Abstract
Background
Hereditary ataxia (HA) represents a group of genetically heterogeneous neurodegenerative diseases caused by dysfunction of the cerebellum or disruption of the connection between the cerebellum and other areas of the central nervous system. Phenotypic manifestation of HA includes unsteadiness of stance and gait, dysarthria, nystagmus, dysmetria and complaints of clumsiness. There are no specific treatments for HA. Management strategies provide supportive treatment to reduce symptoms.
Objectives
This systematic review aimed to identify, evaluate and summarise the published literature on the therapeutic roles of natural remedies in the treatment of HA to provide evidence for clinical practice.
Methods
A systematic literature search was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Web of Science, PubMed and Science Direct Scopus were thoroughly searched for relevant published articles from June 2007 to July 2020.
Results
Ten pre-clinical and two clinical studies were eligible for inclusion in this systematic review. We identified the therapeutic roles of medicinal plants Brassica napus, Gardenia jasminoides, Gastrodia elata, Ginkgo biloba, Glycyrrhiza inflata, Paeonia lactiflora, Pueraria lobata and Rehmannia glutinosa; herbal formulations Shaoyao Gancao Tang and Zhengan Xifeng Tang; and medicinal mushroom Hericium erinaceus in the treatment of HA. In this review, we evaluated the mode of actions contributing to their therapeutic effects, including activation of the ubiquitin–proteasome system, activation of antioxidant pathways, maintenance of intracellular calcium homeostasis and regulation of chaperones. We also briefly highlighted the integral cellular signalling pathways responsible for orchestrating the mode of actions.
Conclusion
We reviewed the therapeutic roles of natural remedies in improving or halting the progression of HA, which warrant further study for applications into clinical practice.
Keywords: Hereditary ataxia, Natural remedy, Medicinal plant, Herbal formulation, Medicinal mushroom
Background
Ataxia is defined as the loss of full control of bodily movements, and can be inherited, acquired, or occur sporadically [1]. Hereditary ataxia (HA) represents a group of genetically heterogeneous neurodegenerative diseases resulting from dysfunction of the cerebellum or disruption of connection between the cerebellum and other areas of the central nervous system [2]. Hereditary ataxia is characterised by pyramidal syndrome, movement disorder, seizure, peripheral neuropathy and cognitive dysfunction [3]. Furthermore, HA patients often present with unsteadiness of stance and gait, dysarthria, nystagmus, dysmetria and clumsiness [4]. Classification of the subtypes of HA is very challenging due to the many neurological and metabolic diseases associated with cerebellar dysfunction, and the phenotypic heterogeneity among the hereditary disorders [5]. Nevertheless, HA can be classified by the pattern of inheritance: autosomal dominant, autosomal recessive and X-linked ataxia [3].
Among the autosomal dominant cerebellar ataxias (ADCA), spinocerebellar ataxia (SCA) 3 is the most common subtype, followed by SCA1, 2, 6, 7 and 17, which are all caused by the expansion of polyglutamine-coding Cytosine-Adenine-Guanine (CAG) repeats [6]. Another ACDA subtype is episodic ataxia (EA), which include the common EA1 and 2 subtypes [2]. Among the autosomal recessive cerebellar ataxias (ARCA), Friedreich’s ataxia (FRDA) is the most common subtype, followed by ataxia-telangiectasia (AT), and ataxia oculomotor apraxia type 1 (AOA1) and type 2 (AOA2) [7]. The most common X-linked ataxia is fragile X-associated tremor ataxia syndrome (FXTAS). The age of onset varies according to types of HA, but onset actually helps in the diagnosis of HA: infancy onset suggests AT; childhood or teenage onsets suggest EA, FRDA, AOA1 and AOA2; and adult onset suggests SCA, FXTAS, or other autosomal recessive cerebellar ataxias [5]. Table 1 summarises the highly prevalent types of HA and their specific ethnic distributions, gene and locus of mutation, and clinical presentations.
Table 1.
Common forms of hereditary ataxia (HA)
| Hereditary ataxia | Ethnicity | Gene and locus of mutation | Clinical presentation | Neuroimaging finding | Neurometabolite finding | Biochemical finding | Reference | |
|---|---|---|---|---|---|---|---|---|
| Motor symptom | Non-motor symptom | |||||||
| Autosomal dominant | ||||||||
| SCA1 | Russian, Polish, Indian, Japanese and Eastern Siberian | ATXN1 on chromosome 6p22.3 | Gait ataxia, pyramidal, extrapyramidal and bulbar signs, dysarthria, nystagmus, chorea and peripheral neuropathy | Cognitive impairment and respiratory failure | Atrophy of the cerebellum, brainstem and cerebral frontal lobe |
Decreased glutamate, tNAA, NAA/Cr ratio and Cho/Cr ratio Increased glutamine, myoinositol and tCr |
Increased lactate | [6–12] |
| SCA2 | Cuban, Korean, South African and Indian | ATXN2 on chromosome 12q24.12 | Gait ataxia, pyramidal and extrapyramidal signs, dysarthria and dysphagia | Cognitive impairment, psychiatric symptoms, sleep disturbances and slow saccadic eye movements | Atrophy of cerebellum, brainstem, cerebral frontal lobe, spinal cord and cranial nerves III to XII |
Decreased glutamate, tNAA, NAA/Cr ratio and Cho/Cr ratio Increased myoinositol and tCr |
Decreased GSH, GST, SOD, CAT, zinc, iron, copper, erythropoietin, dopamine metabolites and ethanolamine Increased MDA |
[6, 8–14] |
| SCA3/MJD |
Portuguese, Japanese, Taiwanese, German and Brazilian |
ATXN3 on chromosome 14q32.12 | Gait ataxia, pyramidal and extrapyramidal signs, nystagmus, lid retraction, decreased saccade velocity, amyotrophy fasciculations and peripheral neuropathy | Mild cognitive impairment, sleep disturbances and involuntary weight loss | Atrophy of cerebellum, brainstem, cerebellar peduncle and cranial nerves III to XII |
Decreased glutamate, tNAA, NAA/Cr ratio, Cho/Cr ratio, tNAA/tCr ratio and Glx/tCr ratio Increased myoinositol and tCr |
Decreased GPx, l-Proline and l-Tryptophan Increased palmitoleic acid (FFA 16:1) and linolenic acid (FFA 18:3) |
[6, 7, 9–12, 15–17] |
| SCA6 | Japanese, American, German, Australian and Dutch | CACNA1A on chromosome 19p13.13 | Gait ataxia, pyramidal signs, dysarthria, nystagmus, intention tremor, dystonia and dysphagia | Diplopia | Atrophy of cerebellum |
Decreased NAA and NAA/Cr ratio Increased myoinositol |
Increased glucose, taurine and lactate | [6, 8, 10–12] |
| SCA7 | South African, Mexican and Scandinavian | ATXN7 on chromosome 3p14.1 | Gait ataxia, pyramidal signs, slow eye movements, dysmetria, hyperreflexia, dysarthria, dysphagia and ophthalmoplegia | Visual loss with retinopathy, cognitive impairment and psychiatric symptoms | Atrophy of cerebellum, brainstem, cerebral peduncle and spinal cord |
Decreased glutamate and tNAA Increased myoinositol and tCr |
Increased lipid hydroperoxides, MDA, protein carbonyls, GR, GPx and paraoxonase | [6, 9–11, 18, 19] |
| SCA17/HDL4 | Japanese, German and Italian | TBP on chromosome 6q27 | Gait ataxia, pyramidal and extrapyramidal signs, chorea and myoclonus | Cognitive impairment and psychiatric symptoms | Atrophy in cerebellum, brainstem and cerebrum | N/A | Decreased presynaptic dopamine transporters | [7, 12, 20, 21] |
| EA1 | Australian, Caucasian, American and Russian | KCNA1 on chromosome 12p13 | Gait ataxia (attack last from seconds to minutes), dysarthria, seizure, myokymia, neuromyotonic and tremor | Vertigo, difficulty in breathing and diaphoresis | Normal brain | N/A | Increased GABA release | [7, 22–24] |
| EA2 | Caucasian and Korean | CACNA1A on chromosome 19p13 | Gait ataxia (attack last from minutes to hours), dysarthria, seizure, nystagmus and dystonia | Vertigo, nausea, headache, diplopia, tonic upward gaze and cognitive impairment | Atrophy of cerebellum | Decreased Cr and high-energy phosphate ratio | Increased lactate | [7, 11, 22, 23, 25, 26] |
| Autosomal recessive | ||||||||
| FRDA | Caucasian, Middle East and North African | FXN on chromosome 9q21.11 | Gait ataxia, eye movement abnormalities, dysarthria, dysphagia, lower extremities weakness and peripheral neuropathy | Skeletal deformities, hypertrophic cardiomyopathy, diabetes, deafness and optic atrophy | Atrophy of cerebellum and cervical spinal cord |
Decreased NAA/Cr ratio and Cho Increased glutamine, myoinositol and tCr |
N/A | [7, 11, 27, 28] |
| AT | Iberian, Polish and Russian | ATM on chromosome 11q22.3 |
Gait ataxia, extrapyramidal signs, oculomotor apraxia, hypotonia, areflexia and peripheral neuropathy |
Oculocutaneous telangiectasia, immunodeficiency, cancer susceptibility, sterility, extreme radio sensitivity, diabetes and pulmonary diseases | Atrophy of cerebellum and brainstem |
Decreased NAA, Cho, NAA/Cho ratio and Cr Increased Cho and Cho/Cr ratio |
Decreased IgA, IgM, IgG2, IgE and absence of ATM protein Increased AFP |
[1, 7, 11, 29–31] |
| AOA1 | Japanese and Portuguese | APTX on chromosome 9p21.1 | Gait ataxia, extrapyramidal signs, oculomotor apraxia, areflexia, dysarthria, choreoathetosis, oculomotor apraxia and peripheral neuropathy | Cognitive impairment and skeletal deformities | Atrophy of cerebellum | N/A |
Decreased albumin level Increased creatine kinase and total cholesterol |
[7, 32, 33] |
| AOA2 | Eastern French | SETX on chromosome 9p34.13 | Gait ataxia, pyramidal and extrapyramidal signs, oculomotor apraxia, chorea, upper motor neuron signs, head tremor, strabismus and peripheral neuropathy | Mild cognitive impairment | Atrophy of cerebellum |
Decreased glutamate, NAA and NAA/Cho ratio Increased myoinositol |
Increased creatinine kinase, total cholesterol, AFP, IgG and IgA | [7, 11, 32, 34] |
| X-linked | ||||||||
| FXTAS | Caucasian | FMR1 on X chromosome | Gait ataxia, intention tremor, eye movement abnormalities and peripheral neuropathy | Cognitive impairment, psychiatric symptoms, hearing loss and dysautonomia | Atrophy of cerebellum and cerebrum | Decreased NAA | N/A | [35–37] |
AFP alpha fetoprotein, AOA ataxia oculomotor apraxia, AT ataxia-telangiectasia, Cho choline compounds, Cr creatine, EA episodic ataxia, FRDA Friedreich’s ataxia, FXTAS fragile X tremor-ataxia syndrome, GABA γ-aminobutyric acid, Glx glutamate + glutamine, GST glutathione S-transferase, GPx glutathione peroxidase, GR glutathione reductase, GSH glutathione, HDL4 Huntington disease-like 4, Ig immunoglobulin, MDA malondialdehyde, MJD Machado-Joseph disease, N/A not applicable, NAA N-acetylaspartate, NAAG N-acetylaspartatylglutamate, PCr phosphocreatine, SOD superoxide distmutase, SCA spinocerebellar ataxia, tCr total creatine (Cr + PCr), tNAA total NAA (NAA + NAAG)
Generally, HA is first diagnosed by assessing the history of the presenting condition, past medical history, and personal history, and then by neurological evaluation, systemic physical evaluation, and pertinent paraclinical tests. Laboratory tests can rule out acquired ataxia and identify biomarkers specific to the HA. Neuroimaging using magnetic resonance imaging (MRI) and computed tomography (CT) are useful to determine the presence of cerebellar atrophy or anomalies. After excluding acquired ataxia, the mode of inheritance is then evaluated. In this context, the age of onset and clinical manifestation can well-describe the HA and may provide crucial clue(s) to identify the mutated gene. Molecular genetic testing for HA with high prevalence such as SCA and FRDA is usually suggested before pursuing other extensive tests [21]. If single gene testing does not provide a molecular diagnosis, then high-throughput next-generation sequencing (NGS) such as multigene panel testing, whole-exome or whole-genome sequencing can be conducted to identify the mutated genes. Specialised genetic counselling and risk assessment can then be provided once the pathogenic mutation has been identified [5].
To date, there are no effective treatments for HA and current therapeutic strategies only focus on symptomatic and supportive management [38]. The complexity in the neurochemistry of HA suggests that there may be multiple therapeutic targets. For example, some pharmacological molecules have been used to target motor and non-motor symptoms associated with HA [39]: riluzole, amantadine and varenicline were demonstrated to reduce the deteriorating symptoms in various ADCA and ARCA [4]; acetazolamide and 4-aminopyridine (4-AP) were reported to be effective in reducing symptoms in EA; and acetyl-DL-leucine was found to improve gait variability in patients with various subtypes of cerebellar ataxia including SCA [40]. N-acetyl-L-leucine is currently being tested in a multinational clinical trial as a treatment for AT [41]. Nevertheless, many pharmacological molecules have yet to reach clinical trials or be translated into breakthrough treatments, mainly due to the limited pre-clinical studies [6]. More efforts are needed to discover safe therapeutic agents for HA [42].
Natural remedies have been used as complementary therapeutic agents for various neurodegenerative diseases [43, 44]. Natural remedies are a good source for the discovery of drugs or agents that have therapeutic potential. In addition, holistic practitioners commonly use natural remedies, as they are well accepted and relatively safe compared to synthetic drugs. One of the key distinguishing attributes of natural remedies is their affinity for the target protein or specific biomolecule in living organisms [44]. The distinct features of biological compounds including more chiral centres; more carbon, hydrogen and oxygen and less nitrogen; higher molecular weight and higher polarities compared to synthetic compounds [45]. Biological compounds also contain more sp3-hybrid carbon atoms allowing the tetrahedron carbons to form flexible chains or cyclic structures, whereas the multi-functional groups make them bind to the biological targets strongly and increase the additional interactions with biological molecules [46]. Natural metabolites with greater structural diversity, bioactivity and complexity are capable of becoming the substrate for one or more of the transporter systems and to be delivered to the targeted intracellular site of action [47].
Besides, with the recent shift in the mode of discovery from “one-target, one-drug” to “network-target, multiple-component-therapeutics”, many natural remedies or compounds with broad safety profiles have great potential as they have proven efficacy on multiple targets in different diseases [48]. The concept of multiple-target compounds involves the merging of active substructures or the fusion of two or more biological active moieties into one compound in the enhancement of biological activities [49]. Curcumin, resveratrol, quercetin, green tea catechins and salicylate are some of the well-studied biological compounds with multiple molecular targets and mechanism of action elucidated [50]. However, a disadvantage of biological compounds with multiple targets is the ability to bind to multiple proteins of unrelated structures or the unintended off-targets, which may result in potential toxicity and adverse effects [51]. Therefore, it is crucial to characterise and optimise the structure of biological compounds to boost their affinity against the disease-related targets and weaken the activity towards undesired adverse targets [50].
Chinese medicines or traditional Chinese medicines (TCM) have a long history of use in Chinese communities in East and Southeast Asia since ancient times. The basic concepts of TCM originated from the ancient naturalistic philosophies namely qi, yin yang and the five phases (wu xing) theory included wood (mu), fire (huo), earth (tu), metal (jin) and water (shui) [52]. TCM is a holistic approach as it focuses on the integrity of the human body and its close relationship with social and natural environment. Numerous natural therapeutic methods such as mind-spiritual methods (qi gong and tai chi) and natural methods (acupuncture, moxibustion and herbal medicine) were applied in TCM to maintain overall health and enhance body’s resistance to disease. The accumulation of empirical experience and collection of observational data in clinical settings are responsible in the development of TCM over the centuries [53].
Demand for TCM continues to grow in the Western world. Application of modern disciplines in TCM has resulted in the isolation and identification of novel and/or active compounds. Some of these were developed to produce new drugs for the treatment of important diseases such as malaria, vascular diseases and cancer which is deemed necessary to improve the quality of TCM materials. However, a majority of TCM has not been elucidated chemically and pharmacologically and most of the TCM-derived compounds were found not to be as active as the original TCM preparation. This points to the possibilities of synergism being involved [48]. Herbal formulation that contains two or more herbs in specific proportions have been used to treat a variety of symptoms in a wide range of diseases, such as forgetfulness, disorientation, insomnia, unconsciousness, cramps and seizures [54].
Therefore, it is a challenging task to develop evidence-based Chinese medicine and new lead compounds and scaffolds from TCM. Combined chemical and biological studies on the effective TCM have provided unprecedented opportunities for the development of new drugs and the modernisation of TCM. In such circumstances, many in vitro and in vivo studies have been conducted to explore the therapeutic effect of the natural remedies in HA. However, the therapeutic efficacy of these natural remedies has yet to be demonstrated or translated into clinical practice. Hence, this review aims to synthesise the evidence in pre-clinical and clinical research on natural remedies published from June 2007 to July 2020 to examine if these experimental studies may provide a mechanistic framework for developing treatments for HA towards clinical trials.
Methods
Search strategy
We conducted a systematic search of Web of Science, PubMed and Science Direct Scopus electronic databases for relevant articles published from June 2007 to July 2020. Google Scholar was used to identify any articles not included in the above databases. The search terms included a combination of the following keywords: algae, alternative medicine, ataxia, basidiomycete, complementary medicine, herbal, herbs, medicinal plants, medicinal mushrooms, mushrooms, natural products, plants and seaweed. The structured search strategy was designed to include all papers that evaluated the use of natural remedies as a treatment for HA published from June 2007 to July 2020.
Eligibility criteria
Studies were considered if they met the following inclusion criteria: (1) pre-clinical (in vitro and in vivo studies) and clinical studies, (2) study model of HA as the primary disorder, (3) full-text available and (4) articles published in English. The exclusion criteria included: (1) studies with ataxia as the secondary symptom, (2) studies targeting non-motor symptoms, (3) studies using chemicals to induce ataxia, (4) review articles, (5) conference abstracts or proceedings, (6) full-text not available and (7) articles published in another language other than English.
Data extraction and analysis
Collection and extraction of data were conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). Two researchers (M.W.L.P and S.Y.L) searched and independently reviewed all the relevant studies and selected the ones meeting the inclusion criteria. Disagreements on the eligibility or on the extraction of data were resolved through discussions between the two researchers. The other team members made the final decision if the two researchers could not reach an agreement. Due to inconsistencies in the methodology or experimental design in the retrieved studies, findings were extracted independently and narrated to the best of our ability.
Results
Study selection
The systematic literature search yielded 3113 results from the three databases. After eliminating duplicate studies, 1829 studies remained and were further evaluated by screening the titles and abstracts. The full-texts of 18 selected studies were retrieved for further assessment and evaluation. Six articles were excluded at this stage for various reasons including language, study design, not ataxia-specific, or ataxia was a secondary symptom. Overall, 12 studies (ten pre-clinical studies and two clinical studies) were eligible for inclusion in this systematic review. Figure 1 shows the PRISMA flow chart for the identification of relevant studies.
Fig. 1.
PRISMA flowchart for identification of relevant studies
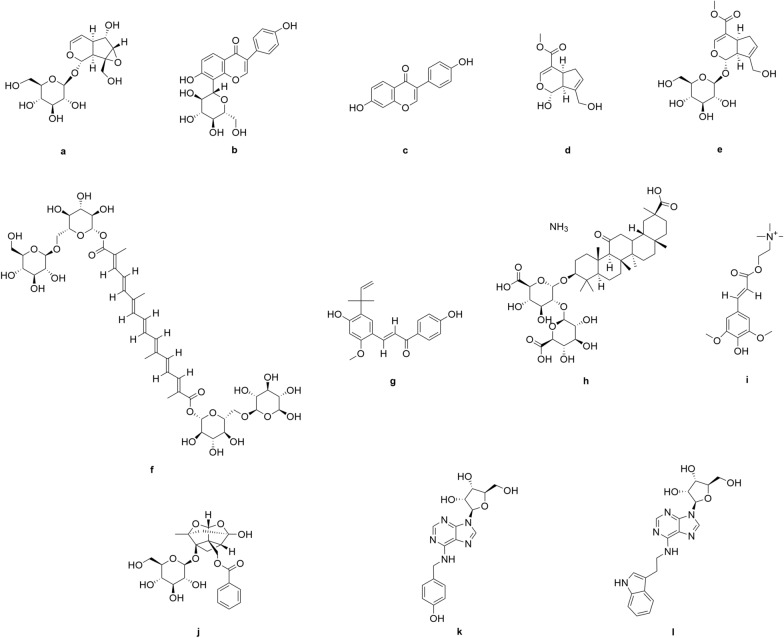
Based on the retrieved studies, we found several natural remedies, including medicinal plants, herbal formulations and medicinal mushrooms, had been investigated as treatments in only two subtypes of HA, SCA and FRDA. The list of natural remedies and their bioactive effects against HA are summarised in Table 2. The chemical constituents of the identified natural remedies are shown in Fig. 2; namely the catalpol (Fig. 2a) from Rehmannia glutinosa; puerarin (Fig. 2b) and daidzein (Fig. 2c) from Pueraria lobata; genipin (Fig. 2d), geniposide (Fig. 2e) and crocin (Fig. 2f) from Gardenia jasminoides; licochalcone A (Fig. 2g) and ammonium glycyrrhizinate (Fig. 2h) from Glycyrrhiza inflata; sinapine (Fig. 2i) in rapeseed pomace; and paeoniflorin (Fig. 2j) from Paeonia lactiflora; and T1-11 (N6-(4-Hydroxybenzyl) adenosine) (Fig. 2k) and JMF-1907 (N6-(3-Indolylethyl) adenosine) (Fig. 2l) from Gastrodia elata.
Table 2.
Natural remedies and their therapeutic effects
| Disorder | Natural Remedies | Compounds | Model | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|---|
| Medicinal plant | ||||||
| SCA3 |
Rehmannia glutinosa
|
Catalpol | HEK-293 and SH-SY5Y cells | Promoted proteasome activity, suppressed polyQ aggregation, prevented cell death | UPS | [55] |
|
Pueraria lobate
|
Puerarin and daidzein | iPSCs | Promoted proteasome activity, suppressed polyQ aggregation, prevented cell death | [56] | ||
|
Gastrodia elata
|
T1-11 and JMF-1907 | Mice | Promoted proteasome activity, prevented cell death, restored motor function | [57] | ||
|
Gardenia jasminoides
|
Genipin, geniposide and crocin | HEK-293 and SH-SY5Y cells | Promoted the expression of antioxidant genes, suppressed ROS level | NRF2 signalling pathway | [58] | |
|
Glycyrrhiza inflata
|
Licochalcone A and AMGZ | Promoted the expression of antioxidant genes, suppressed ROS level, suppressed polyQ aggregation | [59] | |||
|
Paeonia lactiflora
|
Paeoniflorin | Promoted expression of heat shock proteins, suppressed polyQ aggregation, restored motor function | N/A | [60] | ||
|
Brassica napus
|
Sinapine | Roundworm | Promoted expression of antioxidant genes, suppressed ROS level, restored motor function | N/A | [61] | |
| SCA17 |
Ginkgo biloba
|
N/A | SH-SY5Y cells and mice | Prevented calcium influx, prevented cell death | Calcium homeostasis | [62] |
| Herbal formulation | ||||||
| SCA6 | Zhengan Xifeng Tang | N/A | Human | Restored motor function | N/A | [63, 64] |
| SCA17 | Shaoyao Gancao Tang | N/A | HEK-293 and SH-SY5Y cells | Promoted expression of antioxidant genes, prevented cell death, restored mitochondrial biogenesis, restored motor function | N/A | [65] |
| Medicinal mushroom | ||||||
| FRDA |
Hericium erinaceus
|
N/A | Fibroblast | Prevented antioxidant depletion, prevented cell death | N/A | [66] |
SCA spinocerebellar ataxia, HEK-293 human embryonic kidney 293 cells, UPS ubiquitin–proteasome system, iPSCs induced-pluripotent stem cells, T1-11, N6-(4-Hydroxybenzyl) adenosine; JMF-1907, N6-(3-Indolylethyl) adenosine; NRF2 nuclear factor erythroid 2–related factor 2; AMGZ ammonium glycyrrhizinate; FRDA Friedreich’s ataxia; N/A not applicable
Fig. 2.
Chemical structures of the identified compounds with bioactive effects against HA
Discussion
Mode of action
Activation of ubiquitin–proteasome system (UPS)
In SCA, abnormal expansion of CAG repeats in respective coding regions of SCA leads to elongated translation of the polyglutamine (polyQ) tract. When the expansion reaches a specific threshold, it causes the abnormal configuration of the encoded polyQ protein, leading to protein aggregation and nuclear inclusions, a pathological hallmark of SCA [67]. The presence of polyQ aggregates are detrimental to cellular processes, and contributes to cellular dysfunction and death [68, 69]. The UPS plays a pivotal role in intracellular protein homeostasis by clearing misfolded and defective proteins [69]. Furthermore, UPS is one of the major regulators of cell cycle progression and apoptosis [70]. Evidence shows polyQ proteins are susceptible to ubiquitination and proteasome degradation [71–74]. However, it was shown that the accumulation of polyQ aggregates compromised the function of UPS in SCA [55–57] potentially through deubiquitylation [66], leading to further accumulation of aggregates that would otherwise have been removed. Therefore, restoration of UPS function using natural remedies may serve as a therapeutic strategy against polyQ-induced SCA.
In a study by Chen et al. [55], the anti-aggregation effects of catalpol derived from Rehmannia glutinosa, and daidzein and puerarin derived from Pueraria lobata were investigated in in vitro models of SCA3. Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (MG132), a proteasome inhibitor, was used to treat human embryonic kidney 293 cells (HEK-293) and neuroblastoma SH-SY5Y cells expressing mutant ataxin-3 proteins. Pre-treatment with 0.1 μM catalpol, puerarin or daidzein for 24 h showed no cytotoxicity against the cells, as indicated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The compounds were able to restore UPS function and effectively enhance proteasome activity in the cells, as evidenced by low green fluorescent protein (GFP) intensity. Additionally, the compounds suppressed polyQ aggregation induced by mutant ataxin-3 protein, as detected by Western blot analysis. Following pre-treatment with the test compounds, no autophagy activity was observed in the cells, as evidenced by an insignificant increase in red fluorescent protein (DsRed-LC3) intensity, suggesting the anti-aggregation ability of the compounds were due to UPS activation. Furthermore, the compounds also suppressed the expression of pro-apoptotic marker BCL-2-associated X (BAX) protein and promoted the expression of anti-apoptotic marker B-cell lymphoma 2 (BCL2) protein. Caspase-3 activity was also reduced, which prevented polyQ-induced cell death [55]. These findings were in line with another study by Chen et al. [56] using an in vitro model of SCA3 with MG132-induced pluripotent stem cells (iPSCs). Pre-treatment with 50 μM daidzein for 24 h restored proteasome activity in the cells. Additionally, daidzein was able to suppress MG132-induced cell death, as indicated by lactate dehydrogenase (LDH) assay [56].
A study by Chou et al. [57] investigated the proteasome enhancing ability of T1-11 (N6-(4-Hydroxybenzyl) adenosine), a compound derived from Gastrodia elata, and JMF-1907 (N6-(3-Indolylethyl) adenosine), a synthetic analogue of the former. They used an in vivo model of SCA3 in transgenic mice expressing mutant ataxin-3 protein. Oral administration of 50 mg/kg T1-11 or JMF-1907 for 3 months prevented neuronal cell death in the pontine nuclei by reversing the induced expression of BAX protein and decreased expression of B-cell lymphoma-extra large (BCL-xL) protein. Additionally, proteasome activity was significantly enhanced in the cerebellum and pontine nuclei in the treatment group, as indicated by increased levels of 7-amino-4-methylcoumarin (AMC). An improvement in motor functions was also observed in the treatment group compared to untreated animals, as indicated by the rotarod test [57].
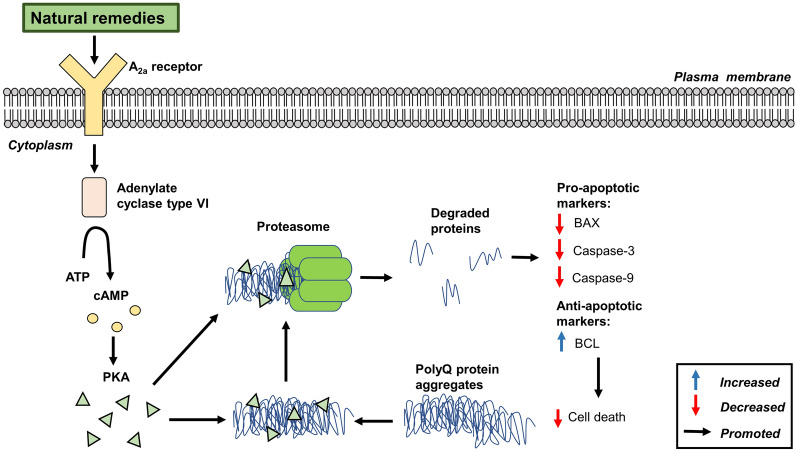
A schematic of the possible activation of the UPS signalling pathway by natural remedies is shown in Fig. 3. Natural remedies have been shown to activate UPS, resulting in the suppression of polyQ aggregation and motor dysfunction associated with SCA [55–57]. The cAMP/PKA pathway is known to activate UPS and promote proteasome activity [75] involving the activation of adenylate cyclase 6 through the A2A receptor [76]. Chou et al. [57] reported that the activation of A2A receptor by T1-11 and JMF-1907 could enhance proteasome activity and promote degradation of ubiquitin proteins. Similar findings were later reported by Chen et al. [55, 56] using puerarin and daidzein compounds extracted from R. glutinosa and P. lobata. Inhibition of A2A receptor using an A2A antagonist blocked the effects induced by T1-11 and JMF-1907. This suggests the stimulation of A2A receptor is required for UPS activation [57]. The suppression of polyQ aggregation was reported to prevent polyQ-induced cell death via suppression of BCL-2-associated X (BAX), and caspase-3 and -9 activities, while enhancing B-cell lymphoma 2 (BCL) expression [55–57].
Fig. 3.
Schematic diagram of the activation of UPS by natural remedies against polyQ-induced SCA. ATP adenosine triphosphate, cAMP cyclic adenosine monophosphate, PKA protein kinase, PolyQ polyglutamine, BAX BCL-2-associated X, BCL B-cell lymphoma
Activation of antioxidant pathways
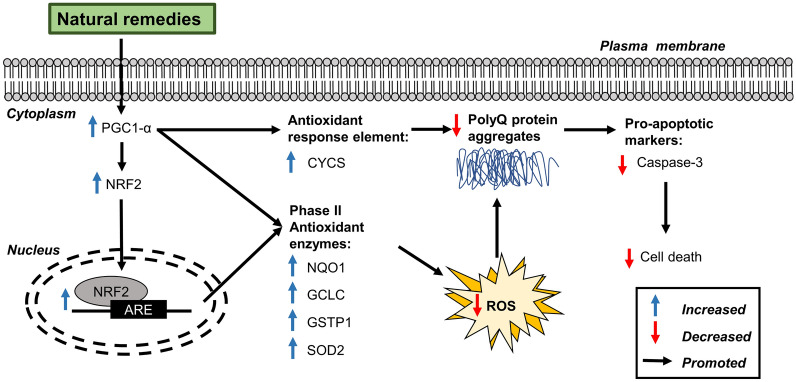
Oxidative damage occurs when reactive oxygen species (ROS) generation exceeds the antioxidant capacity of intracellular antioxidant enzymes [77]. It was shown that the accumulation of polyQ aggregates can cause the depletion of antioxidants including glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT), contributing to the accumulation of ROS and oxidative damage [78, 79]. Patients diagnosed with SCA3 were reported to have reduced levels of glutathione and other thiols, which increased their susceptibility to oxidative damage [16, 80]. This condition may contribute to ROS-induced DNA damage and cell death, leading to motor dysfunctions associated with the disorder [59, 81, 82] Furthermore, the presence of ROS promoted protein aggregation, causing further generation of ROS [59]. Therefore, restoration of endogenous antioxidant levels by natural remedies could serve as a therapeutic strategy against SCAs.
Three derived compounds genipin, geniposide and crocin and the aqueous extract of Gardenia jasminoides were investigated for their antioxidant activities in in vitro models of SCA3 using HEK-293 and SH-SY5Y cells expressing mutant ataxin-3 proteins [58]. The presence of polyQ aggregates in the cells were observed to suppress the expression of nuclear factor erythroid 2-related factor 2 (NRF2), an antioxidant marker. Pre-treatment with aqueous extract (1-10 g/mL), genipin (50–500 nM), geniposide (500 nM) or crocin (100 nM) for 8 h showed no cytotoxicity in the cells, as indicated by MTT assay. Pre-treatment with the extract or compounds restored the depleted expression of NRF2 and downstream antioxidant enzymes NAD(P)H quinone dehydrogenase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutathione S-transferase pi 1 (GSTP1), while suppressing ROS levels and polyQ aggregation, as detected by low ROS fluorescence and GFP intensities. Overexpression of NRF2 in cells suppressed polyQ aggregation, whereas knockdown of its gene promoted polyQ aggregation. These findings suggest the involvement of antioxidant genes in the anti-aggregation effects in cells. Pre-treatment with extract or compounds did not significantly change DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging activity, suggesting DPPH radical scavenging is not a major factor in the anti-aggregation effects [58].
Similar findings were reported by Chen et al. [59] using the aqueous extract of Glycyrrhiza inflata and its compounds licochalcone A and ammonium glycyrrhizinate (AMGZ) in in vitro models of SCA3 using HEK-293 and SH-SY5Y cells expressing mutant ataxin-3 proteins. Pre-treatment with aqueous extract (50 µg/mL), licochalcone A (10 nM) or AMGZ (1 µM) for 8 h showed low cytotoxicity in cells, as indicated by MTT assay. However, the extract or compounds upregulated the expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a regulator of the mitochondrial antioxidant defence system, and its downstream targets, NRF2, NQO1, SOD2, cytochrome c (CYCS) and heme oxygenase 1 (HMOX1), as detected by Western blot analysis. Oxidative damage was reduced through promotion of the glutathione/oxidised glutathione (GSH/GSSG) ratio, suppression of ROS levels and polyQ aggregation [59].
Pohl et al. [61] investigated the antioxidant activities of ethanol extract from rapeseed pomace, a by-product of Brassica napus using Soxhlet extraction. Administration of ethanol extract (1-5 mg/mL) in an in vivo model of SCA3 using transgenic Caenorhabditis elegans expressing mutant ataxin-3 protein showed no toxicity, as detected by food clearance assay. Ethanol extract treatment promoted the expression of glutathione S-transferase 4 (GST4) and SOD3, which are involved in GSH metabolism and mitochondrial manganese SOD synthesis, respectively. Furthermore, ethanol extract improved motor function in transgenic C. elegans comparable with the wild type, as observed by a motility assay. Knockdown of GST4 gene in C. elegans abolished the improvement in motor function by ethanol extract. Pohl et al. [61] suggested that the positive effects of rapeseed pomace extract were dependent on GST4 expression. Moreover, sinapine, a major compound derived from the ethanol extract, also demonstrated improvement in motor function of transgenic C. elegans, but it was less effective than the whole extract. This indicates additional compounds in conjunction with sinapine contributed to the positive effects of ethanol extract [61].
Shaoyao Gancao Tang is a Chinese traditional herbal formulation consisting of Paeonia lactiflora and Glycyrrhiza uralensis in a 1:1 ratio. In a study by Chen et al. [65], the antioxidant activities of the formulation were evaluated in in vitro models of SCA17 using HEK 293 and SH-SY5Y cells expressing mutant TATA-box binding protein (TBP), and in vivo transgenic mouse model of SCA17 overexpressing TBP. Pre-treatment with the formulation (0.001–100 µg/mL) in cells for 8 h demonstrated no cytotoxicity, as indicated by MTT assay. The formulation was able to restore the expression of PGC-1α and NRF2, and their downstream targets, CYCS, GCLC and NQO1, as detected by Western blot analysis. Furthermore, suppression of polyQ aggregation was observed in cells, as shown by low GFP intensity. Administration of 0.4% Shaoyao Gancao Tang in the drinking water of the mouse model improved gait coordination and suppressed hyperactivity, as indicated by footprint and locomotor test, respectively [65].
Zhengan Xifeng Tang is a Chinese traditional herbal formulation consisting of various mixtures of medicinal plants. The positive effects of the formulation were investigated by Okabe et al. [63] in a 60-year-old female patient diagnosed with typical SCA6. Oral administration of the formulation for 2 months showed visible alleviation of ataxia symptoms including vertigo and titubation. Motor function was also observed to be improved, as indicated by the International Cooperative Ataxia Rating Scale (ICARS). At 1 year after treatment discontinuation, the patient experienced a relapse in ataxia symptoms, although re-administration of the formulation alleviated the deteriorating symptoms. However, the patient relapsed a second time after another 15 months after treatment discontinuation, which was again alleviated by the treatment [63]. Similar findings were demonstrated in another study by Okabe [64] in 71-year-old female patient diagnosed with typical SCA6. Although the mechanisms of Zhengan Xifeng Tang on SCA6 were not identified from these studies, it was suggested that the positive effects may be attributed to the antioxidant and anticonvulsant activity of the herb formulation [63, 64].
The activation of the antioxidant pathway by natural remedies is shown in Fig. 4. The antioxidant activities of natural remedies were shown to have therapeutic effects against cell damage in SCA through the activation of NRF2/ARE (antioxidant response element). This antioxidant pathway involved the upregulation of various phase II detoxifying/antioxidant enzymes that attenuated the oxidative damage and prevented ROS-induced cell death [83]. In a study by Chen et al. [65], Shaoyao Gancao Tang upregulated the expression of NRF2 and PGC-1α, a coactivator of NRF2, which suppressed ROS levels in vitro and restored motor function in transgenic SCA17 mice. Studies by Chang et al. [58] and Chen et al. [59] showed that G. jasminoides and G. inflata could activate downstream targets of NRF2 and PGC-1α (e.g., SOD2, CYCS, NAD(P)H, NQO1, GCLC and GSTP1), which effectively suppressed ROS levels and polyQ aggregation. In contrast, knockdown of PGC-1α and/or NRF2 promoted aggregation and attenuated the anti-aggregation activity of Shaoyao Gancao Tang, G. jasminoides and G. inflata, which suggest PGC-1α and NRF2 are involved in the positive effects on SCA [58, 59, 65].
Fig. 4.
Schematic diagram of the activation of the antioxidant signalling pathway by natural remedies against polyQ-induced SCA. PGC-1α peroxisome proliferator-activated receptor γ, NRF2 nuclear factor erythroid 2-related factor 2, ARE antioxidant response element, CYCS cytochrome c, NQO1 NAD(P)H Quinone Dehydrogenase 1, GCLC glutamate-cysteine ligase catalytic subunit, GSTP1 glutathione S-transferase pi 1, SOD2 superoxide dismutase 2, PolyQ polyglutamine, ROS reactive oxygen species
Friedreich’s ataxia (FRDA) is an autosomal recessive disorder caused by the abnormal expansion of GAA repeats contributing to suppressed expression of frataxin (FXN) protein [84]. Evidence shows that frataxin deficiency in FRDA impairs the intracellular antioxidant defence system, resulting in decreased GSH levels [85–87] and expressions of antioxidant enzymes including SOD, CAT, glutaredoxin, and thioredoxin [88]. Additionally, excessive ROS is generated by an iron-dependent Fenton reaction and mitochondrial dysfunction. The compromised antioxidant defence system and ROS accumulation contribute to the unregulated increase in oxidative damage, leading to DNA damage and cell death [89–92].
Lew et al. [66] investigated the antioxidant activities of a standardised aqueous extract of Hericium erinaceus in an in vitro model of FRDA involving L-Buthionine sulfoximine (L-BSO)-induced human dermal fibroblast expressing abnormal expansion of GAA triplet repeat. L-Buthionine sulfoximine is an inhibitor of γ-glutamylcysteine synthetase, which plays a role in GSH biogenesis. Cells treated with L-BSO had reduced levels of GSH, which increased the susceptibility of cells to oxidative damage [93]. Treatment of cells with the extract (32 mg/mL) for 24 h restored the GSH depletion, as shown by an increased GSH/GSSG ratio. The extract also reduced the lactate dehydrogenase (LDH) level and apoptotic body formation, as detected by LDH assay and Hoechst 33258 staining, respectively. The findings suggest the extract was able to prevent oxidative damage-induced cell death by preventing GSH depletion [66].
Maintenance of intracellular calcium homeostasis
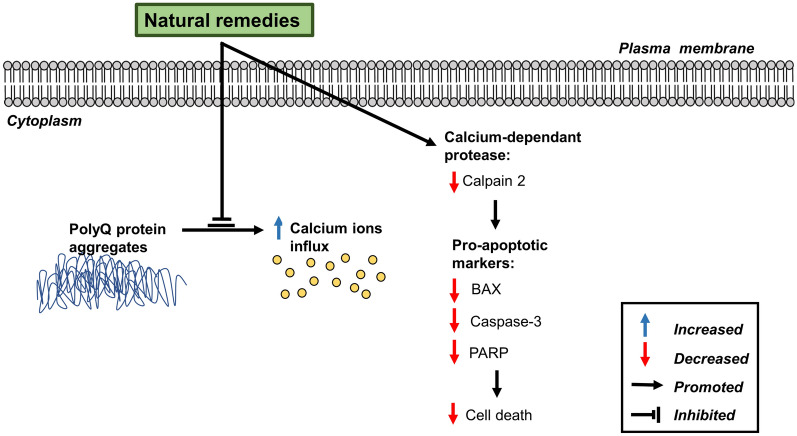
Calcium ions are important signalling molecules that control a wide variety of cellular processes including muscle control, cell signalling and cell growth [94]. Accumulating evidence shows that polyQ aggregates impair calcium homeostasis and induce abnormal calcium ion influx in Purkinje cells through ionotropic α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors and metabotropic glutamate receptors (mGluR) [95–98]. The accumulation of calcium ions leads to the formation of microaggregates, eventually forming larger inclusion bodies, leading to neuronal degeneration and cell death, and compromised cerebellar output and motor function [67]. Natural remedies that can restore calcium homeostasis could potentially treat polyQ disorders.
A study by Huang et al. [62] investigated the positive effects of EGb 761, a standardised extract of Ginkgo biloba, in in vitro models of SCA17 using HEK 293 and SH-SY5Y cells expressing mutant TATA-box binding protein (TBP), and an in vivo transgenic mouse model overexpressing TBP. Pre-treatment with EGb 761 (20 μg/mL) for 1 h restored cellular calcium homeostasis and suppressed calcium influx, as indicated by reduced calcium green fluorescence intensity. Additionally, there was a reduction in calcium-dependent pro-apoptotic marker expressions, including calpain 2, BAX, caspase 3 and poly (adenosine diphosphate-ribose) polymerase (PARP), as detected by Western blot analysis. Intraperitoneal injection of EGb 761 (100 mg/kg) in transgenic mice demonstrated improved motor function by rotarod test. Moreover, EGb 761 prevented the disruption of calbindin integrity, as indicated by immunohistochemistry analysis [62]. Calbindins are major proteins expressed on neurons such as Purkinje cells, which play a role in motor function, calcium homeostasis and cell survival [99, 100]. Similar findings were also reported by Chen et al. [65] using Shaoyao Gancao Tang in Purkinje cells of SCA17 mice detected by calbindin staining. A schematic of the maintenance of intracellular calcium homeostasis by natural remedies is shown in Fig. 5.
Fig. 5.
Schematic diagram of the maintenance of intracellular calcium homeostasis by natural remedies against polyQ-induced SCA. BAX BCL-2 associated X, PARP poly (adenosine diphosphate-ribose) polymerase
Regulation of chaperones
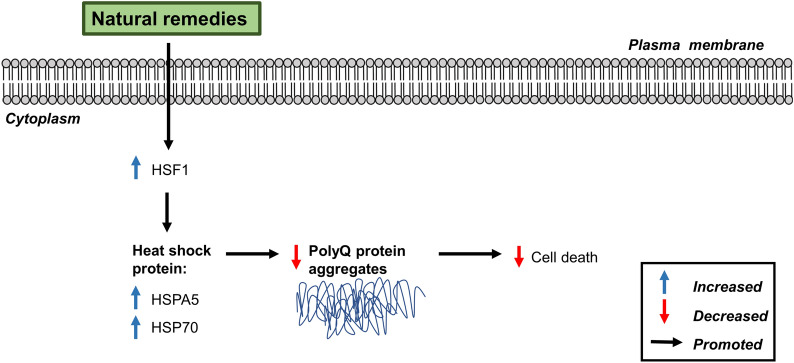
Heat shock proteins (HSPs) are evolutionarily conserved chaperones ubiquitous in organisms and are responsible for cell survival by facilitating protein assembly, folding, translocation, stabilisation and degradation [101, 102]. Previous studies by Chen et al. [103] and Lee et al. [104] demonstrated the presence of TBP induced the sequestration of nuclear transcription factor Y (NFY) complexes and suppressed its downstream targets including the heat shock 70 kDa protein (HSPA) 5 and 8. Studies also showed that upregulation of HSPs could suppress polyQ aggregation and protect against polyQ-induced toxicity [105–109]. Natural remedies that can restore HSP expression and function could serve as an alternative therapeutic option for polyQ-induced SCAs.
Chang et al. [60] investigated the effects of Paeonia lactiflora and its major compound paeoniflorin on HSPs in in vitro models of SCA3 using HEK-293 and SH-SY5Y cells expressing mutant ataxin-3 proteins. Pre-treatment with P. lactiflora (2-50 μg/mL) for 24 h showed no cytotoxicity, as assessed by MTT assay. The extract and compound upregulated the expression of heat shock transcription factor 1 (HSF1) and heat shock protein 70 (HSP70) chaperone in cells. Additionally, suppression of polyQ aggregation was observed in the cells, as shown by low GFP intensity. Overexpression of HSP1 also resulted in suppressed polyQ aggregation, suggesting that the positive effects of the extract and compound were modulated by HSPs. The role of HSPs in suppressing polyQ aggregation was further supported by Chen et al. [65] in an in vivo model of SCA17. Administration of Shaoyao Gancao Tang promoted HSPA5 expression and suppressed TBP protein aggregation in transgenic mice leading to improved motor function in the rotarod test [65]. The regulation of chaperones by natural remedies is shown in Fig. 6.
Fig. 6.
Schematic diagram of the regulation of chaperones by natural remedies against polyQ-induced SCA. HSF1 heat shock transcription factor 1, HSPA5 heat shock 70 kDa protein 5, HSP70 heat shock protein 70
Limitations and future prospects
There are valid concerns on the use of natural remedies in HA drug discovery and development. Natural remedies are often initially experimented as crude extracts and due to the high abundance of non-desirable components, bioassay-guided isolation of target compounds must be performed with subsequent identification [110, 111]. Furthermore, metal impurities, fluorescence-interfering, the presence of non-polar or polar compounds in the extracts may provide inaccurate results for some bioassays. Therefore, such compounds must first be removed depending on the assay system to avoid interference [112]. The process requires more time and effort as compared to high-throughput screening of chemical compound libraries [110, 111].
Another impediment in its acceptance is the lack of standard profile for quality control, cost per sample, complexity of resupply and variation of chemical composition. Natural remedies harvested from the wild are susceptible to environmental factors such as deforestation, pollution, global warming and anthropic pressure which hinders their habitat and limits availability. In addition, discrepancy in growth requirements such as soil composition, weather, altitude and storage conditions contribute to variation of chemical composition. The composition may also be affected during processing and isolation as some compounds may undergo transformation and degradation [110–112]. The ecological issues can be resolved through the generation of a controlled cultivation system which provides optimal yield and uniform composition, preventing batch to batch variation [113]. Nevertheless, majority of the natural remedies worldwide are still harvested from the wild [112]. This is due to the high commercial risk associated with generating the system as the market is unpredictable and the duration required for the establishment is long [113].
It is worthy to emphasise that HA is a rare genetic disorder with severe survival outcomes; hence, development of translational models of human-specific HA is rather limited. Studies of innovative treatment with small sample size by individual laboratories should by no means be substituted by multi-centre pre-clinical mouse studies. Nevertheless, the limitations of such smaller studies should be clearly defined by differentiating between proof-of-concept studies and pre-clinical testing studies.
Although various forms of HA have been identified, only four subtypes of HA (SCA3, 6, 17 and FRDA) were investigated with natural remedies in the reviewed studies. Furthermore, the studies demonstrating the positive effects of natural remedies on HA were largely conducted with pre-clinical models such as in vitro cell culture models and in vivo animal models. While these models have facilitated the understanding of mechanisms and signalling pathways in the treatment of HA, the results need to be validated in human studies. Additionally, although there are no models that can reproduce all symptoms of HA patients or cellular mechanisms involved, mammals (Drosophila, transgenic and knockout mice) and mammalian cell lines (patient-derived cells, iPSCs and iPSC-derived cells) are currently the most useful models, owing to their close physiological features to HA diseases [114, 115]. Due to the complexity and heterogeneity of HA pathogenesis, current approaches are focusing on the development of therapeutic strategies that target different stages of the cellular pathogenesis, thus making comparison across the studies challenging [115]. Moreover, it is difficult to compare transgenic mice derived from different mouse strains as the manifestation of the targeted mutations may be interfered by their phenotypic traits of the original strain [116].
In spite of the fact that natural remedies are shown to produce significant improvement in HA patients, the results of such studies are limited by the sample size and possible bias to ethnicity and gender. Clinical studies by Okabe et al. [63] and Okabe [64] were each conducted with only one test subject. Furthermore, both test subjects were elderly Japanese female aged 60 and 71. The limited number of test subject and lack of diversity in terms of age and ethnicity may affect the reliability of the findings [117]. Another major limitation is the lack of safety and efficacy profile for the natural remedies and compounds studied in this review. It is not known whether the dosage of decoctions with complex formulations and compounds administered may lead to potential toxicity, adverse effects or will be present at the intended site of action in sufficient concentration [118–120]. This is also further complicated with the lack of understanding of the molecular targets and mechanism involving the natural remedies [120]. Greater understanding of the pharmacokinetic and pharmacodynamics profiles of these compounds will support more effective clinical trials in the future.
A more systematic approach in cataloguing the potential effects of natural remedies/compounds will speed up the drug discovery process, in order to have more investigations done on unexplored compounds. Detailed mode of actions for each compound can be delineated using high throughput methods such as NGS coupled with various other confirmative studies such as transcriptomic validation, protein translation and function as well as epigenetic studies. Integrative analysis of such ‘omics’ data will allow researchers with an unprecedented speed in understanding the underlying pathophysiology this rare disease, developing effective biomarkers and pursuing meaningful clinical testing [121]. Studies on specific HA can be undertaken using relevant genetic resources or repositories of biologic samples. NGS can be implemented on HA research in identifying and validating potential targets through epigenetic studies, differential gene expression, genome sequencing and protein translation rates. Moreover, NGS sequencing can be used to discover biomarkers that can reflect the mechanism of action regulated by the natural remedy or compound [122]. These pre-clinical studies can provide proof of concept and mechanism in establishing the pharmacodynamic parameters of these remedies and compounds prior to clinical testing.
As pre-clinical and clinical studies are currently lacking, effort should be directed towards testing using polyQ animal models and randomised controlled trials, respectively, including for forms of HA not yet studied. Improved studies should be done where study conclusions are uncertain due to methodological insufficiencies or discrepancies. Comparative studies should be done with current treatments versus natural remedies (single compound or the mixture) to ascertain the potential of the latter as supplements or alternative treatments for HA. Additionally, further studies can be conducted towards investigating whether the natural remedies could modulate HA through other pathways not discussed in this review.
In addition, a successful planning and execution of a research consortium involving Friedreich’s Ataxia Research Alliance (FARA) and National Ataxia Foundation (NAF) opens new possibilities for HA research. Such effort will foster cross-nationals translational research in the development of alternative treatments for HA. Multidisciplinary team involvement and input form the allied health professionals are also critical in the management of HA, particularly in the palliative stage. Indeed, all these efforts will require strong commitment not only from the researchers, but also the patients diagnosed with HA as well as their family members.
Conclusion
In conclusion, the systematic review provides an overview of the available evidence from pre-clinical and clinical trials pertaining to the natural remedies in improving or halting the progression of HA. Nevertheless, there has been an increasing concern by the clinicians about the potential of interactions of natural remedies with prescribed drugs. The mechanisms underlying natural remedies-drug interactions are always difficult to predict. Another issue is the general lack of understanding on the composition and pharmacological actions of natural remedies. Therefore, research evidence is necessary to ensure its safety and effectiveness and to warrant the integration of natural remedies into evidence-based clinical practice.
Acknowledgements
This work was funded by University of Malaya Faculty of Medicine Research Grant GPF003C-2019. We would like to thank Public Service Department Malaysia for the scholarship support for the postgraduate study and Dr Mohamad Hariz Hamka bin Mahamad of the Faculty of Medicine, University of Malaya for providing hand-drawn images of natural remedies.
Abbreviations
- HA
Hereditary ataxia
- SCA
Spinocerebellar ataxia
- CAG
Cytosine-Adenine-Guanine
- EA
Episodic ataxia
- FRDA
Friedreich’s ataxia
- AT
Ataxia-telangiectasia
- AOA
Ataxia oculomotor apraxia
- FXTAS
Fragile X tremor-ataxia syndrome
- TCM
Traditional Chinese medicine
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analyses
- HEK-293
Human embryonic kidney 293 cells
- PolyQ
Polyglutamine
- UPS
Ubiquitin-proteasome system
- cAMP
Cyclic adenosine monophosphate
- PKA
Protein kinase
- ROS
Reactive oxygen species
- NRF2
Nuclear factor erythroid 2–related factor 2
- AMGZ
Ammonium glycyrrhizinate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- GFP
Green fluorescent protein
- LDH
Lactate dehydrogenase
- BAX
BCL-2-associated X protein
- BCL2
B cell lymphoma 2
- T1-11
N6-(4-Hydroxybenzyl) adenosine
- JMF1907
N6-(3-Indolylethyl) adenosine
- GSH
Glutathione
- SOD
Superoxide dismutase
- CAT
Catalase
- NQO1
NAD(P)H quinone dehydrogenase 1
- GCLC
Glutamate-cysteine ligase catalytic subunit
- GSTP1
Glutathione S-transferase pi 1
- CYCS
Cytochrome c
- GSSG
Oxidised glutathione
- GST4
Glutathione S-transferase 4
- TBP
TATA-box binding protein
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator 1α
- HSP
Heat shock protein
Authors’ contributions
Conceptualisation KHW, Original draft preparation MWLP and SYL. Review and editing KHW, LWL, IC and WKSL. Visualisation MWLP and SYL, Supervision KHW, LWL and IC. All authors read and approved the final manuscript.
Funding
University of Malaya Faculty of Medicine Research Grant GPF003C-2019.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael Weng Lok Phang, Email: michaelphang4572@gmail.com.
Sze Yuen Lew, Email: szeyuenlew@gmail.com.
Ivy Chung, Email: ivychung@ummc.edu.my.
William Kiong-Seng Lim, Email: kslim@unimas.my.
Lee Wei Lim, Email: drlimleewei@gmail.com.
Kah Hui Wong, Email: wkahhui@um.edu.my.
References
- 1.Akbar U, Ashizawa T. Ataxia. Neurol Clin. 2015;33(1):225–248. doi: 10.1016/j.ncl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albernaz PLM, Zuma e Maia FZ, Carmona S, Cal RVR, Zalazar G. The new neurotology: a comprehensive clinical guide. Cham: Springer; 2019. Ataxia; pp. 181–91. [Google Scholar]
- 3.Teive HAG, Ashizawa T. Primary and secondary ataxias. Curr Opin Neurol. 2015;28(4):413–422. doi: 10.1097/WCO.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarva H, Shanker VL. Treatment options in degenerative cerebellar ataxia: a systematic review. Mov Disord Clin Pract. 2014;1(4):291–298. doi: 10.1002/mdc3.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudin M, Matilla-Dueñas A, Soong B-W, Pedroso JL, Barsottini OG, Mitoma H, Tsuji S, Schmahmann JD, Manto M, Rouleau GA, Klein C, Dupre N. The classification of autosomal recessive cerebellar ataxias: a consensus statement from the society for research on the cerebellum and ataxias task force. Cerebellum. 2019;18(6):1098–1125. doi: 10.1007/s12311-019-01052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buijsen RAM, Toonen LJA, Gardiner SL, van Roon-Mom WMC. Genetics, mechanisms, and therapeutic progress in polyglutamine spinocerebellar ataxias. Neurotherapeutics. 2019;16(2):263–286. doi: 10.1007/s13311-018-00696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird TD. Hereditary ataxia overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors. GeneReviews® [Internet] Seattle: University of Washington; 2019. [PubMed] [Google Scholar]
- 8.Öz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM. Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum. 2011;10(2):208–217. doi: 10.1007/s12311-010-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidel K, Siswanto S, Brunt ERP, den Dunnen W, Korf H-W, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124(1):1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 10.Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf H-W, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Piccinin CC, D’Abreu A. Neuroimaging in ataxias. In: Habas C, editor. The neuroimaging of brain diseases: structural and functional advances. Cham: Springer; 2018. pp. 215–32. [Google Scholar]
- 12.Meira AT, Arruda WO, Ono SE, de Carvalho Neto A, Raskin S, Camargo CHF, Teive HAG. Neuroradiological findings in the spinocerebellar ataxias. Tremor Other Hyperkinet Mov (N Y). 2019 doi: 10.7916/tohm.v0.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velázquez-Pérez L, Rodríguez-Labrada R, Freund H-J, Auburger G. Spinocerebellar ataxia type 2. In: Gazulla J, editor. Spinocerebellar ataxia. Rijeka: InTech; 2012. pp. 77–102. [Google Scholar]
- 14.Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J. Spinocerebellar ataxia type 2: clinicogenetic aspects, mechanistic insights, and management approaches. Front Neurol. 2017;8:472. doi: 10.3389/fneur.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vale J, Bugalho P, Silveira I, Sequeiros J, Guimarães J, Coutinho P. Autosomal dominant cerebellar ataxia: frequency analysis and clinical characterization of 45 families from Portugal. Eur J Neurol. 2010;17(1):124–128. doi: 10.1111/j.1468-1331.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 16.de Assis AM, Saute JAM, Longoni A, Haas CB, Torrez VR, Brochier AW, Souza GN, Furtado GV, Gheno TC, Russo A, Monte TL, Castilhos RM, Schumacher-Schuh A, D’ Avila R, Donis KC, de Mello Rieder CR, Souza DO, Camey S, Leotti VB, Jardim LB, Portela LV. Peripheral oxidative stress biomarkers in spinocerebellar ataxia type 3/Machado–Joseph disease. Front Neurol. 2017;8:485. 10.3389/fneur.2017.00485. [DOI] [PMC free article] [PubMed]
- 17.Yang Z, Shi C, Zhou L, Li Y, Yang J, Liu Y, Mao C, Luo H, Xu G, Xu Y. Metabolic profiling reveals biochemical pathways and potential biomarkers of spinocerebellar ataxia 3. Front Mol Neurosci. 2019;12:159. doi: 10.3389/fnmol.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adanyeguh IM, Henry P-G, Nguyen TM, Rinaldi D, Jauffret C, Valabregue R, Emir UE, Deelchand DK, Brice A, Eberly LE, Öz G, Durr A, Mochel F. In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7. Mov Disord. 2015;30(5):662–670. doi: 10.1002/mds.26181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Ramos Y, Montoya-Estrada A, Cisneros B, Tercero-Pérez K, León-Reyes G, Leyva-García N, Hernández-Hernández O, Magaña JJ. Oxidative stress in spinocerebellar ataxia type 7 is associated with disease severity. Cerebellum. 2018;17(5):601–609. doi: 10.1007/s12311-018-0947-0. [DOI] [PubMed] [Google Scholar]
- 20.Brockmann K, Reimold M, Globas C, Hauser TK, Walter U, Machulla H-J, Rolfs A, Schöls L. PET and MRI reveal early evidence of neurodegeneration in spinocerebellar ataxia type 17. J Nucl Med. 2012;53(7):1074–1080. doi: 10.2967/jnumed.111.101543. [DOI] [PubMed] [Google Scholar]
- 21.Mundwiler A, Shakkottai VG. Autosomal-dominant cerebellar ataxias. Handb Clin Neurol. 2018;147:173–185. doi: 10.1016/B978-0-444-63233-3.00012-9. [DOI] [PubMed] [Google Scholar]
- 22.Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW. CINCH investigators. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130(10):2484–2493. doi: 10.1093/brain/awm126. [DOI] [PubMed] [Google Scholar]
- 23.Choi K-D, Choi J-H. Episodic ataxias: clinical and genetic features. J Mov Disord. 2016;9(3):129–135. doi: 10.14802/jmd.16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan SM, D’Adamo MC. Episodic ataxia type 1. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors. GeneReviews® [Internet] Seattle: University of Washington; 2018. [PubMed] [Google Scholar]
- 25.Mantuano E, Veneziano L, Spadaro M, Giunti P, Guida S, Leggio MG, Verriello L, Wood N, Jodice C, Frontali M. Clusters of non-truncating mutations of P/Q type Ca2+ channel subunit Cav2. 1 causing episodic ataxia 2. J Med Genet. 2004;41(6):e82. doi: 10.1136/jmg.2003.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spacey S. Episodic ataxia type 2. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors. GeneReviews® [Internet] Seattle: University of Washington; 2015. [Google Scholar]
- 27.Mascalchi M, Salvi F, Piacentini S, Bartolozzi C. Friedreich’s ataxia: MR findings involving the cervical portion of the spinal cord. AJR Am J Roentgenol. 1994;163(1):187–191. doi: 10.2214/ajr.163.1.8010211. [DOI] [PubMed] [Google Scholar]
- 28.Campuzano V, Montermini L, Molto MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel J-L, Cocozza S, Koenig M, Pandolfo M. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271(5254):1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 29.McKinnon PJ. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol. 2012;7:303–321. doi: 10.1146/annurev-pathol-011811-132509. [DOI] [PubMed] [Google Scholar]
- 30.Sahama I, Sinclair K, Pannek K, Lavin M, Rose S. Radiological imaging in ataxia telangiectasia: a review. Cerebellum. 2014;13(4):521–530. doi: 10.1007/s12311-014-0557-4. [DOI] [PubMed] [Google Scholar]
- 31.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11:159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anheim M, Monga B, Fleury M, Charles P, Barbot C, Salih M, Delaunoy JP, Fritsch M, Arning L, Synofzik M, Schöls L, Sequeiros J, Goizet C, Marelli C, Le Ber I, Koht J, Gazulla J, De Bleecker J, Mukhtar M, Drouot N, Ali-Pacha L, Benhassine T, Chbicheb M, M’Zahem A, Hamri A, Chabrol B, Pouget J, Murphy R, Watanabe M, Coutinho P, Tazir M, Durr A, Brice A, Tranchant C, Koenig M. Ataxia with oculomotor apraxia type 2: clinical, biological and genotype/phenotype correlation study of a cohort of 90 patients. Brain. 2009;132(10):2688–2698. doi: 10.1093/brain/awp211. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho P, Barbot C. Ataxia with oculomotor apraxia type 1. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors. GeneReviews® [Internet] Seattle: University of Washington; 2015. [Google Scholar]
- 34.Moreira M-C, Koenig M. Ataxia with oculomotor apraxia type 2. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors. GeneReviews® [Internet] Seattle: University of Washington; 2018. [PubMed] [Google Scholar]
- 35.Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical phenotype, diagnosis, and treatment. J Investig Med. 2009;57(8):830–836. doi: 10.2310/JIM.0b013e3181af59c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Currie S, Hadjivassiliou M, Craven IJ, Wilkinson ID, Griffiths PD, Hoggard N. Magnetic resonance imaging biomarkers in patients with progressive ataxia: current status and future direction. Cerebellum. 2013;12(2):245–266. doi: 10.1007/s12311-012-0405-3. [DOI] [PubMed] [Google Scholar]
- 37.Tassone F, Hall DA. FXTAS, FXPOI, and other premutation disorders. 2. Cham: Springer; 2016. [Google Scholar]
- 38.Braga-Neto P, Pedroso JL, Kuo S-H, Marcondes Junior CF, Teive HAG, Barsottini OGP. Current concepts in the treatment of hereditary ataxias. Arq Neuropsiquiatr. 2016;74(3):244–252. doi: 10.1590/0004-282X20160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan R, Yau WY, O’Connor E, Houlden H. Spinocerebellar ataxia: an update. J Neurol. 2019;266(2):533–544. doi: 10.1007/s00415-018-9076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schniepp R, Strupp M, Wuehr M, Jahn K, Dieterich M, Brandt T, Feil K. Acetyl-DL-leucine improves gait variability in patients with cerebellar ataxia—a case series. Cerebellum Ataxias. 2016;3:8. doi: 10.1186/s40673-016-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Library of Medicine (U.S.). N-Acetyl-L-Leucine for Ataxia-Telangiectasia (A-T). https://clinicaltrials.gov/ct2/show/NCT03759678 (2018). NLM identifier: NCT03759678. Accessed 25 July 2020
- 42.Butler MS, Fontaine F, Cooper MA. Natural product libraries: assembly, maintenance, and screening. Planta Med. 2014;80(14):1161–1170. doi: 10.1055/s-0033-1360109. [DOI] [PubMed] [Google Scholar]
- 43.Pohl F, Kong Thoo Lin P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. 2018;23(12):3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayaz M, Ullah F, Sadiq A, Kim MO, Ali T. Natural products-based drugs: potential therapeutics against Alzheimer’s disease and other neurological disorders. Front Pharmacol. 2019;10:1417. doi: 10.3389/fphar.2019.01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004;432(7019):829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 46.Guo Z. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7(2):119–136. doi: 10.1016/j.apsb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14(2):111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123. doi: 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr Med Chem. 2013;20(13):1673–1685. doi: 10.2174/0929867311320130007. [DOI] [PubMed] [Google Scholar]
- 50.Koeberle A, Werz O. Multi-target approach for natural products in inflammation. Drug Discov Today. 2014;19(12):1871–1882. doi: 10.1016/j.drudis.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Salvador-Reyes LA, Luesch H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat Prod Rep. 2015;32(3):478–503. doi: 10.1039/c4np00104d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozano F. Basic theories of traditional Chinese medicine. In: Lin Y-C, Hsu ES-Z, editors. Acupuncture for Pain Management. New York: Springer; 2014. [Google Scholar]
- 53.Marshall AC. Traditional Chinese medicine and clinical pharmacology. In: Hock F, Gralinski M, editors. Drug discovery and evaluation: methods in clinical pharmacology. Cham: Springer; 2020. pp. 455–82. [Google Scholar]
- 54.Wu T-Y, Chen C-P, Jinn T-R. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50(2):131–135. doi: 10.1016/j.tjog.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Chen I-C, Chang C-N, Chen W-L, Lin T-H, Chao C-Y, Lin C-H, Lin H-Y, Cheng M-L, Chiang M-C, Lin J-Y, Wu Y-R, Lee-Chen G-J, Chen C-M. Targeting ubiquitin proteasome pathway with traditional Chinese medicine for treatment of spinocerebellar ataxia type 3. Am J Chin Med. 2019;47(1):63–95. doi: 10.1142/s0192415x19500046. [DOI] [PubMed] [Google Scholar]
- 56.Chen I-C, Chang K-H, Chen Y-J, Chen Y-C, Lee-Chen G-J, Chen C-M. Pueraria lobata and daidzein reduce cytotoxicity by enhancing ubiquitin-proteasome system function in SCA3-iPSC-derived neurons. Oxid Med Cell Longev. 2019;2019:8130481. doi: 10.1155/2019/8130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou A-H, Chen Y-L, Chiu C-C, Yuan S-J, Weng Y-H, Yeh T-H, Lin Y-L, Fang J-M, Wang H-L. T1-11 and JMF1907 ameliorate polyglutamine-expanded ataxin-3-induced neurodegeneration, transcriptional dysregulation and symptoms of ataxia in the SCA3 transgenic mouse. Neuropharmacology. 2015;99:308–317. doi: 10.1016/j.neuropharm.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Chang K-H, Chen W-L, Wu Y-R, Lin T-H, Wu Y-C, Chao C-Y, Lin J-Y, Lee L-C, Chen Y-C, Lee-Chen G-J, Chen C-M. Aqueous extract of Gardenia jasminoides targeting oxidative stress to reduce polyQ aggregation in cell models of spinocerebellar ataxia 3. Neuropharmacology. 2014;81:166–175. doi: 10.1016/j.neuropharm.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 59.Chen C-M, Weng Y-T, Chen W-L, Lin T-H, Chao C-Y, Lin C-H, Chen I-C, Lee L-C, Lin H-Y, Wu Y-R, Chen Y-C, Chang K-H, Tang H-Y, Cheng M-L, Lee-Chen G-J, Lin J-Y. Aqueous extract of Glycyrrhiza inflata inhibits aggregation by upregulating PPARGC1A and NFE2L2–ARE pathways in cell models of spinocerebellar ataxia 3. Free Radic Biol Med. 2014;71:339–350. doi: 10.1016/j.freeradbiomed.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Chang K-H, Chen W-L, Lee L-C, Lin C-H, Kung P-J, Lin T-H, Wu Y-C, Wu Y-R, Chen Y-C, Lee G-J, Chen C-M. Aqueous extract of Paeonia lactiflora and paeoniflorin as aggregation reducers targeting chaperones in cell models of spinocerebellar ataxia 3. Evid Based Complement Alternat Med. 2013;2013:471659. doi: 10.1155/2013/471659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pohl F, Teixeira-Castro A, Costa MD, Lindsay V, Fiúza-Fernandes J, Goua M, Bermano G, Russell W, Maciel P, Kong Thoo Lin P. Kong Thoo Lin P. GST-4-dependent suppression of neurodegeneration in C. elegans models of Parkinson’s and Machado-Joseph disease by rapeseed pomace extract supplementation. Front Neurosci. 2019;13:1091. doi: 10.3389/fnins.2019.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang D-S, Lin H-Y, Lee-Chen G-J, Hsieh-Li H-M, Wu C-H, Lin J-Y. Treatment with a Ginkgo biloba extract, EGb 761, inhibits excitotoxicity in an animal model of spinocerebellar ataxia type 17. Drug Des Devel Ther. 2016;10:723–731. doi: 10.2147/DDDT.S98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okabe T, Fujisawa M, Sekiya T, Ichikawa Y, Goto J. Successful treatment of spinocerebellar ataxia 6 with medicinal herbs. Geriatr Gerontol Int. 2007;7(2):195–197. doi: 10.1111/j.1447-0594.2007.00378.x. [DOI] [Google Scholar]
- 64.Okabe T. Remission induction of spinocerebellar ataxia 6 with medicinal herbs. J Aging Neuro Psychol. 2018 doi: 10.29011/janp-108.100008. [DOI] [Google Scholar]
- 65.Chen C-M, Chen W-L, Hung C-T, Lin T-H, Lee M-C, Chen I-C, Lin C-H, Chao C-Y, Wu Y-R, Chang K-H, Hsieh-Li H-M, Lee-Chen G-J. Shaoyao Gancao Tang (SG-Tang), a formulated Chinese medicine, reduces aggregation and exerts neuroprotection in spinocerebellar ataxia type 17 (SCA17) cell and mouse models. Aging. 2019;11(3):986–1007. doi: 10.18632/aging.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lew S-Y, Yow Y-Y, Lim L-W, Wong K-H. Antioxidant-mediated protective role of Hericium erinaceus (Bull.: Fr.) Pers. against oxidative damage in fibroblasts from Friedreich’s ataxia patient. Food Sci Technol. 2020;40(Suppl 1):264–272. doi: 10.1590/fst.09919. [DOI] [Google Scholar]
- 67.McIntosh CS, Aung-Htut MT, Fletcher S, Wilton SD. Polyglutamine ataxias: from clinical and molecular features to current therapeutic strategies. J Genet Syndr Gene Ther. 2017;8(2):1–17. doi: 10.4172/2157-7412.1000319. [DOI] [Google Scholar]
- 68.Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31(10):521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dantuma NP, Bott LC. The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci. 2014;7:70. doi: 10.3389/fnmol.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bader M, Steller H. Regulation of cell death by the ubiquitin–proteasome system. Curr Opin Cell Biol. 2009;21(6):878–884. doi: 10.1016/j.ceb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cummings CJ, Reinstein E, Sun Y, Antalffy B, Jiang Y-H, Ciechanover A, Orr HT, Beaudet AL, Zoghbi HY. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron. 1999;24(4):879–892. doi: 10.1016/S0896-6273(00)81035-1. [DOI] [PubMed] [Google Scholar]
- 72.Matilla A, Gorbea C, Einum DD, Townsend J, Michalik A, van Broeckhoven C, Jensen CC, Murphy KJ, Ptácek LJ, Fu Y-H. Association of ataxin-7 with the proteasome subunit S4 of the 19S regulatory complex. Hum Mol Genet. 2001;10(24):2821–2831. doi: 10.1093/hmg/10.24.2821. [DOI] [PubMed] [Google Scholar]
- 73.Chai Y, Berke SS, Cohen RE, Paulson HL. Poly-ubiquitin binding by the polyglutamine disease protein ataxin-3 links its normal function to protein surveillance pathways. J Biol Chem. 2004;279(5):3605–3611. doi: 10.1074/jbc.M310939200. [DOI] [PubMed] [Google Scholar]
- 74.Dueñas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129(6):1357–1370. doi: 10.1093/brain/awl081. [DOI] [PubMed] [Google Scholar]
- 75.Myeku N, Wang H, Figueiredo-Pereira ME. cAMP stimulates the ubiquitin/proteasome pathway in rat spinal cord neurons. Neurosci Lett. 2012;527(2):126–131. doi: 10.1016/j.neulet.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279(3):F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 77.Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S-J, Kim TS, Hong S, Rhim H, Kim IY, Kang S. Oxidative stimuli affect polyglutamine aggregation and cell death in human mutant ataxin-1-expressing cells. Neurosci Lett. 2003;348(1):21–24. doi: 10.1016/S0304-3940(03)00657-8. [DOI] [PubMed] [Google Scholar]
- 79.Xiao L, Li H, Zhang J, Yang F, Huang A, Deng J, Liang M, Ma F, Hu M, Huang Z. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules. 2014;19(6):7757–7769. doi: 10.3390/molecules19067757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pacheco LS, da Silveira AF, Trott A, Houenou LJ, Algarve TD, Belló C, Lenz AF, Mânica-Cattani MF, da Cruz IBM. Association between Machado-Joseph disease and oxidative stress biomarkers. Mutat Res. 2013;757(2):99–103. doi: 10.1016/j.mrgentox.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 81.Giuliano P, de Cristofaro T, Affaitati A, Pizzulo GM, Feliciello A, Criscuolo C, De Michele G, Filla A, Avvedimento EV, Varrone S. DNA damage induced by polyglutamine-expanded proteins. Hum Mol Genet. 2003;12(18):2301–2309. doi: 10.1093/hmg/ddg242. [DOI] [PubMed] [Google Scholar]
- 82.Bertoni A, Giuliano P, Galgani M, Rotoli D, Ulianich L, Adornetto A, Santillo MR, Porcellini A, Avvedimento VE. Early and late events induced by polyQ-expanded proteins: identification of a common pathogenic property of polyQ-expanded proteins. J Biol Chem. 2011;286(6):4727–4741. doi: 10.1074/jbc.M110.156521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR. p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid Redox Signal. 2013;18(4):386–399. doi: 10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jauslin ML, Vertuani S, Durini E, Buzzoni L, Ciliberti N, Verdecchia S, Palozza P, Meier T, Manfredini S. Protective effects of Fe-Aox29, a novel antioxidant derived from a molecular combination of idebenone and vitamin E, in immortalized fibroblasts and fibroblasts from patients with Friedreich ataxia. Mol Cell Biochem. 2007;302(1–2):79–85. doi: 10.1007/s11010-007-9429-2. [DOI] [PubMed] [Google Scholar]
- 85.Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, Casali C, Damiano M, Federici G, Bertini E. Glutathione in blood of patients with Friedreich’s ataxia. Eur J Clin Invest. 2001;31(11):1007–1011. doi: 10.1046/j.1365-2362.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 86.Auchère F, Santos R, Planamente S, Lesuisse E, Camadro J-M. Glutathione-dependent redox status of frataxin-deficient cells in a yeast model of Friedreich’s ataxia. Hum Mol Genet. 2008;17(18):2790–2802. doi: 10.1093/hmg/ddn178. [DOI] [PubMed] [Google Scholar]
- 87.Clark E, Johnson J, Dong YN, Mercado-Ayon E, Warren N, Zhai M, McMillan E, Salovin A, Lin H, Lynch DR. Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease. Neuronal. Signal. 2018;2(4):NS20180060. doi: 10.1042/NS20180060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomes CM, Santos R. Neurodegeneration in Friedreich’s ataxia: from defective frataxin to oxidative stress. Oxid Med Cell Longev. 2013;2013:487534. doi: 10.1155/2013/487534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson PR, Kirby K, Orr WC, Hilliker AJ, Phillips JP. Hydrogen peroxide scavenging rescues frataxin deficiency in a Drosophila model of Friedreich’s ataxia. Proc Natl Acad Sci U S A. 2008;105(2):611–616. doi: 10.1073/pnas.0709691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos R, Lefevre S, Sliwa D, Seguin A, Camadro J-M, Lesuisse E. Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal. 2010;13(5):651–690. doi: 10.1089/ars.2009.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefevre S, Brossas C, Auchère F, Boggetto N, Camadro J-M, Santos R. Apn1 AP-endonuclease is essential for the repair of oxidatively damaged DNA bases in yeast frataxin-deficient cells. Hum Mol Genet. 2012;21(18):4060–4072. doi: 10.1093/hmg/dds230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carletti B, Piemonte F. Friedreich’s ataxia: a neuronal point of view on the oxidative stress hypothesis. Antioxidants. 2014;3(3):592–603. doi: 10.3390/antiox3030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takahashi K, Tatsunami R, Oba T, Tampo Y. Buthionine sulfoximine promotes methylglyoxal-induced apoptotic cell death and oxidative stress in endothelial cells. Biol Pharm Bull. 2010;33(4):556–560. doi: 10.1248/bpb.33.556. [DOI] [PubMed] [Google Scholar]
- 94.Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22(3):381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang T-S, Tu H, Chan EYW, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1, 4, 5) triphosphate receptor type 1. Neuron. 2003;39(2):227–239. doi: 10.1016/S0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang T-S, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinás R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102(7):2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, Doerr J, Ladewig J, Mertens J, Tüting T, Hoffmann P, Klockgether T, Evert BO, Wüllner U, Brüstle O. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480(7378):543–546. doi: 10.1038/nature10671. [DOI] [PubMed] [Google Scholar]
- 98.Egorova P, Popugaeva E, Bezprozvanny I. Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer’s disease. Semin Cell Dev Biol. 2015;40:127–133. doi: 10.1016/j.semcdb.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barski JJ, Hartmann J, Rose CR, Hoebeek F, Mörl K, Noll-Hussong M, De Zeeuw CI, Konnerth A, Meyer M. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23(8):3469–3477. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmidt H. Three functional facets of calbindin D-28k. Front Mol Neurosci. 2012;5:25. doi: 10.3389/fnmol.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park C-J, Seo Y-S. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J. 2015;31(4):323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller DJ, Fort PE. Heat shock proteins regulatory role in neurodevelopment. Front Neurosci. 2018;12:821. doi: 10.3389/fnins.2018.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen C-M, Lee L-C, Soong B-W, Fung H-C, Hsu W-C, Lin P-Y, Huang H-J, Chen F-L, Lin C-Y, Lee-Chen G-J, Wu Y-R. SCA17 repeat expansion: mildly expanded CAG/CAA repeat alleles in neurological disorders and the functional implications. Clin Chim Acta. 2010;411(5–6):375–380. doi: 10.1016/j.cca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Lee L-C, Chen C-M, Wang H-C, Hsieh H-H, Chiu I-S, Su M-T, Hsieh-Li H-M, Wu C-H, Lee G-C, Lee-Chen G-J, Lin J-Y. Role of the CCAAT-binding protein NFY in SCA17 pathogenesis. PLoS ONE. 2012;7(4):e35302. doi: 10.1371/journal.pone.0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10(14):1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- 106.Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo A-P, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11(9):1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 107.Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, Nakai A. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280(41):34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 108.Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem. 2008;283(38):26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo Y, Ren S, Lao U, Edgar BA, Wang T. Suppression of polyglutamine protein toxicity by co-expression of a heat-shock protein 40 and a heat-shock protein 110. Cell Death Dis. 2013;4(10):e833. doi: 10.1038/cddis.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beutler JA. Natural products as a foundation for drug discovery. Curr Protoc Pharmacol. 2019;86(1):e67. doi: 10.1002/cpph.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krause J, Tobin G. Discovery, development, and regulation of natural products. In: Kulka M, editor. Using old solutions to new problems-natural drug discovery in the 21st century. London: IntechOpen; 2013. pp. 1–35. [Google Scholar]
- 112.Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23(4):180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 114.Perdomini M, Hick A, Puccio H, Pook MA. Animal and cellular models of Friedreich ataxia. J Neurochem. 2013;126(Suppl 1):65–79. doi: 10.1111/jnc.12219. [DOI] [PubMed] [Google Scholar]
- 115.Matos CA, de Almeida LP, Nóbrega C. Machado-Joseph disease/spinocerebellar ataxia type 3: lessons from disease pathogenesis and clues into therapy. J Neurochem. 2019;148(1):8–28. doi: 10.1111/jnc.14541. [DOI] [PubMed] [Google Scholar]
- 116.Cendelin J. From mice to men: lessons from mutant ataxic mice. Cerebellum Ataxias. 2014;1:4. doi: 10.1186/2053-8871-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. 2014;19(4):27–29. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keum J, Yoo S, Lee D, Nam H. Prediction of compound-target interactions of natural products using large-scale drug and protein information. BMC Bioinformatics. 2016;17(Suppl 6):219. doi: 10.1186/s12859-016-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Law BYK, Wu AG, Wang MJ, Zhu YZ. Chinese medicine: a hope for neurodegenerative diseases? J Alzheimers Dis. 2017;60(s1):S151–S160. doi: 10.3233/JAD-170374. [DOI] [PubMed] [Google Scholar]
- 121.Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19(5):299–310. doi: 10.1038/nrg.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woollard PM, Mehta NAL, Vamathevan JJ, Van Horn S, Bonde BK, Dow DJ. The application of next-generation sequencing technologies to drug discovery and development. Drug Discov Today. 2011;16(11–12):512–519. doi: 10.1016/j.drudis.2011.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.