Abstract
Each year there are approximately 7000 out of hospital cardiac arrests in the pediatric population, with 30% resuscitation rate and a 6–10% rate of survival to hospital discharge. Survivors of cardiac arrest exhibit learning and memory deficits that are devastating during the school years. Delayed neuronal cell death occurs in the hippocampus following cardiac arrest and likely contributes to memory impairments. Circulating endogenous estrogen in young adult females has been shown to provide protection against ischemic cell death, as does chronic exogenous administration of 17β-estradiol (E2). Chronic estrogen benefit can have undesirable feminizing effects, particularly in pre-adolescents. Here, we tested if a single-dose of E2 is neuroprotective in our pediatric cardiac arrest mouse model performed in juvenile mice. We subjected P21‒P25 C57Blk6 male and female mice to 8 min of cardiac arrest followed by cardiopulmonary resuscitation (CA/CPR). This developmental stage preceded the hormonal onset and serum estradiol and testosterone levels were not different in males and females. A single dose of E2 (100μg/kg) or vehicle was administered 30 min after resuscitation. Neuronal cell death measured 3 days after CA/CPR showed reduced hippocampal cell death in E2-treated females, but not males. Benefit of E2 in females was blocked by the P38 MAPK inhibitor, SB203580. Hippocampal-dependent memory function was equally impaired in E2-and vehicle-treated females measured in the contextual fear conditioning task at 7 days. Our findings demonstrate female-specific transient neuroprotection with E2 that does not provide sustained functional benefit.
Keywords: Pediatric cardiac arrest, Ischemia, Estrogen, Neuroprotection
1. Introduction
Cardiac arrest (CA) is a major cause of morbidity and mortality in both the adult and pediatric populations (Benjamin et al., 2018; Fink et al., 2016; Michiels et al., 2016; Tress et al., 2010). Although we know several parameters that can worsen brain injury (e.g. hypoxia, hypotension), therapeutic options to improve neurologic outcome after resuscitation remain limited. To date, mild therapeutic hypothermia is the only therapy shown to be effective in increasing survival and decreasing morbidity in adult cardiac arrest patients (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study, 2002), although subsequent studies suggest that avoidance of fever may be as effective as hypothermia (Nielsen et al., 2013). In the pediatric population, therapeutic hypothermia does not improve outcomes over controlled normothermia (Moler et al., 2015), although secondary analyses of the clinical study evaluating efficacy are ongoing (THAPCA, NCT00878644). Thus, there are no therapies that have been proven effective in improving outcomes after cardiac arrest in children. We recently developed a juvenile mouse model of cardiac arrest that allows for interrogation of brain injury mechanisms and therapeutic strategies at this important developmental stage.
Epidemiologic studies in adults have suggested that females have better outcomes after CA when compared to males (Herlitz et al., 2001; Kitamura et al., 2010; Topjian et al., 2010). Numerous experimental studies in adult animal models have recapitulated this clinical data, demonstrating that female animals exhibit significantly less brain injury following cerebral ischemia compared to males (Herson and Hurn, 2010). The sex difference in outcome is due primarily to the high levels of circulating estrogen in adult female animals, as removal of endogenous sex steroids (ovariectomy) increases female brain injury (Fukuda et al., 2000; Rusa et al., 1999). Exogenous estrogen administered to males and females reduces ischemic neuronal injury (Gillies and McArthur, 2010; Jover et al., 2002; Manwani et al., 2015; Nakano et al., 2010; Noppens et al., 2005). We previously demonstrated that pretreatment with estradiol in juveniles subjected to middle cerebral artery occlusion provided neuroprotection only in females (Herson et al., 2013b). This suggests a sexual dimorphism in cell death pathways that precedes the hormonal onset. Feminizing effects of prolonged estrogen treatment are a concern at this developmental stage, limiting translational potential of chronic estrogen treatment that is common in animal models. Here, we used our juvenile cardiac arrest model to determine whether a single dose of estrogen administered at a clinically relevant time point after cardiac arrest can provide histological and functional protection.
2. Methods
2.1. Experimental animals
The Institutional Animal Care and Use Committee (IACUC) approved all experimental protocols in accordance with National Institutes of Health guidelines for the care and use of animals in research. Experiments were performed according to ARRIVE guidelines (Kilkenny et al., 2010) in a blinded, randomized fashion using juvenile male and female C57Bl6 mice 21–25 days old (10–15 g). Mice were obtained from Charles River Laboratories. Animals were randomized to treatment groups with intravenous injection of 17β-estradiol (E2, Sigma) dissolved in 20% 2-Hydroxypropyl-β-cyclodextrin/5% dimethylsulfoxide or vehicle (20% 2-Hydroxypropyl-β-cyclodextrin, Fluka/5% Dimethylsulfoxide) at 30 min following successful resuscitation from CA.
2.2. Mouse cardiac arrest model
Juvenile (P21–25) male and female mice were subjected to CA/CPR as previously described (Deng et al., 2014b; Dietz et al., 2018). Briefly, anesthesia was induced with 3% isoflurane and maintained with 1–1.5% isoflurane in an oxygen-enriched air via facemask. Temperature probes were inserted in the left ear and rectum to monitor head and body temperature simultaneously. An automatic temperature controller was used to maintain body temperature at 37.0 ± 0.2 °C during surgery using a heating pad and heating lamp. A PE-10 catheter was inserted into the right internal jugular vein for drug administration, and flushed with heparinized 0.9% saline. Needle electrodes were placed subcutaneously on the chest for continuous electrocardiograph (EKG) monitoring. Animals were endotracheally intubated, connected to a mouse ventilator (MidiVent Ventilator, Harvard Apparatus), and set to a respiratory rate of 160 min−1 and tidal volume of 150 μL. The head temperature was increased to 37.5 ± 0.2 °C by means of a heated, water-filled coil, which was placed over the animal’s head and connected to an automatic temperature controller.
Cardiac arrest was induced by injection of 30 μL KCl (0.5 M) via the jugular catheter, and confirmed by asystole on EKG and the absence of a precordial pulse. The endotracheal tube was disconnected and anesthesia stopped. During cardiac arrest, body temperature was allowed to decrease to a minimum of 35.5 °C, and head temperature was maintained at 37.5 ± 0.2 °C. CPR was begun 8 min after induction of cardiac arrest by injection of 0.3 mL epinephrine solution (16 μg epinephrine/ml in 0.9% saline), chest compressions at a rate of 300 min−1, and ventilation with 100% oxygen at a respiratory rate of 210 min−1 and 25% greater tidal volume. Chest compressions were stopped as soon as spontaneous circulation was restored. CPR was abandoned if spontaneous circulation was not restored within 3 min. Simultaneous with beginning resuscitation, the head temperature control was adjusted to maintain a temperature of 37.0 ± 0.2 °C. Head and body temperature were recorded every minute for the first 5 min of resuscitation, and then every 5 min thereafter. After 5 min of resuscitation, the ventilator gas mixture was changed from 100% oxygen to 50% oxygen/50% medical air. At 30 min following successful resuscitation, animals were treated with an intravenous dose of E2 (100 μg/kg) or vehicle (equivalent volume, 5 μL/g), and the jugular catheter and temperature probes were removed. The mice were extubated once they recovered an adequate respiratory rate and effort. The animals were placed in their home cages on a heated water blanket (35 °C) for complete recovery, and received soft food and free access to water and regular chow for the three days following CA/CPR.
2.3. Health assessment
Mice were weighed daily, and a health assessment score was calculated for each mouse, as previously described (Deng et al., 2014a) for the first 3 days post-CA/CPR. The graded scoring system ranged from 0 to 2, 0–3, or 0–5 depending on the behavior assessed, with 0 indicating no deficit, and the upper limit indicating the most impaired. The behaviors assessed were consciousness (0–3), interaction (0–2), ability to grab the cage wire top (0–2), motor function (0–5), and activity (0–2). Scores for each category were summed to generate a composite score (Fig. 2B).
Fig. 2. No differences in weight or general health.
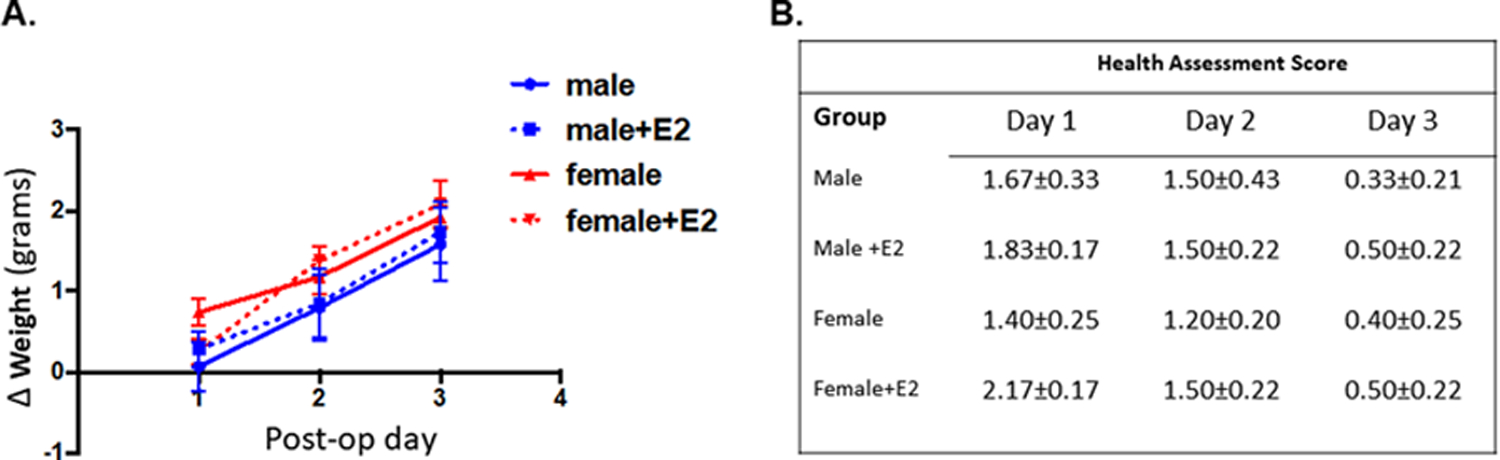
A. Animals were weighed on POD 1–3. The animal weight on the day of (but prior to) cardiac arrest was subtracted from the daily weight to obtain the change in weight (Δ weight) was obtained for each day. There was no statistical difference in daily Δ weight between groups (p = 0.62, 2-way ANOVA). B. Health assessment composite score for each day post-CA. There was no significant difference in daily score between groups (p = 0.57, 2-way ANOVA). Mean ± SEM.
2.4. Histological analysis
Three or seven days after CA/CPR, mice were deeply anesthetized with 4% isoflurane and transcardially perfused and fixed with 4% paraformaldehyde. Brains were removed, embedded in paraffin, and 6 μm coronal sections were serially cut. The CA1 region of the hippocampus was analyzed at three levels (100 μm apart) beginning at −1.5 mm bregma. Sections were stained with hematoxylin and eosin (H&E) for analysis of damaged neurons, determined by the presence of pink eosinophilic cytoplasm and dark pyknotic nucleus. The percentage of damaged neurons was calculated by determining the average injury for three section levels. For animals that were assessed 7 days after CA, the density of live neurons was also assessed. The investigator was blinded to treatment before analyzing neuronal damage.
2.5. Hormone measurements
Serum was collected from mice by cardiac puncture. 50 μL of serum sample was enriched with 10 μL internal standard (10 ng/mL testosterone-d3, estradiol-d5, respectively; Sigma Aldrich, Saint Louis, MO). 40 μL HPLC-grade water and 100 μL acetonitrile were added for protein precipitation, samples were vortexed and then were centrifuged at 16000×g and 4 °C for 30 min. The supernatant was diluted 1:1 with HPLC-grade water prior to LC/LC-MS/MS analysis, and 100 μL were injected for analysis. An Agilent HPLC system consisting of 3 HPLC pumps, a column thermostat and a 6-port switching valve coupled to an ABSciex API5500 was used for the analysis of estrogens and testosterone. A Zorbax XDB-C8 guard column (12 × 4.6 mm) was used for an inline extraction and a Zorbax XDB-C8 analytical column (3.5 μm, 4.6 × 150 mm) was used for the separation of analytes (both columns from Agilent Technologies, Palo Alto, CA). 20% of methanol and 80% of 0.1% formic acid in water were initially pumped at 500 μL/min during the injection for the loading of the analytes onto the inline extraction column. This was followed by a flow increase to 2000 μL/min at 0.7 min. At 0.9 min the switching valve was activated and the analytes were back-flushed with 98% methanol and 2% 0.1% formic acid in water onto the analytical column. The flow rate of the analytical pump was 1000 μL/min throughout the assay. For the first 1.5 min of the assay the composition of methanol and 0.1% formic acid was 60% and 40%, respectively. After 1.5 min the gradient was increased to 98% of methanol at 2. This composition stayed constant for additional 3.5 min. After minute 5.5 the column was re-equilibrated for 1.5 min at the initial solvent composition. Atmospheric pressure photo ionization (APPI) was used for the ionization of steroids. Toluene at the flow rate of 300 μL/min was used as dopant. The API5500 mass spectrometer was operated in positive multiple reaction monitoring (MRM) mode. The following parent-fragment ions were monitored: estrone and estriol 271.1 > 133.1, estradiol 255.1 > 159.1, testosterone 289.1 > 109.1, estradiol-d5 260.3 > 161.2, and testosterone-d3 292.1 > 109.1. Analyst Software version 1.6.2 was used for data acquisition and data processing. All calibration curves had a correlation coefficient R > 0.99.
2.6. Contextual fear conditioning
The contextual fear conditioning (CFC) paradigm was utilized as a hippocampal-dependent memory task (Deng et al., 2017; Dietz et al., 2018). The apparatus consisted of two fear conditioning chambers with shock grid floors, consisting of 16 stainless steel rods connected to a shock generator (Colbourn Instruments, Model H13–15, Whitehall, PA, USA). Female juvenile mice were randomly assigned to groups (sham, vehicle-treated CA/CPR and E2-treated CA/CPR. Behavioral testing was performed 7 days after CA/CPR, at P28‒P32. Mice were transported in white buckets during the training and testing sessions. During training, mice were allowed to habituate to the conditioning chamber for two separate 2-min pre-exposure sessions followed by a foot shock (2-s/1.0 mA electric shock) immediately after the second exposure. Mice were returned to their home cages following shock. Memory was tested 24 h later by transporting mice in white buckets and placing back into the fear conditioning chambers. Memory was determined by percentage of freezing behavior, measured in 10 s intervals across a 5-min test by a blinded observer and was defined as the absence of movement except for heart beat/respiration.
2.7. Statistics
Two-way ANOVA was used to compare E2 neuroprotection and endogenous sex hormone levels in males and females. Comparisons of estrogen neuroprotection with SB203580 and contextual fear conditioning were made using one-way ANOVA with Tukey’s post-hoc analysis. Student’s t-test was used to compare hippocampal injury at 7 days after CA/CPR.
3. Results
3.1. Acute administration of 17β-estradiol reduces hippocampal cell death after cardiac arrest in female juvenile mice
Previous work has shown rapid estrogen signaling can protect against ischemia-induced neuronal cell death (Bryant and Dorsa, 2010; Carpenter et al., 2016; Jover-Mengual et al., 2010; Liu et al., 2010; Tang et al., 2014). In order to assess the acute, rapid protective effects of estrogen, we administered a single dose of E2 after CA/CPR in juvenile mice. We have previously shown that delayed neuronal cell death in juveniles following 8-min of cardiac arrest is maximal at 3 days after resuscitation. In order to determine if a single dose of estrogen is protective to CA1 hippocampal neurons, male and female juvenile (P21–25) mice were given an intravenous injection of E2 (100μg/kg) or saline vehicle 30 min after CA/CPR. This dose was chosen based on previous reports that 50– 100 μg can induce neuroprotection (Noppens et al., 2005). Hippocampal cell death was assessed 3 days later in H&E stained paraffin sections (Fig. 1A). In juvenile male animals there was no difference in the percent injured neurons in the CA1 region in E2 (55.80 ± 5.96, n = 6) and vehicle (43.46 ± 10.21, n = 6) treated mice (p = 0.58, two-way ANOVA, Fig. 1B). In contrast, female animals treated with E2 had a significantly lower percent injured neurons (30.55 ± 5.60, n = 6) compared to vehicle treated females (62.79 ± 1.28, n = 5, p = 0.02, two-way ANOVA). There was no sex difference in hippocampal injury between male and female vehicle treated mice. Importantly, there was no difference in weight change or general health assessment between gender or treatment group (Fig. 2).
Fig. 1. Estradiol reduces CA1 injury at 3 days post-injury.
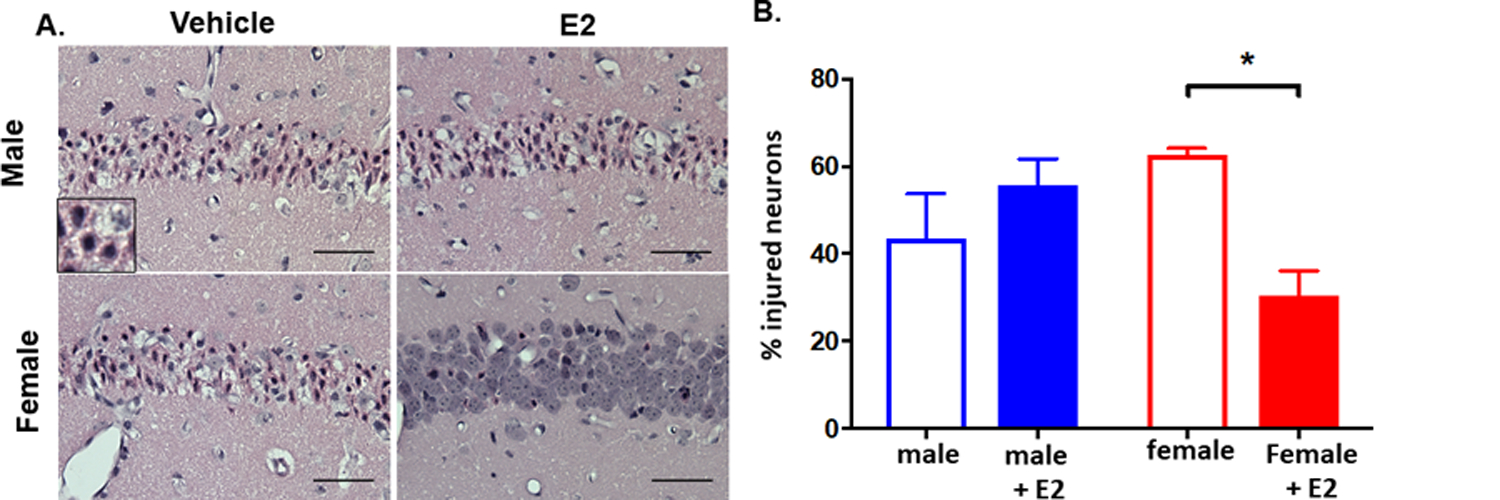
Juvenile (P21–25) male and female mice were subjected to 8 min cardiac arrest/cardiopulmonary resuscitation time and were administered saline vehicle or 17β-estradiol at 30 min after resuscitation. A. Representative H& E images of CA1 injury at 3 days after CA/CPR. Injured neurons were characterized by pyknotic nuclei and hypereosinophylic cytoplasm (inset). Scale bars represent 50 ??m. B. CA1 injury quantified at 3 days after CA/CPR as % injured neurons. Mean ± SEM, n = 5–6 per group. * indicates p < 0.05.
3.2. Juvenile cardiac arrest occurs before divergence of endogenous sex steroids in male and female mice
We compared endogenous estradiol and testosterone levels in male and female mice during the juvenile time period (P21–25), late juvenile period, during the time that neurobehavioral tests were performed (P28–32), and in adults (P56–62). In P21–25 females, endogenous estradiol was below the level of quantification (Fig. 3A). Endogenous estradiol remained low at P28–32 (16.03 ± 6.6 pg/mL, n = 4), and increased significantly by P56–62 (87.78 ± 29.11 pg/mL, n = 5, p < 0.0001 compared to P21–25 and P28–32, respectively). In male animals estradiol levels were low at P21–25 (11.82 ± 17.38 pg/mL, n = 5) and remained low at P28–32 (19.06 ± 5.69 pg/mL, n = 5) and p56–62 (7.5 ± 16.77 pg/mL, n = 5). There was no significant difference in estradiol levels across ages (p > 0.86). There was no difference in estradiol levels between males and females at P21–25 or P28–32 (p = 0.85 and 1.0 respectively). However, adult (P56–62) females had significantly higher estradiol levels compared to adult males (p < 0.001).
Fig. 3. Serum estradiol and testosterone levels.
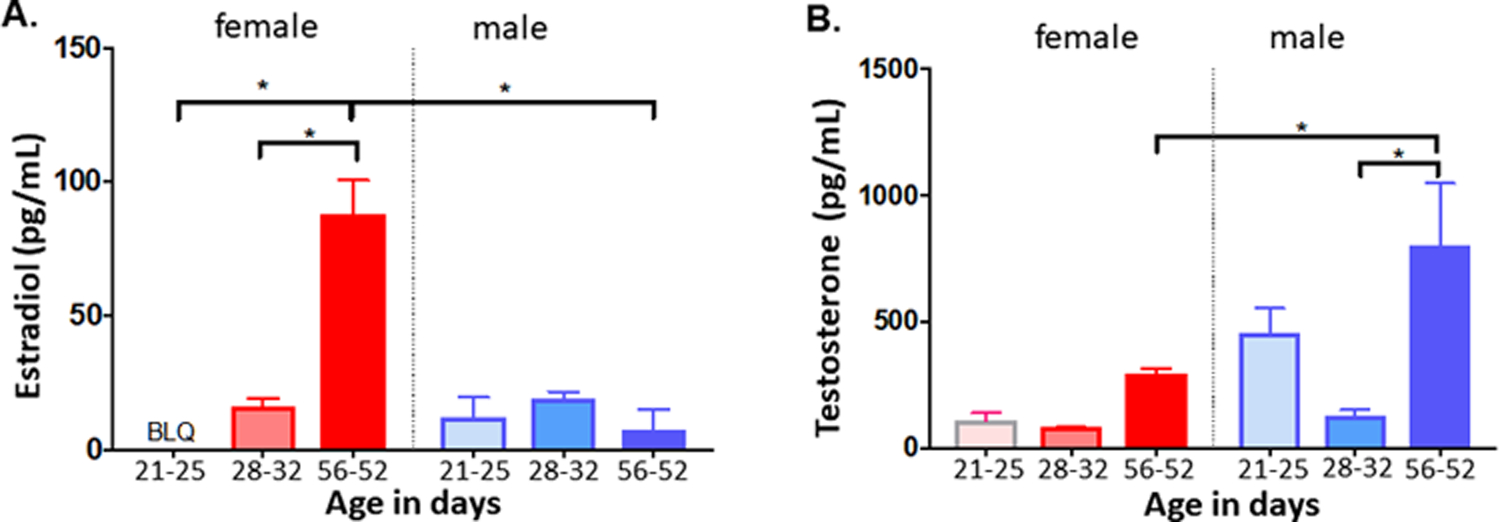
Serum was collected and analyzed by LC/MS to determine estradiol and testosterone concentrations. A) Estradiol levels at ischemia time (P21–P25), at time of behavior (P28–32) and after hormonal maturation (P56–62). B) Testosterone levels in females and males at same ages as in A. Mean ± SEM, n = 5–6 per group. * indicates p < 0.05.
Endogenous testosterone was low in female mice at P21–25 (67.5 ± 30.19 pg/mL, n = 5) and at P28–32 (80 ± 3.06 pg/mL, n = 4) (Fig. 3B). There was an increase in endogenous testosterone at P56–62 (290.8 ± 22.68 pg/mL, n = 5), however the difference across ages was not statistically significant (p > 0.64), in female mice. In male mice there was a dramatic increase in endogenous testosterone at P56–62 (799.4 ± 248.1 pg/mL, n = 5), compared to P21–25 (452.0 ± 101.8 pg/mL, n = 5, p = 0.21) and P28–32 (125.3 ± 24.9 pg/mL, n = 5, p = 0.001). There was not a difference in endogenous testosterone levels between males and females at P21–25 (p = 0.22), or between males and females at P28–32 (p = 1.0, two-way ANOVA). However, adult (P56–62) males had significantly higher testosterone levels compared to adult females (p = 0.02).
3.3. Estrogen protection after cardiac arrest is mediated by the P38 map kinase
Rapid estrogen receptor-mediated signaling involves activation of mitogen-activated protein kinase (MAPK) pathways (Bryant et al., 2006), therefore, we tested the hypothesis that E2-mediated neuroprotection required P38 MAPK activation. In order to determine whether the protective effect of estrogen in females after cardiac arrest is mediated by the P38 MAPK we utilized the selective P38 MAPK inhibitor, SB203580. Female animals underwent cardiac arrest/resuscitation and were treated in one of the following groups 30 min later: Vehicle, E2, SB203580, or E2+SB203580. Percent injured hippocampal CA1 neurons were assessed 3 days after CA/CPR (Fig. 4A). SB203580 administration alone after cardiac arrest did not affect neuronal injury compared to vehicle (62.75 ± 1.26% injured neurons in vehicle treated, n=5 vs 53.66 ± 14.75% injured in SB203580 treated, n=5, p = 0.68, one-way ANOVA, Fig. 4B). When SB203580 was given concurrently with E2, the protection seen with E2 treatment was abolished (30.55 ± 13.71% injured neurons in E2 treated animals, n=6, vs 55.12 ± 16.24% injured in E2+SB203580 treated animals, n=4, p = 0.04, one-way ANOVA).
Fig. 4. Estrogen Protection Observed 3 Days Post-Injury is Mediated by P38-MAPK.
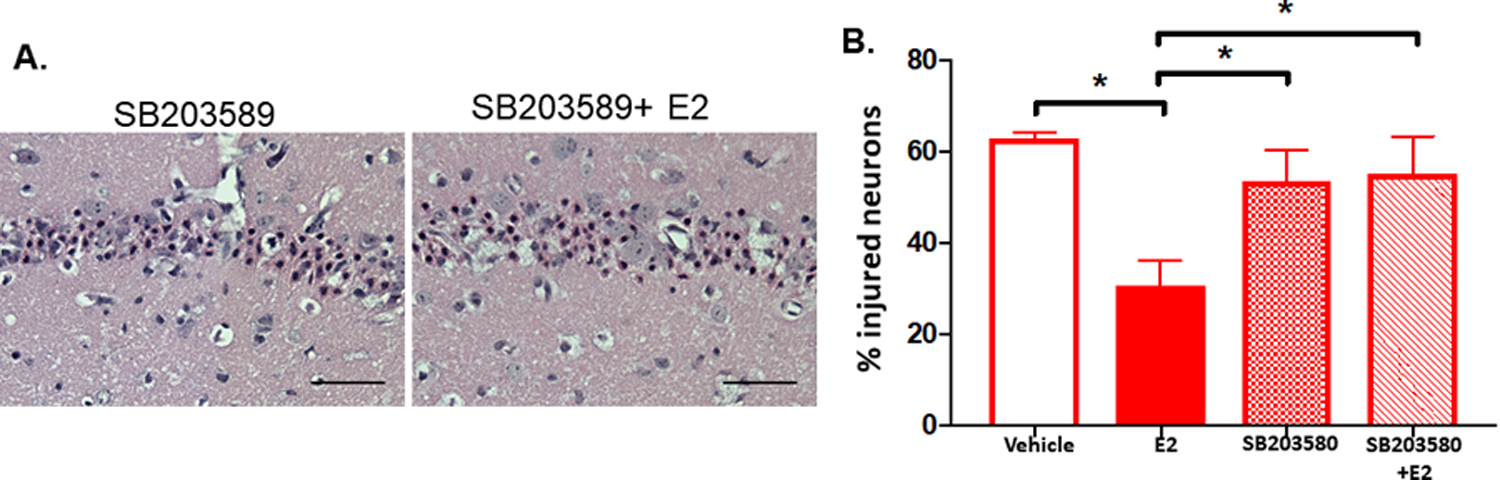
Juvenile (P21–25) female mice were subjected to 8 min CA/CPR and were administered saline vehicle, E2, the p38 MAPK inhibitor, SB203580, or E2+ SB203589 at 30 min after resuscitation, and % injured CA1 hippocampal neurons were determined 3 days later. A. Representative H&E images of CA1 injury at 3 days after CA/CPR, scale bars represent 50 μm. B. quantification of % injured neurons. Female and Female + E2 groups are from Fig. 1. * indicated p < 0.05.
3.4. Neuroprotection by single dose 17β-estradiol after cardiac arrest is not sustained and does not rescue memory deficits sub-acutely
We evaluated a learning and memory task (contextual fear) 7 days after cardiac arrest in female juvenile mice to determine if the neuroprotection seen after a single dose of 17β-Estradiol provides benefits for memory function. In vehicle treated female mice there is impaired contextual memory, measured as reduced % freezing, 7 days after cardiac arrest compared to sham animals (sham 68.33 ± 5.60% freezing, n = 12 vs vehicle 44.21 ± 5.38% freezing, n = 8, p = 0.03, one-way ANOVA, Fig. 5A). In E2 treated animals there was also a significant memory impairment compared to sham animals (42.08 ± 8.379% freezing in E2 treated animals, n = 8, p = 0.02 compared to sham, one-way ANOVA). There was no difference in memory performance between E2-and vehicle-treated animals (p = 0.97, one-way ANOVA). To determine whether a single dose of E2 results in sustained neuroprotection in juvenile female mice, we measured % injured CA1 neurons 7 days after cardiac arrest in animals which had undergone behavioral testing. At this time point we did not see any injured neurons in sham cardiac-arrest animals (data not shown). Contrary to the neuronal protection we saw with E2 treatment in females at 3 days post-cardiac arrest, there was no difference in percent injured neurons at 7 days, with 31.85 ± 9.37% injured neurons in vehicle treated female animals (n = 8) and 37.10 ± 11.53% injured neurons in E2 treated female animals (n = 6, p = 0.72, un-paired t-test, Fig. 5B). Because the % injured neurons does not account for neurons that have died and been cleared by phagocytosis, we also assessed the density of live neurons in the CA1 at 7 days, and did not see a difference between groups (592.1 ± 108.5 cells/mm2 in vehicle treated animals vs 375.6 ± 103.6 cells/mm2 in E2 treated animals, mean ± SEM, p = 0.17, Fig. 5C).
Fig. 5. Neuroprotection is not sustained after a single dose of E2.
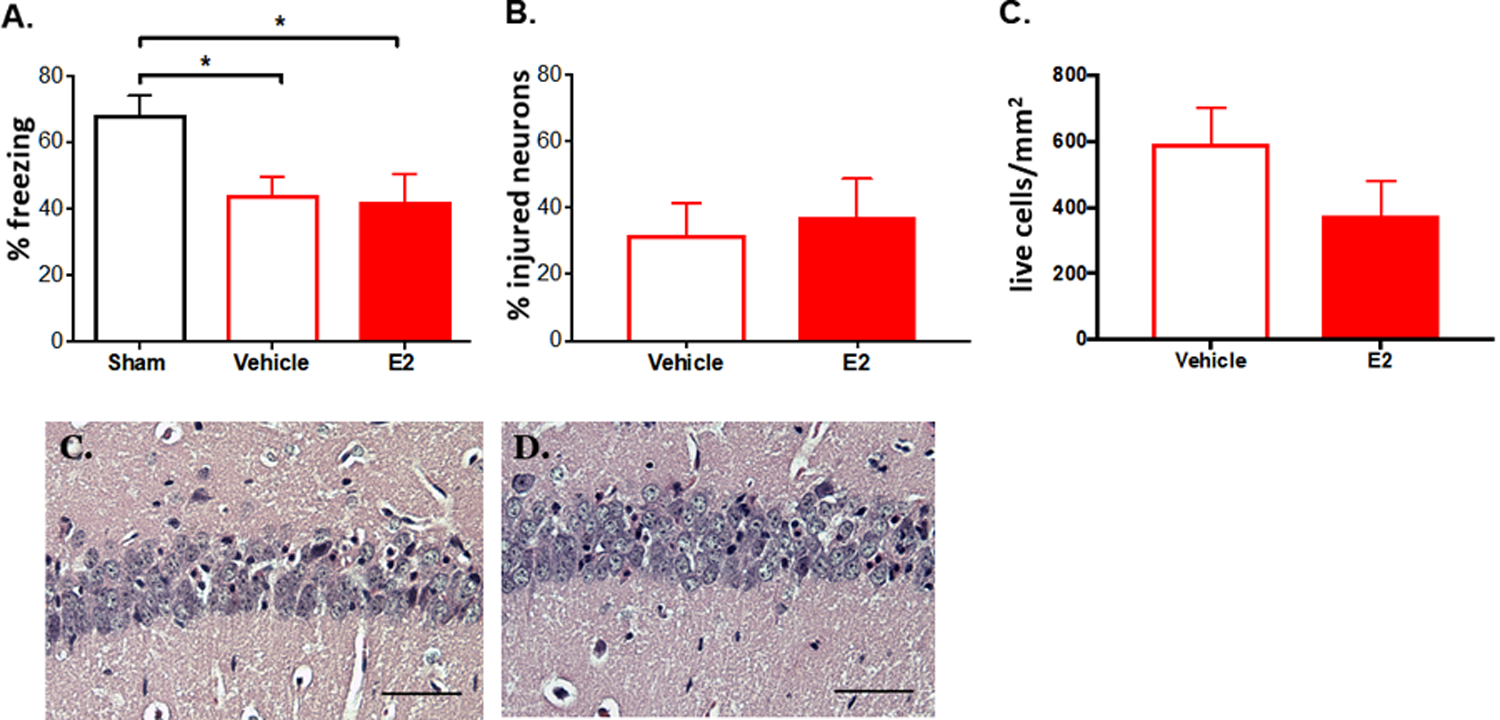
Juvenile female mice underwent 8 min of cardiac arrest/cardiopulmonary resuscitation and were administered E2 or saline vehicle 30 min later. A. Animals underwent the contextual fear behavioral test 7 days later and % freezing was determined. B. The % injured CA1 hippocampal neurons were measured. C. The density of live neurons was also measured. * Representative CA1 are shown from vehicle (D) and E2 (E) treated animals. Scale bars are 50 μm. indicates p < 0.05.
4. Discussion
We demonstrated that estradiol administration provided a transient histological protection in the hippocampal CA1 region that was sex-specific. This protection was blocked by co-administration of the P38-MAPK inhibitor, suggesting estradiol activates this pathway to promote cell survival. Histological protection seen at three days was not sustained and no benefit to memory was observed 1 week after cardiac arrest. These findings suggest that single dose estrogen delayed, rather than prevented, the cell death process.
4.1. Sex differences in injury and neuroprotection specific to E2
Sexual dimorphism in adult animals has been demonstrated in a number of cerebral injury models, with female animals having less brain tissue injury compared to males in cardiac arrest (Nakayama et al., 2013), focal ischemic stroke (Alkayed et al., 1998), and traumatic brain injury (Clevenger et al., 2018). The benefit of female gender is lost in ovariectomized animals and can be rescued with estrogen supplementation (reviewed in (Herson et al., 2009)), strongly implicating estrogen in the neuroprotection seen in mature females. We compared injury in males and females at a time just prior to the human equivalent of puberty; before the marked increase in estrogen and androgens seen after P28. We did not see a difference in injury between genders at this age, which is similar to our prior results in focal transient middle cerebral artery occlusion (MCAO) in juvenile mice (Herson et al., 2013a). The largest body of research regarding gender effects of ischemia in pre-pubertal animals is in models of neonatal hypoxiaischemia (Hurn et al., 2005). These studies largely also show a lack of gender dimorphisms (Hagberg et al., 2004; Waddell et al., 2016), however there is some evidence that injury evolution is different in males and females, and so the finding of a gender effect is dependent on timing of injury evaluation (Mirza et al., 2015). Despite overall similar injury sizes in various models of neonatal and juvenile ischemia, several studies have shown that mechanisms of cell death and inflammation differ, and are differentially influenced by estrogen treatment, in males and females (Hagberg et al., 2004; Herson et al., 2013a; Mirza et al., 2015), and that repair mechanisms may differ between genders (Waddell et al., 2016).
Similar to our prior findings in juvenile MCAO, in our model of juvenile cardiac arrest, estrogen treatment was protective in females, but not in males. These results are contrary to studies in adult mice, in which estrogen is protective in males, (Kosaka et al., 2012; Noppens et al., 2005). The finding of estrogen protection in juvenile females, but not males, suggests a difference in estrogen signaling between the genders. Estrogen receptor (ER) subtypes include ERα and ERβ and the G-protein coupled estrogen receptor, GPER1. In juvenile mice, expression of ERα is highest. We previously found that expression of both ER subtypes was similar in juvenile males and females (Herson et al., 2013a), suggesting that relative receptor expression is not the cause of the gender dimorphism in response to estrogen treatment. In a neonatal hypoxia/ischemic encephalopathy model, ERα expression was induced by ischemia in females only (Cikla, 2016). Thus it possible that there is a differential response to ischemia through estrogen receptor dependent signaling, even in the absence of circulating sex hormones.
Estrogen receptors can signal through genomic and non-genomic pathways to engage neuroprotective signaling. By administering only a single-dose, we think it is more likely that E2 effects were mediated via acute signaling. Our results with the SB203580 block of E2 neuroprotection suggests that estrogen receptor signaling couples to activation of the P38 MAPK pathway. P38 MAPK has been shown to engage pro-survival and pro-apoptotic pathways in different conditions. In the context of ischemia and cell-stress, it has primarily been shown to have a pro-apoptotic role and inhibition of P38 MAPK can be neuroprotective in focal ischemia models (Barone et al., 2001; Piao et al., 2003). We did not observe neuroprotection with SB203580 alone, indicating that P38 MAPK likely does not contribute to cell death mechanisms in juvenile females. Many of the studies that have achieved neuroprotection with P38 MAPK were performed only in males. Male-specific P38 MAPK-mediated neurodegeneration was previously observed in a Parkinson’s mouse model (Saeed et al., 2009). Therefore, P38 MAPK signaling may favor pro-survival signaling in females and pro-apoptotic in males. Indeed, our results are consistent with estrogen engaging pro-survival signaling in females alone. Anti-apoptotic/pro-survival roles of P38 MAPK can be mediated by myocyte enhancer 2 transcription factor pathway (Mao et al., 1999; Okamoto et al., 2000). These results have implications for translational potential of P38 MAPK inhibition as a neuroprotective strategy and highlight the importance of assessing sex-differences in therapeutic strategies.
One of the more surprising findings in our study was the transient nature of neuroprotection. Delayed neuronal cell death is one of key features of neuronal cell death induced by global ischemia. We previously demonstrated that in our model of juvenile cardiac arrest the percentage of injured CA1 neurons peaks at 3 days post-CA and decreases by 7 days, while the density of live cells remains stable in this same time frame (Deng et al., 2014b). Therefore, the decrease in the proportion of neurons with an injured phenotype between 3 and 7 days is presumably due to removal of dead cells via phagocytosis. Similar to our prior findings, in this study we found that in our vehicle treated animals the % of injured neurons was highest at 3 days (62.6%) and decreased by 7 days–31.8%. However, in E2 treated animals we saw an acute protection, with a cell death of only 30.5%, which remained at a similar level by 7 days (37.1%). This suggests that in the E2 treated animals cell death is prolonged, supported by the fact that we see a similar density of live cells in vehicle and E2 treated animals by 7 days post-CA. Our results showing a similar amount of cell death and memory deficit at 7 days indicates that E2 delayed, rather than prevented, the cell death process.
5. Conclusion
Sexual dimorphism in cell death processes continues to pose a challenge to preclinical and clinical researchers. While estrogen receptor-dependent signaling has repeatedly been shown to provide neuroprotection, our finding in juveniles and others in aged animals have shown that this benefit may depend on the sex and age of the animal (Cai et al., 2014; Herson et al., 2013b; Hilton et al., 2003). Our results add to the growing body of literature that demonstrate sex-differences vary across the lifespan and this can have a dramatic impact on the efficacy of pharmacological interventions aimed at providing neuroprotection against ischemic injury and other neurodegenerative processes. Our results also highlight the importance of assessing histological and functional benefits beyond the acute phase of ischemic injury.
Supplementary Material
Funding sources
This work was supported by the National Institute of Health NS046072.
Abbreviations:
- CA/CPR
cardiac arrest and cardiopulmonary resuscitation
- E2
17β-estradiol
- CFC
contextual fear conditioning
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuint.2018.11.013.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, 1998. Gender-linked brain injury in experimental stroke. Stroke 29, 159. [DOI] [PubMed] [Google Scholar]
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, Legos JJ, Erhardt JA, Ohlstein EH, et al. , 2001. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med. Res. Rev 21, 129–145. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. , 2018. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K, 2002. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med 346, 557–563. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Dorsa DM, 2010. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience 170, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM, 2006. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine 29, 199–207. [DOI] [PubMed] [Google Scholar]
- Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, et al. , 2014. The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci. Lett 558, 115–119. [DOI] [PubMed] [Google Scholar]
- Carpenter RS, Iwuchukwu I, Hinkson CL, Reitz S, Lee W, Kukino A, Zhang A, Pike MM, Ardelt AA, 2016. High-dose estrogen treatment at reperfusion reduces lesion volume and accelerates recovery of sensorimotor function after experimental ischemic stroke. Brain Res 1639, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger AC, Kim H, Salcedo E, Yonchek JC, Rodgers KM, Orfila JE, Dietz RM, Quillinan N, Traystman RJ, Herson PS, 2018. Endogenous sex steroids dampen neuroinflammation and improve outcome of traumatic brain injury in mice. J. Mol. Neurosci 64, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Orfila JE, Dietz RM, Moreno-Garcia M, Rodgers KM, Coultrap SJ, Quillinan N, Traystman RJ, Bayer KU, Herson PS, 2017. Autonomous CaMKII activity as a drug target for histological and functional neuroprotection after resuscitation from cardiac arrest. Cell Rep 18, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, Traystman RJ, 2014a. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J. Neurosci. Methods 222, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, Traystman RJ, 2014b. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J. Neurosci. Methods 222, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz RM, Orfila JE, Rodgers KM, Patsos OP, Deng G, Chalmers N, Quillinan N, Traystman RJ, Herson PS, 2018. Juvenile cerebral ischemia reveals age-dependent BDNF–TrkB signaling changes: novel mechanism of recovery and therapeutic intervention J. Cerebr Blood Flow Metabol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink EL, Prince DK, Kaltman JR, Atkins DL, Austin M, Warden C, Hutchison J, Daya M, Goldberg S, Herren H, et al. , 2016. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 107, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, 2000. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke; J. Cerebr. Circ 31, 155–160. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S, 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol. Rev 62, 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Wilson M, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, et al. , 2004. PARP‐1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem 90, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Herlitz J, Rundqvist S, Bang A, Aune S, Lundstrom G, Ekstrom L, Lindkvist J, 2001. Is there a difference between women and men in characteristics and outcome after in hospital cardiac arrest? Resuscitation 49, 15–23. [DOI] [PubMed] [Google Scholar]
- Herson P, Koerner I, Hurn P, 2009. Sex, sex steroids, and brain injury. Semin. Reprod. Med 27, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ, 2013a. Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling. Stroke 44, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ, 2013b. Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling. Stroke; J. Cerebr. Circ 44, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Hurn PD, 2010. Gender and the injured brain. Prog. Brain Res 186, 177–187. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, McCarthy MM, 2003. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience 116, 383–391. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Vannucci SJ, Hagberg H, 2005. Adult or perinatal brain injury. Stroke 36, 193–195. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study, G., 2002. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med 346, 549–556. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS, 2002. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. : Off. J. Soc. Neurosci 22, 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Mengual T, Miyawaki T, Latuszek A, Alborch E, Zukin RS, Etgen AM, 2010. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res 1321, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW, 2010. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J. Gene Med 12, 561–563. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Iwami T, Nichol G, Nishiuchi T, Hayashi Y, Nishiyama C, Sakai T, Kajino K, Hiraide A, Ikeuchi H, et al. , 2010. Reduction in incidence and fatality of out-of-hospital cardiac arrest in females of the reproductive age. Eur. Heart J 31, 1365–1372. [DOI] [PubMed] [Google Scholar]
- Kosaka Y, Quillinan N, Bond CT, Traystman RJ, Hurn PD, Herson PS, 2012. GPER1/GPR30 activation improves neuronal survival following global cerebral ischemia induced by cardiac arrest in mice. Translational stroke research 3, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD, 2010. Neuroprotection of sex steroids. Minerva Endocrinol 35, 127–143. [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD, 2015. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J. Cerebr. Blood Flow Metabol.: Off. J. Int. Soc. Cerebr. Blood Flow Metab 35, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME, 1999. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286, 785–790. [DOI] [PubMed] [Google Scholar]
- Michiels E, Quan L, Dumas F, Rea T, 2016. Long-term neurologic outcomes following paediatric out-of-hospital cardiac arrest. Resuscitation 102, 122–126. [DOI] [PubMed] [Google Scholar]
- Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F, 2015. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflammation 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL, et al. , 2015. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N. Engl. J. Med 372, 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Hurn PD, Herson PS, Traystman RJ, 2010. Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res 1357, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Vest R, Traystman RJ, Herson PS, 2013. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J. Mol. Neurosci 51, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. , 2013. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N. Engl. J. Med 369, 2197–2206. [DOI] [PubMed] [Google Scholar]
- Noppens RR, Kofler J, Hurn PD, Traystman RJ, 2005. Dose-dependent neuroprotection by 17β-estradiol after cardiac arrest and cardiopulmonary resuscitation. Crit. Care Med 33, 1595. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Krainc D, Sherman K, Lipton SA, 2000. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc. Natl. Acad. Sci. U. S. A 97, 7561–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Kim JB, Han PL, Lee JK, 2003. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J. Neurosci. Res 73, 537–544. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD, 1999. 17β-Estradiol reduces stroke injury in estrogen-deficient female animals. Stroke; J. Cerebr. Circ 30, 1665–1670. [DOI] [PubMed] [Google Scholar]
- Saeed U, Karunakaran S, Meka DP, Koumar RC, Ramakrishnan S, Joshi SD, Nidadavolu P, Ravindranath V, 2009. Redox activated MAP kinase death signaling cascade initiated by ASK1 is not activated in female mice following MPTP: novel mechanism of neuroprotection. Neurotox. Res 16, 116–126. [DOI] [PubMed] [Google Scholar]
- Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R, 2014. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol. Cell. Endocrinol 387, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topjian AA, Localio AR, Berg RA, Alessandrini EA, Meaney PA, Pepe PE, Larkin GL, Peberdy MA, Becker LB, Nadkarni VM, 2010. Women of childbearing age have better inhospital cardiac arrest survival outcomes than do equal-aged men. Crit. Care Med 38, 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress E, Kochanek P, Saladino R, Manole M, 2010. Cardiac arrest in children. J. Emergencies, Trauma, Shock 3, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Hanscom M, Edwards SN, McKenna MC, McCarthy MM, 2016. Sex differences in cell genesis, hippocampal volume and behavioral outcomes in a rat model of neonatal HI. Exp. Neurol 275, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


