Abstract
Allostatic load (AL) is the manifestation of cumulative responses to chronic stress exposure. Numerous studies have shown the importance of AL in understanding disease risks. Yet little is known about existing interventions that target AL specifically. We aimed to address this gap by identifying interventions targeting AL and determining the success of these interventions in improving biological functioning. We searched five electronic databases using variations of two concepts: AL and programs or interventions. We included original research reports that focused on AL as an outcome. We excluded work that focused on a single indicator, not written in English or did not implement an intervention. The Template for Intervention Description and Replication checklist guided our intervention critique and synthesis. Six articles were included, with sample size across the interventions ranging between 2 and 733. Despite inconsistencies in the selection of AL indicators and scoring of AL, all four body systems were represented in all the studies. Four interventions showed significant improvement in Al (as indicated by a decrease in AL score) as early as 7 weeks. More interventions targeting Al are needed. The reduction in AL scores among four of the six interventions suggests that Al could be a biological outcome measure that is sensitive to change in response to interventions. This has significant clinical and research implications. Future studies are needed to examine whether AL serves as a mediator in the effects of the intervention on improving clinical manifestations of diseases.
Keywords: Allostatic load, Clinimetric index, literature reviews, interventions, stress pathophysiology
Introduction
Allostatic load (AL) is an important concept that has gained momentum across various disciplines, particularly in the fields of medicine and psychiatry (Liston et al., 2009). AL is the manifestation of cumulative responses to chronic stress exposure (Juster et al., 2010; McEwen, 1998; 2000; McEwen, 2002; McEwen & Seeman, 1999; McEwen & Stellar, 1993; McEwen & Wingfield, 2003, 2010) that can serve as an “early warning system” of wear and tear on the body because it may be more sensitive to effects of stress than a single indicator (Mauss et al., 2016; Seeman et al., 2004). This concept is important because it delineates functioning across not just one but several body systems including sympathetic nervous system (SNS), parasympathetic nervous system (PNS), hypothalamic pituitary adrenal (HPA), cardiovascular, immunologic and inflammatory systems (Beckie, 2012; Johnson et al. 2017; Juster et al., 2010; McEwen, 2000; 2000; McEwen & Wingfield, 2010). Numerous studies have shown the importance of AL in understanding disease risks (Beckie, 2012; von Thiele et al., 2006). Despite studies noting the scientific and clinical relevance of AL, little is known about existing interventions to mitigate stressors and associated cumulative pathophysiologic response that target or measure AL specifically.
There are many intervention approaches to decreasing stressors or improving responses to stress and many biobehavioral approaches to measure their health effects. Given the complexity of the physiologic manifestations of stress on the body, interventions targeting AL—using measures that reflect this complexity—could be fruitful. In their longitudinal cohort study among 171 high-functioning community-dwelling older adults, Karlamangla, and colleagues (Karlamangla et al., 2006), found that those with an increase in AL score had a higher risk for mortality compared to those with decreased AL score (15% vs. 5% = .047). Their study highlighted the potential clinical significance of interventions aimed at decreasing AL (Karlamangla et al., 2006). Given that AL often is driven by one’s perception and direct interpretation or internalization of the stressors, addressing AL itself may be a good approach when a change in stressor is impracticable and maladaptive coping is not the core problem. To this end, we set out to conduct a review by which we explored interventions aimed at ameliorating AL. The purpose of this scoping review was to identify interventions targeting AL and determine the success of these interventions in improving biological functioning. Our primary research question was: Are there any studies aimed at improving AL? Our sub-questions were: What are the key components of these interventions? What were the key AL-related outcomes?
Definition, operationalization, and scoring of AL
AL is the pathophysiologic manifestation of the cumulative effects of stress (McEwen, 1998). Chronic exposure to stress leads to the accumulation of wear and tear across the interacting physiologic systems, a price of adaptation (McEwen, 1998). The cascading event begins with chronic exposure to stress across multiple socio-ecological levels (e.g., structural inequalities, trauma) (Geronimus et al., 2006; Juster et al., 2010; Seeman et al., 2004; von Kanel, 2003). Then primary neuroendocrine responses take place with hormones of the hypothalamic-pituitary-adrenal (HPA) axis (e.g., catecholamines, cortisol) followed by secondary responses which include the dysregulations across the immunologic, metabolic, cardiovascular, and nervous systems. Tertiary outcomes involve clinical conditions (morbidity) and eventually death (McEwen, 2004).
AL is operationalized by a composite score of multiple physiological indicators–anthropometrics and biomarkers—from different body systems (Mauss et al., 2016; Seeman et al., 2004). Some of the most frequently used indicators and biomarkers include cortisol, epinephrine, and norepinephrine from the neuroendocrine system, C-reactive protein (CRP) and interleukin-6 (IL-6) from the immune system, systolic blood pressure (SBP) and diastolic blood pressure (DBP) from the cardiovascular system, high-density lipoprotein (HDL), glycosylated hemoglobin (HbA1c), and total cholesterol (TC) from the metabolic system, and waist/hip ratio (WHR), and body mass index (BMI) as anthropometric measurement (Juster et al., 2010).
The term clinimetrics emphasizes the quality of measurements with a focus on clinical and practice implications (Fava et al., 2012). Specific to AL, studies have used clinimetric approaches to understand socio-ecological factors (e.g., life events), behavioral responses as well as variations across clinical measures of AL and their implications for mortality and morbidity (e.g., cardiovascular disease) (McEwen, 2000; 2019; McEwen & Seeman, 1999). Key clinimetric criteria for AL that can be applied in clinical practice include the presence of a source of stress and stress responses (i.e., psychiatric symptoms, psychosomatic symptoms, significant impairment in social and occupational functioning, significant impairment in psychological well-being) (Fava et al., 2010). Studies have supported the clinical relevance of AL with potential pathways for program implementations to improve health outcomes such as obesity (Ottino-González et al., 2019), other health behavior risks (Suvarna et al., 2020), burnouts (Juster et al., 2011), cardiovascular diseases, and mortality (Seeman et al., 2004). A clear understanding of the stress antecedents (e.g., work stress, trauma, low socio-economic status) and effective measurement of AL will narrow the research-clinical-practice gap thus inform effective upstream and downstream interventions to remediate health disparities (Shonkoff et al., 2009).
Many scoring methods have been used to compute an AL composite score, including the count-based, z-score, canonical correlation, and grade of membership (GOM) methods (Juster et al., 2010). The simple count-based method is the most commonly used method. Using this method, an AL summary score is calculated by summing the number of indicators and biomarkers falling within a high-risk percentile (i.e., upper or lower 25th percentile) based on the sample’s distribution of indicator and biomarker values. The count-based method has demonstrated good predictive performances for predicting self-rated health, hypertension, and diabetes in women of reproductive age (Li et al., 2019). For the Z-Score approach, each indicator is standardized to a mean of zero with one standard deviation. ALI is calculated by adding the sum of the standardized distance of each indicator to the respective mean (Vie et al., 2014). The Canonical correlation emphasizes the ideal linear combination for the AL indicators that are the most highly correlated with the health outcomes (Karlamangla et al., 2002). For the GOM approach, ALI is the sum of N-1. For this approach, each indicator is categorized into low, moderate, or high and excludes the score for the reference group (Seplaki et al., 2005).
There also remains some debate about indicators to include for the measurement of AL. The issue of whether a clinical or sample-based cutoff criteria should be used also is unresolved (Mauss et al., 2015). Despite these debates, studies comparing distinct measurement approaches have found only modest differences in their predictive utility (McEwen & Stellar, 1993; McEwen, 1998; Karlamangla et al., 2002). Moreover, a recent analysis supported the existence of an overarching AL factor comprising physiological dysregulation across six sub-parameters and 18 specific neuroendocrine, metabolic, cardiovascular, and inflammatory indicators (McEwen, 2002).
Clearly, we understand the importance of AL as an early warning-system of disease risks and a catalyst for advancing our understanding of the stress-strain response-morbidity- and mortality trajectory. However, the heterogeneity in how AL is operationalized suggests that clinical intervention research is needed to effectively leverage the utility of AL in practice for optimal health outcomes. This scoping review lays the groundwork toward achieving this goal.
Methods
Design
This is a scoping review design. A scoping review is performed to answer research questions and map key concepts in an area of research that has not been previously comprehensively reviewed. Colquhoun and colleagues (Colquhoun et al., 2014) expanded on Arksey and O’Malley’s work (Arksey & O’Malley, 2005) to define a scoping review as a form of knowledge synthesis that addresses an exploratory research question aimed at mapping key concepts, types of evidence, and gaps in research related to a defined area or field by systematically searching, selecting, and synthesizing existing knowledge (Colquhoun et al., 2014). Arksey & O’Malley (2005)’s five stages of conducting a scoping review guided our approach. The five steps include: 1) identifying the research question, 2) identifying relevant studies, 3) study screening and selection, 4) charting the data, and 5) collating, summarizing, and reporting the results (Arksey & O’Malley, 2005). We proposed three research questions. Our first research question was “Are there any studies aimed at improving AL?” Our second research question was “What are the key components of these interventions?” Our third research question was “What were the key AL-related outcomes?”
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria informed the literature search and record-keeping strategies (see Figure 1). The authors consulted with a health-science library informationist to refine the search protocol. Five electronic databases (PubMed, PsycInfo, Cumulative Index to Nursing and Allied Health Literature [CINAHL], Embase, and Scopus) were searched to identify relevant articles. Search terms included variations on two key concepts of interest: a) programs or intervention research and b) AL.
Figure 1.
PRISMA model to guiding our search and record-keeping approach.
Inclusion and exclusion criteria
Articles were included if: 1) they were original research reports; 2) included a cumulative AL index; 3) had AL as an outcome. We did not have a timeframe restriction because we understood that such a review had not been completed previously and wanted to capture all studies. Articles were excluded if they 1) did not have a composite score for AL. For example, some articles mentioned that they looked at AL yet only focused on one indicator; 2) did not implement an intervention; 3) were not written in English or 4) there was no access to the full text.
Data extraction
Three researchers extracted the data using two steps. First, a table (Table 1) was developed to record the indicators that made up the AL index for each article. Second, the Template for Intervention Description and Replication (TIDieR) checklist and guide (see Table 2) was used to evaluate the interventions (see Table 3). TIDieR was inspired by the Consolidated Standards of Reporting Trials (CONSORT) and SPIRIT (Standard Protocol Items: Recommendations for Intervention Trials) both of which emphasize the importance of clarity and transparency in the description and reporting of intervention protocols and intervention-related outcomes (Campbell et al., 2018; Hoffmann et al., 2014; 2017).
Table 1.
Indicators used across all six interventions.
| Biomarker | Berger et al. (2018) | Carroll et al. (2015) | McClain et al. (2018) | Nuño et al. ( 2019) | Soltani et al. (2018) | Ye et al. (2017) | |
|---|---|---|---|---|---|---|---|
| Cardiovascular | Heart rate | x | x | ||||
| Systolic blood pressure | x | x | x | X | x | ||
| Diastolic blood pressure | x | x | x | X | x | ||
| Creatinine kinase | x | ||||||
| Fibrinogen | x | ||||||
| Standard deviation of R-R interval | x | ||||||
| Neuroendocrine | Hemoglobin | x | |||||
| Cortisol | x | x | x | x | x | ||
| Copeptin | x | ||||||
| Epinephrine/metanephrine | x | x | x | x | |||
| Norepinephrine/normetanephrine | x | x | x | ||||
| DHEA-S* | x | x | x | ||||
| Immune | Serotonin | x | |||||
| Interleukin-6 (IL-6) | x | x | |||||
| TNFα** | x | ||||||
| C-reactive protein (CRP) | x | x | x | x | x | ||
| E-selectin | x | ||||||
| White blood ell count | x | ||||||
| Red blood cell count | x | ||||||
| Metabolic | CD4+/CD8+ | x | |||||
| Triglycerides | x | x | |||||
| High-density lipoprotein (HDL) | x | x | x | x | x | ||
| Low-density lipoprotein (LDL) | x | x | |||||
| Total cholesterol | x | x | x | ||||
| Insulin | x | x | |||||
| Fasting glucose | x | x | |||||
| HbA1c *** | x | x | x | x | x | ||
| enRAGE**** | x | ||||||
| Waist-to-hip ratio | x | x | x | x | |||
| Body mass index | x | x | x | ||||
| Waist circumference | x | ||||||
| AL Score Computation | Number of markers | 22 | 8 | 11 | 10 | 14 | |
| Cutoffs | Above 75th percentile based on distribution in healthy control group | Defined by existing clinical target guidelines for each biomarker | Clinically defined, or previously defined quartile and high-risk cutoffs | Above 75th percentile based on population distribution of normative biomarker values used in clinical practice | Markers were divided into quartiles based on score distribution in the sample | Clinically diagnosed as abnormal by primary oncologist or cardiologist | |
| AL Score Generation Approach | Used a scaling approach: for each category, the number of values above the cutoff for each individual was divided by the total number of markers in that system. The scores for each system were then summed. | Generated z scores to estimate the distance of each marker from the cutoff, which were averaged and multiplied by the total # of markers, creating a multisystem biological risk score representing the summed standard distance from the cutoffs. | One point assigned for each marker exceeding the cutoff point or if a healthy value was maintained through medication use. Points were summed across the 11 markers to generate a composite AL score, with scores ranging from 0–11. | A score of 1 was assigned to biomarkers falling within high-risk percentile (75th percentile for all expect for HDL and Dehydroepiandrosterone for which the lowest 25th percentile corresponds to highest risks). AL score had a numerical value from 0–20. | One point was assigned for each marker for which the subject fell into the highest risk quartile. Points were summed across the 10 markers to generate a composite AL score. | One point assigned for each marker diagnosed as abnormal. Points were summed across the 14 markers to generate a composite AL score, with scores ranging from 0–14. |
Dehydroepiandrosterone sulfate
Tumor necrosis factor alpha
glycated hemoglobin
Ligand for the receptor for advanced glycation end products.
Table 2.
TIDieR criteria evaluation.
| Criteria | Berger et al. (2018) | Carroll et al. (2015) | McClain et al. (2018) | Nuño et al. (2019) | Soltani et al. (2018) | Ye et al. (2017) |
|---|---|---|---|---|---|---|
| Provided the name or a phrase that describes the intervention | X | X | X | X | X | X |
| Described any rationale, theory, or goal of the elements essential to the intervention | X | X | X | X | X | X |
| Gave details on materials and information on where the materials can be accessed (for example, online appendix, URL) | X | X | X | X | ||
| Gave details on procedures used in the intervention | X | X | X | X | X | |
| Provided details on intervention team members | X | X | X | X | ||
| Provided details on mode of delivery such as face to face or by some other mechanism, and whether it was provided individually or in a group | X | X | X | X | X | |
| Provided details on intervention location including any necessary infrastructure or relevant features | X | X | X | X | X | |
| Described the schedule, duration, intensity or dose | X | X | X | X | X | |
| Explained if the intervention was planned to be personalized, titrated or adapted | X | X | ||||
| Explained if the intervention was modified during the course of the study | ||||||
| Explained how intervention adherence or fidelity was assessed | ||||||
| IF intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned |
Table 3.
Intervention details.
| Author and year | Study purposes | Study design | Sample characteristics | Interventions | Primary outcomes | Secondary outcomes | Assessment time | Main findings |
|---|---|---|---|---|---|---|---|---|
| Berger et al. (2018) | 1) To investigate if AL is higher in patients with SCZ and FEP and associated with clinical relevant outcomes; 2) To examine the temporal dynamics of AL in response to treatment with second-generation anti-psychotics. |
Longitudinal study design | 28 patients with SCZ (32% female, average age of 40 yrs), 28 patients with FEP (46% female, average age of 33 yrs), and 53 HC (32% female, average age of 36 yrs). | One of the second-generation antipsychotics (risperidone, olanzapine and quetiapine) were given to patients after baseline assessment. | AL | NA | Baseline and 6 and 12 weeks | Adjusting for age, sex and smoking, positive SCZ symptoms were positively correlated with AL (adjusted R = 0.510 (95%CI 0.247–0.715), p < .001). Psychosocial functioning was negatively correlated with AL (adjusted R = −0.224 (95% CI −0.441–0.016), p = .103). In patients with SCZ or and FEP, AL decreased significantly after treatment (between baseline and 6 and 12-week follow-up assessments; p < .001). |
| Carroll et al. (2015) | To compare the efficacy of CBT, TCC, and SS controls to improve sleep quality and reduce AL in older adults with insomnia. | Secondary analysis of data from a Randomized controlled comparative efficacy trial | 47 older adults with insomnia in CBT (79% female, average age of 65 yrs, 87% White), 39 in TCC (64% female, average age of 67 yrs, 84% White), and 23 SS controls (70% female, average age of 66 yrs, 86% White). | CBT taught behavioral strategies to improve mood and cognitive activity, TCC taught slow-paced movements designed to control physical function and arousal, and SS taught sleep hygiene and factors contributing to sleep issues. All groups received 120-minutes classes each week for 4 months. | Sleep quality measure by the PSQI | AL | Baseline, after intervention at 4-months, 1 year after intervention completion, and at 16-months. |
Both TCC (p = .04) and CBT (p = .001) had significantly lower AL scores than SS at 16-months. CBT reduced risk of being in the high risk AL group at 4-months (odds ratio [OR] = .21 [95% CI, .03—1.47], p < .10) and at 16-months (OR = .06 [95% CI, .005—.669]; p<.01). TCC reduced the risk at 16-months (OR = .10 [95% CI, .008—1.29]; p<.05) but not at 4 months. For participants with high risk AL scores at baseline, sleep quality improvements decreased the likelihood of being in the high risk group at 16-months, OR = .08 (95% CI, .008—.78); p = .01. |
| McClain et al. (2018) | To test the longitudinal effect of FI on AL and to examine the moderation by SNAP participation. | Secondary analysis of data from a longitudinal cohort study | 733 Puerto Rican adults: aged 45 to 75 yrs, 71% female |
Federal program that provides nutrition benefits to low-income individuals and families were used to purchase food for the household. EBT cards were distributed to participants to purchase food at stores. | AL | NA | Baseline, 2 years, and 5 years. | Adjusting for covariates, FI was not associated with AL (OR = 1.07 [0.70–1.64]). However, AL was associated with high neuroendocrine/inflammation scores (1.71 [1.25–2.36]), but not metabolic/cardiovascular scores (0.82 [0.48–1.40]). SNAP participation moderated the relationship between FI and neuroendocrine/ inflammation scores (p = .06). FI participants who had never received SNAP had higher neuroendocrine/inflammation scores than food-secure participants not in SNAP or FI participants in SNAP. |
| Nuño et al. (2019) | To examine the efficacy of osteopathic manipulative treatment (OMT) on graduate student’s overall health through an objective index of representative AL biomarkers |
Within-Subject Study design | One man (age 22 years) and one woman (age 23). Both participants were enrolled in a Masters of Science in Medical Health Sciences program at Touro University California College of Osteopathic Medicine (TUCOM). |
The intervention involved four visits scheduled every 2–4 weeks. Baseline data collection included perceived stress, blood pressure, blood and urine samples and anthropometric measures. Participants then received the OMT following the ABC protocol (autonomics, biomechanics, circulation, screening) for 30 minutes. The OMT was repeated at each of the four visits. |
AL and Perceived stress via the Trier Inventory for Chronic Stress (TICS) | N/A | Baseline and at the end of the intervention after 7 weeks |
AL score decreased from 7 to 4 for the male participant, and from 9 to 7 for the female participant. TICS scores lowered after 7 weeks from 18 to 15 for the male participant and from 40 to 13 for the female participant. |
| Soltani et al. (2018) | To examine the effects of a DGA-based diet on chronic stress load. | Secondary analysis of data from a double-blind RCT | Obese women, ages 20–64; 22 in DGA and 22 in TAD |
Over 8 weeks, both TAD and DGA received daily food delivery with meal checklist with foods to document amount and time consumed; and confirm that no other foods or medications were consumed. They were also instructed on managing social situations and the value of honesty over perfection. | AL; perceived stress measured by the PSS-10 | NA | Baseline and at the end of intervention at 8 weeks. | Perceived stress did not differ after 8 weeks between the diet groups (p = 0.45), and neither did AL (p = 0.79). There were inverse associations between change in stress and diet quality (lower sodium and higher vegetable consumption). Increased sodium consumption was significantly associated with decreased AL across after 8 weeks (ß = −0.21 ± 0.07; p = 0.007). Increased vegetable consumption was significantly associated with a decreased perceived stress after 8 weeks in both diet groups (ß = −1.45 ± 0.66; p = 0.034). As HEI (healthy eating index) increased, AL decreased. |
| Ye et al. (2017) | To test the effects of the BRBC on resilience, QoL, emotional and physical distress (AL), and longevity among women with MBC. | RCT | 226 women with MBC, aged ≥ 40 yrs; 113 in BRBC and 113 controls. No significant demographic differences between groups except for income (P > 0.0254). | Over 12 months, weekly 120-minute face-to-face group sessions, including 45 minutes of education on breast cancer topics and 45–75 minutes of group discussion. Discussion time began with mentors sharing their experiences and continued with participant discussions regarding life changes since diagnosis. | 3- and 5-year cancer-specific survival | anxiety and depression measured by HADS, QoL measured by QLQ-C30, resilience measured by CD-RISC-10, and AL | Survival data were collected every 1–2 months till the end of the study. Secondary outcomes were only collected at baseline, 2 months, 6 months, and 12 months. | At baseline, the two groups did not show any significant difference in AL (P = 0.1817). After 2 months, AL had improved, but the ES was not significant (ES = 0.45, P = 0.1345). After 12 months, the effect size increased significantly (from 0.75 to 0.90). |
Note. AL: allostatic load; SCZ: schizophrenia; FEP: first-episode psychosis; HC: healthy controls; NA: not applicable/not available; CBT: cognitive behavioral therapy; TCC: tai chi chih, SS: sleep seminar; PSQI: Pittsburgh Sleep Quality Index; FI: food insecurity; SNAP: Supplemental Nutrition Assistance Program; EBT: Electronic Benefit Transfer; DGA: Dietary Guidelines for Americans; TAD: Typical American Diet; RCT: randomized controlled trial; PSS-10 : Perceived Stress Scale-10; BRBC: Be Resilient to Breast Cancer; QoL: quality of life; MBC: metastatic breast cancer; HADS: Hospital Anxiety and Depression Scale; CD-RISC-10 : Conner-Davison Resilience Scale.
Results
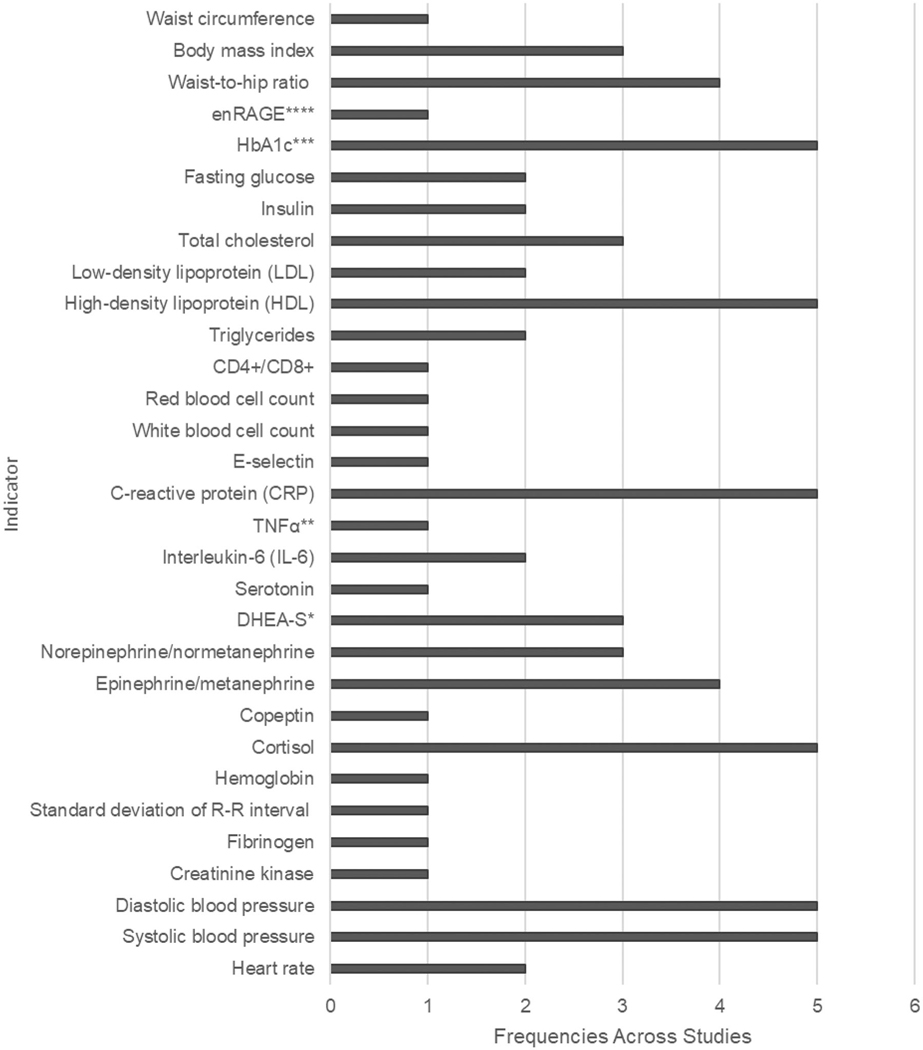
A total of six articles were included in this review (Berger et al., 2018; Carroll et al., 2015; McClain et al., 2018; Nuño et al., 2019; Soltani et al., 2018; Ye et al., 2017). Sample sizes varied between 2 and 733 across all interventions. AL was either a primary (e.g., McClain et al., 2018) or secondary outcome (i.e., Ye et al., 2017) for the intervention. Various combinations of indicators made up the AL index across the six interventions. Carroll et al (Carroll et al., 2015) had eight indicators; Soltani et al (Soltani et al., 2018) had 10; McClain et al (McClain et al., 2018) had 11; Ye et al. (Ye et al., 2017) had 14, Nuño et al (Nuño et al., 2019) had 20, and Berger et al (Berger et al., 2018)had 22 indicators. There were no component indicators of AL consistently used across all the five studies. However, SBP and DBP from the cardiovascular system, cortisol from the neuroendocrine system, CRP from the immune system, and HDL and HbA1c from the metabolic system were included in four of the five studies (Table 1 and Figure 2). Despite different combinations of AL component indicators, all of the four body systems, including the cardiovascular, neuroendocrine, immune, and metabolic systems, were represented in the AL index in the six studies. Four of the five articles scored AL using the within-sample percentile ranking to score AL, while the remaining two used clinical cutoffs.
Figure 2.
Frequencies of AL indicators across studies.
As seen in table two, none of the studies addressed all the 12 components of the TiDeR checklist. For example, none addressed adherence or fidelity to the intervention nor whether the intervention was modified during the course of the project. The interventions included drug therapy (Berger et al., 2018); comparative efficacy of cognitive behavioral therapy (CBT), tai chi chih (TCC), and a sleep seminar (Carroll et al., 2015); a federal nutrition assistance program (McClain et al., 2018); osteopathic manipulative treatment (OMT) (Nuño et al., 2019); a whole foods diet (Soltani et al., 2018); and mentor-based supportive expressive program (Ye et al., 2017). Follow up post-intervention began as early as 1.75 month (Nuño et al., 2019) and occurred as late as 5 years (McClain et al., 2018) post-intervention.
Four of the six studies indicated significant improvement in AL (Table 3) (Berger et al., 2018; Carroll et al., 2015; Nuño et al., 2019; Ye et al., 2017). In Berger et al’s study (Berger et al., 2018) AL decreased significantly after treatment (between baseline and 6 and 12-week follow-up assessments; p < .001) (Berger et al., 2018). In Carroll et al’s study (Carroll et al., 2015) AL scores decreased significantly among the group that received the Tai Chi (p = 0.04) and cognitive behavioral theory (p = 0.001). The improvement (reduction in AL scores) began to show as early as 4 months in the CBT group (odds ratio [OR] = .21 [95% CI, .03—1.47], p < .10) (Carroll et al., 2015). In Ye et al’s study (McClain et al., 2018), the effect size for the AL index increased significantly at 12 months (From 0.75 to 0.90) (Ye et al., 2017). Nuño and colleagues’ study included two participants (one man and one woman). They showed an improvement in the AL score from 7 to 4 for the man and from 9 to 7 for the woman (Nuño et al., 2019). McClain and colleagues’ federal nutrition assistance program study did not show improvement in AL (McClain et al., 2018). Soltani and colleagues’ study (Soltani et al., 2018) showed no significant difference between the two diet intervention groups (p = 0.79).
Discussion
The goal of this scoping review was to identify and synthesize studies aimed at improving AL. AL is a concept used to describe pathophysiologic functioning across all body systems as a response to chronic stress exposure. The potential significant clinical implications of AL in understanding health risks and pathways for morbidity and mortality has been shown. Yet we know little about approaches that have been developed and implemented to address AL, capitalizing on this early warning system metric. We found only six studies, but four of them showed significant AL improvement (Figure 3).
Figure 3.
Intervention targeting AL.
+ indicate interventions yielding significant decrease in AL
- indicate intervention with no change in AL
Among the six studies reviewed, the AL index was made up of different combinations of physiological indicators. But all the four body systems (e.g., the cardiovascular, neuroendocrine, immune, and metabolic systems) were captured in the AL measures of the six studies. The AL scoring approaches also varied across the six studies. Future studies may determine an optimal combination of AL indicators from the four body systems and lead to a consensus about how to best score AL to improve ease of comparison across studies; however, comparison of effect size provides an alternative to AL measurement and scoring consistency.
Despite that there were only six intervention studies examining AL as the primary or secondary outcome, four of the six studies showed efficacy of the interventions in reducing AL. In Carroll et al’s study (Carroll et al., 2015), the significant reduction in AL was found as early as 4 months after the CBT group. This suggests that AL could be a biological outcome measure that is sensitive to change in response to the intervention or treatment. Ye and colleagues (Ye et al., 2017) found the effect size of BRBC on AL increased throughout the intervention from 0.49 (p = .13) at 2 months to 0.90 (p < 0.001) at the end of the intervention at 12 months. Nuño et al. (2019) OMT intervention yielded a decrease from 7 to 4 and 9 to 7 in AL score in the first and second participants respectively. In contrast, Soltani and colleagues (Soltani et al., 2018) did not find significant changes in AL before and after the 8-week DGA diet intervention. There may be several reasons for the heterogeneity in intervention results across the studies. These reasons include but are not limited to discrepancies in the follow-up time, the type of interventions implemented, the process involved in the implementation, the population involved, sample size, and other extraneous factors such as environment, family/social support, and coping behaviors.
Studies are needed to replicate these interventions. More long-term studies are needed in order to determine whether benefits are sustained after interventions end, and whether repair in system function may continue independently. Future studies are needed to examine whether AL serves as a mediator in the effects of the intervention or treatment on improving clinical manifestations of diseases. The full promise of AL lies in the clinical implications of observing a reduction in AL as an early indicator of the effectiveness of an intervention in relation to clinical outcomes.
Limitations
The concept of AL is relatively new to the literature. Limiting our inclusion criteria to studies which specifically include a cumulative AL index means that studies that measure components of allostatic load, even several of them without creating a cumulative index, were not included. Also, this study focused on change in AL as an outcome of intervention. Assessment of AL in observational studies was not examined, and knowing the range of distributions across samples with similar characteristics and stressors may have further illuminated the effect of interventions on AL.
Strengths
Despite these limitations, this study has several strengths. First, to our knowledge, this is the first review of its kind to synthesize interventions targeting AL. This endeavor is of upmost importance, especially given the growing awareness of the significance of AL in our understanding of the relationship between stress (across socio-ecological levels) and morbidity, and mortality. Second, because there are a small number of studies that specify AL as an outcome, this review likely identified them all. Therefore, the review will be instrumental for future research examining AL. In addition, several of the interventions in this review have prior evidence of effectiveness in stress reduction, antipsychotics in schizophrenia (Gispen-de Wied, 2000), CBT (Bryant et al., 1998), Tai chi and a whole foods diet, indicating that observed effect sizes on AL are likely to be valid.
Conclusion
This scoping review was the first to examine the use of a cumulative allostatic load index as an intervention outcome. While earlier literature has explored the measurement and conceptual validity of allostatic load, the cumulative ‘wear and tear’ of chronic stress on the body, its utility as an intervention outcome has been heretofore unknown. This study shows that a cumulative allostatic load index is sensitive to interventions. This has significant clinical and research implications. Measurement of chronic stress and its impact on health is immensely challenging, as there are numerous confounding individual and environmental factors. The use of allostatic load as an intervention outcome is a promising solution.
Footnotes
Impact on the field
This work contributes significantly to the field because it is the first to present collated evidence in support of the fact that allostatic load is amenable to change and is responsive to interventions. This finding is promising for future work aimed at improving how the body responds to chronic stress exposure.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arksey H, & O’Malley L. (2005). Scoping studies: towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- Beckie TM (2012). A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing, 14(4), 311–346. 10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- Berger M, Juster R-P, Westphal S, Amminger GP, Bogerts B, Schiltz K, Bahn S, Steiner J, & Sarnyai Z. (2018). Allostatic load is associated with psychotic symptoms and decreases with antipsychotic treatment in patients with schizophrenia and first-episode psychosis. Psychoneuroendocrinology, 90, 35–42. 10.1016/j.psy-neuen.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG, Dang ST, Sackville T, & Basten C. (1998). Treatment of acute stress disorder: A comparison of cognitive-behavioral therapy and supportive counseling. Journal of Consulting and Clinical Psychology, 66(5), 862–866. 10.1037/0022-006X.66.5.862 [DOI] [PubMed] [Google Scholar]
- Campbell M, Katikireddi SV, Hoffmann T, Armstrong R, Waters E, & Craig P. (2018). TIDieR-PHP: a reporting guideline for population health and policy interventions. BMJ (Clinical Research ed.), 361, k1079 10.1136/bmj.k1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R, Nicassio P, & Irwin MR (2015). Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: Pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology, 55, 184–192. 10.1016/j.psy-neuen.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun HL, Levac D, O’Brien KK, Straus S, Tricco AC, Perrier L, Kastner M, & Moher D. (2014). Scoping reviews: Time for clarity in definition, methods, and reporting. Journal of Clinical Epidemiology, 67(12), 1291–1294. 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Fava GA, Guidi J, Semprini F, Tomba E, & Sonino N. (2010). Clinical assessment of allostatic load and clinimetric criteria. Psychotherapy and Psychosomatics, 79(5), 280–284. 10.1159/000318294 [DOI] [PubMed] [Google Scholar]
- Fava G, Tomba E, & Sonino N. (2012). Clinimetrics: The science of clinical measurements. International Journal of Clinical Practice, 66(1), 11–15. 10.1111/j.1742-1241.2011.02825.x [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J. (2006). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States”. American Journal of Public Health, 96(5), 826–833. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen-de Wied CC (2000). Stress in schizophrenia: An integrative view. European Journal of Pharmacology, 405(1–3), 375–384. 10.1016/S0014-2999(00)00567-7 [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan A-W, & Michie S. (2014). Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clinical Research ed.), 348, g1687 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, Stein K, Sutcliffe K, Ravaud P, Altman DG, Perera R, & Glasziou P. (2017). Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ (Clinical Research ed.), 358, j2998 10.1136/bmj.j2998 [DOI] [PubMed] [Google Scholar]
- Johnson SC, Cavallaro FL, & Leon DA (2017). A systematic review of allostatic load in relation to socioeconomic position: Poor fidelity and major inconsistencies in biomarkers employed. Social Science & Medicine (1982), 192, 66–73. 10.1016/j.socscimed.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Juster R-P, Sindi S, Marin M-F, Perna A, Hashemi A, Pruessner JC, & Lupien SJ (2011). A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology, 36(6), 797–805. 10.1016/j.psyneuen.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, & Seeman TE (2006). Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic Medicine, 68(3), 500–507. 10.1097/01.psy.0000221270.93985.82 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, & Seeman TE (2002). Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. Journal of Clinical Epidemiology, 55(7), 696–710. 10.1016/S0895-4356(02)00399-2 [DOI] [PubMed] [Google Scholar]
- Li Y, Rosemberg M-AS , Dalton VK, Lee SJ, & Seng JS (2019). Exploring the optimal allostatic load scoring method in women of reproductive age. Journal of Advanced Nursing, 75(11), 2548–2558. 10.1111/jan.14014 [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–917. 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss D, Jarczok MN, & Fischer JE (2016). The streamlined Allostatic Load Index: A replication of study results. Stress, 19(6), 553–558. 10.1080/10253890.2016.1219718 [DOI] [PubMed] [Google Scholar]
- Mauss D, Li J, Schmidt B, Angerer P, & Jarczok MN (2015). Measuring allostatic load in the workforce—A systematic review. Industrial Health, 53(1), 5–122. 10.2486/indhealth.20140122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain AC, Xiao RS, Gao X, Tucker KL, Falcon LM, & Mattei J. (2018). Food insecurity and odds of high allostatic load in Puerto Rican adults: The role of participation in the Supplemental Nutrition Assistance Program during 5 years of follow-up. Psychosomatic Medicine, 80(8), 733–741. 10.1097/PSY.0000000000000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. (2000). Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology: Neuropsychopharmacology, 22(2), 108–124 10.1016/S0893-133X(99)00129-3 [DOI] [PubMed] [Google Scholar]
- McEwen B. (2000). Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochemical Research, 25(9–10), 1219–1231. [DOI] [PubMed] [Google Scholar]
- McEwen B. (2002). Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of Aging, 23(5), 921–939. 10.1016/S0197-4580(02)00027-1 [DOI] [PubMed] [Google Scholar]
- McEwen B. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032, 1–7. 10.1196/annals.1314.001 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS (2019). From serendipity to clinical relevance: How clinical psychology and neuroscience converged to illuminate psychoneuroendocrinology. Psychoneuroendocrinology, 105, 36–43. 10.1016/j.psyneuen.2018.09.011 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Wingfield JC (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- McEwen B, & Seeman T. (1999). Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences, 896(1), 30–47. 10.1111/j.1749-6632.1999.tb08103.x [DOI] [PubMed] [Google Scholar]
- McEwen B, & Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- McEwen B, & Wingfield JC (2010). Allostasis and allostatic load In Encyclopedia of stress. Elsevier; (p. 135–141). [Google Scholar]
- Nuño V, Siu A, Deol N, & Juster R-P (2019). Osteopathic manipulative treatment for allostatic load lowering. The Journal of the American Osteopathic Association, 119(10), 646–654. 10.7556/jaoa.2019.112 [DOI] [PubMed] [Google Scholar]
- Ottino-Gonzalez J, Jurado MA, Garcıa-Garcıa I, Caldu X, PratsSoteras X, Tor E, Sender-Palacios MJ, & Garolera M. (2019). Allostatic load and executive functions in overweight adults. Psychoneuroendocrinology, 106, 165–170. 10.1016/j.psyneuen.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang M-H, Singer B, Bucur A, Gruenewald T, Berkman LF, & Reuben DB (2004). Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine (1982)), 58(10), 1985–1997. 10.1016/S0277-9536(03)00402-7 [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, & Weinstein M. (2005). A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology, 40(5), 438–449. 10.1016/j.exger.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, & McEwen BS (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA, 301(21), 2252–2259. 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Soltani H, Keim NL, & Laugero KD (2018). Diet quality for sodium and vegetables mediate effects of whole food diets on 8-week changes in stress load. Nutrients, 10(11), 1606 10.3390/nu10111606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna B, Suvarna A, Phillips R, Juster R-P, McDermott B, & Sarnyai Z. (2020). Health risk behaviours and allostatic load: A systematic review. Neuroscience and Biobehavioral Reviews, 108, 694–711. 10.1016/j.neubiorev.2019.12.020 [DOI] [PubMed] [Google Scholar]
- Vie TL, Hufthammer KO, Holmen TL, Meland E, & Breidablik HJ (2014). Is self-rated health a stable and predictive factor for allostatic load in early adulthood? Findings from the Nord Trøndelag Health Study (HUNT). Social Science & Medicine, 117, 1–9. 10.1016/j.socscimed.2014.07.019 [DOI] [PubMed] [Google Scholar]
- von Kanel R. (2003). Acute procoagulant stress response as a dynamic measure of allostatic load in Alzheimer caregivers. Annals of Behavioral Medicine, 26(1), 42–48. [DOI] [PubMed] [Google Scholar]
- von Thiele U, Lindfors P, & Lundberg U. (2006). Self-rated recovery from work stress and allostatic load in women. Journal of Psychosomatic Research, 61(2), 237–242. 10.1016/j.jpsychores.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Ye ZJ, Qiu HZ, Liang MZ, Liu ML, Li PF, Chen P, Sun Z, Yu YL, Wang SN, Zhang Z, Liao KL, Peng CF, Huang H, Hu GY, Zhu YF, Zeng Z, Hu Q, & Zhao JJ (2017). Effect of a mentor-based, supportive-expressive program, Be Resilient to Breast Cancer, on survival in metastatic breast cancer: a randomised, controlled intervention trial. British Journal of Cancer, 117(10), 1486–1494. 10.1038/bjc.2017.325 [DOI] [PMC free article] [PubMed] [Google Scholar]