year follow-up. Table 1 also presents descriptive statistics for all functioning measures by study. Participants in UKATT had significantly worse functioning on nearly all outcomes, including alcohol dependence severity, WHO risk levels, GGT, and mental health scores.
Table 1.
Descriptive Statistics for WHO Risk Levels and Functional Outcomes by Study
| Outcome | COMBINE | UKATT | χ2 / t-statistic |
|---|---|---|---|
| N (%) / M (SD) | N (%) / M (SD) | (df) | |
| WHO Risk Levela | |||
| At Trial Entry | 50.60 | ||
| Abstinence | 0 (0%) | 0 (0%) | (3), p<0.001 |
| Low risk | 45 (3.3%) | 41 (5.7%) | |
| Medium risk | 154 (11.1%) | 49 (6.8%) | |
| High risk | 354 (25.6%) | 110 (15.2%) | |
| Very high risk | 830 (60.0%) | 523 (72.3%) | |
| Last Month of Treatment | 241.48 | ||
| Abstinence | 459 (35.6%) | 119 (17.7%) | (4), p<0.001 |
| Low risk | 504 (39.1%) | 170 (25.3%) | |
| Medium risk | 126 (9.8%) | 80 (11.9%) | |
| High risk | 88 (6.8%) | 84 (12.5%) | |
| Very high risk | 111 (8.6%) | 219 (32.6%) | |
| One-year Post-Treatment | 66.45 | ||
| Abstinence | 366 (33.3%) | 121 (21.2%) | (4), p<0.001 |
| Low risk | 319 (29.0%) | 140 (24.5%) | |
| Medium risk | 135 (12.3%) | 65 (11.4%) | |
| High risk | 116 (10.6%) | 76 (13.3%) | |
| Very high risk | 163 (14.8%) | 170 (29.7%) | |
| Alcohol Dependence Severity Scoresb | |||
| At Trial Entry | −0.23 (0.79) | 0.04 (0.89) | −7.29 |
| (2110), p<0.001 | |||
| Alcohol Dependence Severity Cutoffsc | |||
| Mild | 228 (16.7%) | 365 (49.9%) | |
| Moderate | 782 (57.4%) | 214 (29.2%) | |
| Severe | 352 (25.8%) | 153 (20.9%) | |
| ALT (IU/L) | |||
| At Trial Entry | 40.14 (37.31) | 39.53 (42.85) | 0.33 (1918) |
| End of Treatment | 31.53 (24.40) | 36.80 (56.16) | −2.54 (1555), p=.01 |
| GGT (IU/L) | |||
| At Trial Entry | 74.50 (126.97) | 113.40 (216.80) | −0.49 (1919), p<0.001 |
| End of Treatment | 51.05 (142.28) | 97.80 (178.35) | −5.75 (1571), p<0.001 |
| Alcohol Related Consequences | DrInC (max. 124) | APQ (max. 60) | |
| At Trial Entry | 48.03 (20.44) | 26.24 (10.93) | |
| End of Treatment | 12.90 (18.69) | 16.08 (12.86) | |
| Short Form Health Survey Mental Health | |||
| At Trial Entry | 41.40 (11.00) | 31.36 (12.44) | 18.24 (1890), p<0.001 |
| End of Treatment | 49.50 (9.56) | 38.05 (14.29) | 19.29 (1613), p<0.001 |
Note. All numbers are observed (percentages are based on valid number of cases) with no imputation for missing data. ALT=alanine aminotransferase; GGT=γ-glutamyltransferase; IU/L=International Units per Liter; DrInC=Drinker Inventory of Consequences; APQ=Alcohol Problems Questionnaire.
WHO risk Levels: low risk: 1–40 g for males/1–20 g for females, medium risk: 41–60 g for males/21–40 g for females, high risk: 61–100 g for males/41–60 g for females, very high risk: 101+g for males/61+g for females
Alcohol Dependence Severity Expected a Posteriori Scores (range: −1.97 to 2.87), based on MNLFA representing standardized scores across studies with higher scores indicating greater severity dependence [36]
In COMBINE, clinician-rated “severity of alcohol dependence for the worst week of the past month” on the Structured Clinical Interview for DSM-IV (SCID) [41] was used with ratings of “mild”, “moderate”, or “severe.” For the UKATT study, we used cutoffs derived from the LDQ [42], with LDQ scores less than 15=“mild”, LDQ scores from 16–23=“moderate”, and LDQ scores from 24–30=“severe”.
The majority of patients in both studies reduced their drinking from baseline to the last month of treatment by at least one level (COMBINE: 88.5%; UKATT: 59.8%) and fewer reduced by at least two levels (COMBINE: 77.1%; UKATT: 46.1%). At one-year post-treatment most achieved at least one-level (COMBINE: 80.0%; UKATT: 63.3%) and fewer reduced by two levels (COMBINE: 66.3%; UKATT: 46.7%). We then examined WHO risk level reductions and maintenance of WHO risk level reductions by severity categories of “mild”, “moderate”, and “severe” alcohol dependence (Table 2). Within each study, prevalence of achieving and maintaining at least one- and two-level reductions were similar for the three severity categories. In COMBINE, the prevalence of achieving a two-level reduction was 77.4%, 79.0% and 73.7% (χ2(2)=3.20, p=.20) and the prevalence of maintaining this reduction was 80.5%, 77.4%, and 77.0% for “mild”, “moderate”, and “severe” dependence, respectively (χ2(2)=0.62, p=.73). In UKATT, the three severity groups were comparable in the prevalence for achieving (“mild”=47.8%, “moderate”=48.4%, “severe”=39.4%; χ2(2)=3.25, p=.20) and maintaining (“mild”=69.1%, “moderate”=68.8%, “severe”=71.1%; χ2(2)=0.07, p=.97) a two-level reduction.
Table 2.
WHO Risk Level Reductions and Maintenance of WHO Risk Level Reductions by Alcohol Dependence Severity Categories
| COMBINE | UKATT | |||||
|---|---|---|---|---|---|---|
| Outcome among those who achieved WHO Risk Level Reductions in the Last Month of Treatment | Mild Alcohol Dependence | Moderate Alcohol Dependence | Severe Alcohol Dependence | Mild Alcohol Dependence | Moderate Alcohol Dependence | Severe Alcohol Dependence |
| Total Sample | ||||||
| % Achieved | % Achieved | % Achieved | % Achieved | % Achieved | % Achieved | |
| At Least 1-Level Reduction Last Month of Treatment | 90.9% | 90.2% | 83.7% | 62.2% | 60.6% | 54.0% |
| Maintained at least 1-level reduction at 1 year | 88.1% | 85.0% | 85.6% | 79.0% | 80.0% | 79.3% |
| At Least 2-Level Reduction Last Month of Treatment | 77.4% | 79.0% | 73.7% | 47.8% | 48.4% | 39.4% |
| Maintained at least 2-level reduction at 1 year | 80.5% | 77.4% | 77.0% | 69.1% | 68.8% | 71.1% |
| Abstainers Excluded | ||||||
| % Achieved | % Achieved | % Achieved | % Achieved | % Achieved | % Achieved | |
| At Least 1-Level Reduction Last Month of Treatment | 85.2% | 84.7% | 74.3% | 54.1% | 52.6% | 43.2% |
| Maintained at least 1-level reduction at 1 year | 84.1% | 79.1% | 82.5% | 74.4% | 79.4% | 71.1% |
| At Least 2-Level Reduction Last Month of Treatment | 67.8% | 69.5% | 60.7% | 38.4% | 37.8% | 25.2% |
| Maintained at least 2-level reduction at 1 year | 71.0% | 68.0% | 72.7% | 63.5% | 68.9% | 55.6% |
Note. All percentages are based on the number of valid cases with no imputation for missing data.
Linear Regression Models
Next, we examined whether achieving at least one- and two-level reductions in WHO risk levels in the last month of treatment was associated with liver enzyme concentrations (GGT, ALT), alcohol-related consequences, and mental health at the end of treatment, and whether alcohol dependence severity interacted with WHO risk level reductions in predicting these outcomes. Results from the regression models are provided in Table 3. Unstandardized adjusted (controlling for covariates) regression coefficients can be interpreted as the change in outcomes based on achieving at least a one- and two-level reduction in the last month of treatment. For example, in COMBINE, at least a one-level reduction in WHO risk level, as compared to no change or an increase, corresponded to an average reduction of 26.22 for the score of alcohol-related consequences (B (SE)=−26.22 (2.22), p<0.001) on the DrInC and average improvement in mental health of 9.42 (B(SE)=9.42 (0.97) , p<0.001) on the SF-12. In both the COMBINE and UKATT studies, at least one- and two-level reductions were associated with significantly lower AST and lower GGT, greater mental health, and fewer alcohol-related consequences at end of treatment. Alcohol dependence severity was also significantly associated with alcohol-related consequences in COMBINE (DrInC score) and UKATT (APQ score) and mental health (SF-12 in COMBINE), such that greater dependence severity was associated with greater consequences and worse mental health.
Table 3.
Linear Regression Models Results for Functioning Outcomes at End of Treatment as Predicted from WHO One- and Two-Level Reductions in the Last Month of Treatment, Alcohol Dependence Severity Scores, and their Interaction
| COMBINE (n=1383) | UKATT (n=742) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome: | Outcome: | Outcome: | Outcome: Alcohol-related | Outcome: | Outcome: | Outcome: | Outcome: Alcohol-related | ||
| ALT (IU/L) | GGT (IU/L) | Mental health | Consequences | ALT (IU/L) | GGT (IU/L) | Mental health | Consequences | ||
| Predictors | (DrInC) | (APQ) | |||||||
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | ||
| At least one-level reduction last month of treatment | |||||||||
| 1+-level reduction | −12.75 (−20.56, −4.93), p=.001 | −64.94 (−94.18, −35.69), p<.001 | 9.42 (7.51, 11.33), p<.001 | −26.22 (−30.62, −21.82), p<.001 | −17.88 (−25.28, −10.48), p<.001 | −89.77 (−122.50, −57.04), p<.001 | 9.53 (7.36, 11.73), p<.001 | −10.38 (−11.57, −9.20), p<.001 | |
| Dependence severity | 3.00 (−4.14, 10.15), p=.41 | 26.63 (−10.94, 64.21), p=.17 | −2.77 (−4.93, −0.60), p=.01 | 6.43 (1.75, 11.11), p=.007 | −6.57 (−12.10, −1.03), p=.02 | −31.67 (−69.82, 6.47), p=.10 | −0.68 (−2.16, 0.79), p=.36 | 1.51 (0.39, 2.63), p=.008 | |
| Dependence severity × 1+-level reduction | −2.35 (−9.99, 5.29), p=.55 | −30.19 (−71.54, 11.17), p=.15 | 2.42 (0.01, 4.83), p=.049 | −6.35 (−11.75, −0.94), p=.02 | 4.24 (−0.57, 9.05), p=.08 | 24.34 (−13.64, 62.32), p=.21 | 0.06 (−1.65, 1.76), p=.95 | −0.33 (−1.56, 0.90), p=.60 | |
| At least two-level reduction last month of treatment | |||||||||
| 2+-level reduction | −10.91 (−16.59, −5.24), p<.001 | −49.62 (−70.56, −28.67), p<.001 | 8.16 (6.64, 9.67), p<.001 | −21.34 (−24.26, −18.41), p<.001 | −17.21(−23.37, −11.05), p<.001 | −86.69 (−112.81, −60.57), p<.001 | 9.74 (7.65, 11.82), p<.001 | −10.45 (−11.88, −9.02), p<.001 | |
| Dependence severity | 2.25 (−2.84, 7.35), p=.39 | 14.02 (−6.88, 34.92), p=.19 | −2.61 (−4.01, −1.21), p<.001 | 5.95 (3.02, 8.88), p<.001 | −5.34 (−9.33, −1.36), p=.009 | −27.89 (−60.10, 4.32), p=.09 | −0.52 (−2.21, 1.17), p=.54 | 1.09 (0.05, 2.12), p=.04 | |
| Dependence severity × 2+-level reduction | −1.74 (−7.02, 3.54), p=.52 | −17.80 (−41.89, 5.93), p=.14 | 2.54 (0.93, 4.14), p=.002 | −6.45 (−9.86, −3.04), p<.001 | 2.51 (−2.47, 7.48), p=.32 | 20.82 (−11.06, 52.71), p=.20 | 0.08 (−2.07, 2.24), p=.94 | −0.07 (−1.24, 1.10), p=.91 | |
Note. B (SE)=Unstandardized adjusted regression coefficients (95% CI=95% confidence intervals), which can be interpreted as the decrease in outcomes based on achieving at least a 1- or 2-level reduction, at the average of all covariates (age, sex, initial WHO risk level, initial level of the outcome); ALT=alanine aminotransferase; GGT=γ-glutamyltransferase; IU/L=International Units per Liter. DrInC=Drinker Inventory of Consequences; APQ=Alcohol Problems Questionnaire. The reference group for the one-level reduction was no change or an increase in the WHO risk level from baseline to the last month of treatment, and the reference group for the two-level reduction was the one-level reduction, no change, or increase in the WHO risk level from baseline to the last month of treatment. Negative values indicate an improvement in the outcome variable, except mental health where it indicated a worsening. Positive values indicate a worsening in the variable except mental health where it indicates an improvement. All analyses controlled for age, sex, initial WHO risk level, and initial level of the outcomes.
Initial alcohol dependence severity interacted with WHO-level reductions at the end of treatment in the prediction of alcohol-related consequences and mental health in COMBINE. Simple slopes analyses, using SCID severity categories to probe the interactions, indicated that as the level of dependence severity increased, at least one- and two-level reductions in WHO risk in the last month of treatment corresponded to larger reductions in alcohol-related consequences (DrInC scores) and greater improvements in mental health (SF-12) at the end of treatment, compared to the referent group (at least one-level reduction predicting DrInC scores: low severity: B (SE)=−16.44 (4.20); moderate severity: B (SE)=−25.00 (2.51); severe severity: B (SE)=−30.55 (4.76) and SF-12 scores: low severity: B (SE)=5.69 (2.71); moderate severity: B (SE)=9.31 (1.47); severe severity: B (SE)= 10.64 (1.94); at least two-level reduction predicting DrInC scores: low severity: B (SE)=−12.99 (2.63); moderate severity: B (SE)=−19.36 (1.85); severe severity: B (SE)=−25.77 (3.48) and SF-12 scores: low severity: B (SE)=4.85 (1.59); moderate severity: B (SE)=7.84 (1.04); severe severity: B (SE)= 9.26 (1.54)).
Logistic Regression
Results from the logistic regression models indicated that a reduction in WHO risk levels in the last month of treatment was significantly associated with at least one- and two-level reductions in WHO risk levels at one-year follow-up in both studies (Table 4). Achieving at least a one- or two-level reduction in the last month of treatment was associated with over 3.2 times greater odds of maintaining at least a one- or two-level reduction at one-year follow-up in COMBINE and over 2.6 times greater odds of maintaining at least one- or two-level reductions in UKATT. Among individuals who achieved at least a one-level reduction by the end of treatment, 85.5% and 84.4% reported at least a one-level reduction at the one-year follow-up in COMBINE and UKATT, respectively. Among individuals who achieved a least a two-level reduction by the end of treatment, 77.8% and 77.7% reported at least a two-level reduction at the one-year follow-up in COMBINE and UKATT, respectively.
Table 4.
Logistic Regression Models Predicting WHO One- and Two-Level Reductions at One-Year Follow-up from WHO One- and Two-Level Reductions in the Last Month of Treatment, Alcohol Dependence Severity Scores, and their Interaction
| Predictor | COMBINE (n=1383) | UKATT (n=742) |
|---|---|---|
| aOR (95% CI) | aOR (95% CI) | |
| Outcome: At least one-level reduction at one-year follow-up | ||
| Sex | 1.07 (0.84, 1.30), p=.57 | 0.75 (0.56, 0.94), p=.02 |
| Age | 1.00 (0.99, 1.01), p=.88 | 1.00 (0.99, 1.01), p=.95 |
| WHO risk level at trial entry | 1.05 (0.91, 1.19), p=.47 | 1.09 (0.97, 1.21), p=.14 |
| Dependence severity | 1.12 (0.83, 1.40), p=.40 | 0.89 (0.72, 1.05), p=.19 |
| 1+-level reduction in last month of treatment | 3.51 (2.73, 4.29), p<.001 | 2.65 (2.32, 2.98), p<.001 |
| Dependence severity × 1+-level reduction in last month of treatment | 0.89 (0.66, 1.13), p=.40 | 1.09 (0.74, 1.44), p=.59 |
| Outcome: At least two-level reduction at one-year follow-up | ||
| Sex | 1.04 (0.82. 1.25), p=.74 | 0.80 (0.57, 1.03), p=.13 |
| Age | 1.00 (0.99, 1.01), p=.92 | 0.99 (0.99, 1.01), p=.70 |
| WHO risk level at trial entry | 0.92 (0.82, 1.03), p=.17 | 1.21 (1.05, 1.38), p=.005 |
| Dependence severity | 1.11 (0.93, 1.29), p=.22 | 1.01 (0.89, 1.14), p=.84 |
| 2+-level reduction in last month of treatment | 3.23 (2.55, 3.92), p<.001 | 2.67 (2.42, 2.92), p<.001 |
| Dependence severity × 2+-level reduction in last month of treatment | 0.88 (0.72, 1.04), p=.16 | 0.95 (0.66, 1.23), p=.72 |
Note. aOR (95% CI)=adjusted odds ratios (95% confidence interval). Alcohol dependence severity score was grand mean centered within study. The reference group for the one-level reduction was no change or an increase in the WHO risk level from baseline to the treatment/follow-up months, and the reference group for the two-level reduction was the one-level reduction, no change, or increase in the WHO risk level from baseline to the treatment/follow-up months. All analyses controlled for age, sex, initial WHO risk level, and initial level of the outcomes.
Interaction tests indicated alcohol dependence severity was not associated with WHO risk level reductions at one-year follow-up in either COMBINE or UKATT. There was also no interaction between alcohol dependence severity and WHO risk level reductions in the last month of treatment in predicting the odds of maintaining at least one- or two-level reductions at one-year follow-up.
Sensitivity Analyses
Sensitivity analyses included only individuals who did not achieve abstinence in the last month of treatment (COMBINE: n=924; UKATT: n=604). In this subgroup, the effects of one- and two-level reductions on functional outcomes were smaller than when abstainers were included (Table 5). In particular, the effects of one- and two-level reductions on ALT in COMBINE were not significant with abstainers excluded, and the interaction between dependence severity and the one- and two-level reduction in WHO risk in predicting mental health in COMBINE was also not significant. However, the effects of one- and two-level reductions on ALT remained significant in UKATT, and in both the COMBINE and UKATT studies, at least one- and two-level non-abstinent reductions were associated with significantly better mental health, lower GGT, and fewer alcohol-related consequences at end of treatment, with no additional interactions with initial alcohol dependence severity.
Table 5.
Regression Models Results for Functioning Outcomes at End of Treatment as Predicted from One- and Two-Level Reductions In the Last Month of Treatment and Alcohol Dependence Severity Scores, Excluding Abstainers
| COMBINE (n=924) | UKATT (n=604) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome: | Outcome: | Outcome: | Outcome: Alcohol-related | Outcome: | Outcome: | Outcome: | Outcome: Alcohol-related | ||
| ALT (IU/L) | GGT (IU/L) | Mental health | Consequences | ALT (IU/L) | GGT (IU/L) | Mental health | Consequences | ||
| Predictors | (DrInC) | (APQ) | |||||||
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | ||
| At least one-level reduction last month of treatment, excluding abstainers | |||||||||
| One-level reduction | −5.99 (−14.13, 2.14), p=.15 | −47.34 (−77.75, −16.93), p=.002 | 8.09 (6.26, 9.92), p<.001 | −20.72 (−24.57, −16.87), p<.001 | −13.08 (−21.10, −5.05), p=.001 | −66.59 (−91.65, −41.53), p<.001 | 8.84 (6.75, 10.92), p<.001 | −8.96 (−10.22, −7.71), p<.001 | |
| Dependence severity | −0.67 (−9.50, 8.15), p=.88 | 12.48 (−30.03, 55.00), p=.57 | −2.80 (−5.07, −0.54), p=.02 | 4.67 (0.55, 8.80), p=.03 | −7.40 (−12.99, −1.80), p=.01 | −29.04 (−56.09, −1.99), p=.04 | −0.11 (−1.70, 1.49), p=.90 | 1.23 (0.06, 2.39), p=.04 | |
| Dependence severity × one-level reduction | 2.01 (−7.48, 11.49), p=.68 | −18.60 (−63.50, 26.30), p=.42 | 1.92 (−0.68, 4.52), p=.15 | −4.20 (−9.01, 0.61), p=.09 | 4.63 (−0.14, 9.39), p=.06 | 14.14 (−13.42, 41.69), p=.32 | −1.16 (−3.05, 0.72), p=.23 | −0.25 (−1.74, 1.24), p=.75 | |
| At least two-level reduction last month of treatment, excluding abstainers | |||||||||
| Two-level reduction | −5.18 (3.16) | −36.20 (12.94)** | 7.31 (5.54, 9.08), p<.001 | −17.63 (−20.85, −14.41), p<.001 | −12.11 (−20.59, −3.63), p=.005 | −59.37 (−84.18, −34.56), p<.001 | 8.85 (6.97, 10.73), p<.001 | −8.87 (−10.65, −7.09), p<.001 | |
| Dependence severity | 0.77 (3.28) | 4.88 (13.27) | −2.45 (−4.11, −0.78), p=.004 | 2.10 (0.85, 6.79), p=.01 | −6.16 (−10.31, −2.02), p=.004 | −25.62 (−47.71, −3.54), p=.02 | −0.14 (−2.05, 1.77), p=.89 | 0.87 (−0.12, 1.86), p=.09 | |
| Dependence severity × two-level reduction | 0.20 (3.67) | −11.20 (14.34) | 1.93 (−0.17, 4.04), p=.07 | −3.82 (−7.17, −0.48), p=.03 | 3.19 (−3.32, 9.70), p=.34 | 10.48 (−13.41, 34.37), p=.39 | −1.34 (−4.07, 1.39), p=.34 | 0.09 (−1.59, 1.76), p=.92 | |
Note. B (SE)=Unstandardized adjusted regression coefficients (95% CI=95% confidence intervals), which can be interpreted as the decrease in outcomes based on achieving at least a 1- and 2-level reduction, at the average of all covariates (age, sex, initial WHO risk level, initial level of the outcome); ALT=alanine aminotransferase; GGT=γ-glutamyltransferase; IU/L=International Units per Liter. DrInC=Drinker Inventory of Consequences; APQ=Alcohol Problems Questionnaire. The reference group for the one-level reduction was no change or an increase in the WHO risk level from baseline to the last month of treatment, and the reference group for the two-level reduction was the one-level reduction, no change, or increase in the WHO risk level from baseline to the last month of treatment. All analyses controlled for age, sex, initial WHO risk level, and initial level of the outcomes.
The results of the logistic regression models were consistent with prior models in which abstainers were included (see Table 2 and Table 6). In both COMBINE and UKATT, achieving at least a one- or two-level non-abstinent reduction during the last month of treatment was associated with approximately 2.5 times the odds of at least a one- or two-level reduction, respectively, at one-year post-treatment, with no differences by alcohol dependence severity (main effects and interactions non-significant).
Table 6.
Logistic Regression Models Predicting WHO One- and Two-Level Reductions at One-Year Follow-up from WHO One- and Two-Level Reductions in the Last Month of Treatment, Alcohol Dependence Severity Scores, and their Interaction, Excluding Abstainers
| Predictor | COMBINE (n=924) | UKATT (n=604) |
|---|---|---|
| aOR (95% CI) | aOR (95% CI) | |
| Outcome: At least one-level reduction at one-year follow-up | ||
| Sex | 1.04 (0.81, 1.23), p=.75 | 0.79 (0.60, 0.95), p=.05 |
| Age | 0.99 (0.99, 1.01), p=.62 | 1.00 (0.99, 1.01), p=.60 |
| WHO risk level at trial entry | 0.99 (0.84, 1.14), p=.92 | 1.08 (0.96, 1.21), p=.19 |
| Dependence severity | 1.18 (0.91, 1.45), p=.16 | 0.88 (0.73, 1.03), p=.13 |
| 1+-level reduction in last month of treatment | 2.80 (2.14, 3.46), p<.001 | 2.46 (2.08, 2.85), p<.001 |
| Dependence severity × 1+-level reduction in last month of treatment | 0.86 (0.64, 1.08), p=.23 | 1.08 (0.76, 1.40), p=.61 |
| Outcome: At least two-level reduction at one-year follow-up | ||
| Sex | 1.01 (0.80. 1.22), p=.96 | 0.82 (0.57, 1.03), p=.20 |
| Age | 1.00 (0.99, 1.01), p=.75 | 0.99 (0.99, 1.01), p=.55 |
| WHO risk level at trial entry | 0.91 (0.79, 1.04), p=.19 | 1.14 (1.01, 1.28), p=.02 |
| Dependence severity | 1.13 (0.93, 1.32), p=.18 | 1.03 (0.89, 1.16), p=.67 |
| 2+-level reduction in last month of treatment | 2.52 (1.96, 3.07), p<.001 | 2.66 (2.23, 3.09), p<.001 |
| Dependence severity × 2+-level reduction in last month of treatment | 0.87 (0.71, 1.04), p=.16 | 0.92 (0.66, 1.18), p=.56 |
Note.
p<0.05;
p<0.01;
p<0.001, aOR (95% CI)=adjusted odds ratios (95% confidence interval). Alcohol dependence severity score was grand mean centered within study. The reference group for the one-level reduction was no change or an increase in the WHO risk level from baseline to the treatment/follow-up months, and the reference group for the two-level reduction was the one-level reduction, no change, or increase in the WHO risk level from baseline to the treatment/follow-up months. All analyses controlled for age, sex, initial WHO risk level, and initial level of the outcomes.
Discussion
The current study replicated recent work [14–16] by demonstrating that at least one- and two-level reductions in WHO risk levels were commonly achieved, were associated with improved functional outcomes, and were maintained over time in a psychotherapy trial of individuals seeking treatment for alcohol problems in UK. The current study also extended recent findings by showing that in both US and UK samples the one- and two-level reductions that were achieved were associated with significant improvements in functioning and maintained over time even among individuals with higher initial levels of alcohol dependence severity.
Contrary to study hypotheses, alcohol dependence severity did not impact the majority of findings, with only two exceptions: as alcohol dependence severity increased among individuals in COMBINE, those who achieved at least one- and two-level reductions at the end of treatment had larger reductions in alcohol related consequences and greater improvements in mental health, as compared to those who did not achieve those reductions in WHO risk level. Results were also robust in sensitivity analyses that excluded abstainers from the models to focus on non-abstinent drinking reductions. However, the effects in these models were smaller, suggesting that abstinence was associated with better outcomes [47].
The results from the current study are consistent with prior work demonstrating the validity and maintenance of the WHO risk level reductions in clinical samples [14–16,19]. The current study makes an important new contribution by replicating prior analyses from US samples in the UKATT data and by extending this research to test whether WHO risk level reductions are associated with improvements in functional outcomes and maintained over time across the range of alcohol dependence severity.
The current study also had limitations. The data available in COMBINE and UKATT differed, requiring that we focus on measures that were similar across studies. For example, we previously found that the WHO risk level reductions correspond to improvements in systolic blood pressure and percent carbohydrate-deficient transferrin in COMBINE [14], but these measures were not assessed in the UKATT. In both studies the drinking data were obtained by verbal report, however both studies also included biomarkers (GGT and ALT), which corresponded to the self-reported drinking data [24,25]. Both samples excluded individuals with severe psychiatric comorbidity and UKATT excluded residentially unstable individuals. Whether the findings generalize to AUD patients with severe psychiatric symptoms is unknown, although epidemiological research finds WHO risk level reductions are associated with reduced risk for depressive/anxiety disorders [20]. Neither study randomized patients to drinking reduction versus abstinence and patients had a mix of abstinence and drinking reduction goals [7,10]. Studying whether drinking reductions are maintained and associated with improvements in outcomes among patients with varying initial treatment goals is an important area for additional research. We conducted multiple tests and did not correct for multiple comparisons. Importantly, substantive conclusions would be similar if we had corrected for multiple comparisons. Finally, the maximum consumption for the “low risk” WHO level is still above many guidelines for “low risk” drinking [48].
Despite these limitations, the current study extends recent work on the WHO risk level reductions in several ways. First, we extended the analysis of WHO risk levels to a clinical trial in the UK among participants with more severe initial dependence severity, mental health, and GGT than those in COMBINE. The UKATT study was defined as an effectiveness study, which increases the generalizability of the findings to real world treatment settings. Second, the findings indicate that WHO risk level reductions are similarly achieved and maintained among individuals with different levels of alcohol dependence severity and that clinically meaningful improvements in functional outcomes are also similarly achieved, even among those who were initially more severe. Third, these data suggest that measures of WHO risk level reduction are applicable as clinical trial endpoints for both pharmacotherapy and psychosocial interventions. Importantly, findings were also consistent when abstainers were excluded, thus ruling out the possibility that abstinence is the critical driver of functional improvement. Many individuals seeking treatment for alcohol problems prefer non-abstinence goals [7,8] and more individuals may become interested in treatment if drinking reduction goals are included as a treatment target[49,50]. Treatment providers may also help reduce harm caused by alcohol by talking with patients about drinking reductions, including non-abstinent reductions. The WHO risk levels can be used clinically to help patients in identifying drinking reduction goals and encourge more individuals to seek care, knowing that these reductions will have clinical value.
Acknowledgments.
In addition to the authors, the following individuals are or were members of the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup and provided intellectual input during attendance at Workgroup meetings: Joanne Fertig, Ph.D. and Megan Ryan, Ph.D., National Institute on Alcohol Abuse and Alcoholism; Tanya Ramey, M.D., Ph.D. and David McCann, Ph.D., National Institute on Drug Abuse; Didier Meulien, M.D., Lundbeck SAS; Anne Andorn, M.D. and Jay Graham, M.D., Indivior; Roger Meyer, M.D., Best Practice Project Management, Inc.; Henri-Jean Aubin, M.D., Paris-Sud Medical School; Charles O’Brien, M.D., Ph.D., University of Pennsylvania; Bernard Silverman, M.D., Alkermes, Inc.; Francoise Trinquet, M.D., Ethypharm; Benjamin Zakine, M.D., Ethypharm. Lindsay Snyder and Sarah Timm (ASCP) provided important administrative support to the ACTIVE workgroup.
Funding: This study was funded by the U.S. National Institute on Alcohol Abuse and Alcoholism (R01AA022328).
Disclosures. Dr. Kranzler is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. Dr. Mann received honoraria for consultancies from Pfizer. Dr. Hasin is Principal Investigator of a study funded by inVentiv Health Consulting that combines support from Actavis, Inc.; Endo Pharmaceuticals; Janssen Pharmaceuticals, Inc.; Mallinckrodt, LLC; Pfizer, Inc.; Purdue Pharma, L.P.; Rhodes Pharmaceuticals, L.P.; Roxane Laboratories, Inc.; and Zogenix, Inc. Dr. O’Malley reports being a consultant or an advisory board member of Alkermes, Amygdala, Indivior, Mitsubishi Tanabe, and Opiant, and a NIDA Clinical Trials Network DSMB member with honorarium from the Emmes Corporation and donated study medications from Astra Zeneca and Novartis. Dr. Anton has been a consultant in recent past for Insys, Allergan, and Life Epigenetics, has received honorarium from Alkermes for grant reviews and currently has grant funding from Laboratorio Farmaceutica CT. Drs. Witkiewitz, Kranzler, Mann, Hasin, Falk, Litten, O’Malley, and Anton are members of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative (ACTIVE Group), which over the time that this paper was developed was supported by Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Ethypharm, Indivior, Lundbeck, Mitsubishi, and Otsuka. Drs. Heather, Falk, and Litten have no disclosures.
Contributor Information
Katie Witkiewitz, Department of Psychology, University of New Mexico, Albuquerque NM, USA.
Nick Heather, Northumbria University, Newcastle upon Tyne, United Kingdom.
Deborah S. Hasin, Department of Epidemiology, Columbia University, New York NY, USA.
Henry R. Kranzler, Department of Psychiatry, University of Pennsylvania, Philadelphia PA, USA.
Karl F. Mann, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Stephanie S. O’Malley, Yale School of Medicine, New Haven, CT, USA.
Raymond F. Anton, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
References
- 1.Sobell MB, Sobell LC. Controlled drinking after 25 years: how important was the great debate? Addiction 1995;90(9):1149–53; discussion 1157–77. [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). International guide for monitoring alcohol consumption and related harm. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 3.European Medicines Agency. Guideline on the development of medicinal products for the treatment of alcohol dependence. United Kingdom: European Medicines Agency; 2010. [Google Scholar]
- 4.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol 1971;27(4):455–6. [DOI] [PubMed] [Google Scholar]
- 5.Maisto SA, Hallgren KA, Roos CR, Witkiewitz K. Course of remission from and relapse to heavy drinking following outpatient treatment of alcohol use disorder. Drug Alcohol Depend 2018;187:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewitz K Lapses following alcohol treatment: modeling the falls from the wagon. J Stud Alcohol Drugs 2008;69(4):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMartini KS, Devine EG, DiClemente CC, Martin DJ, Ray LA, O’Malley SS. Predictors of pretreatment commitment to abstinence: results from the COMBINE study. J Stud Alcohol Drugs 2014;75(3):438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haug S, Castro RP, Eggli P, Schaub MP. Drinking goal trajectories and their association with client characteristics and outcomes among clients in outpatient alcohol treatment. Subst Use Misuse 2018;53(13):2140–51. [DOI] [PubMed] [Google Scholar]
- 9.Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, et al. A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel v1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology 2017;42(5):1012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heather N, Adamson SJ, Raistrick D, Slegg GP, UKATT Research Team. Initial preference for drinking goal in the treatment of alcohol problems: I. Baseline differences between abstinence and non-abstinence groups. Alcohol Alcohol 2010;45(2):128–35. [DOI] [PubMed] [Google Scholar]
- 11.Hodgins DC, Leigh G, Milne R, Gerrish R. Drinking goal selection in behavioral self-manage- ment treatment of chronic alcoholics. Addict Behav 1997;22(2):247–55. [DOI] [PubMed] [Google Scholar]
- 12.Berglund KJ, Rauwolf KK, Berggren U, Balldin J, Fahlke C. Outcome in relation to drinking goals in alcohol-dependent individuals: A follow-up study 2.5 and 5 years after treatment entry. Alcohol Alcohol 2019;54(4):439–45. [DOI] [PubMed] [Google Scholar]
- 13.Gitlow S Guest Editorial Comment: End-point in AUD Treatment. ASAM Wkly 2019;Available from: https://www.asam.org/asamw-editorial-comment/asam-weekly-editorial-comment/2019/02/25/guest-editorial-comment-end-point-in-aud-treatment [Google Scholar]
- 14.Witkiewitz K, Kranzler HR, Hallgren KA, O’Malley SS, Falk DE, Litten RZ, et al. Drinking risk level reductions associated with improvements in physical health and quality of life among individuals with alcohol use disorder. Alcohol Clin Exp Res 2018;42(12):2453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkiewitz K, Hallgren KA, Kranzler HR, Mann K, Hasin DS, Falk DE, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 2017;41(1):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkiewitz K, Falk DE, Litten RZ, Hasin DS, Kranzler HR, Mann KF, et al. Maintenance of World Health Organization risk drinking level reductions and posttreatment functioning following a large alcohol use disorder clinical trial. Alcohol Clin Exp Res 2019;43(5):979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Malley SS, Todtenkopf MS, Du Y, Ehrich E, Silverman BL. Effects of the Opioid System Modulator, Samidorphan, on Measures of Alcohol Consumption and Patient-Reported Outcomes in Adults with Alcohol Dependence. Alcohol Clin Exp Res 2018;42(10):2011–21. [DOI] [PubMed] [Google Scholar]
- 18.Aubin H-J, Reimer J, Nutt DJ, Bladström A, Torup L, François C, et al. Clinical relevance of as-needed treatment with nalmefene in alcohol-dependent patients. Eur Addict Res 2015;21(3):160–8. [DOI] [PubMed] [Google Scholar]
- 19.Falk DE, O’Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, et al. Evaluation of drinking risk levels as outcomes in alcohol pharmacotherapy trials. JAMA Psychiatry 2019;76(4):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox J, Scodes J, Wall M, Witkiewitz K, Kranzler HR, Falk D, et al. Reduction in non-abstinent WHO drinking risk levels and depression/anxiety disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 2019;197:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knox J, Wall M, Witkiewitz K, Kranzler HR, Falk DE, Litten R, et al. Reduction in non-abstinent World Health Organization (WHO) drinking risk levels and drug use disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 2019. ;201:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, et al. Change in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. The Lancet Psychiatry 2017;4(6):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knox J, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, et al. Reduction in nonabstinent WHO drinking risk levels and change in risk for liver disease and positive AUDIT-C Scores: Prospective 3-year follow-up tesults in the U.S. general population. Alcohol Clin Exp Res 2018;42(11):2256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006;295(17):2003–17. [DOI] [PubMed] [Google Scholar]
- 25.UKATT Research Team. Effectiveness of treatment for alcohol problems: findings of the randomised UK alcohol treatment trial (UKATT). BMJ 2005;331(7516):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuerbis A, Morgenstern J, Hail L. Predictors of moderated drinking in a primarily alcohol-dependent sample of men who have sex with men. Psychol Addict Behav 2012;26(3):484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witkiewitz K, Pearson MR, Hallgren KA, Maisto SA, Roos CR, Kirouac M, et al. Who achieves low risk drinking during alcohol treatment? An analysis of patients in three alcohol clinical trials. Addiction 2017;112(12):2112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polich J, Armor D, Braiker H. The Course of Alcoholism: Four Years After Treatment. Santa Monica CA: Rand; 1980. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed.; DSM-IV). 4th ed. Washington, DC, DC: Author; 1994. [Google Scholar]
- 30.Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. [Internet]. Totowa, NJ, US: Human Press; 1992. [Google Scholar]
- 31.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 32.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC). Project MA. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 33.Williams BT, Drummond DC. The Alcohol Problems Questionnaire: reliability and validity. Drug Alcohol Depend 1994;35(3):239–43. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83. [PubMed] [Google Scholar]
- 36.Witkiewitz K, Hallgren KA, O’Sickey AJ, Roos CR, Maisto SA. Reproducibility and differential item functioning of the alcohol dependence syndrome construct across four alcohol treatment studies: An integrative data analysis. Drug Alcohol Depend 2016;158:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer DJ, Hussong AM. Psychometric approaches for developing commensurate measures across independent studies: traditional and new models. Psychol Methods 2009;14(2):101–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussong AM, Curran PJ, Bauer DJ. Integrative data analysis in clinical psychology research. Annu Rev Clin Psychol 2013;9:61–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinner HA, Horn JL. Alcohol Dependence Scale (ADS) user’s guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- 40.Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C. Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 1994;89(5):563–72. [DOI] [PubMed] [Google Scholar]
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C.: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- 42.Heather N, Raistrick D, Tober G, Godfrey C, Parrott S. Leeds Dependence Questionnaire: New data from a large sample of clinic attenders. Addict Res Theory 2001;9:253–69. [Google Scholar]
- 43.Muthén LK, Muthén BO. Mplus users guide (Version 8). Los Angeles, C.A.: Muthén & Muthén; 2017. [Google Scholar]
- 44.Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res 2013;37(12):2152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallgren KA, Witkiewitz K, Kranzler HR, Falk DE, Litten RZ, O’Malley SS, et al. Missing data in alcohol clinical trials with binary outcomes. Alcohol Clin Exp Res 2016;40(7):1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witkiewitz K, Finney JW, Harris AHS, Kivlahan DR, Kranzler HR. Recommendations for the design and analysis of treatment trials for alcohol use disorders. Alcohol Clin Exp Res 2015;39(9):1557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamson SJ, Heather N, Morton V, Raistrick D, UKATT Research Team. Initial preference for drinking goal in the treatment of alcohol problems: II. Treatment outcomes. Alcohol Alcohol 2010;45(2):136–42. [DOI] [PubMed] [Google Scholar]
- 48.Shield KD, Gmel G, Gmel G, Mäkelä P, Probst C, Room R, Rehm J, Life-time risk of mortality due to different levels of alcohol consumption in seven European countries: implications for low-risk drinking guidelines. Addiction 2017; 112(9):1535–1544. [DOI] [PubMed] [Google Scholar]
- 49.Mann K, Aubin H-JH-J, Witkiewitz K. Reduced drinking in alcohol dependence treatment, what is the evidence? Eur Addict Res 2017;23(5):219–30. [DOI] [PubMed] [Google Scholar]
- 50.van Amsterdam J, van den Brink W. Reduced-risk drinking as a viable treatment goal in problematic alcohol use and alcohol dependence. J Psychopharmacol 2013;27(11):987–97. [DOI] [PubMed] [Google Scholar]


