Abstract
Background
Little is known about the long-term lung radiographic changes in convalescent COVID-19 patients, especially the severe cases.
Purpose
To prospectively assess pulmonary sequelae and explore the risk factors for lung fibrotic-like changes on six-month follow-up chest CT of survivors of severe COVID-19 pneumonia.
Materials and Methods
114 patients (80[70%] men; mean age, 54±12 years) were studied prospectively. Initial and follow-up CT scans were obtained on 17±11 days and 175±20 days respectively after symptom onset. Lung changes (opacification, consolidation, reticulation, and fibrotic-like changes) and CT extent scores (score per lobe, 0-5; maximum score, 25) were recorded. Patients were divided into two groups: group#1 presence and group#2 absence of CT evidence of fibrotic-like changes (traction bronchiectasis, parenchymal bands, and/or honeycombing) based on their six-month follow-up CT. Between-group differences were assessed by Fisher’s exact test, two-sample t-test or Mann-Whitney U test. Multiple logistic regression analyses were performed to identify the independent predictive factors of fibrotic-like changes.
Results
On follow-up CT, evidence of fibrotic-like changes was observed in 40/114 (35%) of patients (group#1), while the remaining 74/114 (65%) patients (group#2) showed either complete radiological resolution (43/114, 38%) or residual ground-glass opacification or interstitial thickening (31/114, 27%). Multivariable analysis identified age >50 years (odds ratio [OR]:8.5, 95%CI:1.9-38, p=.01), heart rate >100bpm at admission (OR:5.6, 95%CI:1.1-29, p=.04), duration of in-hospital stay ≥17 days (OR:5.5, 95%CI:1.5-21, p=.01), and acute respiratory distress syndrome (OR:13, 95%CI:3.3-55, p<.001), non-invasive mechanical ventilation (OR:6.3, 95%CI:1.3-30, p=.02) and total CT score ≥18 (OR:4.2, 95%CI:1.2-14, p=.02) on initial CT as independent predictors for lung fibrotic-like changes at 6 months.
Conclusions
Six-month follow-up CT showed lung fibrotic-like changes in more than one-third of patients who survived severe COVID-19 pneumonia. These changes were associated with an older age, acute respiratory distress syndrome, longer in-hospital stays, tachycardia, non-invasive mechanical ventilation and higher initial chest CT score.
See also the editorial by Wells, Devaraj, and Desai.
Summary
This prospective longitudinal study found that approximately one-third of participants showed chest CT findings with pulmonary fibrosis-like changes within 6 months after recovery from severe COVID-19 pneumonia.
Key Results
■ Approximately one third of participants (40/114, 35%) recovered from severe COVID-19 developed lung fibrotic-like changes within 6 months of disease onset.
■ Older age (>50 years old), acute respiratory distress syndrome and higher baseline CT lung involvement score (≥18 out of a possible score of 25) were associated with lung fibrotic-like changes.
■ Twenty-seven of 104(26%) patients had an abnormal DLCO at 6-month follow up, which more frequently occurred in patients with lung fibrotic-like changes than in patients without fibrotic-like changes.
Introduction
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic. By November 19, 2020, this disease has been found in more than 200 countries with 55,659,785 confirmed cases and has caused 1,338,769 deaths(1). Pathological studies (2, 3) have shown that COVID-19 causes injuries in multiple organs and tissues with extensive pulmonary involvement which is similar to the pathology found in other coronavirus infections (i.e. severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome infection (MERS-CoV).
Chest CT plays a crucial role in the diagnosis and follow-up of patients with COVID-19 pneumonia. Numerous studies have documented radiographic changes in the acute course of COVID-19, which range from mild to severe cases (4-7). Recent publications (8,9) have found that approximately 94% of hospitalized patients have persistent lung parenchymal findings on their discharge CT scans at. In addition, Liu et al (10) reported that lung opacities in 53.0% of mild COVID-19 cases resolved with no adverse sequelae within 3 weeks after discharge. Data from previous coronavirus infections (SARS-CoV and MERS-CoV) suggested that there may be substantial fibrotic consequences following COVID-19 patients (11-13). However, little is known about the long-term lung changes after COVID-19 infection. The purpose of this study was to evaluate pulmonary changes on six-month follow-up Chest CT scans and to explore the risk factors for lung fibrotic-like changes in patients who recovered from severe COVID-19 pneumonia.
Methods
Study design and participants
This prospective study obtained ethical approval from the Ethics Commission of Wuhan Jin Yin-tan Hospital and Wuhan Union Hospital. All participants remained anonymous, and written informed content was acquired. This trial was registered with the Chinese Clinical Trial Registry, ChiCTR2000038609.
We prospectively enrolled 114 severe COVID-19 patients who had been discharged from the hospital after treatment for COVID-19 as inpatients between December 25, 2019 and February 20, 2020 at our institutions (Wuhan Jin Yin-tan Hospital, n= 69; Wuhan Union Hospital, n=45, Figure 1).Throat swab samples were collected for confirmation of SARS-CoV-2 by RT-PCR (Sansure Biotech Inc., Changsha, China) as previously described (14, 15). The World Health Organization’s (WHO) interim guidance diagnostic criteria for adults with severe COVID-19 pneumonia were used(16). The discharge criteria were based on the sixth edition of the “Pneumonia Diagnosis and Treatment Plan for New Coronavirus Infection” in China (17).
Figure 1:
Participant flow diagram.
The medical records of each participant were reviewed by one of four physicians (YML, XYH, NL, and XJ, with 7, 5, 4 and 3 years of experience in thoracic radiology, respectively). Age, sex, underlying comorbidities, onset of symptoms, peak acute phase laboratory results and the treatments received by individual patients were recorded. The durations from the onset of disease to hospital admission and chest CT scan were reviewed. The Berlin definition of acute respiratory distress syndrome (ARDS) was used. (18).
The initial CT scans of each participant were done at admission. Within 1 week of the follow-up CT scans, 104 patients underwent standard pulmonary function testing (PFT) for maximum vital capacity (VCmax%), forced expiratory volume in 1s (FEV1%), forced vital capacity (FVC%), diffusion capacity of the lung for carbon monoxide (DLCO), and DLCO divided by the alveolar volume (DLCO/VA) measured in a single breath test. The results were compared with age- and sex-matched control subjects and reported as percentages of predicted values. Pulmonary diffusion was regarded as abnormal when DLCO was < 80% of the predicted value.
CT image acquisition and interpretation
The initial CT examinations were performed in the supine position using one of two CT scanners: SOMATOM Definition AS+ or SOMATOM Perspective (Siemens Healthineers, Forchheim, Germany). Non-contrast Chest CTs were performed with the acquisition from the thoracic inlet to the diaphragm. The following parameters were used: detector collimation widths of 64×0.6 mm or 128×0.6 mm; and a tube voltage of 120 kV. The tube current was regulated by an automatic exposure control system (CARE Dose 4D; Siemens Healthineers). Images of 62/114 (54%) patients were reconstructed with a slice thickness of 5mm and an interval of 5 mm. Images in 52/114 (46%) patients were reconstructed with a slice thickness of 1mm and an interval of 1mm. Images were reconstructed with a pulmonary B70F kernel and a mediastinal B30f kernel (SOMATOM Definition AS+), or pulmonary B80s kernel and a mediastinal B30s kernel (SOMATOM Perspective).
All 114 patients underwent follow-up CT examinations using the same scanners as the initial CT scans. Images of all patients were reconstructed with a slice thickness of 1mm and an interval of 1 mm. Prior to the prospectively planned 6-month follow up scan, 83 of 114 patients (73%) had CT scans at 3 months after symptom onset to monitor the evolution of their lung disease.
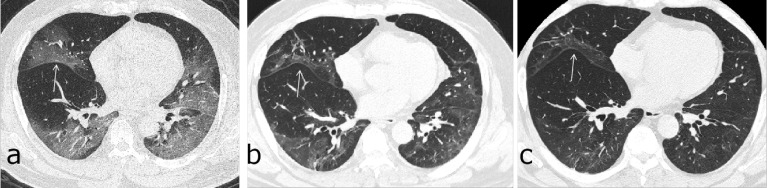
All CT images were reviewed in random order by three senior cardiothoracic radiologists (HSS, YQF, and JG, with 31, 13 and 10 years of experience in thoracic radiology, respectively) who were not aware of any clinical and laboratory findings or patient outcomes. The readers independently assessed the CT features using axial and multiplanar reconstructed images. The mediastinal window (center, 50; width, 350) and lung window(center, -600; width, 1200) were obtained from the picture archiving and communication system (Vue PACS, version 11.3.5.8902, Carestream Health, Canada). After independent evaluation, discussion and consensus resolved any disagreement. For each severe pneumonia patient, the predominant CT patterns according to the Fleischner Society glossary (19) were enumerated as follows: ground-glass opacities (GGO), consolidation, reticulation, emphysema, thickening of the adjacentpleura, pleural effusion, presence of nodules or masses, honey combing, bronchiectasis and interlobar pleural traction (retraction of the interlobar pleura toward the lesions). The CT evidence of fibrotic-like changes was defined as the presence of traction bronchiectasis, parenchymal bands(12, 20), and/or honeycombing(19)(Figure 2).
Figure 2:
Chest follow-up CT findings of COVID-19 pneumonia: (a) traction bronchiectasis; (b) parenchymal bands; (c) honeycombing; (d, e) thickening of the adjacent pleura.
To quantify the extent of pulmonary abnormalities (total lesions, GGO, consolidation, reticulation and fibrotic-like changes), a semiquantitative CT score (21) was assigned on the basis of the area involved in each of the five lung lobes: 0, no involvement; 1, < 5% 2, 5%-25%;3, 26%-49%; 4, 50%-75%;and 5, >75%. The total CT severity score was calculated by summing the individual lobar scores (possible scores range from 0 to 25).
Statistical analysis
The analyses were performed using SAS software (SAS, version 9.4, SAS Institute, Cary, NC, USA). The Kolmogorov–Smirnov test was used to assess the normality of continuous data. Normally and non-normally distributed data, and categorical variables are presented as the means (SD) and the medians (IQR), and numbers (%), respectively. Between-group differences in categorical variables were assessed using by Fisher’s exact test, and continuous variables with normally and non-normally distributed data were assessed using the two-sample t-test or Mann-Whitney U test, respectively. P-values for multiple univariate testing on acute phase data were adjusted by using the Benjamini and Hochberg method. A cutoff CT score value of 18 was selected as suggested by a recent investigation (22), which indicated that chest CT score ≥18 was correlated with the disease severity and increased mortality risk in patients with COVID-19 pneumonia. Multiple logistic regression analyses were performed to identify the independent predictive factors of fibrotic-like changes. The final model was determined using stepwise logistic regression, with significance level for selection set at p=.05. Factors associated with the CT score of fibrotic-like changes were analyzed by calculating the Spearman’s correlation coefficient. Statistical significance was considered at a p value < .05 (two-tailed).
Results
Demographics and patients’ characteristics
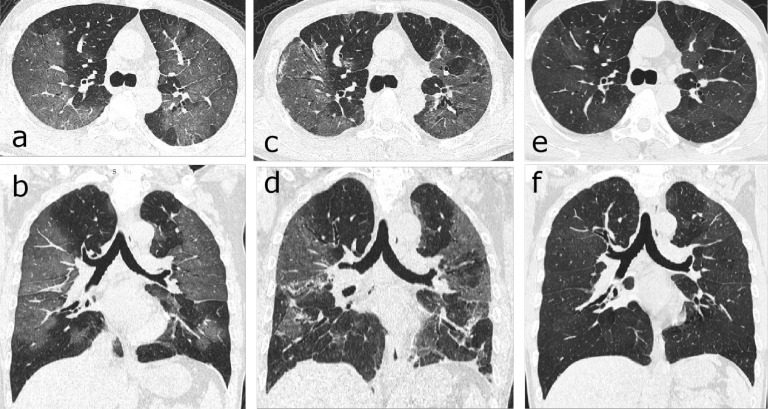
One hundred fourteen patients (80 men, 34 women; mean age, 54±12 years; age range, 24–82 years) were included (Table 1). The initial and follow-up scans were obtained on 17±11 days and 175 ±20 days after disease onset, respectively. Evidence of fibrotic-like changes was observed in 40/114 (35%) patients (group 1) on follow-up CT scans (Figure3), of which the proportion of patients with de novo fibrotic abnormalities was 38/40 (95%). The remaining 74/114 (65%) patients (group 2) showed either complete radiological resolution (43/114, 38%, Figure 4), or residual GGO or interstitial thickening (31/114, 27%, Figure 5).
Table 1:
Comparison of Demographics and Clinical Characteristics between Groups
Figure 3:
Serial CT scans of a 46-year-old woman with severe COVID-19 pneumonia. (a-c) The scan obtained on day 32 after symptom onset showed multiple ground-glass opacities (GGOs) and interstitial thickening with mild cylindrical traction bronchiectasis involving the middle lobe and lower lobe of the right lung. (d-f) The scan obtained on day 198 showed partial absorption of the abnormalities, reduced extension, traction bronchiectasis (thin arrows) and localized “honeycombing” (thick arrow) in the subpleural region of the right middle lobe.
Figure 4:
Serial CT scans of a 63-year-old man with emphysema and severe COVID-19 pneumonia. (a) The axial CT scan obtained on day 27 after onset of symptoms showed multiple ground-glass opacities (GGO) in the subpleural right lung. (b) The scan obtained on day 72 showed obvious absorption of the abnormalities. (c) The scan obtained on day 164 showed complete resolution.
Figure 5:
Serial CT scans of a 57-year-old man with severe COVID-19 pneumonia. (a, b) Axial and coronal thin-section CT scans obtained on day 9 after the onset of symptoms showed extensive ground-glass opacities (GGO) and interstitial thickening bilaterally. (c, d) Scans obtained on day 46 showed evolution to a mixed pattern of ground-glass opacities and consolidation with almost the same extent of lesions. (e, f) Scans obtained on day 159 showed a marked decrease in the density of GGO, with a slightly increased extension of the GGO (“tinted” sign or “melting sugar” sign, which defined as an imaging appearance of increased extension of the GGO or consolidation and decreased density).
After correction for multiple comparisons(Table 1), compared with group 2, patients in group 1 were significantly older (60±12 years vs 51±11 years, p=.003), had a higher heart rate at admission (HR, 96±16 bpm vs 87±12 bpm, p=.03)and greater incidence of acute respiratory distress syndrome (ARDS, 63% vs 8.1%, p<.001) and other comorbidities (73% vs 41%, p=.01), particularly chronic pulmonary disease (28% vs 6.8%, p=.02).The hospital stay was longer for patients in group 1 than patients in group 2 (27 days [IQR, 26] vs 15 days [IQR, 8]), p<.001. Regarding treatment, patients in group 1 were more likely to receive glucocorticosteroids (53% vs 20%, p=.01) and non-invasive mechanical ventilation (45% vs 8.1%, p<.001) than patients in group 2.
Comparison of peak laboratory findings
After correction for multiple comparisons (Table 2), the laboratory findings showed significantly higher peak levels of hypersensitive C-reactive protein (hsCRP, 80 mg/L [IQR, 124] vs 26 mg/L [IQR, 76], p=.03) and D-dimer (8.7 mg/L [IQR, 33] vs 1.0 mg/L [IQR, 1.5], p<.001) in group 1 than in group 2.
Table 2:
Comparison of Peak Laboratory Findings between Groups
Comparison of initial CT findings and scores
The initial CT scans were obtained on 17±11 days after the onset of symptoms, with no difference between the two groups (19±11 days vs 16±11 days, p=1.00, Table 3). The overall median total CT score was 15 [IQR, 9]. After correction for multiple comparisons (Table 3), patients in group 1 had significantly higher scores for total lesions (20 [IQR, 5.5] vs 13 [IQR, 7], p<.001),GGO (16 [IQR,10] vs 10 [IQR, 8], p=.02) than patients in group 2. Thickening of the adjacent pleura (55% vs 24%, p=.02, Figure 2) was more common in group 1.
Table 3:
Comparison of Initial CT Findings and Scores between Groups
Factors associated with lung fibrotic-like changes
The multivariable analysis identified an age>50 years(OR:8.5, 95% CI: 1.9-38 p=.01), HR>100bpm at admission (OR: 5.6, 95% CI: 1.1-29, p=.04), hospital stay ≥ 17 days (OR:5.5, 95% CI: 1.5-21, p=.01), ARDS (OR:13, 95% CI: 3.3-55, p<.001), noninvasive mechanical ventilation (OR: 6.3, 95% CI: 1.3-30, p=.02) and total CT score ≥ 18 (OR: 4.2 95% CI: 1.2-14, p=.02) on initial CT scans as independent predictors of lung fibrotic-like changes (Table 4).
Table 4:
Multivariable Analysis of Predictors of Lung Fibrotic-like Changes in Survivors of Severe COVID-19
Scores for fibrotic-like changes
According to the Spearman’s correlation analysis (Table E1 [Appendix E1]), the score for fibrotic-like changes was correlated with age (r=0.32, p<.001), HR at admission (r=0.24, p=0.01), hospital stay (r=0.49, p<.001), ARDS (r=0.57, p<.001), peak hsCRP level (r=0.37, p<.001), peak D-dimer level (r=0.59, p<.001), noninvasive mechanical ventilation (r=0.49, p<.001), total CT score (r=0.47, p<.001), and CT score for GGO (r=0.38, p<.001). Compared with the initial CT, a significant increase in the CT score for fibrotic-like changes (median, 0; range, 0-4; [IQR, 0] vs median, 0; range, 0-18; [IQR,4], p<.001) was observed in all patients on 6 months follow-up CT (Table E2 [Appendix E1]). In addition, the median score for fibrotic-like changes in patients in group 1 on 6 months follow-up CT was 6; range, 2-18; [IQR, 5].
Comparison of CT findings and scores between initial and follow-up scans
Significant decrease in the CT scores for total lesions (p<.001), GGO (p<.001), and consolidation (p<.001) were observed in all patients (Table E2 [Appendix E1]). Compared with the initial CT scans, the incidence rate of nodules or masses (17% vs 1.8%, p<.001), interlobar pleural traction (17% vs 7.9%, p=.04, Figure 6), pulmonary atelectasis (11% vs 3.5%, p=.02, Figure E1 [Appendix E1]) and bronchiectasis (24% vs 7.0%, p<.001) were significantly higher in the follow-up scans, while pleural effusion was completely resorbed (0 vs 6.1%, p=.01).
Figure 6:
Serial CT scans of a 52-year-old man with severe COVID-19 pneumonia. (a) The axial thin-section CT scan obtained on day 8 after symptom onset showed multiple ground-glass opacities (GGOs) bilaterally, with a slight traction of the right interlobar pleural (arrow). (b, c) Scans obtained on days 79 and 149, respectively, showed continuous absorption of previous opacifications, with the progression of interlobar pleural traction.
Time points of occurrence of fibrotic-like changes or complete resolution
In group 1, of 40 patients who exhibited lung fibrotic-like changes, 2/40 (5%) showed the fibrotic-like changes on initial CT scans, 17/37 (46%) patients who presented for follow up showed fibrotic-like changes at 3 months, and 22/40 (55%) showed these changes at 6 months of follow-up. In group 2, of 43 (58%) patients who demonstrated complete resolution of CT abnormalities, 20/43 (47%) patients who presented for follow up showed resolution at 3 months, and the remaining 23/43 (53%) showed resolution at the 6-month follow-up (Table E3 [Appendix E1]).
Follow-up findings
At the six-month follow-up (Table E4 [Appendix E1]), 7/114 (6.1%) of patients were still complaining of dry cough; 11/114 (10%) had expectoration and 16/114 (14%) experienced slight exertion dyspnea. Patients in group 1 with lung fibrotic-like changes more commonly experienced dry cough (p=.03) than patients in group 2. Of the 104 patients who underwent PFT, 27 (26%) presented with abnormal pulmonary diffusion (DLCO<80% predicted), with patients in group 1 more frequently presenting diffusion abnormalities than patients in group 2 (50% vs 13%, p<.001).
Discussion
In our study, 40/114 (35%) of patients who recovered from severe COVID-19 pneumonia developed lung fibrotic-like changes within 6 months in whom most of the fibrotic-like changes (55%, 22/40) presented on 6 months follow up CT. Using multivariable analysis, we found that age > 50 years (odds ratio [OR]: 8.5, 95% CI: 1.9-38 p=.01), heart rate > 100 bpm at admission (HR, OR: 5.6, 95% CI: 1.1-29, p=.04), duration of in-hospital stay ≥ 17 days(OR: 5.5, 95% CI: 1.5-21, p=.01), and ARDS (OR: 13, 95% CI: 3.3-55, p < .001), non-invasive mechanical ventilation (OR:6.3, 95% CI: 1.3-30, p=.02) and a total chest CT score ≥ 18 (OR: 4.2, 95% CI: 1.2-14, p=.02) on the initial CT scans were independent predictors of the subsequent development of lung fibrotic-like changes after six-months of follow-up.
Patients with lung fibrotic-like changes showed a higher incidence of ARDS (63%,25/40), which was also a predictor of fibrotic-like changes. Previous studies (23, 24) demonstrated that a substantial proportion of patients who survive ARDS may develop progressive fibrotic-like changes on CT scans. Nevertheless, it remains uncertain whether the fibrotic-like changes observed in this study represent true fibrotic lung disease (e.g. at pathology or on longer term follow-up CT). Whether or not these fibrotic-like changes, found at 6 months, reflect permanent change in the lung remains to be investigated. Additionally, the high frequency of non-invasive mechanical ventilation is another risk factor for the development of fibrotic-like changes at 6 months in our study. Based on previously published data (24), mechanical ventilation was strongly related to fibrotic-like changes observed after ARDS. Likewise, the lung fibrotic-like changes in our patients may also be associated with ventilator-induced lung injury. The laboratory results also demonstrated higher D-dimer and hsCRP levels in patients with pulmonary fibrotic-like changes. Emerging evidence of coagulopathy and an over exuberant inflammatory response has been reported in severe COVID-19 patients (25, 26), which are associated with disease severity and may also lead to greater damage to the pulmonary parenchyma.
We found that a higher CT score (≥18) on the initial CT was an independent prognostic factor for the presence of fibrotic-like changes on the 6 months follow up exam. According to a previous study on idiopathic pulmonary fibrosis (27), CT score was correlated with the degree of pulmonary fibrosis in pathological specimens. Moreover, a recent publicationof pulmonary fibrosis in pathological specimens. Moreover, a recent publication revealed an association between a CT score of ≥ 18 was associated with an increased mortality risk in COVID-19 patients (22). Therefore, a greater extent of lung injury in the acute phase may be associated with a higher mortality rate and more severe pulmonary sequelae in survivors. In addition, the correlations of scores for fibrotic-like changes with the aforementioned risk factors were also confirmed in our study.
At the six-month follow-up, a few patients still complained of ongoing respiratory symptoms, and 26% of patients had pulmonary diffusion abnormalities, which more frequently occurred in patients with fibrotic-like changes. Thus, both structural and functional lung impairments may simultaneously occur in patients who survive severe COVID-19 pneumonia. On the follow-up CT, significant decreases in CT scores for total lesions, GGO and consolidation were observed compared with the initial CT. Although the predominant CT pattern on follow up CT was still GGO, but the densities were visually decreased, which might follow the “tinted” sign (10) or “melting sugar” sign (28). Two studies (10, 28) reported an increased extension of the GGO or consolidation and a decreased density on follow up CT of COVID-19 pneumonia, which may indicate the gradual regression of the inflammation and re-expansion of the alveoli. GGO in the acute phase of COVID-19 pneumonia may represent the inflammatory infiltrates, edema or hemorrhaging (2, 3). Moreover, increased D-dimer levels in the acute phase was associated with pulmonary embolism in COVID-19 patients, which might also account for GGO appearance on the chest CT (29, 30), however, CT pulmonary angiography was not routinely performed in our patients to clarify this point. The pathophysiology underlying GGO in the convalescent phase of COVID-19 pneumonia and the correlation with fibrosis is worthy of further investigation.
Our study has several limitations. First, sample size was small and only 6 months of follow up. Patients with fibrotic-like changes require longer follow-up to determine whether the fibrotic-like changes are permanent, progressive or reversible. Second, the extent of lung fibrotic-like changes was not quantified by a computer-based analysis as described in previous study (31). However, we have supplied the semi-quantitative scores for the fibrotic-like changes, which were shown to be correlated with the degree of pulmonary fibrosis in pathological specimens. Third, the inter and intra reader comparison of CT grading was not performed. Fourth, the years of smoking was not evaluated in the present study. Fifth, 62/114 (54.4%) patients had a slice thickness of 5 mm in the initial scan, in which case subtle findings may be occult or overlooked. However, all follow up CT scans were performed with thin slices of 1 mm to assess the lung abnormalities. Finally, the lack of a histological correlation is a limitation. Further studies are warranted to explore whether fibrotic-like changes on CT scans represent true pathological fibrosis.
In summary, follow-up CT scans obtained within 6 months of disease onset showed lung fibrotic-like changes in more than one third of patients who survived severe COVID-19 pneumonia. These patients were older and had more severe disease during the acute phase. However, the long-term lung sequelae of these CT findings are still largely unknown. This report serves as a basis for new prospective large-scale long-term investigations analyzing these high-risk patients.
Acknowledgments
Acknowledgements
We would like to thank all colleagues for helping us during the current study and all the selfless volunteers who participated in the study. We highly appreciate Dr. Hongwei Jiang, PhD (Epidemiology & Biostatistics, Huazhong University of Science and Technology) for his assistance in statistical analysis. We also very grateful to many members of the frontline medical staff for their selfless dedication and heroic dedication in the face of this outbreak, despite the potential threat to their own lives and the lives of their families.
Footnotes
Xiaoyu Han and Yanqing Fan contributed equally to this work.
Funding This study was supported by the National Natural Science Foundation of China (grant numbers: 82071921), Zhejiang University special scientific research fund for COVID-19 prevention and control, and the Fundamental Research Funds for the Central Universities(2020kfyXGYJ019).
Availability of data and material The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate This study had ethics approval of the Ethics Commission of Wuhan Jin Yin-tan Hospital and Wuhan Union Hospital. All participants remained anonymous, and written informed content was acquired.
References
- 1.World Health Organization . Coronavirus disease 2019 (COVID-19) Situation report. https://covid19.who.int/. (Accessed 19 November 2020) [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine 2020;8(4):420-422. doi: 10.1016/s2213-2600(20)30076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, Trilling M, Lu M, Dittmer U, Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol 2020;92(5):491-494. doi: 10.1002/jmv.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases 2020;20(4):425-434. doi: 10.1016/s1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020;295(3):715-721. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandirali M, Sconfienza LM, Serra R, Brembilla R, Albano D, Pregliasco FE, Messina C. Chest Radiograph Findings in Asymptomatic and Minimally Symptomatic Quarantined Patients in Codogno, Italy during COVID-19 Pandemic. Radiology 2020;295(3):E7. doi: 10.1148/radiol.2020201102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, Dong L, Ma X, Zhang HJ, Gao BL. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 2020;80(4):394-400. doi: 10.1016/j.jinf.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Cao Y, Jiang N, Chen Y, Alwalid O, Zhang X, Gu J, Dai M, Liu J, Zhu W, Zheng C, Shi H. Novel Coronavirus Pneumonia (COVID-19) Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section CT Features During Recovery. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2020. doi: 10.1093/cid/ciaa271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020:200843. doi: 10.1148/radiol.2020200843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Zhang W, Pan F, Li L, Yang L, Zheng D, Wang J, Liang B. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res 2020;21(1):125. doi: 10.1186/s12931-020-01385-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, Chen L, Jiang M, Pan F, Zheng Y, Gao Z, Jiang B. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone research 2020;8:8. doi: 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology 2003;228(3):810- 815. doi: 10.1148/radiol.2283030726 [DOI] [PubMed] [Google Scholar]
- 13.Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, Larsson SG, Langer RD. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. The Indian journal of radiology & imaging 2017;27(3):342-349. doi: 10.4103/ijri.IJRI_469_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia Ja, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020;395(10223):507-513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Published on January 12, 2020.
- 17.General Office of National Health Committee . Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial sixth edition) 2020.2.18.http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml (Accessed 19 November 2020) [Google Scholar]
- 18.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008;246(3):697-722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 20.Westcott JL, Cole SR. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology 1986;161(3):665-669. doi: 10.1148/radiology.161.3.3786716 [DOI] [PubMed] [Google Scholar]
- 21.Chang Y-C, Yu C-J, Chang S-C, Galvin JR, Liu H-M, Hsiao C-H, Kuo P-H, Chen K-Y, Franks TJ, Huang K-M, Yang P-C. Pulmonary Sequelae in Convalescent Patients after Severe Acute Respiratory Syndrome: Evaluation with Thin-Section CT. Radiology 2005;236(3):1067-1075. doi: 10.1148/radiol.2363040958 [DOI] [PubMed] [Google Scholar]
- 22.Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, Panebianco V, Andreoli C, Colaiacomo MC, Zingaropoli MA, Ciardi MR, Mastroianni CM, Pugliese F, Alessandri F, Turriziani O, Ricci P, Catalano C. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol 2020. doi: 10.1007/s00330-020-07033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GU Meduri, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107(4):1062-1073. doi: 10.1378/chest.107.4.1062 [DOI] [PubMed] [Google Scholar]
- 24.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology 1999;210(1):29-35. doi: 10.1148/radiology.210.1.r99ja2629 [DOI] [PubMed] [Google Scholar]
- 25.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory medicine 2020. doi: 10.1016/s2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive care medicine 2020;46(6):1089-1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazerooni EA, Martinez FJ, Flint A, Jamadar DA, Gross BH, Spizarny DL, Cascade PN, Whyte RI, Lynch JP, 3rd, Toews G. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169(4):977-983. doi: 10.2214/ajr.169.4.9308447 [DOI] [PubMed] [Google Scholar]
- 28.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30(6):3306-3309. doi: 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud TC, Gommers D, van Dissel J, Ten Cate H, van Beek EJ. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology 2020:201629. doi: 10.1148/radiol.2020201629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thoma P, Rondelet B, Mélot C, Tack D, Naeije R, Gevenois PA. Acute pulmonary embolism: relationships between ground-glass opacification at thin-section CT and hemodynamics in pigs. Radiology 2009;250(3):721-729. doi: 10.1148/radiol.2503081134 [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Kim GH, Salisbury ML, Barber D, Bartholmai BJ, Brown KK, Conoscenti CS, De Backer J, Flaherty KR, Gruden JF, Hoffman EA, Humphries SM, Jacob J, Maher TM, Raghu G, Richeldi L, Ross BD, Schlenker-Herceg R, Sverzellati N, Wells AU, Martinez FJ, Lynch DA, Goldin J, Walsh SLF. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis. American journal of respiratory and critical care medicine 2019;199(1):12-21. doi: 10.1164/rccm.201803-0444PP [DOI] [PubMed] [Google Scholar]