Abstract

Pro-inflammatory cytokines, such as the IL-18-induced inflammatory response and associated damage in fibroblast-like synoviocytes (FLS), play an important role in the pathogenesis of rheumatoid arthritis (RA). Roflumilast, an inhibitor of phosphodiesterase-4 (PDE-4), has been licensed for the treatment of chronic obstructive pulmonary disease (COPD). However, it is unknown whether roflumilast possesses a protective effect against the IL-18-induced inflammatory response in FLS. We found that roflumilast attenuated IL-18-induced oxidative stress by reducing the production of reactive oxygen species and malondialdehyde (MDA) in MH7A fibroblast-like synoviocytes (FLS). Additionally, roflumilast prevented IL-18-induced expressions and secretions of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α. Importantly, we found that roflumilast inhibited IL-18-induced expressions of chemokines such as CCL5, CXCL9, and CXCL10. Further, roflumilast inhibited the expression of extracellular matrix degradative enzymes, such as matrix metalloproteinase-3 (MMP-3) and MMP-13. Mechanistically, we found that roflumilast suppressed the activation of the transcriptional factor AP-1 and NF-κB. Our results suggest that roflumilast might be a potential therapeutic agent for the treatment of RA.
1. Introduction
Rheumatoid arthritis (RA) is a common chronic inflammatory autoimmune disease that may affect all joints of the body, especially the small joints of the extremities.1 The symptoms of RA include swelling and stiffness of the joint. RA is characterized by continuous arthrosynovitis, causing the destruction of cartilage, bone damage, and deformity in joints, ultimately leading to disability, loss of quality of life, and premature death.2,3 The underlying mechanism of RA is complicated. Previous studies have demonstrated that the pathogenesis of RA is related to heredity, infection, hormones, and environmental factors.4 Interleukin-18 (IL-18) is a pro-inflammatory cytokine that belongs to the interleukin-1 (IL-1) family.5 IL-18 is primarily well known as an interferon-γ (IFN-γ)-inducing factor and has been demonstrated to play an important role in the innate and adaptive immunobiology.6 IL-18 can be expressed by a wide range of immune and nonimmune cells.7,8 The main function of IL-18 is to stimulate lymphocytes to produce IFN-γ and regulate cell activity, thereby increasing the expression of pro-inflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α, CCL5, CXCL9, and CXCL10.9,10 Moreover, IL-18 can trigger the transcription of multiple inflammatory genes via the activation of nuclear factor-κB (NF-κB) to promote an inflammatory response. In the clinic, it is observed that the overproduction of IL-18 is closely related to RA.11 Fibroblast-like synoviocytes (FLS) are cells derived from the mesenchyme that line the internal synovial tissues and play an important role in the development of RA. FLS proliferate aberrantly when stimulated with pro-inflammatory cytokines such as IL-1β and TNF-α. When activated, FLS release multiple factors including pro-inflammatory cytokines, chemokines, and matrix-degrading enzymes, resulting in inflammation and destruction of the extracellular matrix (ECM).12,13 Therefore, FLS have been recognized as a potential therapeutic target for the treatment of RA.
Roflumilast is a selective phosphodiesterase-4 (PDE-4) inhibitor and is distinctive to cyclic adenosine 3′,5′-monophosphate (cAMP) degradation. Roflumilast has been approved for the treatment of chronic obstructive pulmonary disease (COPD) to reduce the risk of exacerbations in patients with COPD. Previous studies showed evidence that inhibition of PDE-4 could drive the resolution of inflammation.14 Actually, as a PDE-4 inhibitor, the anti-inflammation effects of roflumilast have been proven in several studies.15,16 However, whether it is therapeutically effective in RA is unknown. This study aims to investigate the protective effects of roflumilast against IL-18-induced inflammation in FLS.
2. Results
2.1. Effects of Roflumilast in Cell Viability of MH7A FLS
The molecular structure of roflumilast is shown in Figure 1A. First, we tested the cytotoxicity of roflumilast in FLS. Cells were treated with six doses of roflumilast ranging from 0.5 to 100 μM for 24 h. The results in Figure 1B show that roflumilast did not affect the cell viability of FLS when its concentration was not more than 10 μM. However, 50 and 100 μM roflumilast significantly reduced the cell viability, 9 and 12%. Therefore, 5 and 10 μM roflumilast were used in subsequent experiments.
Figure 1.

Effects of roflumilast in cell viability of MH7A fibroblast-like synoviocytes (FLS). (A) Molecular structure of roflumilast; (B) MH7A cells were stimulated with roflumilast at concentrations of 0, 0.5, 1, 5, 10, 50, and 100 μM for 24 h. Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (#P < 0.05, ##P < 0.01 vs vehicle group).
2.2. Roflumilast Reduced IL-18-Induced Oxidative Stress in MH7A FLS
To assess the effect of roflumilast on oxidative stress in FLS, the production of reactive oxygen species (ROS) and malondialdehyde (MDA) was measured. As shown in Figure 2A, treatment with IL-18 induced a 3.1-fold increase of the level of intracellular ROS, which were reduced to 2.2- and 1.5-fold by two doses of roflumilast, respectively. Similarly, the results in Figure 2B show that the expression of MDA was significantly inhibited by the same doses of roflumilast, respectively, compared with the exposure to IL-18 only. Taken together, roflumilast shows a strong inhibitory effect on oxidative stress induced by IL-18 in FLS in a dose-dependent manner.
Figure 2.

Roflumilast reduced IL-18-induced oxidative stress in MH7A FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. (A) Intracellular ROS was measured using dihydroethidium (DHE) staining; scale bar, 200 μm. (B) Production of MDA (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
2.3. Roflumilast Reduced IL-18-Induced Expression and Secretions of Pro-inflammatory Cytokines in MH7A FLS
Pro-inflammatory cytokines play a pivotal role in the development of an inflammatory response. We tested the expressions and secretions of IL-6, IL-8, and TNF-α. As shown in Figure 3A–C, stimulation with IL-18 induced a significant increase of these three cytokines at mRNA level; however, 5 and 10 μM roflumilast reduced their expression remarkably, in a dose-dependent manner. As expected, the results in Figure 3D–F indicate a strong inhibitory effect of roflumilast on the secretion of these pro-inflammatory cytokines.
Figure 3.
Roflumilast reduced IL-18-induced expressions and secretions of pro-inflammatory cytokines IL-6, IL-8, and TNF-α in MH7A FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. (A–C) mRNA of IL-6, IL-8, and TNF-α; (D–F) secretions of IL-6, IL-8, and TNF-α (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
2.4. Roflumilast Reduced IL-18-Induced Expression and Secretions of Pro-inflammatory Chemokines
As shown in Figure 4A–F, exposure to IL-18 significantly increased the production of the pro-inflammatory chemokines CCL5, CXCL9, and CXCL10 in FLS, while the presence of 5 and 10 μM roflumilast significantly reduced the IL-18-induced production of CCL5, CXCL9, and CXCL10 in a dose-dependent manner at both mRNA and protein levels, respectively.
Figure 4.
Roflumilast reduced IL-18 induced expression and secretion of pro-inflammatory chemokines CCL5, CXCL9, and CXCL10 in MH7A FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. (A–C) mRNA of CCL5, CXCL9, and CXCL10; (D–F) secretions of CCL5, CXCL9, and CXCL10 (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
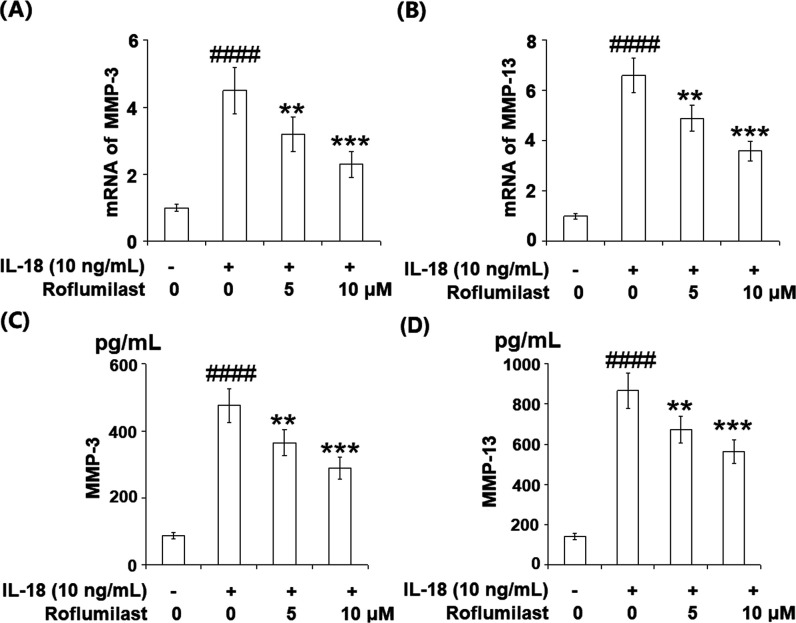
2.5. Roflumilast Reduced IL-18-Induced Expression and Secretions of MMP-3 and MMP-13
MMP-3 and MMP-13 are recognized as the main collagenases responsible for the degradation of the articular extracellular matrix (ECM). The results in Figure 5A–D reveal that treatment with IL-18 resulted in a remarkable increase in expressions of MMP-3 and MMP-13. However, two doses of roflumilast suppressed the IL-18-induced expressions of MMP-3 and MMP-13 in a dose-dependent manner at both mRNA and protein levels.
Figure 5.
Roflumilast reduced IL-18-induced expressions and secretions of MMP-3 and MMP-13 in MH7A FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. mRNA of MMP-3 (A) and MMP-13 (B); protein levels of MMP-3 (C) and MMP-13 (D) (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
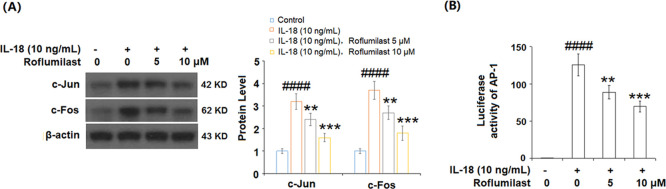
2.6. Roflumilast Reduced IL-18-Induced Activation of AP-1
To evaluate the effect of roflumilast on activation of the transcriptional factor AP-1, we tested the protein expressions of c-Jun and c-Fos and the luciferase activity of AP-1. As illustrated in Figure 6A, expressions of c-Jun and c-Fos were increased at significantly higher levels by exposure to IL-18 only, while roflumilast reduced their expression in a dose-dependent manner. Congruously, the results in Figure 6B show that roflumilast also inhibited the luciferase activity of AP-1. Our data shows that roflumilast has an inhibitory effect on the activation of AP-1.
Figure 6.
Roflumilast reduced IL-18-induced activation of AP-1 in MH7A FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. (A) Protein expression of c-Jun and c-Fos and (B) luciferase activity of AP-1 (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
2.7. Roflumilast Reduced IL-18-Induced Activation of NF-κB
Activation of NF-κB is considered a key mediator in an inflammatory response and has been shown to drive the development of numerous diseases including RA. To determine the effects of roflumilast on IL-18-induced activation of NF-κB, we measured the nuclear translocation of p65 and luciferase activity of NF-κB. The results in Figure 7A,B show that two doses of roflumilast significantly inhibited the nuclear translocation of p65 as well as the luciferase activity of NF-κB, compared with the remarkable increase induced by IL-18.
Figure 7.
Roflumilast reduced IL-18-induced activation of NF-κB in MH7A RA-FLS. Cells were stimulated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast. (A) Nuclear levels of NF-κB p65; (B) luciferase activity of NF-κB (####P < 0.0001 vs vehicle group; **P < 0.01, ***P < 0.001 vs IL-18 group).
3. Discussion
RA is one of the most common chronic inflammatory diseases around the world and brings a critical burden to patients, families, and the society. The risks of RA include not only the destruction of cartilage and deformity in joints but also systemic complications such as rheumatoid vasculitis, atherosclerotic cardiovascular disease, and RA-associated lung disease, which increase the mortality of patients with RA.17−19 Proliferative FLS play crucial roles in joint inflammation, destruction of cartilage, and bone damage.20 Therefore, it is meaningful to find the related crucial inflammatory target molecules in FLS for the research of the RA mechanism. The current study aimed to investigate the anti-inflammatory effects of roflumilast on IL-18-stimulated FLS and to elucidate the underlying mechanism involved. Our results revealed that roflumilast significantly suppressed IL-18-induced oxidative stress through mitigating the production of ROS and MDA, inhibiting the expressions of pro-inflammatory cytokines and chemokines. In addition, roflumilast reduced the production of MMP-3 and MMP-13, which play an important role in the degradation of cartilage. Furthermore, roflumilast suppressed the activation of the transcriptional factor AP-1 and NF-κB.
Oxidative stress is defined as an imbalance between the production of ROS and antioxidants. This imbalance directly results in the damage to cells. Furthermore, oxidative stress can lead to chronic inflammation via activating a variety of transcription factors including NF-κB and AP-1, which mediate the expression of various inflammatory factors and enzymes to promote the development of inflammation.21 Several research studies pointed out that increased levels of ROS production in patients with RA were observed, leading to increased levels of different markers such as MDA.22 Our data shows that expressions of ROS and MDA were significantly increased in IL-18-induced FLS. Meanwhile, we found that 5 and 10 μM roflumilast significantly suppressed the generation of ROS and MDA. Therefore, we demonstrated the inhibitory effect of roflumilast on oxidative stress.
Several relevant pro-inflammatory cytokines have been determined to play important roles in the progression and development of RA, especially IL-6, IL-8, and TNF-α. They can not only activate FLS but also induce the expression of other cytokines, chemokines, and metalloproteinases.23−25 Interestingly, a previous study found that in cultured synovial cells from patients with RA, blockade of TNF-α with antibodies reduced the expressions of IL-6 and IL-8.26 In our study, overexpression of these three cytokines was observed in IL-18-induced FLS. Another research showed that roflumilast has an inhibitory effect on the expression of pro-inflammatory cytokines in a murine model of chronic asthma.16 As expected, in our study, we confirmed that roflumilast could suppress the expression of pro-inflammatory cytokines at both mRNA and protein levels, in a dose-dependent manner. Additionally, roflumilast also reduced the expression of pro-inflammatory chemokines such as CCL5, CXCL9, and CXCL10, which are responsible for recruiting immune cells to the synovial tissue, promoting the development of inflammation. ECM plays an important role in maintaining the structure of cartilage tissues in the joint. It has been demonstrated that ECM can be degraded by metalloproteinases such as MMP-3 and MMP-9 in osteoarthritis.27 Similarly, in our study, we found that treatment with roflumilast significantly reduced the production of MMP-3 and MMP-9 in IL-18-induced FLS. This finding indicates that roflumilast might have a potential effect in preventing the degradation of cartilage.
NF-κB and AP-1 are important regulatory transcription factors that are associated with inflammation and proved to be relevant to RA disease. Upon activation, free NF-κB formed by a heterodimer of p50 and p65, and c-Jun and c-Fos (the subunits of AP-1), pass into the nucleus. Subsequently, they bind to the promoter regions of genes for inflammatory proteins such as cytokines, chemokines, and enzymes.28,29 Besides, in animal arthritis models, inhibition of NF-κB delayed the destruction of joints.30 Meanwhile, evidence shows that activation of AP-1 is related to the production of pro-inflammatory cytokines in RA.31 In the present study, we found that the expressions of p65, c-Jun, and c-Fos were significant in IL-18-induced FLS. Our data demonstrates that roflumilast has a strong inhibitory effect on the activation of NF-κB and AP-1. A recent study also confirmed this viewpoint in a sepsis model.32
Taken together, the present study shows that roflumilast prevents the IL-18-induced inflammatory response in FLS. More research is required to further investigate the protective effects of roflumilast on RA.
4. Materials and Methods
4.1. Cell Culture and Treatment
MH7A fibroblast-like synoviocytes (FLS) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P/S). Cells were stimulated with IL-18 (10 ng/mL, Cat# 10119-HNCE, SinoBiological)6,7,10 in the presence or absence of 5 and 10 μM roflumilast (Cat# A10804, Adooq Bioscience).16,18
4.2. Cell Viability
MH7A FLS (1 × 104/well) were added in a 96-well plate and cultured for 6–8 h. After being treated with roflumilast at different concentrations for 24 h, cell viability was determined using the MTT assay (Cat# ab211091, Abcam). The cells were incubated with the MTT assay at 37 °C for 4 h. The absorbance of each well was then measured at 570 nm using a microplate reader (Thermo Fisher Scientific), and the percentage viability was calculated.
4.3. Dihydroethidium (DHE) Staining
ROS levels were determined using the DHE assay (Cat# ab236206, Abcam). Briefly, the cells were cultured on 4-well chamber slides for 4 h and treated with IL-18 (10 ng/mL) in the presence or absence of 5 and 10 μM roflumilast for 24 h. The cells were then incubated with prepared DHE (5 μM) reagent for 30 min at 37 °C in the dark. After capturing the fluorescent image density using a fluorescence microscope, the fluorescence density of DHE staining was calculated using ImageJ software. Briefly, we characterized the regions of interest in the images. The integrated density value (IDV) of fluorescence and total cells in the regions were recorded. Average levels of intracellular ROS = IDV/the average number of cells.
4.4. mRNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
Cells were plated at a concentration of 2.5 × 105 cells/well in 6-well plates. After the required treatment, mRNA was extracted with an RNeasy Mini Kit (Qiagen). Then, 2 μg of total RNA was reverse-transcribed to cDNA using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific) and then subjected to real-time PCR using the iTaq Universal SYBR Green Supermix (BioRad). The cDNAs were then amplified with specific primers for IL-6, IL-8, TNF-α, CCL5, CXCL9, CXCL10, MMP-13, and MMP-3. All reactions were run in triplicate. A semi-quantitative analysis was performed based on the cycle number (CT) at which the SYBR Green fluorescent signal crossed a threshold in the log-linear range of real-time PCR. The fold change of all target genes was shown, relative to the change in the expressions of GAPDH RNA that was measured as an internal control.
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
The secretion levels of IL-6, IL-8, TNF-α, CCL5, CXCL9, CXCL10, MMP-3, and MMP-13 were determined using the ELISA assay. For ELISA assay, the cells were cultured in a coated 96-well plate. ELISA was performed following the protocol from the manufacturer. The following ELISA kits were used in this study: IL-6 (Cat# E-EL-R0015c, Elabscience), IL-8 (Cat# E-EL-H6008, Elabscience), TNF-α (Cat# kt99985, MSK), CCL5 (Cat# SEKRT-0136, Solarbio), CCL9 E (Cat# EK1225, Boster), MMP-3 (Cat# kt90046, MSK), and MMP-13 (Cat# ab100605, Abcam).
4.6. Protein Isolation and Western Blots
Protein was isolated from the cells using the radioimmunoprecipitation assay (RIPA) buffer. The concentration of protein was determined using the Pierce BCA Protein Assay Kit (Cat# 23225, Thermo Fisher Scientific). Protein (20–40 μg) was loaded in the wells of Nu-Page precast 4–10% sodium dodecyl sulfate (SDS)-poly-acrylamide gels (Invitrogen) and then transferred onto poly(vinylidene fluoride) membranes by a dry electroblotting method using an I-Blot. The membranes were then incubated with specific primary antibodies including c-Jun antibody (#9165, Cell Signaling Technology), c-Fos antibody (#2250, Cell Signaling Technology), NF-κB p65 (#3034, Cell Signaling Technology), secondary antibodies including mouse anti-rabbit IgG-HRP (Santa Cruz, sc-2357, Santa Cruz Biotechnology), and Goat Anti-Mouse IgG-HRP (SE131, Solarbio) step by step and incubated with visualized SuperSignal West Atto Ultimate Sensitivity Substrate (Cat# A38555, Thermo Fisher Scientific) for 5 min before visualizing on the X-ray film (no. C500046, Sango Biotech) in the darkroom.
4.7. Luciferase Activity
The cells (2 × 106) cultured in a 6-well plate were transfected with AP-1 luciferase plasmid, NF-κB luciferase plasmid, or a plasmid encoding β-galactosidase following the product instructions. The cells were collected and centrifuged for 5 s at 12 000 rpm to pellet the cell debris. The supernatant was collected in a new tube. Then, 20 μL of the cell extract was mixed with 100 μL of the luciferase assay reagent at room temperature. The luciferase reporter activity was measured using a luminometer reader, and the result was normalized to the β-galactosidase activity.
4.8. Statistical Analysis
The data was displayed as mean ± standard deviation (SD). The contrast among different groups was analyzed using the analysis of variance (ANOVA), followed by the Student–Newman–Keuls post hoc test. P-value < 0.05 was regarded as a statistically significant difference between the groups.
Acknowledgments
This study is funded by YiLing Hospital.
Author Contributions
∥ B.Z. and S.G. contributed equally to this work.
The authors declare no competing financial interest.
References
- Lerner A.; Matthias T. Rheumatoid arthritis–celiac disease relationship: joints get that gut feeling. Autoimmun. Rev. 2015, 14, 1038–1047. 10.1016/j.autrev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Wang L. F.; Wang F. S.; Gershwin M. E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- Quintero-Ronderos P.; Montoya-Ortiz G. Epigenetics and autoimmune diseases. Autoimmune Dis. 2012, 2012, 593720 10.1155/2012/593720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman A. J.; Pearson J. E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. Ther. 2002, 4, S265–S272. 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H.; Xie M.; Xu L.; Zheng X.; Yang Y.; Lv X. The protective role of interleukin-18 binding protein in a murine model of cardiac ischemia/reperfusion injury. Transplant Int. 2015, 28, 1436–1444. 10.1111/tri.12683. [DOI] [PubMed] [Google Scholar]
- Ono S.; Obara H.; Takayanagi A.; Tanabe M.; Kawachi S.; Itano O.; Shinoda M.; Kitago M.; Hibi T.; Chiba T.; et al. Suppressive effects of interleukin-18 on liver function in rat liver allografts. J. Surg. Res. 2012, 176, 293–300. 10.1016/j.jss.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Pawlus J.; Sierocka A.; Tejchman K.; Ziętek Z.; Romanowski M.; Pawlik A.; Sieńko J.; Żukowski M.; Ciechanowski K.; Ostrowski M.; et al. The impact of interleukin 12B (1188A>C), interleukin 16 (-295T>C), and interleukin 18 (607C>A, 137G>C) gene polymorphisms on long-term renal transplant function and recipient outcomes. Transplant Proc. 2014, 46, 2079–2082. 10.1016/j.transproceed.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Ozsoy M.; Gonul Y.; Bal A.; Ozkececi Z. T.; Celep R. B.; Adali F.; Hazman O.; Koçak A.; Tosun M. Effect of IL-18 binding protein on hepatic ischemia-reperfusion injury induced by infrarenal aortic occlusion. Ann. Surg. Treat. Res. 2015, 88, 92–99. 10.4174/astr.2015.88.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejr S.; Karimi M. H.; Sabet S.; Mohammadi B.; Nikeghbalian S.; Geramizadeh B.; Yaghobi R. Polymorphism of the IL-18 and CD40 genes and liver transplant outcome in Iranian patients. Int. J. Organ Transplant. Med. 2014, 5, 149–154. [PMC free article] [PubMed] [Google Scholar]
- Okamoto H.; Hoshi D.; Kiire A.; Yamanaka H.; Kamatani N. Molecular targets of rheumatoid arthritis. Inflammation Allergy: Drug Targets 2008, 7, 53–66. 10.2174/187152808784165199. [DOI] [PubMed] [Google Scholar]
- Bartok B.; Firestein G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamatawong T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J. Thorac. Dis. 2017, 9, 1144–1154. 10.21037/jtd.2017.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa L. P.; Lopes F.; Silva D. M.; Tavares L. P.; Vieira A. T.; Rezende B. M.; Carmo A. F.; Russo R. C.; Garcia C. C.; et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-κB-independent manner. J. Leukocyte Biol. 2010, 87, 895–904. 10.1189/jlb.0809540. [DOI] [PubMed] [Google Scholar]
- Kim S. W.; Kim J. H.; Park C. K.; Kim T. J.; Lee S. Y.; Kim Y. K.; Kwon S. S.; Rhee C. K.; Yoon H. K. Effect of roflumilast on airway remodelling in a murine model of chronic asthma. Clin. Exp. Allergy 2016, 46, 754–763. 10.1111/cea.12670. [DOI] [PubMed] [Google Scholar]
- Anwar M. M.; Tariq E. F.; Khan U.; Zaheer M.; Ijaz S. H. Rheumatoid Vasculitis: Is It Always a Late Manifestation of Rheumatoid Arthritis?. Cureus 2019, 11, e5790 10.7759/cureus.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Doyle T. J.; Hammer M. M.; Byrne S. C.; Huang W.; Marshall A. A.; Christine K.; Iannaccone ck.; Huang J.; Feathers V.; Weinblatt M. E.; et al. Rheumatoid arthritis-related lung disease detected on clinical chest computed tomography imaging: Prevalence, risk factors, and impact on mortality. Semin. Arthritis Rheum. 2020, 50, 1216–1225. 10.1016/j.semarthrit.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erre G. L.; Buscetta G.; Paliogiannis P.; Mangoni A. A.; Carru C.; Passiu G.; Zinellu A. Coronary flow reserve in systemic rheumatic diseases: A systematic review and meta -analysis. Rheumatol. Int. 2018, 38, 1179–1190. 10.1007/s00296-018-4039-8. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Wang B.; Wang M.; Christine K.; Iannaccone C. K.; Jie Huang J.; Feathers V.; Michael E Weinblatt M. E.; et al. TL1A increased IL-6 production on fibroblast-like synoviocytes by preferentially activating TNF receptor 2 in rheumatoid arthritis. Cytokine 2016, 83, 92–98. 10.1016/j.cyto.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Reuter S.; Gupta S. C.; Chaturvedi M. M.; Aggarwal B. B. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radical Biol. Med. 2010, 49, 1603–1616. 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P.; Swaak A. J.; Penders J. M.; Beindorff C. M.; Koster J. F. Superoxide production by polymorphonuclear leucocytes in rheumatoid arthritis and osteoarthritis: in vivo inhibition by the antirheumatic drug piroxicam due to interference with the activation of the NADPH-oxidase. Ann. Rheum. Dis. 1986, 45, 249–255. 10.1136/ard.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. B.; Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Brennan F. M.; McInnes I. B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 2008, 118, 3537–3545. 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E. H.; Panayi G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Butler D. M.; Maini R. N.; Feldmann M.; Brennan F. M. Modulation of pro-inflammatory cytokine release in rheumatoid synovial membrane cell cultures: comparison of monoclonal anti TNF-a antibody with interleukin-1 receptor antagonist. Eur. Cytokine Network 1995, 6, 225–230. [PubMed] [Google Scholar]
- Bai Y.; Chen K.; Zhan J.; Wu M. miR-122/SIRT1 Axis Regulates Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biosci. Rep. 2020, 40, BSR20191908 10.1042/BSR20191908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha V. T.; Beak H. S.; Kim E.; Baek K. S.; Hossen M. J.; Yang W. S.; Kim Y.; Kim J. H.; Yang S.; Kim J. H.; et al. NF-kappaB/AP-1-targeted inhibition of macrophage-mediated inflammatory responses by depigmenting compound AP736 derived from natural 1,3-diphenylpropane skeleton. Mediators Inflammation 2014, 2014, 354843 10.1155/2014/354843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q.; Cao J.; Fang L.; Zhao H.; Liu Z.; Ran J.; Zheng X.; Li X.; Zhou Y.; Ge D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. 10.1016/j.intimp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Yan H.; Pan H.; Hou K. K.; Akk A.; Springer L. E.; Hu Y.; J Allen S.; Wickline S. A.; Pham C. T.; et al. Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis. J. Clin. Invest. 2014, 124, 4363. 10.1172/JCI75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S. M.; Chen P. C.; Lin C. M.; Fang M. L.; Chi M. C.; Liu J. F. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 2020, 22, 251 10.1186/s13075-020-02331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Liao L.; Hu B.; Jiang H.; Tan M. Roflumilast, a Phosphodiesterases-4 (PDE4) Inhibitor, Alleviates Sepsis-induced Acute Kidney Injury. Med. Sci. Monit. 2020, 26, e921319 10.12659/MSM.921319. [DOI] [PMC free article] [PubMed] [Google Scholar]