Abstract
Sufficient water and fertilizer inputs in agriculture play a major role in crop growth, production, and quality. In this study, the response of sugarcane to limited water irrigation and foliar application of potassium salt of active phosphorus (PSAP) for photosynthetic responses were examined, and PSAP’s role in limited water irrigation management was assessed. Sugarcane plants were subjected to limited irrigation (95–90 and 45–40% FC) after three months of germination, followed by a foliar spray (0, 2, 4, 6, and 10 M) of PSAP. The obtained results indicated that limited water irrigation negatively affected sugarcane growth and reduced leaf gas exchange activities. However, the application of PSAP increased the photosynthetic activities by protecting the photosynthetic machinery during unfavorable conditions. Mathematical modeling, a Skewed model, was developed and compared with the existing Gaussian model to describe the photosynthetic responses of sugarcane leaves under the limited irrigation with and without PSAP application. The models fitted well with the observed values, and the predicted photosynthetic parameters were in close relationship with the obtained results. The Skewed model was found to be better than the Gaussian model in describing the photosynthetic parameters of plant leaves positioned over a stem of limited water irrigation and applied PSAP application and is recommended for further application.
1. Introduction
Sugarcane (Saccharum spp.) is one of the major cash crops in the globe, mainly cultivated in dry and semidry regions.1 China is the third largest cane producer worldwide,2,3 and the Guangxi province is the leading sugarcane producer, which produces 6–9 million tons of cane sugar, amounting to over 60% of the total production of sugarcane in the country,3 mainly for sugar and ethanol production. Cane production has rapidly enhanced and gained attention as a feedstock for 2-G ethanol, considered as a source of cleaner energy as relative to fossil fuels.4
Limited water is one of the main limiting factors for agricultural crop production. The loss of yield by limited irrigation of crops exceeds about 60% for a variety of plants/crops.4−6 Limited water which inhibits plant leaf gas exchange and growth traits1,7,8 is responsible for the loss in crop production.9,10 However, the impacts of limited water supply vary according to the growth phases.
Photosynthetic capacity is the main physiological process for crop growth and productivity.11,12 Other related studies have reported that the leaf photosynthetic performance in C4 crops is very sensitive to fluctuations in soil moisture capacity.13−18 The inhibitory impacts of insufficient water supply on photosynthetic performance can be linked with low CO2 levels in the stroma of chloroplasts caused by diffusion limitations through the stomata and the mesophyll,19 the variation of enzymatic carbon assimilation, and phloem transport limitations.17,20,21 The closure of stomatal openings is an initial effect to limited irrigation and an efficient way to decrease the loss of water when stress is not too severe; however, it limits carbon dioxide diffusion in the plant leaves for photosynthetic capacity.18,22,23 The requirement of water under field conditions has been a serious issue since most agricultural areas suffer from seasonal water stress conditions.4,10,24
Potassium (K) plays an important role in plant development.25 The research evidence indicated that the plants subjected to limited irrigation have a more internal requirement for K element,26,27 and crop productivity-limiting effects of limited water supply could be overcome by enhancing K supplementation.28−30 Under limited water supply, more K is required for the balance of photosynthetic CO2 assimilation rates, defense of chloroplasts from oxidative damage, impairment of related disruption in carbohydrate metabolism, regulation of stomatal openings, and relations of water status.31
Phosphorus (P) is an essential element for optimum plant growth and development, but its slow mobility in soil results in poor uptake by plant roots, which consequently hinders the growth and metabolism activities.32,33 Previous studies indicated that P contributes to the enlargement of root morphology, and P deficiency will exacerbate limited water irrigation.34,35 The application of P decreases its deficiency in soil, enhances the stress-tolerance mechanism of plants,36 and results in adaptations of morpho-physiological and biochemical activities that upregulate plant performance.33,37−41
However, knowledge about how potassium salt of active phosphorus (PSAP) regulates the photosynthetic variation in sugarcane plants subjected to limited water irrigation remains elusive. In addition, available information concerning the specific dose of PSAP for its application method in sugarcane crops is very limited and thus warrants an in-depth assessment. Exposure to severe water stress may affect the photosynthetic capacity of sugarcane plants with the effects on the leaves varying with leaf position (+1–6th, top to bottom). The plant performance/productivity is actually associated with the accumulated photosynthetic activities and hence with the cumulative photosynthesis, so the response of sugarcane plants to limited irrigation in relation to plant leaf position should be better understood. This study was devoted to develop a mathematical modeling for correlating the photosynthetic activities against leaf position over the main stem that could be helpful in integrating the photosynthetic parameters in each leaf of the main stem.
2. Results
Sugarcane plants (Saccharum hybrid cv. GT 42) were used to examine the photosynthetic traits to limited irrigation and impact of PSAP by foliar application. The observed position-wise (from top to bottom since leaf + 1) leaf area expansion is given in Table 1. The model constants (regression coefficient) for the Skewed model, that is, α, β, and γ, and for the Gaussian model, that is, a, b, and c, of the control and limited irrigation (95–90 and 45–40% of FC) with PSAP (0, 2, 4, 6, and 10 M) in sugarcane plants are shown in Table 2. The calculated values of the photosynthetic parameters such as the net photosynthetic rate (PN), stomatal conductance to water vapor (gs), and transpiration rate (E) with the Skewed and Gaussian models of normal and treated plants with PSAP application are represented in Tables 3 and 4 and Figures 1–6.
Table 1. Influence of PSAP on Leaf Area Expansion (cm2) in Sugarcane Plants Subjected to Limited Water Irrigationa.
| leaf
position vs leaf area expansion (cm2) |
|||||||
|---|---|---|---|---|---|---|---|
| irrigation level (% FC) | PSAP (M) | 1 | 2 | 3 | 4 | 5 | 6 |
| 95–90% | 0 | 235.25 | 251.66 | 268.32 | 316.33 | 317.31 | 317.39 |
| 2 | 246.89 | 263.19 | 279.19 | 319.09 | 320.13 | 320.98 | |
| 4 | 251.05 | 273.98 | 293.01 | 321.12 | 323.79 | 325.02 | |
| 6 | 270.21 | 298.36 | 317.61 | 324.08 | 325.18 | 326.61 | |
| 10 | 278.74 | 302.13 | 327.31 | 339.91 | 342.67 | 346.09 | |
| 45–40% | 0 | 172.52 | 187.8 | 201.38 | 206.41 | 211.02 | 213.15 |
| 2 | 184.02 | 208.18 | 221.13 | 227.43 | 229.19 | 231.09 | |
| 4 | 185.69 | 217.09 | 232.27 | 239.49 | 241.01 | 244.06 | |
| 6 | 199.11 | 224.16 | 239.8 | 251.21 | 257.94 | 259.07 | |
| 10 | 209.05 | 237.96 | 257.11 | 264.13 | 268.09 | 269.17 | |
Each set of data represents mean of at least five biological replicates. FC = field capacity.
Table 2. Model Constants for the Skewed and Gaussian Models of Control (A) and Limited Irrigation (B) with Foliar Application of PSAP in Sugarcane Plantsa.
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| control (95–90% of FC) |
|||||||
| Skewed
model |
Gaussian
model |
||||||
| photosynthetic responses | PSAP (M) | α | β | γ | a | b | c |
| PN | 0 | 21.60 | 83.27 | 17.58 | 21.97 | 1.71 | 3.99 |
| 2 | 21.97 | 1634.09 | 15.78 | 22.33 | 1.91 | 4.14 | |
| 4 | 24.14 | 104.69 | 11.92 | 24.22 | 1.46 | 5.05 | |
| 6 | 28.80 | 14.24 | 47.96 | 29.32 | 0.73 | 5.67 | |
| 10 | 28.89 | 34.65 | 92.81 | 29.49 | 0.017 | 6.27 | |
| gs | 0 | 146.38 | 107.68 | 5.98 | 158.13 | –1.79 | 6.43 |
| 2 | 205.52 | 156.24 | 2.71 | 306.89 | –4.09 | 17.33 | |
| 4 | 259.95 | 151.97 | 2.33 | 199.51 | –95.63 | 25.83 | |
| 6 | 245.28 | 603.60 | 2.88 | 100.48 | –109.31 | 26.79 | |
| 10 | 295.67 | 722.28 | 2.12 | 233.27 | –35.47 | 2.07 | |
| E | 0 | 1.98 | 1.48 | 18.94 | 2.03 | 0.47 | 4.60 |
| 2 | 2.04 | 2.46 | 14.33 | 2.07 | 0.95 | 4.02 | |
| 4 | 2.27 | 47.36 | 8.54 | 2.27 | 0.69 | 4.59 | |
| 6 | 2.90 | 2.84 | 10.32 | 3.04 | –0.14 | 4.55 | |
| 10 | 2.86 | 7.19 | 5.74 | 3.06 | –0.89 | 5.06 | |
| (B) | |||||||
|---|---|---|---|---|---|---|---|
| drought (45–40% of FC) |
|||||||
| Skewed
model |
Gaussian
model |
||||||
| photosynthetic responses | PSAP (M) | α | β | γ | a | b | c |
| PN | 0 | 13.38 | 5.38 | 258.18 | 14.81 | –1.41 | 6.69 |
| 2 | 15.77 | 8.39 | 42.42 | 17.13 | –0.86 | 5.84 | |
| 4 | 17.57 | 17.18 | 7.81 | 19.18 | –1.32 | 5.96 | |
| 6 | 20.08 | 22.94 | 7.37 | 21.66 | –1.05 | 5.67 | |
| 10 | 20.32 | 62.38 | 5.39 | 22.78 | –2.17 | 6.35 | |
| gs | 0 | 91.27 | 60.04 | 43.07 | 92.95 | 7.28 | 4.16 |
| 2 | 97.25 | 64.25 | 59.28 | 98.23 | 1.26 | 3.99 | |
| 4 | 123.37 | 194.81 | 9.14 | 124.71 | 0.63 | 4.03 | |
| 6 | 128.98 | 142.99 | 17.43 | 130.95 | 0.99 | 4.09 | |
| 10 | 129.27 | 273.39 | 8.52 | 130.61 | 0.62 | 4.32 | |
| E | 0 | 1.13 | 0.83 | 5.76 | 1.39 | –6.78 | 12.41 |
| 2 | 1.21 | 2.58 | 6.07 | 1.301 | –2.84 | 10.14 | |
| 4 | 1.22 | 0.81 | 41.47 | 1.24 | 1.69 | 5.24 | |
| 6 | 1.45 | 10.42 | 7.30 | 1.49 | –0.34 | 6.45 | |
| 10 | 1.28 | 30.83 | 11.15 | 1.30 | 0.75 | 6.30 | |
PN = photosynthesis, gs = stomatal conductance to water vapor, and E = transpiration rate.
Table 3. Calculated Values of the Photosynthetic Parameters, That Is, Net Photosynthetic Rate (PN), Stomatal Conductance to Water Vapor (gs), and Transpiration Rate (E) by the Skewed and Gaussian Models for Different Leaf Positions under Normal Growth Conditions with Foliar Application of PSAP in Sugarcane Plants.
| Skewed
model |
Gaussian
model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PSAP
(M) |
PSAP
(M) |
|||||||||
| leaf position | 0 | 2 | 4 | 6 | 10 | 0 | 2 | 4 | 6 | 10 |
| PN (μmol CO2 m–2 s–1) | ||||||||||
| 1 | 21.601 | 21.975 | 24.141 | 28.803 | 28.802 | 21.623 | 21.795 | 24.121 | 29.287 | 29.166 |
| 2 | 21.567 | 21.920 | 23.996 | 28.796 | 28.415 | 21.908 | 22.320 | 24.078 | 28.592 | 28.125 |
| 3 | 20.955 | 21.440 | 23.141 | 27.753 | 26.616 | 20.847 | 21.563 | 23.110 | 27.058 | 26.440 |
| 4 | 19.009 | 20.006 | 21.378 | 24.582 | 24.081 | 18.632 | 19.654 | 21.328 | 24.822 | 24.232 |
| 5 | 15.845 | 17.308 | 18.940 | 21.536 | 21.455 | 15.641 | 16.899 | 18.925 | 22.074 | 21.650 |
| 6 | 11.981 | 13.282 | 16.112 | 19.397 | 19.018 | 12.331 | 13.709 | 16.147 | 19.029 | 18.858 |
| gs (mmol H2O m–2 s–1) | ||||||||||
| 1 | 143.66 | 168.671 | 187.230 | 211.566 | 209.307 | 143.85 | 165.301 | 182.529 | 209.025 | 206.113 |
| 2 | 133.35 | 141.335 | 154.428 | 177.398 | 169.841 | 132.82 | 143.547 | 157.801 | 179.119 | 173.372 |
| 3 | 119.65 | 121.407 | 132.394 | 151.068 | 143.751 | 119.71 | 124.242 | 136.220 | 153.278 | 145.650 |
| 4 | 104.97 | 105.716 | 115.650 | 129.946 | 124.170 | 105.32 | 107.176 | 117.413 | 130.982 | 123.301 |
| 5 | 90.294 | 92.773 | 102.103 | 112.401 | 108.487 | 90.453 | 92.146 | 101.052 | 111.774 | 106.611 |
| 6 | 76.011 | 81.762 | 90.712 | 97.445 | 95.410 | 75.824 | 78.962 | 86.840 | 95.249 | 95.793 |
| E (mmol H2O m–2 s–1) | ||||||||||
| 1 | 1.985 | 2.042 | 2.265 | 2.903 | 2.839 | 2.013 | 2.067 | 2.267 | 2.945 | 2.851 |
| 2 | 1.968 | 2.020 | 2.179 | 2.803 | 2.621 | 1.918 | 1.998 | 2.182 | 2.720 | 2.597 |
| 3 | 1.763 | 1.838 | 2.001 | 2.401 | 2.272 | 1.743 | 1.816 | 2.002 | 2.394 | 2.275 |
| 4 | 1.461 | 1.532 | 1.756 | 1.943 | 1.899 | 1.511 | 1.551 | 1.752 | 2.007 | 1.916 |
| 5 | 1.211 | 1.226 | 1.470 | 1.567 | 1.546 | 1.249 | 1.246 | 1.462 | 1.604 | 1.552 |
| 6 | 1.033 | 0.964 | 1.158 | 1.281 | 1.224 | 0.985 | 0.941 | 1.164 | 1.221 | 1.209 |
Table 4. Calculated Values of the Photosynthetic Parameters, That Is, Net Photosynthetic Rate (PN), Stomatal Conductance to Water Vapor (gs), and Transpiration Rate (E) by the Skewed and Gaussian Models for Different Leaf Positions during Limited Water Irrigation with Foliar Application of PSAP in Sugarcane Plants.
| Skewed
model |
Gaussian
model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PSAP
(M) |
PSAP
(M) |
|||||||||
| leaf position | 0 | 2 | 4 | 6 | 10 | 0 | 2 | 4 | 6 | 10 |
| PN (μmol CO2 m–2 s–1) | ||||||||||
| 1 | 13.382 | 15.775 | 17.564 | 20.069 | 20.040 | 13.883 | 16.283 | 17.776 | 20.293 | 20.110 |
| 2 | 13.382 | 15.740 | 16.808 | 19.140 | 18.493 | 13.010 | 15.193 | 16.418 | 18.748 | 18.360 |
| 3 | 12.569 | 14.179 | 14.783 | 16.837 | 16.348 | 11.922 | 13.767 | 14.743 | 16.791 | 16.351 |
| 4 | 10.187 | 11.650 | 12.606 | 14.321 | 14.123 | 10.685 | 12.113 | 12.871 | 14.579 | 14.206 |
| 5 | 8.940 | 9.963 | 10.747 | 12.097 | 11.998 | 9.364 | 10.351 | 10.925 | 12.271 | 12.041 |
| 6 | 8.437 | 9.001 | 9.249 | 10.245 | 10.025 | 8.026 | 8.588 | 9.016 | 10.012 | 9.955 |
| gs (mmol H2O m–2 s–1) | ||||||||||
| 1 | 91.275 | 97.256 | 123.354 | 128.989 | 129.225 | 92.749 | 98.018 | 124.207 | 130.959 | 130.112 |
| 2 | 91.117 | 97.234 | 119.442 | 128.222 | 125.263 | 88.697 | 96.556 | 117.753 | 127.067 | 124.123 |
| 3 | 81.961 | 91.825 | 105.198 | 118.202 | 112.618 | 80.054 | 89.327 | 104.959 | 116.142 | 112.239 |
| 4 | 64.798 | 75.326 | 86.551 | 99.170 | 95.464 | 68.191 | 77.611 | 87.959 | 100.000 | 96.203 |
| 5 | 52.320 | 60.691 | 68.478 | 79.633 | 77.651 | 54.820 | 63.329 | 69.306 | 81.109 | 78.160 |
| 6 | 44.836 | 51.186 | 52.596 | 63.104 | 60.878 | 41.594 | 48.530 | 51.342 | 61.972 | 60.192 |
| E (mmol H2O m–2 s–1) | ||||||||||
| 1 | 1.135 | 1.207 | 1.225 | 1.452 | 1.287 | 1.140 | 1.210 | 1.225 | 1.456 | 1.301 |
| 2 | 1.092 | 1.166 | 1.225 | 1.399 | 1.274 | 1.080 | 1.160 | 1.233 | 1.393 | 1.276 |
| 3 | 1.018 | 1.103 | 1.208 | 1.303 | 1.231 | 1.017 | 1.101 | 1.198 | 1.301 | 1.221 |
| 4 | 0.944 | 1.032 | 1.124 | 1.183 | 1.152 | 0.952 | 1.035 | 1.121 | 1.186 | 1.139 |
| 5 | 0.879 | 0.961 | 1.001 | 1.053 | 1.042 | 0.884 | 0.964 | 1.012 | 1.056 | 1.036 |
| 6 | 0.824 | 0.892 | 0.885 | 0.975 | 0.908 | 0.817 | 0.889 | 0.880 | 0.917 | 0.919 |
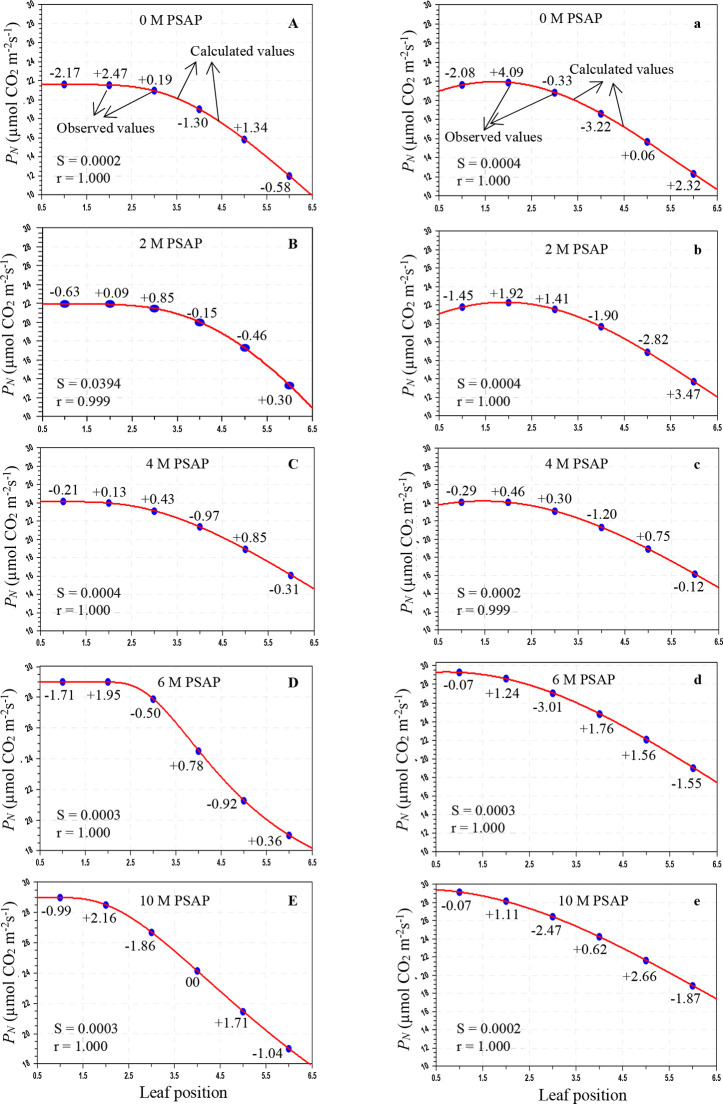
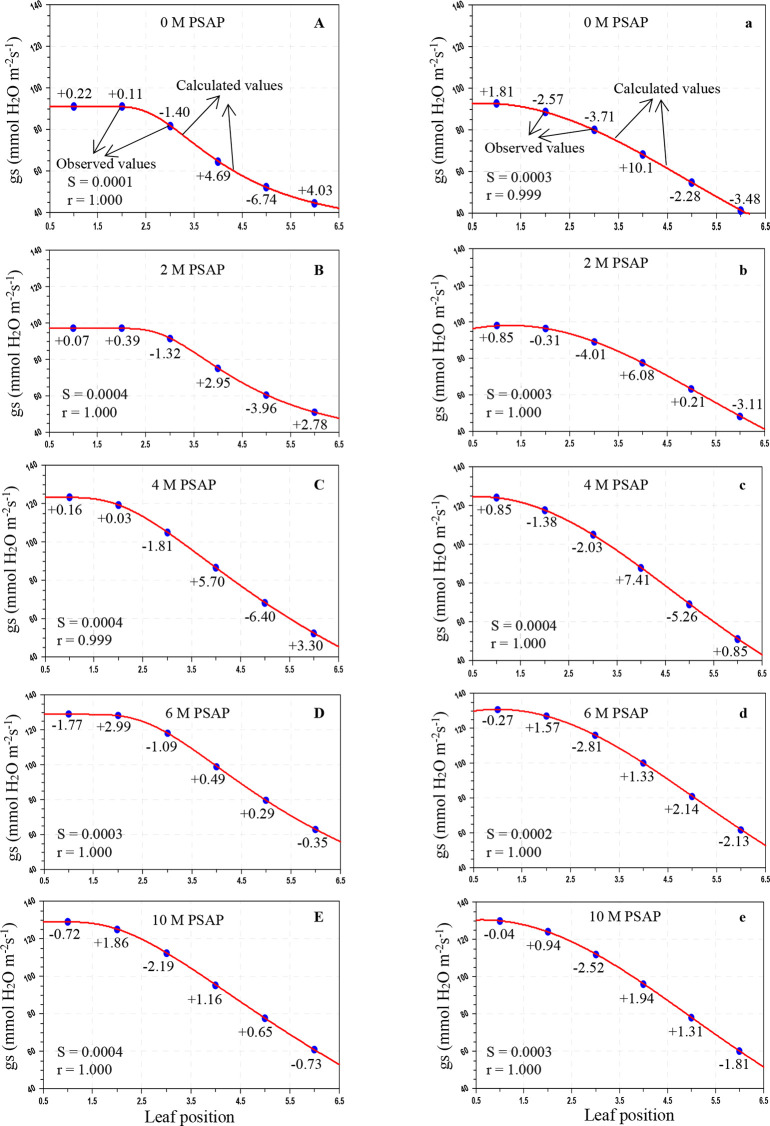
Figure 1.
Variation of photosynthesis (PN; μmol CO2 m–2 s–1) in sugarcane leaves after the application of PSAP (0, 2, 4, 6, and 10 M) under normal irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
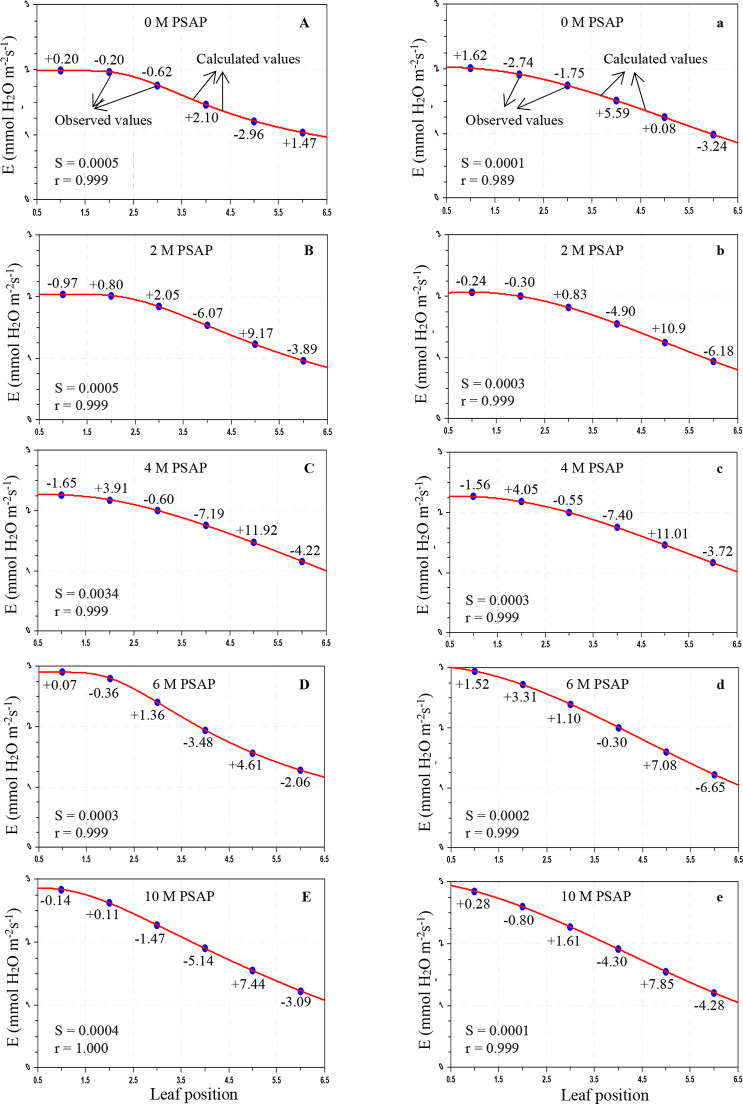
Figure 6.
Variation of transpiration rate (E; mmol H2O m–2 s–1) in sugarcane leaves after application of PSAP (0, 2, 4, 6, and 10 M) under limited water irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
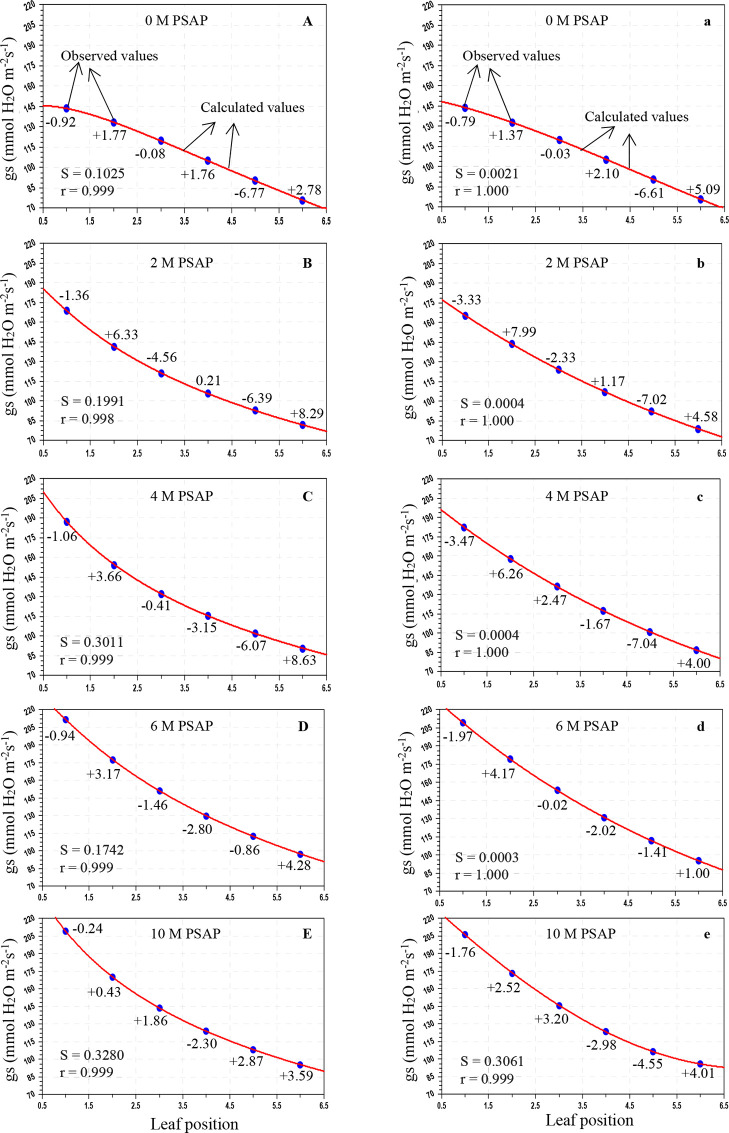
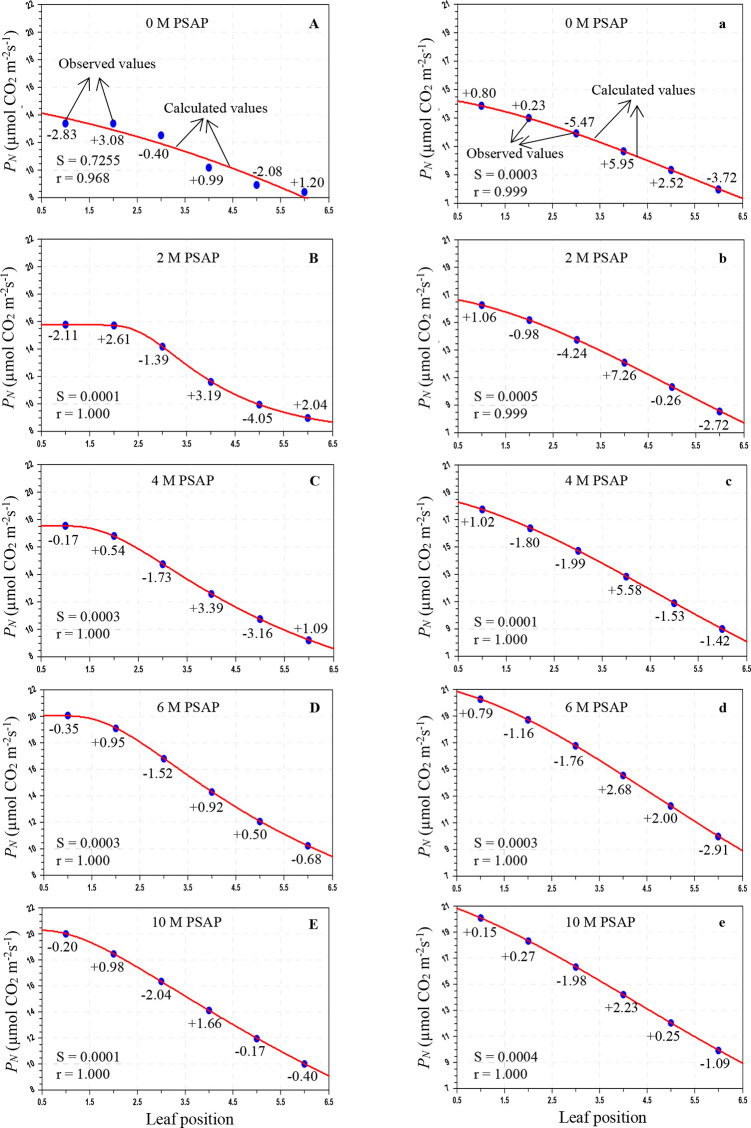
Figure 2.
Variation of stomatal conductance to water vapor (gs; mmol H2O m–2 s–1) in sugarcane leaves after application of PSAP (0, 2, 4, 6, and 10 M) under normal irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
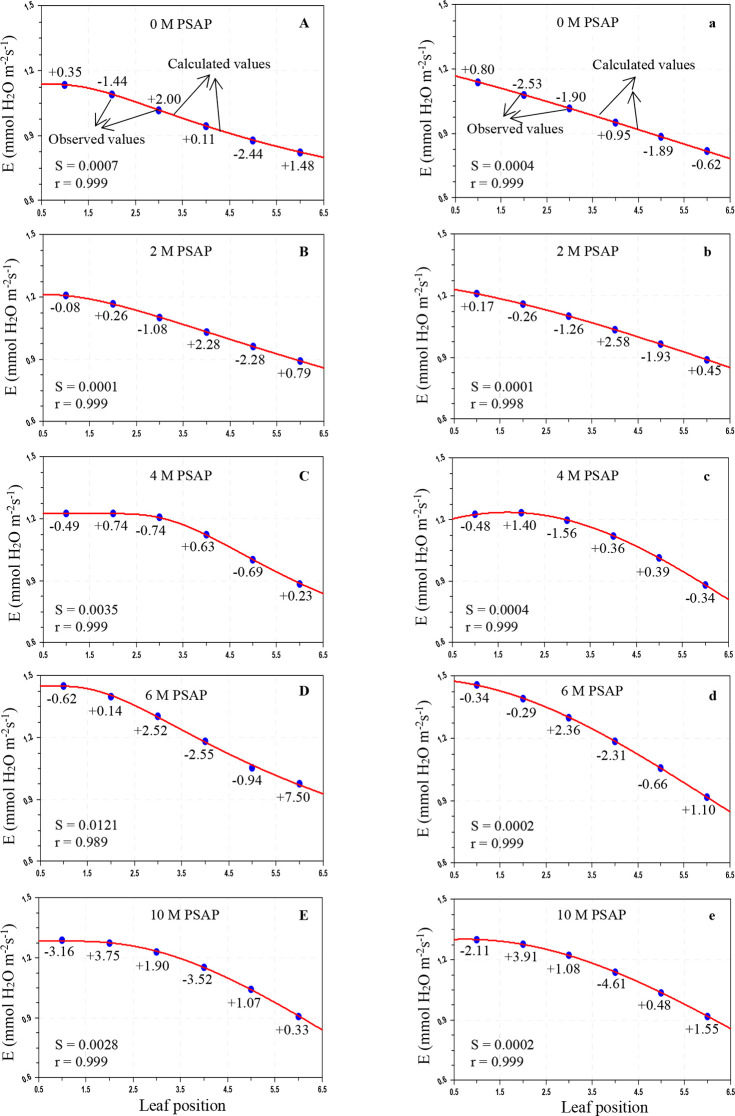
Figure 3.
Variation of the transpiration rate (E; mmol H2O m–2 s–1) in sugarcane leaves after application of PSAP (0, 2, 4, 6, and 10 M) under normal irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
Figure 5.
Variation of stomatal conductance to water vapor (gs; mmol H2O m–2 s–1) in sugarcane leaves after application of PSAP (0, 2, 4, 6, and 10 M) under limited water irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
As shown in Table 3, under normal irrigation with different concentrations of PSAP, the calculated values of the PN for the Skewed model were in the range of 28.803–11.981 μmol CO2 m–2 s–1, and for the Gaussian model, the range was 29.287–12.331 μmol CO2 m–2 s–1; the predicted gs for the Skewed model ranges from 211.566 to 76.011 mmol H2O m–2 s–1 and that for the Gaussian model ranges from 209.025 to 75.824 mmol H2O m–2 s–1; and the E for the Skewed model ranges from 2.903 to 0.964 mmol H2O m–2 s–1 and that for the Gaussian model ranges from 2.945 to 0.941 mmol H2O m–2 s–1. For drought stress (45–40% of FC) with foliar application of PSAP, the calculated values of PN for the Skewed model range from 20.069 to 8.437 μmol CO2 m–2 s–1 and those for the Gaussian model range from 20.293 to 8.026 μmol CO2 m–2 s–1; the predicted gs for the Skewed model ranges from 128.989 to 44.836 mmol H2O m–2 s–1 and that for the Gaussian model ranges from 130.959 to 41.594 mmol H2O m–2 s–1; and the E for the Skewed model ranges from 1.452 to 0.824 mmol H2O m–2 s–1 and that for the Gaussian model ranges from 1.456 to 0.817 mmol H2O m–2 s–1.
The average percent (%) deviation was maximum for the predicted values of the net photosynthetic rate, stomatal conductance to water vapor, and transpiration rate by the Skewed and Gaussian models in the control and stressed plants with PSAP application (Tables 5 and 6). As may be seen from Table 5, for normal irrigation with PSAP application, the present deviations of the predicted PN for the Skewed model range from +2.47 to −2.17% and those for the Gaussian model range from +4.09 to −3.22%, and the stomatal conductance and transpiration rate for the Skewed model range from +8.63 to −6.77 and +11.62 to −7.19%, respectively, and those for the Gaussian model range from +7.99 to −7.04 and +11.01 to −7.40%, respectively. When PSAP was supplied as foliar application with limited water irrigation of sugarcane plants, the deviations of the predicted photosynthetic capacity were enhanced for both models. With the Skewed model, the % deviations of the ranges of the calculated PN, gs, and E were +3.36 to −4.05, +5.70 to −6.74, and +7.50 to −3.52%, respectively. Similarly, with the Gaussian model, the ranges of the percent deviations of the predicted values of PN, gs, and E were +7.26 to −5.47, +10.18 to −5.26, and +3.91 to −4.61%, respectively.
Table 5. The Percentage Deviations (±) of the Calculated Values of Photosynthetic Responses by the Skewed and Gaussian Models for Different Leaf Positions under Control Conditions (95–90% FC) with PSAP Application in Sugarcane Plants.
| Skewed
model |
Gaussian
model |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSAP
(M) |
PSAP
(M) |
|||||||||||
| leaf position | 0 | 2 | 4 | 6 | 10 | average | 0 | 2 | 4 | 6 | 10 | average |
| PN (μmol CO2 m–2 s–1) | ||||||||||||
| 1 | –2.17 | –0.63 | –0.21 | –1.71 | –0.99 | 1.142 | –2.08 | –1.45 | –0.29 | –0.07 | –0.07 | 0.792 |
| 2 | +2.47 | +0.09 | +0.13 | +1.95 | +2.16 | 1.36 | +4.09 | +1.92 | +0.46 | +1.24 | +1.11 | 1.764 |
| 3 | +0.19 | +0.85 | +0.43 | –0.50 | –1.84 | 0.762 | –0.33 | +1.41 | +0.30 | –3.01 | –2.47 | 1.504 |
| 4 | –1.30 | –0.15 | –0.97 | +0.78 | 0.8 | –3.22 | –1.90 | –1.20 | +1.76 | +0.62 | 1.74 | |
| 5 | +1.34 | –0.46 | +0.85 | –0.92 | +1.71 | 1.056 | +0.06 | –2.82 | +0.75 | +1.56 | +2.66 | 1.57 |
| 6 | –0.58 | +0.30 | –0.31 | +0.36 | –1.04 | 0.518 | +2.32 | +3.47 | –0.12 | –1.55 | –1.87 | 1.866 |
| average | 1.342 | 0.413 | 0.483 | 1.037 | 1.29 | 0.913 | 2.017 | 2.162 | 0.52 | 1.532 | 1.467 | 1.539 |
| gs (mmol H2O m–2 s–1) | ||||||||||||
| 1 | –0.92 | –1.36 | –1.06 | –0.94 | –0.24 | 0.904 | –0.79 | –3.33 | –3.47 | –1.97 | –1.76 | 2.264 |
| 2 | +1.77 | +6.33 | +3.99 | +3.17 | +0.43 | 3.318 | +1.37 | +7.99 | +6.26 | +4.17 | +2.52 | 4.462 |
| 3 | –0.08 | –4.56 | –0.41 | –1.46 | +1.86 | 1.674 | –0.03 | –2.33 | +2.47 | –0.02 | +3.20 | 1.61 |
| 4 | +1.76 | –0.21 | –3.15 | –2.80 | –2.30 | 2.044 | +2.10 | +1.17 | –1.67 | –2.02 | –2.98 | 1.988 |
| 5 | –6.77 | –6.39 | –6.07 | –0.86 | –2.87 | 4.592 | –6.61 | –7.02 | –7.04 | –1.41 | –4.55 | 5.326 |
| 6 | +2.78 | +8.29 | +8.63 | +4.28 | +3.59 | 5.514 | +5.09 | +4.58 | +4.00 | +1.00 | +4.01 | 3.736 |
| average | 2.347 | 4.523 | 3.885 | 2.252 | 1.882 | 2.978 | 2.665 | 4.403 | 4.152 | 1.765 | 3.17 | 3.231 |
| E (mmol H2O m–2 s–1) | ||||||||||||
| 1 | +0.20 | –0.97 | –1.65 | +0.07 | –0.14 | 0.606 | +1.62 | –0.24 | –1.56 | +1.52 | +0.28 | 1.044 |
| 2 | –0.20 | +0.80 | +3.91 | –0.36 | +0.11 | 1.076 | –2.74 | –0.30 | +4.05 | +3.31 | –0.80 | 2.24 |
| 3 | –0.62 | +2.05 | –0.60 | +1.39 | +1.47 | 1.226 | –1.75 | +0.83 | –0.55 | +1.10 | +1.61 | 1.168 |
| 4 | +2.10 | –6.07 | –7.19 | –3.48 | –5.14 | 4.796 | +5.59 | –4.90 | –7.40 | –0.30 | –4.30 | 4.498 |
| 5 | –2.96 | +9.17 | +11.62 | +4.61 | +7.44 | 7.16 | +0.08 | +10.95 | +11.01 | +7.08 | +7.85 | 7.394 |
| 6 | +1.47 | –3.89 | –4.22 | –2.06 | –3.09 | 2.946 | –3.24 | –6.18 | –3.72 | –6.65 | –4.28 | 4.814 |
| average | 1.258 | 3.825 | 4.865 | 1.995 | 2.898 | 2.968 | 2.503 | 3.9 | 4.715 | 3.327 | 3.187 | 3.526 |
Table 6. Percentage Deviations (±) of the Calculated Values of Photosynthetic Parameters by the Skewed and Gaussian Models for Different Leaf Positions during Limited Irrigation (45–40% FC) with PSAP Application in Sugarcane Plants.
| Skewed
model |
Gaussian
model |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSAP
(M) |
PSAP
(M) |
|||||||||||
| leaf position | 0 | 2 | 4 | 6 | 10 | average | 0 | 2 | 4 | 6 | 10 | average |
| PN (μmol CO2 m–2 s–1) | ||||||||||||
| 1 | –2.83 | –2.11 | –0.17 | –0.35 | –0.20 | 1.132 | +0.80 | +1.06 | +1.02 | +0.79 | +0.15 | 0.764 |
| 2 | +3.08 | +2.61 | +0.54 | +0.95 | +0.98 | 1.632 | +0.23 | –0.98 | –1.80 | –1.16 | +0.27 | 0.888 |
| 3 | –0.40 | –1.39 | –1.73 | –1.52 | –2.04 | 1.416 | –5.47 | –4.24 | –1.99 | –1.76 | –1.98 | 3.088 |
| 4 | +0.99 | +3.19 | +3.36 | +0.92 | +1.66 | 2.024 | +5.95 | +7.26 | +5.58 | +2.68 | +2.23 | 4.74 |
| 5 | –2.08 | –4.05 | –3.16 | +0.50 | –0.17 | 1.992 | +2.52 | –0.29 | –1.53 | +2.00 | +0.25 | 1.318 |
| 6 | +1.20 | +2.04 | +1.09 | –0.68 | –0.40 | 1.082 | –3.72 | –2.72 | –1.42 | –2.91 | –1.09 | 2.372 |
| average | 1.763 | 2.565 | 1.675 | 0.82 | 0.908 | 1.546 | 3.115 | 2.758 | 2.223 | 1.883 | 0.995 | 2.195 |
| gs (mmol H2O m–2 s–1) | ||||||||||||
| 1 | +0.22 | +0.07 | +0.16 | –1.77 | –0.72 | 0.588 | +1.81 | +0.85 | +0.85 | –0.27 | –0.04 | 0.764 |
| 2 | +0.11 | +0.39 | +0.03 | +2.49 | +1.86 | 0.976 | –2.57 | –0.31 | –1.38 | +1.57 | +0.94 | 1.354 |
| 3 | –1.40 | –1.32 | –1.81 | –1.09 | –2.19 | 1.562 | –3.71 | –4.01 | –2.03 | –2.81 | –2.52 | 3.016 |
| 4 | +4.69 | +2.95 | +5.70 | +0.49 | +1.16 | 2.998 | +10.18 | +6.08 | +7.41 | +1.33 | +1.94 | 5.388 |
| 5 | –6.74 | –3.96 | –6.40 | +0.29 | +0.65 | 3.608 | –2.28 | +0.21 | –5.26 | +2.14 | +1.31 | 2.24 |
| 6 | +4.03 | +2.78 | +3.30 | –0.35 | –0.73 | 2.238 | –3.48 | –3.11 | +0.85 | –2.13 | –1.81 | 2.276 |
| average | 2.865 | 1.911 | 2.9 | 1.08 | 1.218 | 1.995 | 4.005 | 2.428 | 2.963 | 1.708 | 1.427 | 2.506 |
| E (mmol H2O m–2 s–1) | ||||||||||||
| 1 | +0.35 | –0.08 | –0.49 | –0.62 | –3.16 | 0.94 | +0.80 | +0.17 | –0.48 | –0.34 | –2.11 | 0.78 |
| 2 | –1.44 | +0.26 | +0.74 | +0.14 | +3.75 | 1.266 | –2.53 | –0.26 | +1.40 | –0.29 | +3.91 | 1.678 |
| 3 | +2.00 | –1.08 | –0.74 | +2.52 | +1.90 | 1.648 | +1.90 | –1.26 | –1.56 | +2.36 | +1.08 | 1.632 |
| 4 | +0.11 | +2.28 | +0.63 | –2.55 | –3.52 | 1.818 | +0.95 | +2.58 | +0.36 | –2.31 | –4.61 | 2.162 |
| 5 | –2.44 | –2.24 | –0.69 | –0.94 | +1.07 | 1.476 | –1.89 | –1.93 | +0.39 | –0.66 | +0.48 | 1.07 |
| 6 | +1.48 | +0.79 | +0.23 | +7.50 | +0.33 | 2.066 | –0.62 | +0.45 | –0.34 | +1.10 | +1.55 | 0.812 |
| average | 1.303 | 1.122 | 0.587 | 2.378 | 2.288 | 1.536 | 1.448 | 1.108 | 0.755 | 1.177 | 2.29 | 1.356 |
Overall mean percent deviations of the Skewed and Gaussian models were 0.913, 2.978, and 2.968% and 1.539, 3.231, and 3.526%, respectively, for PN, gs, and E of PSAP application under control conditions, and 1.546, 1.995, and 1.536% and 2.195, 2.506, and 1.356%, respectively, under limited water with PSAP. The Skewed model should be used for future studies for modeling the photosynthetic responses of sugarcane against leaf positions.
Under control conditions with foliar application of PSAP, the correlation coefficients (r) for PN, gs, and E in the Skewed model were found to be 0.999–1.000, 0.998–0.0999, and 0.999–1.000, respectively and those in the Gaussian model were found to be 0.999–1.000, 0.999–1.000, and 0.989–0.999, respectively. Under limited water irrigation with PSAP application, the r values for PN, gs, and E were found to be 0.968–1.000, 0.999–1.000, and 0.989–0.999, respectively, in the Skewed model, and 0.999–1.000, 0.999–1.000, and 0.998–0.999, respectively, in the Gaussian model. The “r” values were higher in the Skewed model than in the Gaussian model for control and stressed plants with different levels of PSAP. The Skewed model is superior to the Gaussian model. The Skewed model predicted more closely, the values of the photosynthetic traits of control and limited irrigation with PSAP application and may hence be recommended for further application.
3. Discussion
Insufficient water irrigation is well-known for its inhibitory effects. It reduces crop growth, development, and ultimately productivity.42−44 Limited water irrigation decreases photosynthetic responses due to the reduction in leaf area expansion and linked damage to the photosynthetic apparatus.45 Plants have developed numerous types of adaptive mechanisms to respond to stresses. In this experiment, the protective role of the PSAP fertilizer was assessed in sugarcane plants subjected to limited water irrigation. However, the application of PSAP as foliar spraying decreased the severity of limited irrigation-induced growth inhibition. It increased sugarcane tolerance to limited irrigation in terms of maintaining and/or improving the photosynthetic responses. During limited irrigation, stomatal closure is one of the initial plant responses to reduce the loss of water, accompanied by a remarkable reduction in stomatal conductance to water vapor and consequently, stomatal limitation of photosynthetic CO2 assimilation rates.18,43,46,47 The PSAP application also resulted in an enhanced rate of transpiration, possibly driven by the increased gs to improve a steady state of PN subjected to limited irrigation (Figures 1–6).
Stomatal closure is considered a major factor in reducing the photosynthetic CO2 assimilation rate subjected to limited irrigation.18,43,48 Stomatal closure in response to limited soil moisture occurs because roots release higher abscisic acid (ABA) concentration to the xylem, and as a result, the enhanced pH of the xylem sap promotes ABA loading and subsequent uptake to the shoots.49 The loss of gs limits leaf gas exchange activities and reduces Ci levels and photosynthetic rates due to downregulation of Rubisco activity.50 The present findings are consistent with previous observations that have demonstrated increased photosynthetic responses in various plant varieties/cultivars treated with P application subjected to stress conditions.33,41,51,52
Plant leaves are the most important factors for the photosynthetic activities, and the area of leaves depends on light harvesting, which affects gas exchange activities and the accumulation of photosynthetic products.12,18,53 The present study noted that on all limited irrigation levels, photosynthetic capacity with PSAP was higher than that without PSAP application (Figures 4–6). The photosynthetic CO2 assimilation rate reflects leaf gas exchange characteristics of plants and is the important factor to achieve the maximum crop productivity.6,12,54 Limited water irrigation levels could significantly downregulate sugarcane photosynthesis and productivity.18,55
Figure 4.
Variation of photosynthesis (PN; μmol CO2 m–2 s–1) in sugarcane leaves after application of PSAP (0, 2, 4, 6, and 10 M) under limited water irrigation in the Skewed and Gaussian models. Data are represented as the arithmetic mean (n = 3). Blue ovals denote the observed values and red lines show the calculated values. Parenthesis values indicate percent deviation. (A–E) Skewed model, (a–e) Gaussian model, S = standard error, and r = correlation coefficient.
Soil irrigation is a common technique for the application of essential nutrients to plants. However, plants can also absorb mineral nutrients when supplied as a foliar spray in the required dose.27,56 The foliar application facilitates the continued absorption of mineral elements, and it can be performed throughout the growth period, particularly during the apex phase of nutrient requirement without the interaction with soil particles.57 There are limited studies on the impact of foliar application of K to correct deficiency signs and upgrade plant performance and production.58 The status of water in plant leaves depends on stomatal regulation and supply of water from the vasculature to internal plant organs.27,59
Modeling of photosynthetic responses of plants is essentially required for assessing overall growth and productivity of agricultural crops. Photosynthetic responses can be integrated in terms of productivity of the leaf area expansion, and temporal variations of photoassimilation are known in response to leaf positions. The Gaussian and Skewed models have been used for explaining variations of physiological responses against leaf positions. The best performing model was the Skewed model, which explained the variations of physiological responses against leaf positions of sugarcane under normal and limited water irrigation with PSAP application. The model may be quite useful for future studies in relating crop responses against any type of nutrient and water treatment.
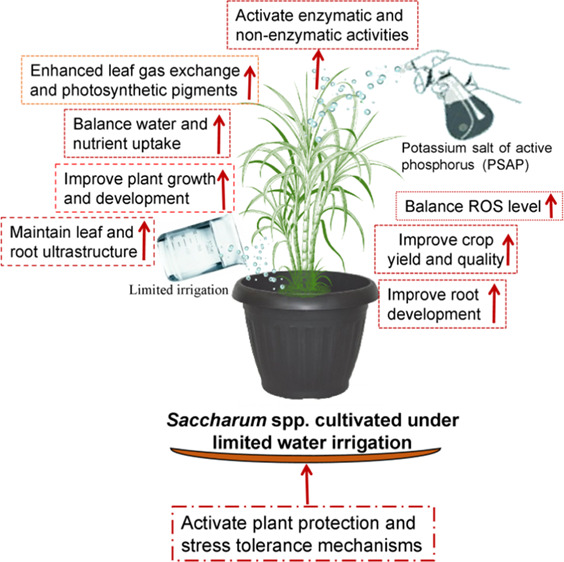
In conclusion, overall, the present results revealed that the PSAP application might be an efficient technique for improving the tolerance of sugarcane plants subjected to limited water irrigation. It also upregulated the photosynthetic capacity by protecting the negative impacts of sugarcane plants during limited irrigation. Taken together, PSAP has a significant role in sugarcane cultivation under insufficient water availability for irrigation and its optimum dose will be supportive in mitigation of limited irrigation in a variety of crops for sugar and bioenergy sectors. This combination also greatly improved the photosynthetic activities and plant growth. However, to suggest an optimum dose of PSAP concentration, a large-scale demonstration under field conditions should be assessed in later studies.
4. Experimental Section
4.1. Plant Material, Experimental Site, and Design
The sugarcane (Saccharum spp. cv. GT 42) plants were provided by Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China (22°49′ N, 108°18′ E, 800–1731 masl), and the experiment was conducted in an open greenhouse during 2020 with three replications of each treatment as a completely randomized block design. The soil of the experiment was silty clay soil. One-bud cane sets were planted in the month of mid-March 2020, following the farmer’s standard practices. Row-to-row spacing was maintained (about 75 cm). Recommended basal dose of fertilizers (N/P/K) was applied. Plants were raised with a standard dose of fertilizer for three months and then exposed to limited water irrigation (drought stress) by withholding irrigation, while control plants were watered regularly and manually. Uniform plants were selected and maintained for each treatment (control and limited irrigation), and the solution of PSAP (0, 2, 4, 6, and 10 M) was applied on the upper parts (canopies) of the plant manually using a sprayer. The spraying was done only with distilled water (without PSAP) over control and stressed plants. The water treatment included normal water irrigation (95–90% of field capacity) and limited water irrigation (45–40% of FC). Soil field capacity (moisture level, %) was measured using a soil moisture meter (0–10 cm soil depth) during experiment. The PSAP source was 85% salt of potassium and phosphorus and 15% other nutrients. The PSAP solution was prepared by dissolving the appropriate concentration of PSAP in distilled water. PSAP was applied once at one-month intervals up to three months during limited irrigation. PSAP is non-poisonous and environment friendly. This salt is manufactured by the Isha Agro-Sciences Private Limited, Pune, India.
4.2. Leaf Gas Exchange
Plant leaf gas exchange characteristics such as the net photosynthetic rate (PN), stomatal conductance to water vapor (gs), and transpiration rate (E) were observed on 90 days subjected to limited water irrigation with foliar application of PSAP in sugarcane plants using an Li-6800 portable photosynthesis system (Li-COR Biosciences, Lincoln, NE, US). For each treatment, leaf photosynthetic parameters were recorded between 09:30 and 11:00 h on both treated and non-treated plants (three replicates). In each treatment, position-wise leaves (+1 to +6 from the top to middle part of the leaf) were used for photosynthetic responses without changing the leaf angle. The photosynthetic photon flux density, air temperature, and CO2 concentration were set at 1200 μmol m–2 s–1, 25 °C, and 400 ppm, respectively, inside the leaf chamber. As photosynthetic response rates change linearly along the length of the leaf, observing at the middle of the leaf provides an estimate of the integrated whole photosynthetic rate.
4.3. Models
Verma et al.60 developed the first model to describe physiological responses of plant leaves over a stem/twig, which followed the normal distribution pattern. Measured values of the CO2 assimilation (PN), stomatal conductance to water vapor (gs), and transpiration rate (E) of sugarcane leaves with respect to their positions fitted best in the model. The Gaussian model given by Verma et al.61 is written as below.
| 1 |
where b and c = constants, pn = the physiological response against the leaf position, n, and pm = the maximum physiological response.
Verma et al.61 developed the second model by combining the following hypotheses: (a) the rate of change of physiological responses with respect to the leaf position (dp/dn) is directly proportional to the physiological response (p) and (b) the rate of change of physiological responses with respect to the leaf position is directly proportional to the physiological response and inversely proportional to the leaf position. The following governing equation was developed.
| 2 |
where, p = physiological response, n = leaf position, and μ and λ are the model constants.
They solved the above equation and obtained the following solution.
| 3 |
where, λ = loge ω and γ = eC. The derived model was called as the Skewed model.
Both the models were fitted in the present study for a comparative study to find the best one.
4.4. Model Validations and Comparison with the Existing Model
Measured values of CO2 assimilation (PN), stomatal conductance to water vapor (gs), and transpiration rate (E) of sugarcane leaves with respect to their position were fitted in the derived “Skewed model” for validation. The same data were also fitted in the Gaussian model of Verma et al.61 for comparison purposes.
4.5. Statistical Analyses
Statistical analyses were performed between or within limited water irrigations, depending on parameters using CurveExpert 1.4 software.
Acknowledgments
We are thankful to the Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China for providing the necessary facilities for this study. This study was financially supported by the Youth Program of National Natural Science Foundation of China (31901594), Fund for Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03-01), Fund of Guangxi Academy of Agricultural Sciences (2015YT02), and Guangxi R and D Program Fund (GK17195100).
Author Contributions
∇ K.K.V. and X.P.S. have contributed equally to this work.
The authors declare no competing financial interest.
References
- Verma K. K.; Wu K.-C.; Verma C. L.; Li D.-M.; Malviya M. K.; Singh R. K.; Singh P.; Chen G.-L.; Song X. P.; Li Y. R. Developing mathematical model for diurnal dynamics of photosynthesis in Saccharum officinarum responsive to different irrigation and silicon application. PeerJ 2020, 8, e10154 10.7717/peerj.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Govindaraju M.. Sugarcane production in China. Sugarcane—Technology and Research; de Oliveira A. B. Ed.; IntechOpen, 2018. [Google Scholar]
- Chen G.-L.; Zheng F.-J.; Lin B.; Lao S.-B.; He J.; Huang Z.; Zeng Y.; Sun J.; Verma K. K. Phenolic and Volatile Compounds in the Production of Sugarcane Vinegar. ACS Omega 2020, 5, 30587. 10.1021/acsomega.0c04524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela R. D.; Bezerra B. K. L.; Froehlich A.; Endres L. Antioxidant system is essential to increase drought tolerance of sugarcane. Ann. Appl. Biol. 2017, 171, 451–463. 10.1111/aab.12387. [DOI] [Google Scholar]
- Boyer J. S. Plant productivity and environment. Science 1982, 218, 443–448. 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Verma K. K.; Singh P.; Song X.-P.; Malviya M. K.; Singh R. K.; Chen G.-L.; Solomon S.; Li Y.-R. Mitigating climate change for sugarcane improvement: role of silicon in alleviating abiotic stresses. Sugar Tech 2020a, 22, 741–749. 10.1007/s12355-020-00831-0. [DOI] [Google Scholar]
- Barbosa A. M.; Guidorizi K. A.; Catuchi T. A.; Marques T. A.; Ribeiro R. V.; Souza G. M. Biomass and bioenergy partitioning of sugarcane plants under water deficit. Acta Physiol. Plant. 2015, 37, 142. 10.1007/s11738-015-1887-7. [DOI] [Google Scholar]
- Dinh T. H.; Watanabe K.; Takaragawa H.; Nakabaru M.; Kawamitsu Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. 10.1080/1343943x.2017.1371570. [DOI] [Google Scholar]
- Zhao D.; Li Y.-R. Climate change and sugarcane production: potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 547386. 10.1155/2015/547386. [DOI] [Google Scholar]
- Tripathi P.; Chandra A.; Prakash J. Physio-biochemical assessment and expression analysis of genes associated with drought tolerance in sugarcane (Saccharumspp. hybrids) exposed toGA3at grand growth stage. Plant Biol. 2019, 21, 45–53. 10.1111/plb.12919. [DOI] [PubMed] [Google Scholar]
- Chaves M. M.; Flexas J.; Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. H.; Wang Y. Y.; Li N. N.; Zhao R. H.; Khan A.; Wang J.; Luo H. H. Cotton leaf photosynthetic characteristics, biomass production, and their correlation analysis under different irrigation and phosphorus application. Photosynthetica 2019, 57, 1066–1075. 10.32615/ps.2019.118. [DOI] [Google Scholar]
- Ripley B. S.; Gilbert M. E.; Ibrahim D. G.; Osborne C. P. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. J. Exp. Bot. 2007, 58, 1351–1363. 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Ripley B.; Frole K.; Gilbert M. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Ann. Bot. 2010, 105, 493–503. 10.1093/aob/mcp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D. G.; Gilbert M. E.; Ripley B. S.; Osborne C. P. Seasonal differences in photosynthesis between the C3and C4subspecies of Alloteropsis semialata are offset by frost and drought. Plant Cell Environ. 2008, 31, 1038–1050. 10.1111/j.1365-3040.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. H.; Ripley B. S.; Woodward F. I.; Osborne C. P. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell Environ. 2011, 34, 65–75. 10.1111/j.1365-3040.2010.02226.x. [DOI] [PubMed] [Google Scholar]
- Zhong S.; Xu Y.; Meng B.; Loik M. E.; Ma J.-Y.; Sun W. Nitrogen Addition Increases the Sensitivity of Photosynthesis to Drought and Re-watering Differentially in C3 Versus C4 Grass Species. Front. Plant Sci. 2019, 10, 815. 10.3389/fpls.2019.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K. K.; Song X.-P.; Zeng Y.; Li D.-M.; Guo D.-J.; Rajput V. D.; Chen G.-L.; Barakhov A.; Minkina T. M.; Li Y.-R. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 2020b, 5, 24145–24153. 10.1021/acsomega.0c03820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J.; Bota J.; Loreto F.; Cornic G.; Sharkey T. D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Lawlor D. W.; Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Mencuccini M.; Hölttä T. The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytol. 2010, 185, 189–203. 10.1111/j.1469-8137.2009.03050.x. [DOI] [PubMed] [Google Scholar]
- Cornic G.; Bukhov N. G.; Wiese C.; Bligny R.; Heber U. Flexible coupling between light-dependent electron and vectorial proton transport in illuminated leaves of C3 plants. Role of photosystem I-dependent proton pumping. Planta 2000, 210, 468–477. 10.1007/pl00008154. [DOI] [PubMed] [Google Scholar]
- Rajput V. D.; Chen Y.; Ayup M. Effects of high salinity on physiological and anatomical indices in the early stages of Populus euphratica growth. Russ. J. Plant Physiol. 2015, 62, 229–236. 10.1134/s1021443715020168. [DOI] [Google Scholar]
- Chaves M. M.; Pereira J. S.; Maroco J. P.; Rodrigues M. L.; Ricardo C. P. P.; Osorio M. L.; Carvalho I.; Faria T.; Pinheiro C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. 10.1093/aob/mcf105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford M.; Horst W.; Kichey T.; Lambers H.; Schjoerring J.; Skrumsager-Moller I.; White P.. Function of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner P., Ed.; Academic Press: London, 2012; pp 135–189. [Google Scholar]
- Wang M.; Zheng Q.; Shen Q.; Guo S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. 10.3390/ijms14047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami-Rad S.; Hajiboland R. Effect of potassium application in drought-stressed tobacco ( Nicotiana rustica L.) plants: Comparison of root with foliar application. Ann. Agric. Sci. 2017, 62, 121–130. 10.1016/j.aoas.2017.08.001. [DOI] [Google Scholar]
- Sangakkara U. R.; Frehner M.; Nosberger J. Effect of soil moisture and potassium fertilizer on shoot water potential, photosynthesis and partitioning of carbon in mungbean and cowpea. J. Agron. Crop Sci. 2000, 185, 201–207. 10.1046/j.1439-037x.2000.00422.x. [DOI] [Google Scholar]
- Zhao D.; Oosterhuis D. M.; Bednarz C. W. Influences of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosyntetica 2001, 39, 103–109. 10.1023/a:1012404204910. [DOI] [Google Scholar]
- Damon P. M.; Rengel Z. Wheat genotypes differ in potassium efficiency under glasshouse and field conditions. Aust. J. Agric. Res. 2007, 58, 816–823. 10.1071/ar06402. [DOI] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. 10.1002/jpln.200420485. [DOI] [Google Scholar]
- Suriyagoda L. D. B.; Ryan M. H.; Renton M.; Lambers H. Above- and below-ground interactions of grass and pasture legume species when grown together under drought and low phosphorus availability. Plant Soil 2011, 348, 281–297. 10.1007/s11104-011-0754-6. [DOI] [Google Scholar]
- Tariq A.; Pan K.; Olatunji O. A.; Graciano C.; Li Z.; Sun F.; Sun X.; Song D.; Chen W.; Zhang A.; Wu X.; Zhang L.; Mingrui D.; Xiong Q.; Liu C. Phosphorous Application Improves Drought Tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 1561. 10.3389/fpls.2017.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer M. D.; Hawkins H.-J.; Verboom G. A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. 10.1007/s00442-009-1364-3. [DOI] [PubMed] [Google Scholar]
- Sardans J.; Peñuelas J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. 10.1104/pp.112.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina J.; Vilagrosa A.; Trubat R. The role of nutrients for improving seedling quality in drylands. New Form. 2013, 44, 719–732. 10.1007/s11056-013-9379-3. [DOI] [Google Scholar]
- dos Santos M. G.; Ribeiro R. V.; de Oliveira R. F.; Pimentel C. Gas exchange and yield response to foliar phosphorus application in Phaseolus vulgaris L. under drought. Braz. J. Plant Physiol. 2004, 16, 171–179. 10.1590/s1677-04202004000300007. [DOI] [Google Scholar]
- Jones C.; Jacobsen J.; Wraith J. Response of malt barley to phosphorus fertilization under drought conditions. J. Plant Nutr. 2005, 28, 1605–1617. 10.1080/01904160500203531. [DOI] [Google Scholar]
- Campbell C. D.; Sage R. F. Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.). Plant Cell Environ. 2006, 29, 844–853. 10.1111/j.1365-3040.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- Faustino L. I.; Bulfe N. M. L.; Pinazo M. A.; Monteoliva S. E.; Graciano C. Dry weight partitioning and hydraulic traits in young Pinus taeda trees fertilized with nitrogen and phosphorus in a subtropical area. Tree Physiol. 2013, 33, 241–251. 10.1093/treephys/tps129. [DOI] [PubMed] [Google Scholar]
- Liu C.; Wang Y.; Pan K.; Jin Y.; Li W.; Zhang L. Effects of phosphorus application on photosynthetic carbon and nitrogen metabolism, water use efficiency and growth of dwarf bamboo (Fargesia rufa) subjected to water deficit. Plant Physiol. Biochem. 2015, 96, 20–28. 10.1016/j.plaphy.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Munns R.; Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Ahmad S.; Kamran M.; Ding R.; Meng X.; Wang H.; Ahmad I.; Fahad S.; Han Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793 10.7717/peerj.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K. K.; Wu K.-C.; Singh P.; Malviya M. K.; Singh R. K.; Song X.-P.; Li Y. R. The protective role of silicon in sugarcane under water stress: photosynthesis and antioxidant enzymes. Biomed. J. Sci. Tech. Res. 2019, 15, 002685. 10.26717/BJSTR.2019.15.002685. [DOI] [Google Scholar]
- Wahid A.; Rasul E.. Photosynthesis in leaf, stem, flower and fruit. In Handbook of Photosynthesis, 2nd ed.; Pessarakli M., Ed.; CRC Press: Boca Raton, FL, 2005; pp 479–497. [Google Scholar]
- Meloni D. A.; Oliva M. A.; Martinez C. A.; Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. 10.1016/s0098-8472(02)00058-8. [DOI] [Google Scholar]
- Liu D.; Wu L.; Naeem M. S.; Liu H.; Deng X.; Xu L.; Zhang F.; Zhou W. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013, 35, 2747–2759. 10.1007/s11738-013-1307-9. [DOI] [Google Scholar]
- Anjum S. A.; Xie X.; Wang L.; Saleem M. F.; Man C.; Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. 10.5897/AJAR10.027. [DOI] [Google Scholar]
- Hartung W.; Sauter A.; Hose E. Abscisic acid in the xylem: where does it come from, where does it go to?. J. Exp. Bot. 2002, 53, 27–32. 10.1093/jexbot/53.366.27. [DOI] [PubMed] [Google Scholar]
- Reddy A. R.; Chaitanya K. V.; Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Burman U.; Garg B. K.; Kathju S. Effect of Phosphorus Application on Clusterbean under Different Intensities of Water Stress. J. Plant Nutr. 2009, 32, 668–680. 10.1080/01904160802715620. [DOI] [Google Scholar]
- Singh S. K.; Badgujar G.; Reddy V. R.; Fleisher D. H.; Bunce J. A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. 10.1016/j.jplph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Lawlor D. W. Photosynthesis, productivity and environment. J. Exp. Bot. 1995, 46, 1449–1461. 10.1093/jxb/46.special_issue.1449. [DOI] [Google Scholar]
- Wang J.; Chen Y.; Wang P.; Li Y. S.; Wang G.; Liu P.; Khan A. Leaf gas exchange, phosphorus uptake, growth and yield responses of cotton cultivars to different phosphorus rates. Photosynthetica 2018, 56, 1414–1421. 10.1007/s11099-018-0845-1. [DOI] [Google Scholar]
- Kaiser W. M. Effects of water deficit on photosynthetic capacity. Physiol. Plantarum 1987, 71, 142–149. 10.1111/j.1399-3054.1987.tb04631.x. [DOI] [Google Scholar]
- Eichert T.; Fernández V.. Uptake and release of elements by leaves and other aerial plant parts. In Marschner’s Mineral Nutrition of Higher Plants; Marschner P., Ed.; Academic Press: London, 2012; pp 71–84. [Google Scholar]
- Fernández V.; Brown P. H. From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. 10.3389/fpls.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewdar M. D. H.; Rady M. M. Influence of soil and foliar applications of potassium fertilization on potential and fiber quality traits for two (Gossypium barbadense L.) varieties. J. Agric. Res. 2013, 8, 2211–2215. 10.5897/ajar12.1861. [DOI] [Google Scholar]
- Pantin F.; Simonneau T.; Muller B. Coming of leaf age: control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. 10.1111/j.1469-8137.2012.04273.x. [DOI] [PubMed] [Google Scholar]
- Verma K. K.; Singh M.; Verma C. L. Developing a mathematical model for variation of physiological responses of Jatropha curcas leaves depending on leaf positions under soil flooding. Acta Physiol. Plant. 2012, 34, 1435–1443. 10.1007/s11738-012-0941-y. [DOI] [Google Scholar]
- Verma K. K.; Singh M.; Verma C. L. The effect of leaf position on the physiological responses in poplar leaves (clone S7C15) irrigated with fluoride-laden water. Fluoride 2020c, 53, 312–334. [Google Scholar]