Abstract
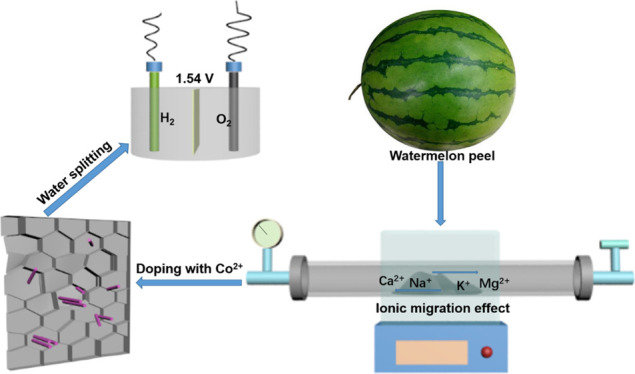
Water splitting is the most potential method to produce hydrogen energy, however, the conventional electrocatalysts encounter the hindrances of high overpotential and low hydrogen production efficiency. Herein, we report a carbon-based nanocomposite (denoted as CCW-x, x stands for the calcination temperature) derived from watermelon peels and CoCl2, and the as-synthesized CCW-x is used as the electrocatalyst. The overpotential and the Tafel slope of CCW-700 for oxygen evolution reaction (OER) is 237 mV at 10 mA cm–2 and 69.8 mV dec–1, respectively, both of which are lower than those of commercial RuO2. For hydrogen evolution reaction (HER), the overpotential of CCW-700 (111 mV) is higher than that of the widely studied Pt/C (73 mV) but still lower than those of lots of carbon-based nanomaterials (122–177 mV). In the light of CCW-700 is highly active for both OER and HER, we assembled a water-splitting electrocatalyst by employing nickel foam loaded with CCW-700 as the anode and cathode in 1 M KOH. The water-splitting voltage is only 1.54 V for the CCW-700//CCW-700 electrodes and 1.62 V for the RuO2//Pt/C ones. Therefore, the so-denoted CCW-x powder possesses good electrocatalytic hydrogen production efficiency.
1. Introduction
As a global problem, energy crisis has urged people to exploit sustainable renewable energy in recent years. Compared with solar energy, wind energy, and tidal energy, hydrogen energy has attracted scientists’ attention because of the less external interference.1 Water splitting is the cleanest and most potential way to produce hydrogen, however, the hydrogen production efficiency is low because of the high overpotential of the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode.2,3
Traditional electrocatalysts mainly include noble metals and their oxides, such as RuO2 and IrO2.4 However, their prohibitive cost and instability in an alkaline medium largely limit their applications, which makes people seek for other materials to replace noble metal series.5−7 As an earth-abundant material, carbon has attracted much attention in the electrocatalyst field because of its low-cost and good electrical conductivity.8,9 Amorphous carbon black, carbon nanotubes, graphene, and mesoporous graphite all have been used as metal-free electrodes, but the overpotential for OER is still high and the hydrogen production efficiency is not ideal.10−13 In order to decrease the overpotential and enhance hydrogen production efficiency of carbon materials, heteroatom doping with transition metal compounds is one of the most efficient ways.14,15 The transition metal compounds can create more active sites to make ions and electrons transfer more quickly.16 Guo et al.17 designed a bimetal–N–C framework (Co0.75Fe0.25) with a low overpotential of 303 mV and a small Tafel slope for OER. Dasog et al.18 synthesized porous Mo2C nanosheets derived from biochar, showing excellent electrocatalyst performances and stable operation for HER.
In this study, we have chosen a biowaste, watermelon peel as the carbon source. Compared with other biomass carbon sources, watermelon peel contains large amounts of alkali metal and alkaline earth metal, which can activate carbon and promote the formation of porous structures via the ionic migration effect at high temperature.19 As the elements in the VIII family, Fe, Co, and Ni have some positive influences on water splitting because of the special electronic structure.20,21 Herein, nanoparticles of cobalt oxide were grown on the carbon derived from watermelon peels to obtain CCW-x nanocomposites. The as-synthesized CCW-700 was used as the electrocatalytic material, and its overpotential for OER was only 237 mV at 10 mA cm–2, which was superior to that of commercial RuO2. In addition, the hydrogen production efficiency was fast on account of the low Tafel slope of 69.8 mV dec–1, as well as good stability within 20 h. Moreover, we assembled the CCW-700//CCW-700 electrocatalyst system and the water-splitting voltage was 1.54 V, which was superior to that of the commercial RuO2//Pt/C system. Our work supplies a facile and sustainable approach for low-cost and high-efficient electrocatalysts in the field of harvesting hydrogen energy by water splitting.
2. Results and Discussion
The microstructures of different samples are shown in Figure 1. The surface of the bulk carbon derived from watermelon peels at 400 °C is smooth (Figure 1a). Porous microstructures (Figure 1b) are formed by activation of the smooth bulk carbon in KOH and subsequent calcination at 700 °C. The abundant alkali and alkaline earth ions in the watermelon peels are supposed to cause an ionic migration effect to promote the formation of such porous microstructures.22 On the porous structures, nanoparticles are deposited by further hydrothermal treatment with CoCl2 (Figure 1c,d). Such nanoparticles are CoO as revealed by X-ray diffraction (XRD; to be discussed later in Figure 2a) and the average diameter is 58 nm or so (Figure 1e). From the transmission electron microscopy (TEM) images of CCW-700 (Figure 1f), we can clearly see that CoO nanoparticles are attached on the biomass carbon substrate. The high-resolution TEM (HR-TEM) image in Figure 1g reveals the crystal stripes of CCW-700; the red mark reflects typical carbon lattice (002) and the distance is 0.34 nm, while the yellow ones represent lattices (111) and (200) of CoO, and the spaces are 0.25 nm and 0.21 nm, respectively. Figure 1h shows the scanning electron microscopy (SEM) image and the elemental map of CCW-800. The uniformly distributed elements of C, O, and P are all originated from watermelon peels and Co is from CoO nanoparticles.
Figure 1.
SEM images of (a) precarbonized powder from watermelon peel at 400 °C; (b) CW-700; (c) CCW-600; (d) CCW-700; and (e) CoO nanoparticles of CCW-700. (f) TEM image of CCW-700. (g) HR-TEM image of CCW-700. (h) SEM and elemental mapping images of CCW-800.
Figure 2.
(a) XRD patterns, (b) Raman spectra, and (c) isothermal adsorption/desorption curves in N2 of CW-700, CCW-600, CCW-700, and CCW-800.
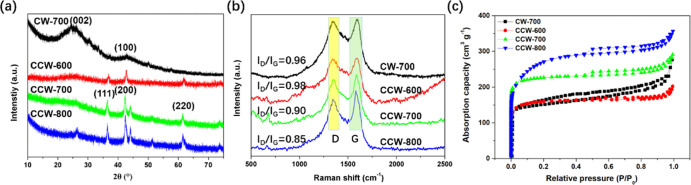
Figure 2a depicts the XRD patterns of CW-700, CCW-600, CCW-700, and CCW-800. CW-700 shows only two obvious peaks at 2θ = 25.8 and 42.8°, corresponding to the (002) and (100) crystal planes of the carbon, respectively. XRD patterns of CCW-x (x = 600, 700, and 800 °C) show three sharp peaks at 2θ = 36.5, 42.3, and 61.3°, which are attributed to the (111), (200), and (220) lattice planes of CoO, respectively. Therefore, we can say that the so-deposited nanoparticles are CoO.23
As shown in Figure 2b, two obvious peaks at 1341 and 1592 cm–1 are ascribed to disordered sp3 (D band) and graphitic sp2 (G band) of carbon, respectively.24 We calculated the intensity ratio of D and G bands (ID/IG), 0.98 for CCW-600, 0.90 for CCW-700, and 0.85 for CCW-800, which represent the degree of carbon disorder. This suggests that as the calcination temperature of CCW-x increased, the degree of graphitization is enhanced.25 Meanwhile, the graphitic degree of CCW-700 is also higher than that of CW-700, indicating that the introduction of CoO can enhance the graphitic degree of biochar materials. It is worth mentioning that the sharp peaks of CoO may cover the carbon lattices of CCW-600 and CCW-700 in Figure 2a, while the graphitic degree of CCW-800 is higher than those of CCW-600 and CCW-700, thus we can clearly see the peak of the (002) lattice in the XRD pattern of CCW-800.
Figure 2c displays the isothermal adsorption/desorption curves of the as-synthesized electrocatalyst materials examined by N2 at 77K. Intriguingly, all of the CCW-x and CW-700 exhibit type I isothermal adsorption/desorption curves, suggesting these carbon materials contain an amount of micropores. It should be noted that the curve of CW-700 shows obvious type H4 hysteresis loops at the middle P/P0, which indicates that mesopores maybe existed in CW-700.26 The specific surface areas calculated through the BET method are 736 m2 g–1 for CW-700, 724 m2 g–1 for CCW-600, 1025 m2 g–1 for CCW-700, and 1331 m2 g–1 for CCW-800, respectively. It follows that the existence of CoO indeed enhances the surface area of carbon derived from watermelon peels at the same calcination temperature.
Figure 3a displays X-ray photoelectron spectroscopy (XPS) survey spectra of samples. All of the CCW-x spectra depict three sharp peaks at 285.0, 531.8, and 781.7 eV, which belong to C 1s, O 1s, and Co 2p, respectively, while CW-700 does not have any peak in the region of 600–800 eV because of no doping of Co. We have separately analyzed C 1s, O 1s, Co 2p, and P 2p peaks of the materials. As can be seen in Figure 3b, the deconvoluted C 1s peaks of CCW-700 located at 284.5, 286.6, and 288.0 eV represent C–C, C–O, and C=O, respectively.27,28Figure 3e displays the complicated peaks of the Co 2p XPS spectra in CCW-700, among them 781.0, 783.8, and 786.6 e are assigned to Co 2p3/2, while 796.7, 798.3, and 803.3 eV are ascribed to Co 2p1/2.29Figure 4d,f shows the Co 2p XPS profiles of CCW-600 and CCW-800, respectively. Compared with CCW-600, Co 2p3/2 and Co 2p1/2 peaks of CCW-700 and CCW-800 shift to left in different degrees, suggesting higher calcination temperature leads to a higher binding energy of CoO.
Figure 3.
XPS survey curves of CW-700, CCW-600, CCW-700, and CCW-800 (a). XPS fitting curves, (b) C 1s of CCW-700, (c) P 2p of CW-700 and CCW-700, (d) Co 2p of CCW-600, (e) Co 2p of CCW-700, and (f) Co 2p of CCW-800.
Figure 4.
The electrocatalyst performances of CCW-600, CCW-700, CCW-800, CW-700, and RuO2 electrodes. (a) LSV curves for OER at the scan rate of 2 mV s–1; (b) Tafel curves for OER; (c) LSV curves for HER at the scan rate of 2 mV s–1; and (d) Tafel curves for HER.
The relative coarse peak in the 130–140 eV region in Figure 3a corresponds to P 2p, which is influenced by the low content in the samples. Figure 3c shows the XPS curve of P 2p of CW-700 and CCW-700, wherein two peaks at 132.9 and 133.9 eV in CCW-700 can be ascribed to P–C and P–O, respectively.30,31 The content of P in CW-700 and CCW-700 is 2.59 and 2.33 at. %, respectively, demonstrating that the doping with Co does not affect the ratio of P in carbon materials. However, the P 2p of CCW-700 slightly shifts toward a higher binding energy compared with CW-700 (Figure 3c), which may be related to doping of transition metal Co.32 Because the electronegativity of P (2.19) is different from that of C (2.55), the existence of P can change the electron distribution of the adjacent carbon skeleton.33,34 In periodic table of elements, phosphorus has the same number of valence electrons as nitrogen, but phosphorus has a larger atomic radius and higher electron-donating ability than nitrogen, which also plays a good role in activation of carbon materials.33 Herein, the calcination temperature exerts an influence on the ratio of P in carbon, 1.48 at. % for CCW-600 and 2.06 at. % for CCW-800, which may also modulate the electrocatalysis properties of the carbon materials.
The OER performances of the as-obtained electrocatalysts and commercial RuO2 were measured in 1 M KOH. The slopes of linear sweep voltammetry (LSV) curves in Figure 4a for CCW-x are all greater than that of RuO2. The overpotential of CCW-700 is only 237 mV at 10 mA cm–2, the result is much lower than that of RuO2 (380 mV). The overpotentials of CCW-600 and CCW-800 are also 300 and 252 mV, respectively. However, the CW-700 electrode almost has no OER capacity when the voltage is lower than 1.8 V, from the flat line in Figure 4a, suggesting that the introduction of CoO in carbon materials has much positive influence on OER. The Co2p curve of CCW-700 has more satellite peaks compared with those of CCW-600 and CCW-800, and the binding energies of CCW-700 and CCW-800 are a little higher than that of CCW-600 (Figure 3d–f), which leads that the overpotentials of CCW-700 and CCW-800 are much lower than that of CCW-600, and CCW-700 exhibits the lowest overpotential of 237 mV. Coincidently, the Tafel curves in Figure 4b reflect the kinetic rate of H2 production, which is in accordance with the data shown in Figure 4a. The Tafel slopes of CCW-600, CCW-700, and CCW-800 are 96.3, 69.8, and 77.2 mV dec–1, respectively, which are all smaller than that of RuO2 (104.7 mV dec–1). It is reported that doping with P tends to hybridize with C, thus increasing the active surface and enhancing the OER performances.35,36 Besides, we also compared the electrochemical impedance of CCW-x and RuO2, the results are shown in Figure 5a. Clearly, CCW-x enable smaller solution resistance (Rs) than that of RuO2 (Rs = 3.25 Ω), and Rs of CCW-700 is only 2.12 Ω. Meanwhile, the charge transfer resistance (Rct) of CCW-700 is 0.50 Ω, which is also smaller than that of RuO2 (0.68 Ω). In addition, we calculated the electrochemically active surface (ECSA) via double layer capacitance (Cdl). The fitted line of current density (j) at various scan rates (0.02, 0.04, 0.06, 0.08, 0.10, and 0.12 V s–1) is shown in Figure 5b, and the slope can be regarded as 2Cdl. ECSA can be calculated according to the following equation
where Cs is 0.04 mF cm–2.37
Figure 5.
(a) Nyquist plots of CCW-600, CCW-700, CCW-800, CW-700, and RuO2 electrodes; (b) plots of current density to scan rate (0.02–0.12 V s–1); (c) ECSA of CCW-600, CCW-700, and CCW-800 electrodes.
According to the equation, ECSAs of CCW-600, CCW-700, and CCW-800 are 219, 489, and 381 cm2, respectively, the change tendency is in agreement with the results of overpotential and the Tafel curve, as shown in Figure 5c. Among them, the ECSA of CCW-700 is superior to others, which also suggests CCW-700 has most active sites.38 The analogous honeycomb structure of CCW-700 exposes more active sites for the electrochemical reaction between the electrode and the electrolyte, and a short path for rapid exchange of ions.39
We also analyzed the HER performances of CCW-x as compared with the Pt/C electrode. Similarly, the CW-700 electrode still has little HER activation effect when the voltage is lower than 0.6 V (Figure 4c). The overpotentials of CCW-600, CCW-700, and CCW-800 are 153, 111, and 94 mV at 10 mA cm–2, respectively. Unfortunately, all the overpotentials of CCW-x are higher than that of Pt/C (73 mV), which is related to weak interaction between hydrogen and Co.40 As shown in Figure 4d, the Tafel slope of CCW-800 and CCW-700 is 66.5 and 93.9 mV dec–1, respectively, both of which are larger than that of Pt/C (45.7 mV dec–1). Although the electrocatalytic properties of CCW-700 and CCW-800 cannot be equal to that of Pt/C, the overpotentials are still lower than those of most carbon nanomaterials as listed in Table 1.23,41−44
Table 1. Comparison of HER Overpotentials with Other Carbon Electrocatalysts in the Three-Electrode System.
In the light of CCW-700 is highly active for both OER and HER, we assembled a water-splitting electrocatalyst by employing nickel foams loaded with CCW-700 as the anode and cathode in 1 M KOH, and the electrocatalytic performances are shown in Figure 6. The water-splitting voltage of the CCW-700//CCW-700 electrocatalyst system is only 1.54 V at 10 mA cm–2, which is lower than that of the commercial RuO2//Pt/C system (1.62 V).45 As depicted in Figure 6b, plenty of bubbles emerged from the nickel foams, O2 on the anode and H2 on the cathode, respectively. Apart from that, we also tested the stability of the CCW-700//CCW-700 electrocatalyst, the results are shown in Figure 6c,d. The primary water-splitting voltage is 1.54 V at 10 mA cm–2, after 20 h, the voltage just increases to 1.56 V (Figure 6c). The I–t plot is shown in Figure 6d, the current still remains 10 mA cm–2 after 10 h, demonstrating good stability of the CCW-700//CCW-700 electrocatalyst system.
Figure 6.
(a) LSV curves for OER at a scan rate of 2 mV s–1 by two-electrode method; (b) picture of the CCW-700//CCW-700 electrocatalyst; (c) V–t plot of the CCW-700//CCW-700 electrocatalyst at 10 mA cm–2; and (d) I–t plot of the CCW-700//CCW-700 electrocatalyst at 1.54 V.
3. Conclusions
In summary, the porous carbon doped by cobalt from the biowaste of watermelon has been produced via simple carbonization and abridged as CCW-x (x stands for the carbonization temperature). Because of the honeycomb-like porous micro-structures and the doping with cobalt, CCW-700 exhibits a lower OER overpotential (237 mV vs. 380 mV for RuO2) and a Tafel slope (69.8 vs 104.7 mV dec–1 for RuO2) as compared with the commercial RuO2. Regarding HER, the overpotential of CCW-700 (111 mV) is unfortunately higher than that of the widely studied Pt/C (73 mV) but still lower than the amounts of carbon-based nanomaterials as listed in Table 1 (122–177 mV). The water-splitting voltage obtained using CCW-700//CCW-700 as the electrode is only 1.54 V and 1.62 V by using the RuO2//Pt/C system. Our work provides a facile and green avenue for fabricating of the biomass carbon materials for hydrogen production.
4. Experimental Section
4.1. Materials
Watermelon was purchased from the market. CoCl2.6H2O (99.99%), RuO2 (99.9%), Pt/C (20% Pt), and 5% Nafion perfluorinated resin were bought from Aladdin chemical company. Ethanol (AR), KOH, and HCl were purchased from Jiangsu Qiangsheng chemical company.
4.2. Preparation
The watermelon was peeled off and cleaned, then chopped into small fragments and dried. The dried fragments were then precarbonized in a tube furnace at 400 °C for 2 h under nitrogen (100 mL min–1) to obtain the carbon powder. 2 g of the so-obtained carbon was immersed into the CoCl2 solution (50 mL, 0.1 M), and then transferred into a hydrothermal reactor and kept at 120 °C for 10 h. Afterward, the as-synthesized carbon and Co composites were activated by KOH (1:3), and then calcined in the tube furnace at 600, 700, and 800 °C for 2 h under the nitrogen (100 mL min–1), respectively. The powder was then dispersed in water and neutralized with HCl until the pH of the dispersion reaches 7 and followed by washing with water and finally dried at 60 °C for 10 h. The so-obtained composite is abridged as CCW-x; the first C stands for carbon, the second C for Co, W for watermelon peel, and x for the calcination temperature. Meanwhile, the CW-700 sample without CoCl2 was also fabricated and set as the control sample.
4.3. Characterization
The microstructures of the so-obtained product were characterized by TEM (JEM-2100) and SEM (Sigma 500). The chemical compositions of the samples were characterized using XRD, BET (2MP, Quantachrome), Raman spectroscopy (XploRA), and XPS (PHI 5000CESCA).
The electrochemical properties of the product were assessed by electrochemical workstation (Chenhua, CHI 760E) by measurements of LSV, cyclic voltammetry, chronopotentiometry, and electrochemical impedance spectroscopy. The nickel foam loaded with the powder was used as the testing electrode, the graphite counter electrode and the Hg/HgO reference electrode were also used. Aqueous solution of KOH (1 M) was used as the electrolyte. The working electrode was assembled as follows. 2 mg of powder was dispersed in a mixture of water, ethanol, and Nafion with a volume ratio of 2:7:1. Then, 40 μL of the dispersion was coated on a piece of nickel foam (1 × 1 cm) with a mass loading of 0.4 mg cm–2.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31800495 and 21908086), the Natural Science Foundation of Jiangsu Province (BK20181040), Natural Science Foundation of the Jiangsu Higher Education Institutions (19KJB610011), and China Postdoctoral Science Foundation (2019M653654).
The authors declare no competing financial interest.
References
- Jamesh M.-I.; Sun X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting - A review. J. Power Sources 2018, 400, 31–68. 10.1016/j.jpowsour.2018.07.125. [DOI] [Google Scholar]
- Kuang M.; Han P.; Wang Q.; Li J.; Zheng G. CuCo Hybrid Oxides as Bifunctional Electrocatalyst for Efficient Water Splitting. Adv. Funct. Mater. 2016, 26, 8555–8561. 10.1002/adfm.201604804. [DOI] [Google Scholar]
- Han G.-Q.; Shang X.; Lu S.-S.; Dong B.; Li X.; Liu Y.-R.; Hu W.-H.; Zeng J.-B.; Chai Y.-M.; Liu C.-G. Electrodeposited MoSx films assisted by liquid crystal template with ultrahigh electrocatalytic activity for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 5132–5138. 10.1016/j.ijhydene.2017.01.009. [DOI] [Google Scholar]
- Abbott D. F.; Pittkowski R. K.; Macounová K.; Nebel R.; Marelli E.; Fabbri E.; Castelli I. E.; Krtil P.; Schmidt T. J. Design and Synthesis of Ir/Ru Pyrochlore Catalysts for the Oxygen Evolution Reaction Based on Their Bulk Thermodynamic Properties. ACS Appl. Mater. Interfaces 2019, 11, 37748–37760. 10.1021/acsami.9b13220. [DOI] [PubMed] [Google Scholar]
- Schäfer H.; Chevrier D. M.; Zhang P.; Stangl J.; Mueller-Buschbaum K.; Hardege J. D.; Kuepper K.; Wollschlaeger J.; Krupp U.; Duehnen S.; Steinhart M.; Walder L.; Sadaf S.; Schmidt M. Electro-Oxidation of Ni42 Steel: A Highly Active Bifunctional Electrocatalyst. Adv. Funct. Mater. 2016, 26, 6402–6417. 10.1002/adfm.201601581. [DOI] [Google Scholar]
- Lv L.; Yang Z.; Chen K.; Wang C.; Xiong Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. 10.1002/aenm.201803358. [DOI] [Google Scholar]
- Park J.; Cho J. Advances in Understanding Mechanisms of Perovskites and Pyrochlores as Electrocatalysts using In-Situ X-ray Absorption Spectroscopy. Angew. Chem., Int. Ed. Engl. 2020, 59, 15314–15324. 10.1002/anie.202000768. [DOI] [PubMed] [Google Scholar]
- Wang Y.-J.; Fang B.; Zhang D.; Li A.; Wilkinson D. P.; Ignaszak A.; Zhang L.; Zhang J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal-Air Batteries. Electrochem. Energy Rev. 2018, 1, 1–34. 10.1007/s41918-018-0002-3. [DOI] [Google Scholar]
- Wu G.; Santandreu A.; Kellogg W.; Gupta S.; Ogoke O.; Zhang H.; Wang H.-L.; Dai L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. 10.1016/j.nanoen.2015.12.032. [DOI] [Google Scholar]
- Kang B. K.; Im S. Y.; Lee J.; Kwag S. H.; Kwon S. B.; Tiruneh S.; Kim M.-J.; Kim J. H.; Yang W. S.; Lim B.; Yoon D. H. In-situ formation of MOF derived mesoporous Co3N/amorphous N-doped carbon nanocubes as an efficient electrocatalytic oxygen evolution reaction. Nano Res. 2019, 12, 1605–1611. 10.1007/s12274-019-2399-3. [DOI] [Google Scholar]
- Wang J.-Y.; Liu W.-T.; Li X.-P.; Ouyang T.; Liu Z.-Q. Strong hydrophilicity NiS2/Fe7S8 heterojunctions encapsulated in N-doped carbon nanotubes for enhanced oxygen evolution reaction. Chem. Commun. 2020, 56, 1489–1492. 10.1039/c9cc09303f. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang X.; Yang Z.; Yan S.; Zhang C.; Liu S. Space-Confined Synthesis of Lasagna-like N-Doped Graphene-Wrapped Copper-Cobalt Sulfides as Efficient and Durable Electrocatalysts for Oxygen Reduction and Oxygen Evolution Reactions. ACS Sustainable Chem. Eng. 2020, 8, 1004–1014. 10.1021/acssuschemeng.9b05710. [DOI] [Google Scholar]
- Popovicheva O.; Timofeev M.; Persiantseva N.; Jefferson M. A.; Johnson M.; Rogak S. N.; Baldelli A. Microstructure and Chemical Composition of Particles from Small-scale Gas Flaring. Aerosol Air Qual. Res. 2019, 19, 2205–2221. 10.4209/aaqr.2019.04.0177. [DOI] [Google Scholar]
- Tang C.; Titirici M.-M.; Zhang Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093. 10.1016/j.jechem.2017.08.008. [DOI] [Google Scholar]
- Huang X.; Shen T.; Zhang T.; Qiu H.; Gu X.; Ali Z.; Hou Y. Efficient Oxygen Reduction Catalysts of Porous Carbon Nanostructures Decorated with Transition Metal Species. Adv. Energy Mater. 2019, 10, 1900375. 10.1002/aenm.201900375. [DOI] [Google Scholar]
- Wang Y.-J.; Fan H.; Ignaszak A.; Zhang L.; Shao S.; Wilkinson D. P.; Zhang J. Compositing doped-carbon with metals, non-metals, metal oxides, metal nitrides and other materials to form bifunctional electrocatalysts to enhance metal-air battery oxygen reduction and evolution reactions. Chem. Eng. J. 2018, 348, 416–437. 10.1016/j.cej.2018.04.208. [DOI] [Google Scholar]
- Feng X.; Bo X.; Guo L. CoM(M=Fe,Cu,Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 2018, 389, 249–259. 10.1016/j.jpowsour.2018.04.027. [DOI] [Google Scholar]
- Humagain G.; MacDougal K.; MacInnis J.; Lowe J. M.; Coridan R. H.; MacQuarrie S.; Dasog M. Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2018, 8, 1801461. 10.1002/aenm.201801461. [DOI] [Google Scholar]
- Zhang Y.; Liu S.; Zheng X.; Wang X.; Xu Y.; Tang H.; Kang F.; Yang Q.-H.; Luo J. Biomass Organs Control the Porosity of Their Pyrolyzed Carbon. Adv. Funct. Mater. 2017, 27, 1604687. 10.1002/adfm.201604687. [DOI] [Google Scholar]
- Stelmachowski P.; Monteverde Videla A. H. A.; Jakubek T.; Kotarba A.; Specchia S. The Effect of Fe, Co, and Ni Structural Promotion of Cryptomelane (KMn8O16) on the Catalytic Activity in Oxygen Evolution Reaction. Electrocatalysis 2018, 9, 762–769. 10.1007/s12678-018-0488-9. [DOI] [Google Scholar]
- Xu J.; Li J.; Xiong D.; Zhang B.; Liu Y.; Wu K.-H.; Amorim I.; Li W.; Liu L. Trends in activity for the oxygen evolution reaction on transition metal (M = Fe, Co, Ni) phosphide pre-catalysts. Chem. Sci. 2018, 9, 3470–3476. 10.1039/c7sc05033j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli A.; Trivanovic U.; Sipkens T. A.; Rogak S. N. On determining soot maturity: A review of the role of microscopy- and spectroscopy-based techniques. Chemosphere 2020, 252, 126532. 10.1016/j.chemosphere.2020.126532. [DOI] [PubMed] [Google Scholar]
- Hu Q.; Li G.; Han Z.; Wang Z.; Huang X.; Chai X.; Zhang Q.; Liu J.; He C. General Synthesis of Ultrathin Metal Borate Nanomeshes Enabled by 3D Bark-Like N-Doped Carbon for Electrocatalysis. Adv. Energy Mater. 2019, 9, 1901130. 10.1002/aenm.201970109. [DOI] [Google Scholar]
- Baldelli A.; Rogak S. N. Morphology and Raman spectra of aerodynamically classified soot samples. Atmos. Meas. Tech. 2019, 12, 4339–4346. 10.5194/amt-12-4339-2019. [DOI] [Google Scholar]
- Chen Q.; Tan X.; Liu Y.; Liu S.; Li M.; Gu Y.; Zhang P.; Ye S.; Yang Z.; Yang Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. 10.1039/c9ta11618d. [DOI] [Google Scholar]
- Yang Z.; Xiang M.; Zhu W.; Hui J.; Qin H. Biomass Heteroatom Carbon/Cerium Dioxide Composite Nanomaterials Electrode for High-Performance Supercapacitors. ACS Sustainable Chem. Eng. 2020, 8, 6675–6681. 10.1021/acssuschemeng.0c00188. [DOI] [Google Scholar]
- Peng Z.; Zou Y.; Xu S.; Zhong W.; Yang W. High-Performance Biomass-Based Flexible Solid-State Supercapacitor Constructed of Pressure-Sensitive Lignin-Based and Cellulose Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 22190–22200. 10.1021/acsami.8b05171. [DOI] [PubMed] [Google Scholar]
- Peng H.; Yao B.; Wei X.; Liu T.; Kou T.; Xiao P.; Zhang Y.; Li Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. 10.1002/aenm.201803665. [DOI] [Google Scholar]
- Menezes P. W.; Panda C.; Walter C.; Schwarze M.; Driess M. A Cobalt-Based Amorphous Bifunctional Electrocatalysts for Water-Splitting Evolved from a Single-Source Lazulite Cobalt Phosphate. Adv. Funct. Mater. 2019, 29, 1808632. 10.1002/adfm.201808632. [DOI] [Google Scholar]
- Chen Z.; Zhong H.; Hu W.; Yin H.; Cao G.; Wen H.; Wang J.; Wang P. Highly dispersed Ni2–MoP nanoparticles on oxygen-defect-rich NiMoO4– nanosheets as an active electrocatalyst for alkaline hydrogen evolution reaction. J. Power Sources 2019, 444, 227311. 10.1016/j.jpowsour.2019.227311. [DOI] [Google Scholar]
- Tan H.; Zhao Y.; Xia W.; Zhao J.; Xu X.; Wood K.; Sugahara Y.; Yamauchi Y.; Tang J. Phosphorus- and Nitrogen-Doped Carbon Nanosheets Constructed with Monolayered Mesoporous Architectures. Chem. Mater. 2020, 32, 4248–4256. 10.1021/acs.chemmater.0c00731. [DOI] [Google Scholar]
- Liu Y.-T.; Tang L.; Dai J.; Yu J.; Ding B. Promoted Electrocatalytic Nitrogen Fixation in Fe-Ni Layered Double Hydroxide Arrays Coupled to Carbon Nanofibers: The Role of Phosphorus Doping. Angew. Chem., Int. Ed. Engl. 2020, 59, 13623. 10.1002/anie.202005579. [DOI] [PubMed] [Google Scholar]
- Yang N.; Zheng X.; Li L.; Li J.; Wei Z. Influence of Phosphorus Configuration on Electronic Structure and Oxygen Reduction Reactions of Phosphorus-Doped Graphene. J. Phys. Chem. C 2017, 121, 19321–19328. 10.1021/acs.jpcc.7b06748. [DOI] [Google Scholar]
- Zhang X.; Lu Z.; Fu Z.; Tang Y.; Ma D.; Yang Z. The mechanisms of oxygen reduction reaction on phosphorus doped graphene: A first-principles study. J. Power Sources 2015, 276, 222–229. 10.1016/j.jpowsour.2014.11.105. [DOI] [Google Scholar]
- Zhu Y. P.; Jing Y.; Vasileff A.; Heine T.; Qiao S.-Z. 3D Synergistically Active Carbon Nanofibers for Improved Oxygen Evolution. Adv. Energy Mater. 2017, 7, 1602928. 10.1002/aenm.201602928. [DOI] [Google Scholar]
- Jorge A. B.; Jervis R.; Periasamy A. P.; Qiao M.; Feng J.; Tran L. N.; Titirici M. M. 3D Carbon Materials for Efficient Oxygen and Hydrogen Electrocatalysis. Adv. Energy Mater. 2020, 10, 1902494. 10.1002/aenm.201902494. [DOI] [Google Scholar]
- McCrory C. C. L.; Jung S.; Peters J. C.; Jaramillo T. F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. 10.1021/ja407115p. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Ge Z.; Li X.; Zhao J.; Ma B.; Chen Y. Cu2S nanorod arrays with coarse surfaces to enhance the electrochemically active surface area for water oxidation. J. Colloid Interface Sci. 2020, 567, 308–315. 10.1016/j.jcis.2020.02.030. [DOI] [PubMed] [Google Scholar]
- Peng L.; Wang Y.; Masood I.; Zhou B.; Wang Y.; Lin J.; Qiao J.; Zhang F.-Y. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction. Appl. Catal., B 2020, 264, 118447. 10.1016/j.apcatb.2019.118447. [DOI] [Google Scholar]
- Zheng Y.; Jiao Y.; Jaroniec M.; Qiao S. Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem., Int. Ed. Engl. 2015, 54, 52–65. 10.1002/anie.201407031. [DOI] [PubMed] [Google Scholar]
- Chen W.-F.; Iyer S.; Iyer S.; Sasaki K.; Wang C.-H.; Zhu Y.; Muckerman J. T.; Fujita E. Biomass-derived electrocatalytic composites for hydrogen evolution. Energy Environ. Sci. 2013, 6, 1818–1826. 10.1039/c3ee40596f. [DOI] [Google Scholar]
- Feng X.; Bo X.; Guo L. An advanced hollow bimetallic carbide/nitrogen-doped carbon nanotube for efficient catalysis of oxygen reduction and hydrogen evolution and oxygen evolution reaction. J. Colloid Interface Sci. 2020, 575, 69–77. 10.1016/j.jcis.2020.04.093. [DOI] [PubMed] [Google Scholar]
- Li C.; Wang J.; Wang Y.; Li J.; Yao Z.; Jiang Z. Enhancing Hydrogen Evolution Reaction by Synergistically Coupling NiMo Alloy with Ni(OH)2 Nanosheet on Carbon Cloth. Chemistryselect 2020, 5, 6774–6779. 10.1002/slct.202000955. [DOI] [Google Scholar]
- Zhang P.; Wang R.; Xiao T.; Chang Z.; Fang Z.; Zhu Z.; Xu C.; Wang L.; Cheng J. The High-Performance Bifunctional Catalyst Pd/Ti3C2Tx-Carbon Nanotube for Oxygen Reduction Reaction and Hydrogen Evolution Reaction in Alkaline Medium. Energy Technol. 2020, 8, 2000306. 10.1002/ente.202000306. [DOI] [Google Scholar]
- Baldelli A.; Ou J.; Li W.; Amirfazli A. Spray-On Nanocomposite Coatings: Wettability and Conductivity. Langmuir 2020, 36, 11393–11410. 10.1021/acs.langmuir.0c01020. [DOI] [PubMed] [Google Scholar]