Abstract

The thyroid gland and thyroid hormones control a multitude of homeostatic functions including maintenance of fluid and electrolyte balance and normal functioning of the kidneys. Thyroid dysfunction alters the sytemic hemodynamic and metabolic balance, thereby affecting the kidney. In this study, we aimed to identify and characterize the urinary proteome of the patients with hypothyroidism. An untargeted proteomic approach with network analysis was used to identify changes in total urinary proteome in patients with newly diagnosed overt hypothyroidism. Urine samples were collected from nine age-matched patients’ before and after l-thyroxine treatment. Differences in the abundance of urinary proteins between hypothyroid and euthyroid states were determined using a two-dimensional difference in gel electrophoresis (2D-DIGE) coupled to matrix-assisted laser desorption and ionization time-of-flight (MALDI TOF) mass spectrometry. Alterations in the abundance of urinary proteins, analyzed by Progenesis software, revealed statistically significant differential abundance in a total of 49 spots corresponding to 42 proteins, 28 up and 14 down (≥1.5-fold change, analysis of variance (ANOVA), p ≤ 0.05). The proteins identified in the study are known to regulate processes related to transport, acute phase response, oxidative stress, generation of reactive oxygen species, cellular proliferation, and endocytosis. Bioinformatic analysis using Ingenuity Pathway Analysis (IPA) identified dysregulation of pathways related to amino acid metabolism, molecular transport, and small-molecule biochemistry and involved the MAPK kinase, vascular endothelial growth factor (VEGF), PI3 kinase/Akt, protein kinase C (PKC), signaling pathways. The identified proteins were involved in the regulation of thyroglobulin (Tg) and thyrotropin (TSH) metabolism. Alterations in their levels indicate the presence of a compensatory mechanism aimed at increasing the regulation of Tg in the hypothyroid state.
1. Introduction
The thyroid gland is the master controller of a multitude of homeostatic functions including development, differentiation, proliferation, and nearly all of the physiological and metabolic activities of the human body. A strong interrelationship is known to exist between the thyroid hormone (TH) and the kidney due to their entwined interactions, including control of fluid and electrolyte balance. TH plays an important role in kidney growth and development, its normal physiological function, renal blood flow, glomerular filtration rate (GFR), tubular function, and water and electrolyte homeostasis.1 Kidney in turn plays a crucial role in both the metabolism and elimination of thyroid hormone (TH) and its metabolites. Likewise, patients with chronic kidney disease (CKD) or those with nephrotic syndrome are known to present with hypothyroidism and/or subclinical hypothyroidism that is characterized as a low T3 syndrome and considered part of an atypical nonthyroidal illness.2−4 It is understandable that thyroid dysfunction and its effects on the tissues and kidney due to altered TH metabolic activity will be reflected by changes in the secretion and elimination of proteins in the urine.
TH directly influences the filtering capacity of the kidneys and formation of urine by regulating the activity of the renin–angiotensin–aldosterone system and tubular absorption of bicarbonate, sodium, chloride, and water.5 Hypothyroidism, or low TH levels, pathologically changes the kidney morphology, specifically the nephron through thickening of the glomerular basement membrane, expansion of the mesangial matrix, and loss of medullary hypertonicity. These alterations lead to impairments in urinary concentrating capability, reduction in renal blood flow, decrease in GFR, and increased glomerular capillary permeability to proteins.6−8 The urinary proteins reflect changes in proteins arising from the tissues and circulation (30%) in addition to those proteins arising from the glomerulus, renal tubules, and the urinary tract (70%).9 The consequent proteinuria along with the proteolytic fragments, generated from small circulating proteins or peptides and vesicles, passing through the glomerulus change the urinary proteome due to the hypothyroid state. Although thyroid dysfunction is extensively studied, the differences characterizing the proteome of these patients are limited and more commonly carried out in the plasma. In our previous study, we described the changes in the plasma proteome of patients in the hypothyroid and euthyroid states. Although analyzing serum helps us to identify the changes, it still remains challenging due to its dynamic range of abundant proteins and the complex composition of blood serum.10 We did not find any study to date that has characterized the urinary proteome alterations with hypothyroidism.
Proteomic profiling or whole-expression proteomics, using a two-dimensional difference in gel electrophoresis (2D-DIGE), allows the determination of differences in the proteomes between normal and disease conditions. The technique is advantageous over the traditional two-dimensional polyacrylamide gel electrophoresis (PAGE) as the fluorescence pre-labeling of proteins increases the sensitivity and detection of low-abundance proteins, and the use of pooled internal standard allows for quantitative accuracy and reduces gel-to-gel variability.11 Being an ultrafiltrate of the plasma, urine is less complex than the plasma and a more appropriate diagnostic biological fluid that can be easily studied.2,3 Urine proteomics is a noninvasive technique that employs a simple mode of sampling to characterize the disease-related proteome.12,13 Several authors including our group have studied differences in urinary proteome between normal and disease states, in renal and nonrenal systemic conditions,14−18 and for identifying biomarkers of cancer.19−23 The influence of thyroid dysfunction on the urinary proteome and urine was assessed in the majority of the studies using rodent models.24 Although these studies have undoubtedly added to our understanding of TH action on metabolism, translation of these results to humans is still missing.
In this study, we aimed to characterize the urinary proteome of the patients with hypothyroidism, before and after treatment with l-thyroxine using quantitative 2D-DIGE followed by matrix-assisted laser desorption and ionization time-of-flight (MALDI TOF) mass spectrometry (MS). Identification of these urinary proteins and their interactions will help understand the biochemical and physiological changes, their associations with the disease state, and can further help identify urinary protein biomarkers of the disease.
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Declaration of Helsinki and the universal ICH-GCP guidelines. The study protocol was approved by the Institutional Review Board, College of Medicine, King Saud University Hospital (no E-10-172). Written informed consent was obtained from all participants.
2.2. Study Design and Participant Selection
We studied nine patients (six females and three males, age: 39 ± 12.9 years) who were referred to our endocrine outpatient clinic at KKUH with newly diagnosed overt hypothyroidism. Blood samples were obtained from each patient after 10 h fasting and before starting thyroxine (pretreatment sample). The sample size was determined by carrying out a power analysis using the Progenesis SameSpots nonlinear dynamics statistical software for the determination of the minimum number of required biological replicate (Figure S1, Supporting information). Hypothyroidism was defined as a thyrotropin (TSH) level higher than 10 mIU/L and FT4 levels lower than 12 pmol/L. Samples were obtained from each patient at two time points: pretreatment samples (hypothyroid) were collected before starting treatment and the post-treatment samples (euthyroid) were obtained from those with TSH level normalized after treatment with the appropriate dose of l-thyroxine treated 6 weeks duration or until the thyroid hormones normalized to euthyroid levels. None of the patients recruited in the study had a history of hypertension, diabetes mellitus, inflammatory, or other autoimmune. Blood samples were collected after a standard 10 h fasting by venipuncture into ethylenediaminetetraacetic acid (EDTA)-coated tubes, and plasma was obtained by centrifugation (15 min, 3000g), then aliquoted, and stored in multiple aliquots at −80 °C until analysis.25
2.3. Urine Collection and Protein Extraction
Midstream spot urine samples (50–100 mL) were obtained from participants after a standard 10 h fasting, into a sterile urine container and transported immediately on ice to prevent microbe contamination and proteolysis. Urine analysis was carried out to determine the presence of urinary protein, infection, sugar, or occult blood using a urine test strip (Combur10 Test, Roche).
The samples were then processed and insoluble materials were removed by centrifugation at 2000g (4000 rpm) at 4 °C for 10 min, within 30 min of collection, to prevent protein release from these artifacts. The supernatant (5 mL) was carefully removed, aliquoted, and stored at −80 °C for long-term storage. Proteins were isolated from the urine samples, as described previously.26 The protein pellets were solubilized in labeling buffer (7 M urea, 2 M thiourea, 30 mM Tris–HCl, and 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), pH 8.5). Insoluble material was pelleted by centrifugation (12 000g, room temperature (RT), 5 min), and protein concentrations were determined in triplicate using the 2D-Quant kit (GE Healthcare).
2.4. Two-Dimensional DIGE and MALDI TOF/TOF MS Analysis
DIGE analysis was performed to determine differentially expressed proteins between the hypothyroid vs euthyroid groups as described.25 Briefly, proteins extracted (50 μg) from each sample were labeled with either Cy3 or Cy5, respectively. Labeling was performed for 30 min on ice in the dark. Reactions were then quenched by the addition of 1 μL of lysine (10 mM) for 10 min on ice in the dark. A mixture of equal amounts of protein isolated from each sample in the experiment was labeled with Cy2 and used as an internal standard (see Table S1, Supporting Information). After 2D-DIGE, the gels were scanned on a Typhoon 9410 scanner with Ettan DALT gel alignment guides using excitation/emission wavelengths specific for Cy2 (488/520 nm), Cy3 (532/580 nm), and Cy5 (633/670 nm). DIGE images were analyzed using Progenesis Samespots v3.3 software (Nonlinear Dynamics Ltd., U.K.). Independent direct comparisons were made between hypothyroid and euthyroid states, fold change was calculated, and p-values were determined using one-way analysis of variance (ANOVA) to quantify differential expression. All spots were prefiltered and manually checked before applying the statistical criteria (ANOVA test, p ≤ 0.05 and fold change ≥1.5). Furthermore, the spots showing a statistical significance between the groups were manually excised and in-gel digestion with trypsin was carried out as described earlier.25,27 Briefly, preparative gel, total protein (1 mg) was obtained from a pool of equal protein amounts of the 20 urine samples. This sample was denatured in lysis buffer, then mixed in a rehydration buffer. Then, the protein samples were separated by first and second dimensions with same conditions in the DIGE section. Then, the gels were fixed in 40% (v/v) ethanol containing 10% (v/v) acetic acid (overnight) and then washed (3×, 30 min each, ddH2O). The gels were incubated (1 h, 34% (v/v) CH3OH containing 17% (w/v) ammonium sulfate and 3% (v/v) phosphoric acid) prior to the addition of 0.5 g/L Coomassie G-250. After 5 days, the stained gels were briefly rinsed with Milli-Q water and stored until the spots could be picked and identified by MS. MALDI-MS(/MS) data were obtained using an UltraflexTerm time-of-flight (TOF) mass spectrometer equipped with an LIFT-MS/MS device (Bruker Daltonics) instrument as described.25,27−29
Coomassie-stained gel spots were excised manually, washed, and digested according to previously described methods. Briefly, the mixture of tryptic peptides (0.5 μL) derived from each protein was spotted onto a MALDI target (384 anchorchip MTP 800 μm Anchorchip; Bruker Daltonik, Germany) together with 0.5 μL of matrix (10 mg of α-cyano-4-hydroxycinnamic acid (CHCA) in 1 mL of 30% CH3CN and 0.1% aqueous CF3COOH) and left to dry (RT) before MS analysis. Spectra were acquired on a MALDI-TOF MS (UltraFlexTrem, Bruker Daltonics, Germany) in the positive mode (target voltage, 25 kV; pulsed ion extraction voltage, 20 kV). The reflector voltage was set to 21 kV, and the detector voltage was set to 17 kV. Peptide mass fingerprints (PMFs) were calibrated against a standard mixture by assigning appropriate monoisotopic masses to the peaks: i.e., bradykinin (1–7), m/z 757.399; angiotensin I, m/z 1296.685; angiotensin II, m/z 1046.54; rennin-substrate, m/z 1758.93; ACTH clip (1–17), m/z 2093.086; and somatostatin, m/z 3147.471 (peptide calibration standard II, Bruker Daltonics, Germany). The PMFs were processed using Flex Analysis software (version 2.4, Bruker Daltonics, Germany), and the SNAP algorithms were used for peak detection (S/N, 3; maximum number of peaks, 100; quality factor threshold, 30). MS spectra were recorded automatically across the mass range m/z of 700–3000, and the spectra were typically the sum of 400 laser shots. MS data were interpreted using BioTools v3.2 (Bruker Daltonics, Germany), together with the Mascot search algorithm (version 2.0.04 updated 09/05/2018; Matrix Science Ltd., U.K.). Mascot parameters were as follows: fixed cysteine modification with propionamide, variable modification due to methionine oxidation, one missed cleavage site (i.e., in the case of incomplete trypsin hydrolysis), and a mass tolerance of 100 ppm. Identified proteins were accepted as correct if they showed a Mascot score greater than 56 and p < 0.05. Not all spots of interest could be identified because some proteins were of low abundance and did not yield sufficiently intense mass fingerprints, whereas others were mixtures of multiple proteins. The detailed information about protein identification is provided in Table S2, Supporting Information.
2.5. Immunoblotting
To independently confirm the findings of the 2D-DIGE studies, statistically significant proteins with differential abundance were selected and examined by immunoblotting. Monoclonal antibodies against transferrin (mouse, cat # SC-365871), retinol-binding protein (RBP, mouse, cat # SC-69795), and β-actin (goat, N-18, cat # SC-1616), were purchased from Santa Cruz Biotechnology (Santa Cruz). An equal amount of protein from each sample (50 μg) was separated by one-dimensional discontinuous slab gel electrophoresis (12% sodium dodecyl sulfate (SDS)-polyacrylamide gel). Proteins were electrotransferred to an Immobilon-P, poly(vinylidene difluoride) (PVDF) transfer membrane (Millipore) using a mini trans-blot electrotransfer cell (BioRad). Following the transfer, the membrane was stained with Ponceau-S to confirm the transfer efficiency. The membrane was then blocked (5% fat-free milk (FFM) in Tris-buffered saline (TBS), 1 h, RT), and rinsed (three changes of TBS-T in 10 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20 buffer). The samples were then incubated with the specified primary antibodies (1:200 dilution) in a blocking buffer. Blots were incubated with the appropriate immunoglobulin G (IgG)-horseradish peroxidase (HRP)-conjugated secondary antibody, the immunoreactive bands were detected by enhanced chemiluminescence (ECL, Thermo Scientific), visualized by scanning with Fluorchem Q (Cell Biosciences), and digitalized using the image analysis software AlphaView Q 3.0 (Cell Biosciences).
2.6. Bioinformatic Analysis: Functional Classification of Proteins and Pathway Analysis
The successfully identified protein dataset was next uploaded into the Ingenuity Pathway Analysis (IPA) software program (Ingenuity Systems, http://www.ingenuity.com) to determine the protein interactions and pathways, depicted as networks, most strongly associated with them. The associations are determined by overlaying the experimental expression data on networks constructed from published interactions. The identified proteins were additionally grouped and classified into different categories based on their molecular function and biological processes using the information provided in the Gene Ontology (GO) database (http://www.geneontology.org/).
2.7. Statistical Analyses
The results on the biochemical parameters in the hypothyroid and euthyroid group are presented as mean ± standard deviation (SD), and significant differences between the mean values were assessed using Student’s t-test. All statistical analyses were conducted using GraphPad Prism software, version 5.0 for Windows (GraphPad software, San Diego, CA).
Statistical analyses for gel image analysis: raw gel images were uploaded into Progenesis “SameSpots” software (Nonlinear Dynamics, U.K.) and an automated spot detection method was performed. Three different experimental designs were set in Progenesis for spot detection. One design compared between the hypothyroid and euthyroid groups at each sample automatic analysis was performed to detect all of the spots in all of the gels samples. Each selected spot was verified and manually edited if necessary. Normalized volumes were used to identify spots that were differentially expressed. A cutoff ratio greater than 1.5-fold was imposed. Student’s t-test was used to calculate statistically significant differences between groups. p < 0.05 was considered statistically significant. A principal component analysis (PCA) of the log-transformed spot data was performed.
3. Results and Discussion
3.1. Anthropometric and Biochemical Data
The parameters and biochemical data of the recruited patients are summarized in Table S3. As expected, statistically significant changes (p-value <0.001) in biochemical profile were noted for FT4 and TSH values after becoming euthyroid post-treatment with l-thyroxine.
3.2. Two-Dimensional DIGE Analysis and Identification of Differentially Expressed Proteins
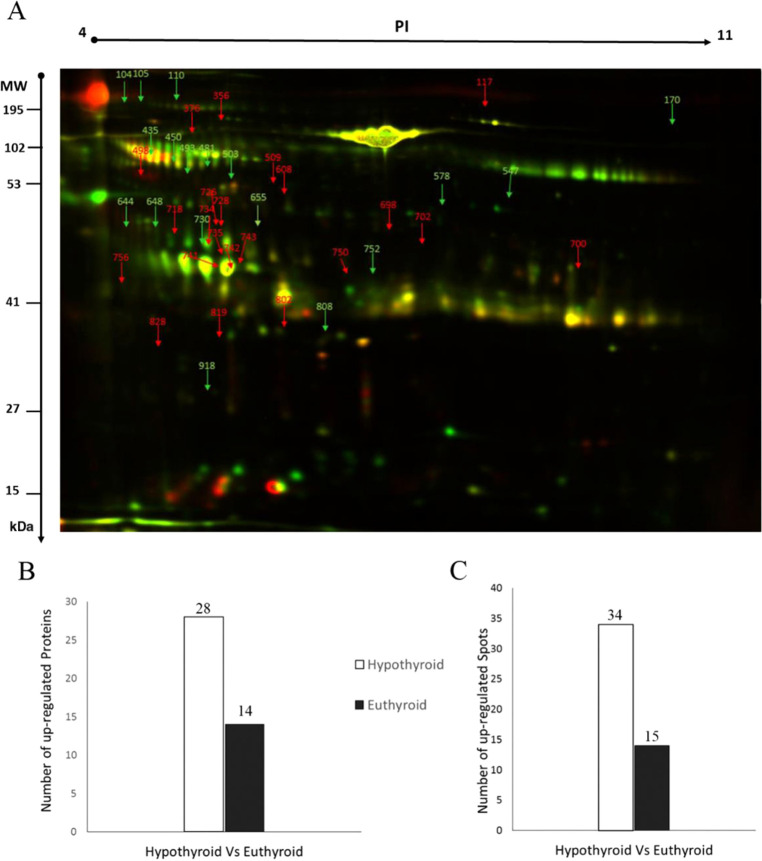
In this study, we evaluated differential protein expression in pairwise samples from nine hypothyroid and nine euthyroid urine samples (18 samples from 9 gels) through analytical 2D-DIGE technique followed by statistical analysis using Progenesis software. For these nine pairs of biological replicates, the internal standard was composed of all 18 samples. A representative 2D-DIGE image for one pair of samples is shown in Figure 1: Cy3 (Euth) (A), Cy5 (Hypo) (B), and Cy2 (pooled internal standard) (C).
Figure 1.
Two-dimensional DIGE analysis and identification of differentially expressed proteins. Representative fluorescent protein profiles of a 2D-DIGE containing euthyroid samples labeled with Cy3 (A), hypothyroid labeled with Cy5 (B), and pooled internal control labeled with Cy2 (C). Urine proteins were separated on immobilized pH gradient (IPG) strip (pH 3–11) in the first dimension followed by 12.5% PAGE in the second-dimensional gel electrophoresis. Images were captured using a Typhoon 9400 variable mode image.
All gel images were matched, aligned, and considered for analysis. We detected a total of 1100 matched protein spots present consistently in all individual gels. A total of 49 protein spots showed a significant differential change in protein abundance (ANOVA test, p ≤ 0.05, and fold change ≥1.5) between the two groups. The spot patterns across all of the nine gels had high reproducibility permitting further analysis. In some cases, variants of the same protein were found at several locations on the gel. MALDI-TOF mass spectrometry found these 42 spots to be unique protein sequences that were matched to entries in the SWISS-PROT database by Mascot with high confidence scores (Figure 2A, Tables S2 and S4, Supporting Information). Peptide mass fingerprints (PMFs) successfully identified 42 out of the 49 protein spots that are for 28 proteins out of 34 differential expressed upregulated protein spots, and 14 proteins out of 15 differential expressed downregulated protein spots (Figure 2B,C). Among 42 proteins, 28 protein spots were upregulated and 14 were downregulated in hypothyroid compared to the euthyroid state (Figure 2A and Table 1). In Figure 2A, the arrow indicates the differentially abundant spots that are either decreased (green arrow) or increased (red arrow) between the groups, and the yellow spots represent proteins with the same isoelectric point, molecular weight, and nearly equal fluorescence intensity. We were not able to determine and pick all differential expressed protein spots on the Coomassie-stained gel.28 The significantly upregulated proteins in the hypothyroid state were identified as serum albumin (up 1.9-fold, p = 0.009), serotransferrin (up 4.5-fold, p = 0.044), huntingtin-interacting protein M (up 3.3-fold, p = 0.002), V-type proton ATPase subunit B, brain isoform (up 2.3-fold, p = 0.006), β-1,3-galactosyl-O-glycosyl-glycoprotein β-1,6-N-acetylglucosaminyltransferase 3 (up 2.8-fold, p = 0.028), E3 ubiquitin-protein ligase DCST1 (up 2.4-fold, p = 0.014), cathepsin D (up 2.5-fold, p = 0.026), putative E3 ubiquitin-protein ligase UBR7 (up 1.9-fold, p = 0.008), keratin, type I cytoskeletal 10 (up 1.5-fold, p = 0.016), probable E3 ubiquitin-protein ligase TRIML2 (up 1.5-fold, p = 0.036), AN1-type zinc finger protein 6 (up 2.1-fold, p = 0.0.38), keratin, type II cytoskeletal 5 (up 1.6-fold, p = 0.044), protein AMBP (up 2.8-fold, p = 0.021), vesicular integral membrane protein VIP36 (up 2.2-fold, p = 0.018), kininogen-1 (up 1.8-fold, p = 0.0015), cleft lip and palate transmembrane protein 1-like protein (up 1.7-fold, p = 0.021), zinc finger protein 839 (up 2.5-fold, p = 0.026), homogentisate 1,2-dioxygenase (up 1.6-fold, p = 0.053), protein OS-9 (up 1.9-fold, p = 0.058), and retinol-binding protein 4 (up 1.9-fold, p = 0.058). The significantly downregulated proteins in the hypothyroid state were identified as Kin of IRRE-like protein 3 (down 3-fold, p = 0.014), phosphopantothenoylcysteine decarboxylase (down 2.3-fold, p = 0.021), syntaxin-binding protein 6 (down 1.7-fold, p = 0.042), aryl hydrocarbon receptor nuclear translocator 2 (down 2.1-fold, p = 0.04), GTPase HRas (down 2.2-fold, p = 0.05), nesprin-1 (down 3.4-fold, p = 0.04), serine/arginine repetitive matrix protein 2 (down 2.6-fold, p = 0.006), protein-glutamine γ-glutamyltransferase Z (down 3.9-fold, p = 0.022), IgA-inducing protein homolog (down 2.1-fold, p = 0.023), a disintegrin and metalloproteinase with thrombospondin motifs 8 (down 1.9-fold, p = 0.042), and prefoldin subunit 6 (down 2.1-fold, p = 0.030) (Tables S2 and S4, Supporting Information).
Figure 2.
Representative image of a gel depicting the protein spots identified with MALDI-TOF/TOF in the urine samples. Numbered spots indicate those which were significantly differentially abundant (over 1.5-fold change, p < 0.05) (A) between hypothyroid and euthyroid states. The red and green arrows indicate the differentially abundant proteins that are upregulated and downregulated, respectively, between the two states. Graphs showing the number of proteins successively identified by MALDI-TOF (B) and the number of statistically significantly upregulated spots in the hypothyroid compared to the euthyroid state (C).
Table 1. List of Upregulated and Downregulated Proteins in Hyperthyroid States Compared to Euthyroid States in Urine Samples.
| upregulated proteins | downregulated proteins |
|---|---|
| •serum albumin | •kin of IRRE-like protein 3 |
| •huntingtin-interacting protein M | •phosphopantothenoylcysteine decarboxylase |
| •serotransferrin | •syntaxin-binding protein 6 |
| •V-type proton ATPase subunit B, brain isoform | •aryl hydrocarbon receptor nuclear translocator 2 |
| •β-1,3-galactosyl-O-glycosyl-glycoprotein-β-1,6-N-acetyl glucosaminyl transferase 3 | •GTPase HRas |
| •E3 ubiquitin-protein ligase DCST1 | •nesprin-1 |
| •cathepsin D | •serine/arginine repetitive matrix protein 2 |
| •putative E3 ubiquitin-protein ligase UBR7 | •AN1-type zinc finger protein 6 |
| •keratin, type I cytoskeletal 10 | •protein-glutamine γ-glutamyltransferase Z |
| •probable E3 ubiquitin-protein ligase TRIML2 | •IgA-inducing protein homolog |
| •AN1-type zinc finger protein 6 | •a disintegrin and metalloproteinase with thrombospondin motifs 8 |
| •keratin, type II cytoskeletal 5 | •prefoldin subunit 6 |
| •protein AMBP | |
| •vesicular integral membrane protein VIP36 | |
| •kininogen-1 | |
| •cleft lip and palate transmembrane protein 1-like protein | |
| •zinc finger protein 8393 | |
| •homogentisate 1,2-dioxygenase | |
| •protein OS-9 |
3.3. Confirmation of Changes in Selected Proteins by Immunoblotting
Key proteins found to be differentially abundant between the groups were selected for confirmatory testing by immunoblot analysis (Figure 3). The proteins targeted for confirmation included: transferrin and RBP. Immunoblots confirmed the significantly (p ≤ 0.05) differential expression of these proteins in the human urine samples collected from the hypothyroid state in comparison to the euthyroid state. Immunoblot data were normalized to the housekeeping protein β-actin (Figure 3A,B).
Figure 3.
Confirmation of the proteomic data using immunoblot analysis of selected proteins, identified by 2D-DIGE analysis. Results obtained by immunoblotting were similar to the results obtained by 2D-DIGE (A). Graphical representation of the relative intensity values of normalized protein bands between the hypothyroid and euthyroid states. The data are reported as histograms of the mean ± SD (B).
3.4. Mapping of Protein–Protein Interaction Networks
The protein–protein interaction analysis was also performed for all of these 42 differentially regulated proteins using ingenuity pathway analysis The analysis revealed that among the 42 proteins, 13 proteins interact either directly or indirectly through protein networks (Figure 4A). The software computes a score based on the best fit obtained from the input dataset of proteins and from the biological functions database, to generate a protein–protein interactions network. The generated network is preferentially enriched for proteins with specific and extensive interactions, the interacting proteins are represented as nodes and their biological relationships as a line. Based on the data, three interaction networks were identified for the proteins exhibiting differential expression profiles. The highest-scoring network (score = 26) (Figure 3) incorporated 13 focus molecules. The proposed highest-interaction-network pathway is related to amino acid metabolism, molecular transport, and small-molecule biochemistry. Only the top pathways are shown (Figure 4A).
Figure 4.
Most enriched interaction network of the differentially expressed proteins in hypothyroid compared to the euthyroid states. The red nodes indicate upregulated proteins, and the green nodes indicate downregulated proteins. The central nodes of the pathway related to signaling of the MAP kinases, Pkc, vascular endothelial growth factor (VEGF), PI3 kinase/Akt were found to be deregulated between the two states. Uncolored nodes are proposed by IPA and indicate potential targets that were functionally coordinated with the differentially expressed proteins. The solid lines indicate direct molecular interactions, and the dashed lines represent indirect interactions (A). Diagram showing the 14 top canonical pathways ranked by the p-values obtained by the IPA (B).
The canonical pathways enriched in the current dataset are shown in Figure 4B. The three most interesting enriched canonical pathways included thyroid hormone biosynthesis (30% overlap), LXR/RXR Activation (4.1% overlap), FXR/RXR Activation (4% overlap), and acute phase response signaling (2.8% overlap). The details of the canonical pathways identified in this study are summarized in Figure S2, Supporting Information.
3.5. Principal Component Analysis (PCA)
The PCA, an unsupervised exploratory data analysis, was performed using Progenesis SameSpots software. The biplot helps us to determine and visualize the distinct separation of the samples coming from the hypothyroid and euthyroid states. The PCA was performed on all 62 spot features, which exhibited statistically significant (ANOVA p < 0.05) changes in abundance, identified by MS, the analyses revealed that the two groups clustered distinctly from one another based on different urine proteins with 64% of the variability (Figure 5).
Figure 5.
Principal component analysis of the proteomic dataset. The purple dots indicate euthyroid, and the blue dots represent hypothyroid. Together they explained 64% of the selected spot’s variability values. The colored dots and numbers are the representation of gels and spots, respectively.
3.6. Subcellular and Functional Characterization of the Differentially Expressed Proteins
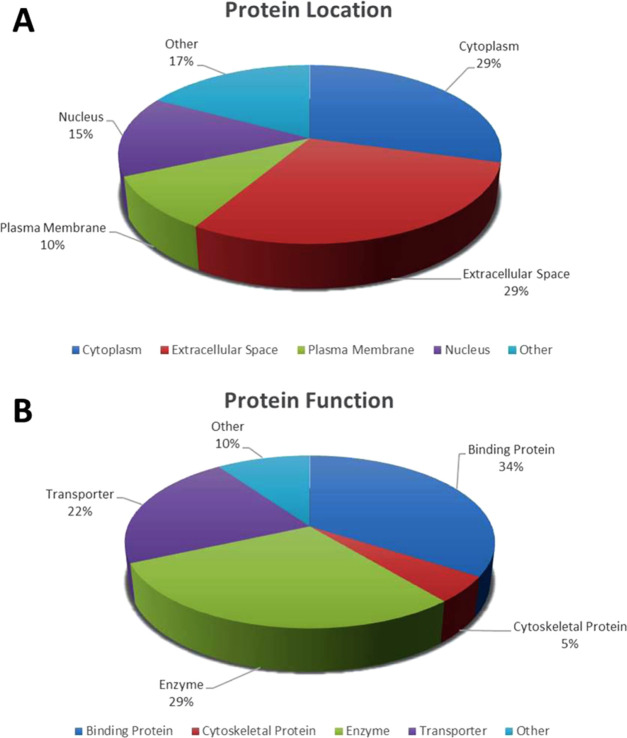
Following MS analysis, all successful 42 differentially abundant proteins identified between the hypothyroid and euthyroid were subjected to functional classification using Gene Ontology (GO) terms using UniProtKB. The identified proteins were classified to gain more information about their location in Figure 6A and the molecular function in Figure 6B in which they are involved. The dominant functional categories identified were binding protein (34%), enzyme (29%), transporter (22%), cytoskeletal (5%) protein, and others (10%).
Figure 6.
Comparative depiction (%) of the significantly identified proteins using MALDI-TOF/TOF-MS: categorized into groups according to their location (A) and function (B).
The metabolic functioning of nearly all tissues depends on the optimal levels of circulating serum TH levels. This level is in turn tightly regulated by feedback control mechanisms via the hypothalamo-pituitary thyroid axis (through actions of thyrotropin (TSH) and thyroglobulin (Tg)), and also by metabolic processes (peripheral enzymatic deiodination) in the peripheral organs including the kidney. Hypothyroidism directly and indirectly affects the kidney and increases peripheral vascular resistance due to reductions in renal blood flow, decreased glomerular filtration, altered tubular function, hyponatremia, and decreased free-water excretion. The protein content within the urine, an ultrafiltrate of the plasma, is a reflection of proteins and products derived from blood perfusing peripheral organs, from the different metabolisms, and from kidney and the urinary tract.30 In this study, we compared the urinary proteomic profiles between patients with hypothyroidism, before and after treatment with levothyroxine using the 2D-DIGE and MALDI-TOF untargeted proteomics approach. In our previous study, we characterize the plasma proteomic profile between the hypothyroid and euthyroid state in the same group of patients.20 Overall, our results showed alterations in a total of 42 proteins, with a >1.5-fold statistically significant fold change (p = 0.05) in abundance between the two states. A total of 28 proteins were upregulated, and 14 proteins were downregulated in the hypothyroid state compared to the euthyroid state. On grouping these proteins based on their biological processes to understand their roles, we found that they were involved in the regulation of processes related to (i) transport (ALB, TF, RBP4, ATP6V1B2, HRAS, KNG1, LMAN2, ARNT2); (ii) acute phase response proteins (ALB, TF, protein AMBP); (iii) generation of reactive oxygen species (ALB, TF, RBP, HRAS, KNG1); (iv) proliferation of connective tissue cells (TF, CTSD, HRAS, KNG1, KRT10); (v) proteolysis and endocytosis (ALB, TF, AMBP, ATP6V1B2, HRAS, LMAN2, SYNE1 CTSD, GCNT3); and (iv) others. Our proteomic analysis revealed that the majority of the proteins in the hypothyroid patient’s urine represented extracellular proteins and plasma membrane proteins and were identified in the urinary proteome map.12,31 As a majority of the identified proteins are endowed with multiple functional properties, as well as participate in and regulate different metabolic processes at the same time, they are discussed according to their individual significance.
Eight proteins identified in this study (ALB, TF, RBP4, ATP6V1B2, HRAS, KNG1, LMAN2, ARNT2) were involved in the transport of TH. Transport of the TH from its site of synthesis to the target organs mediated via the TH binding proteins is critical for its physiological actions. TH binding and transport via different proteins having different binding capacities ensures a balanced source of bioavailable TH to the different organs and acts as a mechanism that senses and determines the extent of their release. We found an increase in the levels of human serum albumin (HSA) in the urinary proteome of the hypothyroid group in comparison to the euthyroid. HSA is a 66.5 kDa protein synthesized by the liver and associates with a wide variety of substances and hormones including circulating TH. It is a low-affinity, high-capacity TH binding protein binding 10% of the circulating TH and preventing its filtration by the renal tissue.1,32 Albumin secretion in the urine, above 150 mg/dL, is considered pathological (proteinuria) and is a feature of systemic diseases, such as hypertension, diabetes, or more locally kidney diseases (example glomerulonephritis). An increase of albumin in the urine proteome with no evidence of urinary microalbuminuria has been previously documented in other proteomic studies.27,32 The increase of urinary albumin levels may reflect increased serum levels as a compensatory mechanism aimed at increasing the binding and uptake of the TH for increasing their availability to the peripheral tissues and to prevent filtration by the glomerulus and renal loss.
The protein spots relating to RBP, an adipocytokine, were also found to be significantly increased in the urinary proteome between the hypothyroid and euthyroid states. RBP circulates as a part of transthyretin (TTR), another TH binding protein involved in its transport.32 Unlike albumin, RBP and TTR are known to have an intermediate binding affinity for TH, binding approximately 20% of the hormones.33 RBP TTR complex selectively binds to T4 (80%) rather than T3 and facilitates its transport to the brain through the blood–brain barrier and the cerebrospinal fluid (CSF). Differences in the binding affinities of the different proteins helps maintain the balance between the protein-bound hormone and its free state. Besides transport, elevated levels of RBP also indicate alterations in the intermediary metabolism, insulin resistance, increased oxidative stress, and inflammation that are characteristically seen with hypothyroidism.34 RBP levels were also significantly and positively associated with TSH in obesity and markers of oxidative stress and inflammation.35 RBP is normally not excreted in the urine, suggesting that its increased urinary values are probably a measure of increased synthesis or acute stress resulting from expanded extracellular free pools that undergo overflow into the kidney. The exact role of RBP and thyroid disease needs to be further evaluated.
Another multifunctional protein identified in our study with a significantly increased abundance in the urine proteome was transferrin. TF is known to function as an iron transport protein, an acute phase protein, and an antioxidant whose levels increase in response to increasing iron demand and during oxidative stress. Iron deficiency without development of anemia adversely affects thyroid function. Previous studies have shown that decreased TF levels are associated with lower catalytic action of the heme-containing enzyme thyroid peroxidase, which is crucial for thyroid hormone synthesis and reduces peripheral conversion of T4 to T3, thereby leading to hypothyroidism.36,37 The increase in the levels of transferrin was also documented in our previous proteomic study in the plasma of hypothyroid patients.25 As hypothyroidism is characterized by a state of low-grade chronic inflammation,38 we identified a number of APPs along with albumin TF, another AMBP serving as an index of oxidative stress and inflammation. AMBP is a large glycoprotein containing two subunits α1-microglobulin and bikunin, which together impart the proteins anti-inflammatory properties that modulates inflammatory events. Urinary secretion of α1-microglobulin occurs at the proximal tubule, and an increase in AMBP may serve as markers of an increased anti-inflammatory response and of early proximal tubular changes39 in hypothyroidism.
Aside from the transport protein, we identified proteins involved in the regulation and synthesis of thyroglobulin (Tg), the storage form of TH. The canonical pathway analysis also showed that the proteins identified in our study were involved in upregulating TH synthesis. Serum levels of TH are tightly regulated by the levels of TSH and Tg and vice versa. Decreased circulating TH levels lead to a reciprocal increase in TSH and Tg. In line with this, we found an increase in the protein spots relating to LMAN, a vesicular integral membrane type I transmembrane lectin, involved in intracellular protein trafficking sorting, vesicle-mediated transport, of Tg in the vesicles. LMAN helps us to transport exocytic vesicles containing mature Tg to the apical cell surface and deliver them to follicular lumen for release via secretory endocytosis.40 An increase in LMAN may indicate activation of compensatory mechanisms for higher Tg processing to increase the circulating levels of TH. We also found a decrease in the abundance of nesprin-1, a nuclear envelope spectrin-repeat protein that links the nuclear lamina to the cytoskeleton, regulates the endothelial migration, and functions as the intracellular scaffold to maintain the cellular shape and structure.41 These connections importantly connect the nuclear metabolism to the cytoplasmic actions for the regulation of proteins such as Tg. Other proteomic studies have also documented the presence of nesprin in the urine from patients with prostate cancer and kidney disease.42,43
Hypothyroidism often leads to an enlargement of the thyroid gland as a compensatory mechanism to overcome the decrease in the circulating TH and in response to an increased TSH stimulation. A number of proteins known to increase Tg processing endocytosis and proteolysis to release TH were found to be significantly differentially abundant between the hypothyroid and euthyroid state. These included CTSD, E3 ubiquitin-protein ligase DCST1, putative E3 ubiquitin-protein ligase UBR7, probable E3 ubiquitin-protein ligase TRIML2, and protein OS-9. Tg plays a role in thyroid hormogenesis through its degradation via the endosomal lysosomal pathway by cysteine cathepsins (thiol proteases) and the ubiquitin-dependent proteolytic pathways. CTSD along with cathepsin B breaks down the covalently cross-linked Tg in the acidic endosomal environment to release soluble Tg for TH liberation and reutilization.44,45 On similar lines, we also found an increase in proteins belonging to the ubiquitin–proteasome system of proteins involved in cellular processing, maturation, and proteasomal degradation of the Tg molecule. These included the E3 ubiquitin ligases involved in the ubiquitin-dependent proteasomal degradation of Tg and the tripartite motif (TRIM) proteins, which are the largest subfamilies of E3 ubiquitin ligases, UBR7, and ARNT2 E3 ubiquitin ligase.46 With the increase in all of the enzymes involved in the proteolysis, endocytosis aims to liberate TH from the Tg, leading ultimately to their secretion in the circulation.
It is interesting to note that the urinary proteome in our study also identified the enzymes GCNT3, TGM7, and protein OS-9 with an increased abundance in the hypothyroid group. These enzymes are responsible for post-translational glycosylation of Tg and increasing the covalent cross-links between the Tg molecules to increase its storage within the thyrocyte.47 Tg glycosylation is important for its correct folding and trafficking, iodination, and TH synthesis,48 while unglycosylated Tg tends to lose its ability to synthesize TH.49 Protein OS-9 helps recognize the glycosylated extended mannose residues on the Tg molecule via its mannose 6-phosphate receptor homology domains for targeted delivery to ER for cellular processing and quality control.50 We found a significant decrease in the abundance of Hras in the hypothyroid versus euthyroid states. Hras is a member of the membrane-bound guanine nucleotide-binding proteins that function as signal transducers from cell membrane to nucleus for TH and other growth factor receptors. Besides the thyroid tissue, Hras is expressed in the kidney and is abundant in the distal collecting duct.51 Ras interacts with multiple downstream effectors including PI3K and leads to the activation of MAPK kinases resulting in gene induction.52 Another protein CLPTM1L identified in our dataset is known to work in conjunction with the RAS proteins through the phosphoinositide 3-kinase (PI3 Kinase) pathway and is an essential molecule for their signaling functions.53
TH are synthesized from the amino acids tyrosine and phenylalanine. Interestingly, in our study, we observed an increase in the levels of the enzyme homogentisate 1,2-dioxygenase. The enzyme is known to act on the metabolic breakdown product of tyrosine homogentisic acid, indicating a probable increase in the oxidation of tyrosine. The other proteins of interest identified in the study with increased abundance between the hypothyroid and euthyroid state were zinc finger proteins and the keratins that are involved in several cellular processes with a key role in development and differentiation, tumorigenesis, cancer progression, and metastasis.
The biological significance of the identified proteins in our proteomic analysis was explored by IPA and the network pathway identified the highest-scoring interaction network pathway related to amino acid metabolism, molecular transport, small-molecule biochemistry. This is true considering the fact that THs are peptide hormones synthesized from the aromatic amino acids, tyrosine, and phenylalanine. The central nodes with the highest connectivity to the identified proteins in the network pathway map included the MAP kinase family p38MAPK, ERK1/2, JNK, PI3 Kinase/Akt, PKC, Nfkb, and VEGF. This reiterated the involvement of the identified proteins in pathways related to cell proliferation, inflammation, and endothelial function. The proteome analysis also points to the fact that there is a concerted action to increase the TH circulating levels through an increase of Tg synthesis in the hypothyroid state.
4. Conclusions
In conclusion, our comparative proteomics study using 2D-DIGE analysis of the urine proteome profile between the hypothyroid and the euthyroid states revealed significant changes in the proteins related to TH and Tg metabolism. The alterations in the different proteins identified in the study demonstrate a compensatory increase in the regulation of Tg as an attempt to increase its synthesis to circumvent the decrease in circulating levels of TH in the hypothyroid state.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Saudi Arabia, (Project no. 13-MED920-02).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05686.
Power calculation, canonical pathways, experimental design, mass spectrometry list, biochemical parameters, and list of identified proteins (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Iglesias P.; Diez J. J. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009, 160, 503–515. 10.1530/EJE-08-0837. [DOI] [PubMed] [Google Scholar]
- Shin D. H.; Lee M. J.; Kim S. J.; Oh H. J.; Kim H. R.; Han J. H.; Koo H. M.; Doh F. M.; Park J. T.; Han S. H.; Yoo T. H.; Kang S. W. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 2732–2740. 10.1210/jc.2012-1663. [DOI] [PubMed] [Google Scholar]
- Li L. Z.; Hu Y.; Ai S. L.; Cheng L.; Liu J.; Morris E.; Li Y.; Gou S. J.; Fu P. The relationship between thyroid dysfunction and nephrotic syndrome: a clinicopathological study. Sci. Rep. 2019, 9, 6421 10.1038/s41598-019-42905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr M. R.; Kalogiros J.; Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol., Dial., Transplant. 2004, 19, 1190–1197. 10.1093/ndt/gfh096. [DOI] [PubMed] [Google Scholar]
- Asmah B. J.; Wan Nazaimoon W. M.; Norazmi K.; Tan T. T.; Khalid B. A. Plasma renin and aldosterone in thyroid diseases. Horm. Metab. Res. 1997, 29, 580–583. 10.1055/s-2007-979105. [DOI] [PubMed] [Google Scholar]
- Michael U. F.; Barenberg R. L.; Chavez R.; Vaamonde C. A.; Papper S. Renal handling of sodium and water in the hypothyroid rat. Clearance and micropuncture studies. J. Clin. Invest. 1972, 51, 1405–1412. 10.1172/JCI106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. E.; Coelho J. B.; Sealey J. E.; Edwards K. D.; Stephan F. Changes in glomerulotubular dimensions, single nephron glomerular filtration rates and the renin-angiotensin system in hypothyroid rats. Life Sci. 1982, 30, 633–639. 10.1016/0024-3205(82)90279-X. [DOI] [PubMed] [Google Scholar]
- Wheatley T.; Edwards O. M. Mild hypothyroidism and oedema: evidence for increased capillary permeability to protein. Clin. Endocrinol. 1983, 18, 627–635. 10.1111/j.1365-2265.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Thongboonkerd V.; Malasit P. Renal and urinary proteomics: current applications and challenges. Proteomics 2005, 5, 1033–1042. 10.1002/pmic.200401012. [DOI] [PubMed] [Google Scholar]
- Ignjatovic V.; Geyer P. E.; Palaniappan K. K.; Chaaban J. E.; Omenn G. S.; Baker M. S.; Deutsch E. W.; Schwenk J. M. Mass Spectrometry-Based Plasma Proteomics: Considerations from Sample Collection to Achieving Translational Data. J. Proteome Res. 2019, 18, 4085–4097. 10.1021/acs.jproteome.9b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood A.; Benabdelkamel H.; Alfadda A. A. Obesity Proteomics: An Update on the Strategies and Tools Employed in the Study of Human Obesity. High-Throughput 2018, 7, 27 10.3390/ht7030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi J.; Kumar C.; Zhang Y.; Olsen J. V.; Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006, 7, R80 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimuthu A.; O’Meally R. N.; Chaerkady R.; Subbannayya Y.; Nanjappa V.; Kumar P.; Kelkar D. S.; Pinto S. M.; Sharma R.; Renuse S.; Goel R.; Christopher R.; Delanghe B.; Cole R. N.; Harsha H. C.; Pandey A. A comprehensive map of the human urinary proteome. J. Proteome Res. 2011, 10, 2734–2743. 10.1021/pr2003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L. U.; Schiffer E.; Zurbig P.; Good D. M.; Kellmann M.; Mouls L.; Pitt A. R.; Coon J. J.; Schmieder R. E.; Peter K. H.; Mischak H.; Kolch W.; Delles C.; Dominiczak A. F. Urinary proteomic biomarkers in coronary artery disease. Mol. Cell. Proteomics 2008, 7, 290–298. 10.1074/mcp.M700394-MCP200. [DOI] [PubMed] [Google Scholar]
- Neisius U.; Koeck T.; Mischak H.; Rossi S. H.; Olson E.; Carty D. M.; Dymott J. A.; Dominiczak A. F.; Berry C.; Oldroyd K. G.; Delles C. Urine proteomics in the diagnosis of stable angina. BMC Cardiovasc. Disord. 2016, 16, 70 10.1186/s12872-016-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. X.; Zhu Y. B.; Wu C. P.; Zhong M.; Hu S. W. Potential urine biomarkers for gestational hypertension and preeclampsia. Mol. Med. Rep. 2019, 19, 2463–2470. 10.3892/mmr.2019.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y.; Nkuipou-Kenfack E.; Staessen J. A. Urinary Peptidomic Biomarker for Personalized Prevention and Treatment of Diastolic Left Ventricular Dysfunction. Proteomics: Clin. Appl. 2019, 13, e1800174 10.1002/prca.201800174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaburagi Y.; Takahashi E.; Kajio H.; Yamashita S.; Yamamoto-Honda R.; Shiga T.; Okumura A.; Goto A.; Fukazawa Y.; Seki N.; Tobe K.; Matsumoto M.; Noda M.; Unoki-Kubota H. Urinary afamin levels are associated with the progression of diabetic nephropathy. Diabetes Res. Clin. Pract. 2019, 147, 37–46. 10.1016/j.diabres.2018.02.034. [DOI] [PubMed] [Google Scholar]
- Sandow J. J.; Rainczuk A.; Infusini G.; Makanji M.; Bilandzic M.; Wilson A. L.; Fairweather N.; Stanton P. G.; Garama D.; Gough D.; Jobling T. W.; Webb A. I.; Stephens A. N. Discovery and Validation of Novel Protein Biomarkers in Ovarian Cancer Patient Urine. Proteomics: Clin. Appl. 2018, 12, e1700135 10.1002/prca.201700135. [DOI] [PubMed] [Google Scholar]
- Wu D.; Ni J.; Beretov J.; Cozzi P.; Willcox M.; Wasinger V.; Walsh B.; Graham P.; Li Y. Urinary biomarkers in prostate cancer detection and monitoring progression. Crit. Rev. Oncol./Hematol. 2017, 118, 15–26. 10.1016/j.critrevonc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Papale M.; Vocino G.; Lucarelli G.; Rutigliano M.; Gigante M.; Rocchetti M. T.; Pesce F.; Sanguedolce F.; Bufo P.; Battaglia M.; Stallone G.; Grandaliano G.; Carrieri G.; Gesualdo L.; Ranieri E. Urinary RKIP/p-RKIP is a potential diagnostic and prognostic marker of clear cell renal cell carcinoma. Oncotarget 2017, 8, 40412–40424. 10.18632/oncotarget.16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Wang S.; Zhang M. Identification of urine biomarkers associated with lung adenocarcinoma. Oncotarget 2017, 8, 38517–38529. 10.18632/oncotarget.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye A.; Dabhi R.; Taunk K.; Vannuruswamy G.; RoyChoudhury S.; Adhav R.; Seal S.; Mane A.; Bayatigeri S.; Santra M. K.; Chaudhury K.; Rapole S. Urinary proteome alterations in HER2 enriched breast cancer revealed by multipronged quantitative proteomics. Proteomics 2016, 16, 2403–2418. 10.1002/pmic.201600015. [DOI] [PubMed] [Google Scholar]
- Rhee C. M. The interaction between thyroid and kidney disease: an overview of the evidence. Curr. Opin. Endocrinol., Diabetes Obes. 2016, 23, 407–415. 10.1097/MED.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadda A. A.; Benabdelkamel H.; Masood A.; Jammah A. A.; Ekhzaimy A. A. Differences in the Plasma Proteome of Patients with Hypothyroidism before and after Thyroid Hormone Replacement: A Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 88 10.3390/ijms19010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D.; Flugge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Benabdelkamel H.; Masood A.; Okla M.; Al-Naami M. Y.; Alfadda A. A. A Proteomics-Based Approach Reveals Differential Regulation of Urine Proteins between Metabolically Healthy and Unhealthy Obese Patients. Int. J. Mol. Sci. 2019, 20, 4905 10.3390/ijms20194905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadda A. A.; Benabdelkamel H.; Masood A.; Moustafa A.; Sallam R.; Bassas A.; Duncan M. Proteomic analysis of mature adipocytes from obese patients in relation to aging. Exp. Gerontol. 2013, 48, 1196–1203. 10.1016/j.exger.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Benabdelkamel H.; Masood A.; Almidani G. M.; Alsadhan A. A.; Bassas A. F.; Duncan M. W.; Alfadda A. A. Mature adipocyte proteome reveals differentially altered protein abundances between lean, overweight and morbidly obese human subjects. Mol. Cell. Endocrinol. 2015, 401, 142–154. 10.1016/j.mce.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Vall-Palomar M.; Arevalo J.; Ariceta G.; Meseguer A. Establishment of urinary exosome-like vesicles isolation protocol for FHHNC patients and evaluation of different exosomal RNA extraction methods. J. Transl. Med. 2018, 16, 278 10.1186/s12967-018-1651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiano G.; Santucci L.; Petretto A.; Bruschi M.; Dimuccio V.; Urbani A.; Bagnasco S.; Ghiggeri G. M. 2D-electrophoresis and the urine proteome map: where do we stand?. J. Proteomics 2010, 73, 829–844. 10.1016/j.jprot.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Hennemann G.; Krenning E. P.; Docter R.. Thyroid Hormone-Binding Plasma Proteins. In Frontiers in Thyroidology; Medeiros-Neto G.; Gaitan E., Eds.; Springer US: Boston, MA, 1986; Vol. 1, pp 97–101. [Google Scholar]
- Southwell B. R.; Duan W.; Alcorn D.; Brack C.; Richardson S. J.; Kohrle J.; Schreiber G. Thyroxine transport to the brain: role of protein synthesis by the choroid plexus. Endocrinology 1993, 133, 2116–2126. 10.1210/endo.133.5.8404661. [DOI] [PubMed] [Google Scholar]
- Kokkinos S.; Papazoglou D.; Zisimopoulos A.; Papanas N.; Tiaka E.; Antonoglou C.; Maltezos E. Retinol Binding Protein-4 and Adiponectin Levels in Thyroid Overt and Subclinical Dysfunction. Exp. Clin. Endocrinol. Diabetes 2016, 124, 87–92. 10.1055/s-0035-1564199. [DOI] [PubMed] [Google Scholar]
- Benabdelkamel H.; Masood A.; Alanazi I. O.; Alfadda A. A. Comparison of protein precipitation methods from adipose tissue using difference gel electrophoresis. Electrophoresis 2018, 1745. 10.1002/elps.201800124. [DOI] [PubMed] [Google Scholar]
- Hu X.; Teng X.; Zheng H.; Shan Z.; Li J.; Jin T.; Xiong C.; Zhang H.; Fan C.; Teng W. Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr. Res. 2014, 34, 604–612. 10.1016/j.nutres.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Hess S. Y.; Zimmermann M. B.; Arnold M.; Langhans W.; Hurrell R. F. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J. Nutr. 2002, 132, 1951–1955. 10.1093/jn/132.7.1951. [DOI] [PubMed] [Google Scholar]
- Türemen E. E.; Cetinarslan B.; Sahin T.; Canturk Z.; Tarkun I. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocr. J. 2011, 58, 349–354. 10.1507/endocrj.K10E-333. [DOI] [PubMed] [Google Scholar]
- Rao P. V.; Lu X.; Standley M.; Pattee P.; Neelima G.; Girisesh G.; Dakshinamurthy K.; Roberts C. T.; Nagalla S. R. Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care 2007, 30, 629–637. 10.2337/dc06-2056. [DOI] [PubMed] [Google Scholar]
- Hara-Kuge S.; Ohkura T.; Seko A.; Yamashita K. Vesicular-integral membrane protein, VIP36, recognizes high-mannose type glycans containing α1→ 2 mannosyl residues in MDCK cells. Glycobiology 1999, 9, 833–839. 10.1093/glycob/9.8.833. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Skepper J. N.; Yang F.; Davies J. D.; Hegyi L.; Roberts R. G.; Weissberg P. L.; Ellis J. A.; Shanahan C. M. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 2001, 114, 4485–4498. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zheng C.; Wang X.; Zuo K.; Liu Z. Proteomic profilebased screening of potential protein biomarkers in the urine of patients with nephrotic syndrome. Mol. Med. Rep. 2017, 16, 6276–6284. 10.3892/mmr.2017.7329. [DOI] [PubMed] [Google Scholar]
- Kim K. H.; Moon M. H. High speed two-dimensional protein separation without gel by isoelectric focusing-asymmetrical flow field flow fractionation: application to urinary proteome. J. Proteome Res. 2009, 8, 4272–4278. 10.1021/pr900363s. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Chen Y.; Xia F.; Wang N.; Chen C.; Nie X.; Li Q.; Han B.; Zhai H.; Jiang B.; et al. A higher ratio of estradiol to testosterone is associated with autoimmune thyroid disease in males. Thyroid 2017, 27, 960–966. 10.1089/thy.2016.0661. [DOI] [PubMed] [Google Scholar]
- Weber J.; McInnes J.; Kizilirmak C.; Rehders M.; Qatato M.; Wirth E. K.; Schweizer U.; Verrey F.; Heuer H.; Brix K. Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland. Eur. J. Cell Biol. 2017, 96, 440–456. 10.1016/j.ejcb.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Di Jeso B.; Arvan P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr. Rev. 2016, 37, 2–36. 10.1210/er.2015-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber-Lichtenberg Y.; Brix K.; Schmitz A.; Heuser J. E.; Wilson J. H.; Lorand L.; Herzog V. Covalent cross-linking of secreted bovine thyroglobulin by transglutaminase. FASEB J. 2000, 14, 1005–1014. 10.1096/fasebj.14.7.1005. [DOI] [PubMed] [Google Scholar]
- Zabczynska M.; Kozlowska K.; Pochec E. Glycosylation in the Thyroid Gland: Vital Aspects of Glycoprotein Function in Thyrocyte Physiology and Thyroid Disorders. Int. J. Mol. Sci. 2018, 19, 2792 10.3390/ijms19092792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citterio C. E.; Veluswamy B.; Morgan S. J.; Galton V. A.; Banga J. P.; Atkins S.; Morishita Y.; Neumann S.; Latif R.; Gershengorn M. C.; Smith T. J.; Arvan P. De novo triiodothyronine formation from thyrocytes activated by thyroid-stimulating hormone. J. Biol. Chem. 2017, 292, 15434–15444. 10.1074/jbc.M117.784447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N.; Kamiya Y.; Kamiya D.; Kato K.; Nagata K. Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans. J. Biol. Chem. 2009, 284, 17061–17068. 10.1074/jbc.M809725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. H.; Song S. H.; Cook D. I.; Dinudom A. H-Ras mediates the inhibitory effect of epidermal growth factor on the epithelial Na+ channel. PLoS One 2015, 10, e0116938 10.1371/journal.pone.0116938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou R.; Zhang L.; Lu T.; Liu D.; Mei F.; Huang J.; Qian L. Identification of a novel HRAS variant and its association with papillary thyroid carcinoma. Oncol. Lett. 2018, 15, 4511–4516. 10.3892/ol.2018.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. A.; Vikis H. G.; Tate E.; Rymaszewski A. L.; You M. CRR9/CLPTM1L regulates cell survival signaling and is required for Ras transformation and lung tumorigenesis. Cancer Res. 2014, 74, 1116–1127. 10.1158/0008-5472.CAN-13-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.