Abstract
Aims
This post hoc analysis of ELIMINATE-AF evaluated requirements of unfractionated heparin (UFH) and procedure-related bleeding in atrial fibrillation (AF) patients undergoing ablation with uninterrupted edoxaban or vitamin K antagonist (VKA) therapy.
Methods and results
Patients were randomized 2:1 to once-daily edoxaban 60 mg (or dose-reduced 30 mg) or dose-adjusted VKA (target international normalized ratio: 2.0–3.0). Uninterrupted anticoagulation was mandated for 21–28 days’ pre-ablation and 90 days’ post-ablation. During ablation, UFH administration targeted an activated clotting time (ACT) of 300–400 s. Periprocedural bleeding was differentiated between procedure-related (bleeding at puncture side, cardiac tamponade) and unrelated events. Of 614 randomized patients, 553 received study drug and underwent catheter ablation (edoxaban n = 375; VKA n = 178). The median (Q1–Q3) time from last dose to ablation procedure was 14.8 (13.3–16.5) vs. 16.5 (14.8–19.5) h (edoxaban vs. VKA group, respectively). Mean ACT (SD) ≥300 s was observed in 52% edoxaban- vs. 76% VKA-treated patients, despite a higher mean (SD) UFH dose in the edoxaban vs. VKA group [14 261 (6397) IU vs. 11 473 (4300) IU; exploratory P-value < 0.0001]. In the edoxaban group, 13 patients (3.5%) had procedure-related bleeds of whom 9 had received an UFH dose above the median (13 000 IU). In the VKA arm, 7 patients (3.9%) had procedure-related bleeds of whom 3 had received an UFH dose above the median (10 225 IU).
Conclusion
The rate of procedure-related major/clinically relevant non-major bleeding did not differ between the treatment arms despite higher doses of UFH used with edoxaban vs. VKA to achieve a target ACT during AF ablation.
Keywords: Edoxaban, Non-vitamin K antagonist oral anticoagulants, Anticoagulant, Atrial fibrillation, Ablation, Periprocedural anticoagulation
What’s new?
This post hoc analysis of ELIMINATE-AF showed that patients anticoagulated with edoxaban during atrial fibrillation ablation received a higher dose of unfractionated heparin (UFH) to achieve recommended level of activated clotting time (ACT) than those treated with vitamin K antagonist.
Despite the higher UFH dose, the mean ACT was lower in patients treated with edoxaban.
The number of patients with procedure-related major/clinically relevant non-major bleeding did not differ between the treatment arms despite higher doses of UFH used in combination with edoxaban.
Introduction
Catheter ablation of atrial fibrillation (AF) is the most frequently performed ablation procedure world-wide and constitutes an effective treatment modality. However, the procedure can entail serious complications, including stroke, transient ischaemic attack, and cardiac tamponade.1 To minimize these complications, current guidelines recommend continuation of oral anticoagulation with vitamin K antagonists (VKAs) or non-VKA oral anticoagulants (NOACs) during the procedure, maintaining effective anticoagulation.2 Four randomized controlled trials compared uninterrupted VKA vs. uninterrupted NOAC therapy in patients undergoing AF ablation.3–6 Each of these trials demonstrated low rates of ischaemic and bleeding events in patients treated with uninterrupted NOAC therapy. These results have prompted the guideline recommendation to perform AF ablation without interruption of warfarin or NOAC therapy.2 The guidelines also recommend systemic anticoagulation with unfractionated heparin (UFH) during the ablation procedure to achieve an activated clotting time (ACT) of >300 s to avoid procedure-related thromboembolic events.1,2 Recent observations indicate that in order to achieve this target, heparin requirements may differ in patients receiving different NOACs compared to VKA.7–11 While Calkins et al.10 demonstrated that the amount of UFH required in subjects treated with the factor II inhibitor dabigatran or warfarin was similar, mean UFH doses tended to be higher in patients receiving a factor Xa inhibitor.3,5
This post hoc analysis of the ELIMINATE-AF trial evaluated the correlation between UFH doses and ACT in patients undergoing AF ablation on an uninterrupted edoxaban- or VKA-based anticoagulation regimen. Particularly, the relationship between UFH doses and periprocedural major or clinically relevant non-major (CRNM) bleeding events was evaluated in detail.
Methods
Study design
The design, methodology, and primary results of ELIMINATE-AF have been previously reported in detail.6,12 In brief, ELIMINATE-AF (ClinicalTrials.gov: NCT02942576) was a multinational, multicentre, randomized, open-label, parallel-group, blinded-endpoint evaluation (PROBE) study. The protocol was approved by the institutional review board or independent ethics committee at each participating study centre. All patients provided written informed consent prior to enrolment. Adult patients (≥18 years of age) with documented non-valvular AF (paroxysmal, ≤7 days; persistent, >7 days but ≤12 months; long-standing persistent, >12 months) scheduled for their first or repeated catheter ablation for AF were eligible. Patients were randomized in a 2:1 ratio to receive edoxaban or VKA using a block randomization method. Patients randomized to edoxaban received once-daily edoxaban 60 mg (30 mg if they met 1 or more of the criteria for dose reduction).12 The preferred VKA varied across countries (phenprocoumon in Germany and Belgium, acenocoumarol in Spain, and warfarin in Belgium and in all other countries). Patients randomized to a VKA were required to maintain an international normalized ratio of 2.0–3.0 at least for the last 10 days prior to ablation.
Uninterrupted anticoagulation was mandated for 21–28 days pre-ablation and continued for 90 days post-ablation. The maximum interval between the last pre-ablation edoxaban dose and the ablation procedure was 18 h in order to maintain sufficient inhibition of endogenous factor Xa. During ablation, UFH was used according to contemporary guideline recommendations1,2 targeting an ACT of 300–400 s. The first dose of UFH was given after sheath placement before or immediately after the transseptal puncture. For the duration of the ablation when catheters were in the left atrium, investigators were instructed to maintain an ACT ≥300 s by administering repeated boluses of UFH. Activated clotting time was assessed within 15 min after the administration of the bolus dose and regularly thereafter. Study medication was restarted at least 6 h post-sheath removal after achieving adequate haemostasis.
Parameters of coagulation were correlated with major or CRNM bleeding events (ISTH definition). For this purpose, only those events were considered which occurred from start of ablation up to 48 h after the end of the ablation procedure. Periprocedural bleeding was differentiated between procedure-related (bleeding at puncture side and cardiac tamponade) and unrelated events.
Statistical analysis
The administered heparin doses and ACT values in both treatment groups are presented descriptively. All P-values for group comparisons are exploratory.
Results
Patient population
A total of 632 patients were enrolled at 58 sites across 11 countries in Europe, Asia, and Canada. Of these, 614 patients were randomized, and 602 received at least one dose of study medication. A total of 553 subjects (375 in the edoxaban group and 178 in the VKA group) received study drug and underwent catheter ablation. The baseline characteristics of the two patient groups were well balanced (Table 1) and represented a typical AF ablation population with a male preponderance and a mean age of 60.5 years. The median (Q1–Q3) time interval between the last intake of study medication and start of the ablation procedure was 14.8 (13.3–16.5) h in the edoxaban group vs. 16.5 (14.8–19.5) h in the VKA group.
Table 1.
Patient characteristics
| Total | Edoxaban | VKA | |
|---|---|---|---|
| ITT analysis set | N = 614 | N = 411 | N = 203 |
| Age (years) | 60.5 (53–67) | 60.0 (53–67) | 61.0 (52–67) |
| Male, n (%) | 439 (71.5) | 290 (70.6) | 149 (73.4) |
| CHA2DS2-VASc score | |||
| Mean (SD) | 1.7 (1.5) | 1.8 (1.6) | 1.7 (1.4) |
| Median (Q1–Q3) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–2.0) |
| Medical history, n (%) | |||
| Congestive heart failure | 110 (17.9) | 71 (17.3) | 39 (19.2) |
| Previous CAD (prior MI, prior PCI, or prior CABG) | 117 (19.2) | 76 (18.6) | 41 (20.3) |
| Previous MI | 24 (3.9) | 19 (4.6) | 5 (2.5) |
| Previous stroke/TIAa | 30 (4.9) | 22 (5.4) | 8 (3.9) |
| Peripheral artery disease | 10 (1.6) | 7 (1.7) | 3 (1.5) |
| Diabetes mellitus | 87 (14.2) | 55 (13.4) | 32 (15.8) |
| Hypertension | 371 (60.4) | 250 (60.8) | 121 (59.6) |
| Mild valvular heart disease | 52 (8.5) | 32 (7.8) | 20 (9.9) |
| AF type, n (%) | |||
| Paroxysmal | 415 (67.6) | 284 (69.1) | 131 (64.5) |
| Persistent | 166 (27.0) | 105 (25.5) | 61 (30.0) |
| Long-standing persistent | 33 (5.4) | 22 (5.4) | 11 (5.4) |
| Ablation population, mITT with ablation analysis set | N = 553 | N = 375 | N = 178 |
| Last dose of IP to start of CA procedure (h) | |||
| Median (Q1–Q3) | 15.4 (13.5–17.1) | 14.8 (13.3–16.5) | 16.5 (14.8–19.5) |
| Sheath removal to next dose of IP (h) | |||
| Median (Q1–Q3) | 6.7 (6.1–8.0) | 6.7 (6.1–8.0) | 6.6 (6.1–7.9) |
| Type of CA performed, n (%) | |||
| De novo CA | 460 (83.2) | 315 (84.0) | 145 (81.5) |
| Re-do CA | 93 (16.8) | 60 (16.0) | 33 (18.5) |
| CA technique used, n (%) | |||
| Radiofrequency | 368 (66.7) | 247 (65.9) | 121 (68.4) |
| Cryoballoon | 181 (32.8) | 127 (33.9) | 54 (30.5) |
| Other | 3 (0.5) | 1 (0.3) | 2 (1.1) |
| Percentage time in TTR (2.0–3.0) with VKA | |||
| N | 178 | ||
| Mean (SD) | 64.1 (21.1) | ||
| Median | 64.8 | ||
| Q1–Q3 | 48.3–82.1 | ||
| Percentage time in TTR (1.8–3.2) with VKA | |||
| N | 178 | ||
| Mean (SD) | 80.9 (17.0) | ||
| Median | 84.7 | ||
| Q1–Q3 | 70.8–95.2 |
CA, catheter ablation; CABG, coronary artery bypass grafting; CAD, coronary artery disease; IP, investigational product; ITT, intent to treat; mITT, modified intent to treat; PCI, percutaneous coronary intention; TIA, transient ischaemic attack; TTR, time in therapeutic range; VKA, vitamin K antagonist.
Includes ischaemic, embolic, and undetermined; haemorrhagic stroke prohibited.
Heparin dosing and activated clotting time measurements
Data on UFH use on the day of ablation were collected in 552 patients, 374 assigned to edoxaban therapy and 178 assigned to receive VKA. The median (Q1–Q3) UFH dose administered was higher in the edoxaban group than in the VKA group (Table 2). Heparin dosing tended to be lower in patients with a first ACT measurement of ≥300 vs. <300 s for all patients (Table 2).
Table 2.
Heparin dose requirements in patients with ACT <300 s vs. ≥300 s
| Heparin dose (IU) | Total (N = 553) | Edoxaban (N = 375) | VKA (N = 178) |
|---|---|---|---|
| N | 552 | 374 | 178 |
| Mean (SD) | 13 361.8 (5945.3) | 14 260.9 (6397.2) | 11 472.6 (4300.4) |
| Median | 12 301 | 13 000 | 10 225 |
| Q1–Q3 | 10 000–16 000 | 10 000–17 500 | 8541–14 000 |
| First ACT | |||
| <300 s | |||
| N | 256 | 195 | 61 |
| Mean (SD) | 14 679.6 (6510.8) | 15 423.0 (6766.6) | 12 303.2 (4957.6) |
| Median | 14 000 | 14 250 | 12 000 |
| Q1–Q3 | 10 000–18 000 | 11 000–18 000 | 9000–15 500 |
| ≥300 s | |||
| N | 292 | 176 | 116 |
| Mean (SD) | 12 264.4 (5136.7) | 13 051.0 (5697.9) | 11 070.0 (3869.7) |
| Median | 11 000 | 12 500 | 10 000 |
| Q1–Q3 | 9000–15 000 | 10 000–15 875 | 8520–13 000 |
| Minimum ACT | |||
| <300 s | |||
| N | 417 | 301 | 116 |
| Mean (SD) | 13 737.5 (6138.9) | 14 515.9 (6445.5) | 11 717.8 (4716.6) |
| Median | 12 500 | 13 000 | 10 500 |
| Q1–Q3 | 10 000–17 000 | 10 000–18 000 | 8400–15 000 |
| ≥300 s | |||
| N | 131 | 70 | 61 |
| Mean (SD) | 12 295.1 (5113.1) | 13 361.5 (6073.5) | 11 071.4 (3372.7) |
| Median | 12 000 | 12 500 | 10 250 |
| Q1–Q3 | 9000–14 500 | 10 000–15 750 | 9000–13 000 |
| Maximum ACT | |||
| <300 s | |||
| N | 50 | 43 | 7 |
| Mean (SD) | 12 265.2 (5131.7) | 12 889.8 (5184.4) | 8428.6 (2636.7) |
| Median | 11 000 | 11 720 | 8000 |
| Q1–Q3 | 10 000–15 000 | 10 000–16 000 | 8000–9000 |
| ≥300 s | |||
| N | 498 | 328 | 170 |
| Mean (SD) | 13 505.9 (6005.2) | 14 482.7 (6510.2) | 11 621.3 (4315.4) |
| Median | 12 500 | 13 000 | 10 800 |
| Q1–Q3 | 10 000–16 000 | 10 000–18 000 | 9000–14 000 |
| Mean ACT | |||
| <300 s | |||
| N | 219 | 177 | 42 |
| Mean (SD) | 13 833.8 (6432.1) | 14 427.3 (6689.9) | 11 332.7 (4463.9) |
| Median | 12 500 | 13 000 | 10 000 |
| Q1–Q3 | 10 000–16 331 | 10 000–17 000 | 8000–13 000 |
| ≥300 s | |||
| N | 329 | 194 | 135 |
| Mean (SD) | 13 099.0 (5574.7) | 14 180.1 (6108.6) | 11 545.5 (4266.4) |
| Median | 12 000 | 13 000 | 10 600 |
| Q1–Q3 | 10 000–16 000 | 10 000–17 500 | 9000–14 000 |
ACT, activated clotting time; IU, international units; SD, standard deviation.
The median (Q1–Q3) ACT over the course of the ablation was lower in the edoxaban group than in the VKA group (Table 3; exploratory P-value <0.0001). An individual mean ACT of ≥300 s over the course of the procedure was achieved in 52% of patients in the edoxaban group vs. 76% in the VKA group. Median (Q1–Q3) heparin dosing was similar in patients with mean ACT measurement of <300 vs. ≥300 s (Table 3). In the edoxaban treatment group, the first ACT was ≥300 s for 47% and in the VKA group for 66% of patients (exploratory P < 0.0001; Table 4).
Table 3.
Overview of ACT
| Total (N = 553) | Edoxaban (N = 375) | VKA (N = 178) | |
|---|---|---|---|
| Individual mean ACT (s) | |||
| N | 548 | 371 | 177 |
| Mean (SD) | 314.2 (51.4) | 302.8 (41.6) | 338.0 (61.2) |
| Median | 307.7 | 301.4 | 332.6 |
| Q1–Q3 | 281.6–341.5 | 277.0–330.4 | 300.5–371.0 |
| Mean ACT categories, n (%) | |||
| <300 s | 219 (40.0) | 177 (47.7) | 42 (23.7) |
| ≥300 s | 329 (60.0) | 194 (52.3) | 135 (76.3) |
| Individual first ACT (s) | |||
| N | 548 | 371 | 177 |
| Mean (SD) | 307.3 (89.5) | 293.9 (76.7) | 335.2 (106.7) |
| Median | 304.0 | 293.0 | 348.0 |
| Q1–Q3 | 250.0–365.0 | 246.0–350.0 | 256.0–400.0 |
| First ACT categories, n (%) | |||
| <300 s | 256 (46.7) | 195 (52.6) | 61 (34.5) |
| ≥300 s | 292 (53.3) | 176 (47.4) | 116 (65.5) |
| Individual minimum ACT (s) | |||
| N | 548 | 371 | 177 |
| Mean (SD) | 246.3 (75.8) | 240.3 (65.3) | 258.8 (93.0) |
| Median | 240.0 | 240.0 | 246.0 |
| Q1–Q3 | 182.0–296.5 | 187.0–284.0 | 179.0–320.0 |
| Minimum ACT categories, n (%) | |||
| <300 s | 417 (76.1) | 301 (81.1) | 116 (65.5) |
| ≥300 s | 131 (23.9) | 70 (18.9) | 61 (34.5) |
| Individual maximum ACT (s) | |||
| N | 548 | 371 | 177 |
| Mean (SD) | 365.8 (65.2) | 350.7 (53.7) | 397.5 (75.2) |
| Median | 361.0 | 351.0 | 390.0 |
| Q1–Q3 | 328.5–395.0 | 317.0–376.0 | 357.0–426.0 |
| Maximum ACT categories, n (%) | |||
| <300 s | 50 (9.1) | 43 (11.6) | 7 (4.0) |
| ≥300 s | 498 (90.9) | 328 (88.4) | 170 (96.0) |
ACT, activated clotting time; SD, standard deviation.
Table 4.
ACT and heparin dose according to the time from the last dose of study medication edoxaban/VKA to start of the ablation procedure (for sheath insertion and trans-septal puncture)
| CA procedure performed, n (%) | Time from edoxaban dose to septal puncture (N = 375, 100%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 to <8 h | 8 to <16 h | 16 to <24 h | ≥24 h | Total | ||||
| N (%) | 3 (0.8) | 248 (66.1) | 118 (31.5) | 6 (1.6) | 375 (100.0) | |||
| Heparin dose (IU) | ||||||||
| N | 3 | 248 | 117 | 6 | 374 | |||
| Mean (SD) | 14 300.0 (2696.3) | 14 280.8 (6428.4) | 14 262.4 (6477.0) | 13 388.8 (5865.7) | 14 260.9 (6397.2) | |||
| Median | 13 000.0 | 13 000.0 | 12 500.0 | 12 750.0 | 13 000.0 | |||
| Q1–Q3 | 12 500.0–17 400.0 | 10 000.0–17 750.0 | 10 000.0–17 000.0 | 8000.0–19 000.0 | 10 000.0–17 500.0 | |||
| First ACT, n (%) | ||||||||
| 0 to <100 s | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 100 to <200 s | 0 (0.0) | 32 (12.9) | 7 (6.1) | 1 (20.0) | 40 (10.8) | |||
| 200 to <300 s | 0 (0.0) | 104 (41.9) | 49 (42.6) | 2 (40.0) | 155 (41.8) | |||
| ≥300 s | 3 (100.0) | 112 (45.2) | 59 (51.30) | 2 (40.0) | 176 (47.4) | |||
| Total | 3 (100.0) | 248 (100.0) | 115 (100.0) | 5 (100.0) | 371 (100.0) | |||
| Mean ACT, n (%) | ||||||||
| 0 to <100 s | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 100 to <200 s | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 200 to <300 s | 1 (33.3) | 127 (51.2) | 46 (40.0) | 3 (60.0) | 177 (47.7) | |||
| ≥300 s | 2 (66.7) | 121 (48.8) | 69 (60.0) | 2 (40.0) | 194 (52.3) | |||
| Total | 3 (100.0) | 248 (100.0) | 115 (100.0) | 5 (100.0) | 371 (100.0) | |||
| Time from VKA dose to septal puncture (N = 178, 100%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 to <8 h | 8 to <16 h | 16 to <24 h | ≥24 h | Total | ||||
| N (%) | 2 (1.1) | 77 (43.3) | 70 (39.3) | 29 (16.3) | 178 (100.0) | |||
| Heparin dose (IU) | ||||||||
| N | 2 | 77 | 70 | 29 | 178 | |||
| Mean (SD) | 11 197.5 (5377.6) | 11 000.5 (4468.9) | 10 767.6 (3517.2) | 14 446.6 (4490.0) | 11 472.6 (4300.4) | |||
| Median | 11 197.5 | 10 000.0 | 10 000.0 | 14 000.0 | 10 225.0 | |||
| Q1–Q3 | 7395.0–15 000.0 | 8000.0–12 500.0 | 8500.0–12 500.0 | 12 000.0–16 000.0 | 8541.0–14 000.0 | |||
| First ACT, n (%) | ||||||||
| 0 to <100 s | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 100 to <200 s | 0 (0.0) | 13 (16.9) | 7 (10.0) | 4 (14.3) | 24 (13.6) | |||
| 200 to <300 s | 0 (0.0) | 13 (16.9) | 15 (21.4) | 9 (32.1) | 37 (20.9) | |||
| ≥300 s | 2 (100.0) | 51 (66.2) | 48 (68.6) | 15 (53.6) | 116 (65.5) | |||
| Total | 2 (100.0) | 77 (100.0) | 70 (100.0) | 28 (100.0) | 177 (100.0) | |||
| Mean ACT, n (%) | ||||||||
| 0 to <100 s | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 100 to <200 s | 0 (0.0) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |||
| 200 to <300 s | 0 (0.0) | 18 (23.4) | 14 (20.0) | 9 (32.1) | 41 (23.2) | |||
| ≥300 s | 2 (100.0) | 58 (75.3) | 56 (80.0) | 19 (67.9) | 135 (76.3) | |||
| Total | 2 (100.0) | 77 (100.0) | 70 (100.0) | 28 (100.0) | 177 (100.0) | |||
ACT, activated clotting time; IU, international units; SD, standard deviation; VKA, vitamin K antagonist.
Periprocedural anticoagulation and bleeding events
Between sheath insertion and up to 48 h after sheath removal, major, or CRNM bleeding events occurred in 17 (4.5%) edoxaban- compared with 7 (3.9%) VKA-treated patients (Table 5). In the edoxaban group, 11 of the 17 patients (65%) with bleeding events had received an UFH dose above the median (13 000 IU) while in the VKA arm, 3 of 7 patients (43%) with bleeding events had received an UFH dose above the median (10 225 IU) (Table 5). Procedure-related (bleeding at puncture site and cardiac tamponade) major or CRNM bleeding events occurred in 13 (3.5%) edoxaban- vs. 7 (3.9%) VKA-treated patients (Table 6). In the edoxaban group, 9 of 13 patients with procedure-related bleeds had received an UFH dose above the median (13 000 IU) while in the VKA arm, 3 of 7 patients with procedure-related bleedings received an UFH dose above the median (10 225 IU) (Figure 1).
Table 5.
Major or CRNM bleeding events (ISTH) occurring from start of ablation up to 48 h after the end of the ablation procedure
| Total (N = 553), n (%) | Edoxaban (N = 375), n (%) | VKA (N = 178), n (%) | |
|---|---|---|---|
| Major or CRNM bleeding (ISTH) | 24 (4.3) | 17 (4.5) | 7 (3.9) |
| Heparin dose < median of treatment arma | 6 | 4 | |
| Heparin dose ≥ median of treatment arma | 11 | 3 | |
| Major bleeding (ISTH) | 10 (1.8) | 7 (1.9) | 3 (1.7) |
| Heparin dose < median of treatment arma | 2 | 1 | |
| Heparin dose ≥ median of treatment arma | 5 | 2 |
CRNM, clinically relevant non-major bleeding; ISTH, International Society on Thrombosis and Haemostasis.
Median heparin dose was 13 000 IU in edoxaban arm and 10 225 IU in VKA arm.
Table 6.
Procedure-related major or CRNM bleeding events (ISTH) occurring from start of ablation up to 48 h after the end of the ablation procedure
| Total (N = 553), n (%) | Edoxaban (N = 375), n (%) | VKA (N = 178), n (%) | |
|---|---|---|---|
| Major or CRNM bleeding (ISTH) | 20 (3.6) | 13 (3.5) | 7 (3.9) |
| Heparin dose < median of treatment arma | 4 | 4 | |
| Heparin dose ≥ median of treatment arma | 9 | 3 | |
| Major bleeding (ISTH) | 9 (1.6) | 6 (1.6) | 3 (1.7) |
| Heparin dose < median of treatment arma | 1 | 1 | |
| Heparin dose ≥ median of treatment arma | 5 | 2 |
CRNM, clinically relevant non-major bleeding; ISTH, International Society on Thrombosis and Haemostasis; IU, international units.
Median heparin dose was 13 000 IU in edoxaban arm and 10 225 IU in VKA arm.
Figure 1.
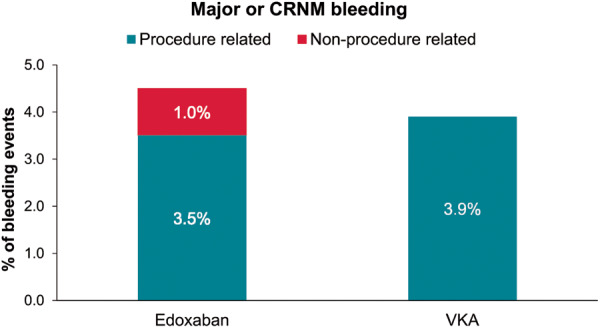
Procedure- and non-procedure-related major or CRNM bleeding events (ISTH) occurring from start of ablation up to 48 h after the end of the ablation procedure. CRNM, clinically relevant non-major; ISTH, International Society on Thrombosis and Haemostasis; VKA, vitamin K antagonist.
Discussion
The present post hoc analysis of the ELIMINATE-AF trial shows that patients assigned to edoxaban during AF ablation procedure received a higher dose of UFH than those treated with VKA. Despite the higher administered UFH dose, the mean ACT was lower in the group of subjects treated with edoxaban. The number of patients with major or CRNM bleeding events related to the ablation procedure did not differ between the treatment arms despite higher doses of UFH used in combination with edoxaban. Of note, in the edoxaban group, 9 of 13 major or CRNM bleeding events occurred in patients who received an UFH dose above the median.
Our observations confirm those reported from other studies using uninterrupted factor Xa therapy around the time of AF ablation and extend these findings specifically to patients anticoagulated with edoxaban. For instance, in the VENTURE-AF study, the average total heparin dose used to manage ACT in patients undergoing catheter ablation for AF was higher in patients receiving rivaroxaban vs. those receiving VKA (13.871 vs. 10.964 units; P < 0.001).3 Similarly, a larger dose of UFH was required with uninterrupted apixaban compared with VKA to achieve the target ACT ≥300 s, and the ACT during the procedure was lower (342.1 ± 23.1 vs. 363 ± 26.5 s, P < 0.001) with apixaban.13 The comparable observation was made in the AXAFA-AFNET 5 trial.5 In contrast, a sub-analysis of the RE-CIRCUIT trial has recently shown that a similar amount of UFH was needed in patients anticoagulated with dabigatran vs. those treated with warfarin to achieve an ACT of >300 s during ablation.10 These studies, however, did not report on bleeding events in relation to UFH dose or ACT.
The reason for this difference in UFH requirements in patients’ anticoagulated with different NOACs is not entirely clear.11 A likely explanation could be that dabigatran, as a direct thrombin inhibitor, can prolong activated partial thromboplastin time (aPTT) and ACT in a dose-dependent manner, similar to VKAs.14 Factor Xa inhibitors, on the other hand, may affect ACT or aPTT to a lesser degree.11 Our observations are in line with other reports which also found shorter baseline ACT in patients on edoxaban therapy.15 This suggests that patients treated with edoxaban, rivaroxaban, or apixaban are expected to need higher doses of UFH to maintain ACT. It has therefore been speculated that use of ACT to reflect global intraprocedural anticoagulation is limited in the presence of uninterrupted NOAC therapy, particularly when using factor Xa inhibitors.11
In almost all patients in the edoxaban arm, the time interval between the last edoxaban dose to septal puncture was <24 h, hence anticoagulation was truly uninterrupted (Table 4). The findings from this analysis attest to the safety of an uninterrupted NOAC strategy in patients undergoing AF ablation. Specifically, the low incidence of major or CRNM bleeding endpoints in the edoxaban group despite higher doses of UFH used, is reassuring.
The present analysis has some limitations. Among those is the relatively small sample size, residual bias due to the non-blinded nature of the study, and other inherent inadequacies of post hoc analyses. In addition, it is important to emphasize that the occurrence of vascular access bleeding after the ablation procedure may not depend only on anticoagulation drugs but also on multiple other factors such as the mode of puncture (e.g. ultrasound-guided vs. landmark-guided), sheath management after the procedure (e.g. immediate removal with Z stitch vs. delayed removal with compression) or the use of protamine.
Conclusions
The present analysis of the ELIMINATE-AF trial shows that considerably higher doses of UFH were needed with edoxaban vs. VKA to achieve a target ACT during AF ablation. Nevertheless, the number of patients with major or CRNM bleeding events related to the ablation procedure did not differ between treatments despite higher doses of UFH used in combination with edoxaban. However, in the edoxaban group, 9/13 major or CRNM bleeding events occurred in patients who received an UFH dose above the median. Careful post-operative surveillance appears to be indicated in patients in whom higher UFH doses had been administered during ablation.
Funding
This study was supported by Daiichi Sankyo Europe GmbH, Munich, Germany. Editorial support was provided by Shelley Narula from inScience Communications, Springer Healthcare Ltd, UK, and was funded by Daiichi Sankyo Europe GmbH, Munich, Germany in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Conflict of interest: S.H.H. reports personal fees from Bayer Healthcare, BI, BMS, Daiichi Sankyo, Medtronic, Pfizer, SJM, and Zoll, outside the submitted work. J.C. has received personal fees for attending advisory committees and lecturing and his institution has received research grants from Daiichi Sankyo, and for work outside this project has received personal payments and institutional grants from Bayer, Boehringer Ingelheim, Pfizer/BMS, and Portola. R.C. reports research grants from and was a speaker consultant for Boston Scientific, Bayer, Medtronic, Abbott, St. Jude Medical, Pfizer, Daiichi Sankyo, Boehringer Ingelheim, Biosense Webster, and Johnson & Johnson, during the conduct of this study. H.-C.D. received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from Abbott, Bayer Vital, BMS, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Pfizer, Portola, Sanofi-Aventis, and WebMD Global. Financial support for research projects was provided by Boehringer Ingelheim. H.C.D. received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. H.C.D. served as editor of Neurologie up2date, Info Neurologie & Psychiatrie, Arzneimitteltherapie, as co-editor of Cephalalgia and on the editorial board of Lancet Neurology. H.C.D. chairs the Treatment Guidelines Committee of the German Society of Neurology and contributed to the EHRA and ESC guidelines for the treatment of AF. H.H. has received no personal funding, and discloses institutional support as unconditional research grants from Medtronic, Daiichi Sankyo, Boehringer-Ingelheim, Bayer, Pfizer-BMS, Biotronik, Abbott and Bracco Imaging. Lluís Mont reports personal fees from Daiichi Sankyo. C.A.M. reports grants and personal fees from Bayer; personal fees from Pfizer and Servier; and was on a steering committee for Daiichi Sankyo during the conduct of this work. H.-J.L., H.R., P.-E.R., and R.S. are employees of Daiichi Sankyo Europe GmbH, Munich, Germany. J.K. reports personal fees and other from Daiichi Sankyo, during the conduct of the study; advisory boards, proctoring, and education events and received personal fees from Biosense Webster, personal fees from Boston Scientific; lectures, educational events and personal fees from Biotronik, advisory board, educational events, and personal fees Medtronic, and advisory board and educational fees from Abbott (St. Jude Medical), personal fees from Bayer, Boehringer Ingelheim and Pfizer outside the submitted work.
Data availability
De-identified individual participant data and applicable supporting clinical study documents are available on request, depending on circumstances, at https://vivli.org. In cases in which clinical study data and supporting documents are provided pursuant to the sponsor’s policies and procedures, the sponsor will continue to protect the privacy of the clinical study participants. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo/.
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA. et al. ; TASK Force Members. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS . Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ. et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH. et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A. et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L. et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M. et al. Adequate initial heparin dosage for atrial fibrillation ablation in patients receiving non-vitamin K antagonist oral anticoagulants. Clin Drug Investig 2016;36:837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M. et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagao T, Inden Y, Yanagisawa S, Kato H, Ishikawa S, Okumura S. et al. Differences in activated clotting time among uninterrupted anticoagulants during the periprocedural period of atrial fibrillation ablation. Heart Rhythm 2015;12:1972–8. [DOI] [PubMed] [Google Scholar]
- 10. Calkins H, Willems S, Verma A, Schilling R, Hohnloser SH, Okumura K. et al. Heparin dosing in uninterrupted anticoagulation with dabigatran vs. warfarin in atrial fibrillation ablation: RE-CIRCUIT study. Europace 2019;21:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin AC, Godier A, Narayanan K, Smadja DM, Marijon E.. Management of intraprocedural anticoagulation in patients on non-vitamin K antagonist oral anticoagulants undergoing catheter ablation for atrial fibrillation: understanding the gaps in evidence. Circulation 2018;138:627–33. [DOI] [PubMed] [Google Scholar]
- 12. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Lanz HJ. et al. Uninterrupted administration of edoxaban vs vitamin K antagonists in patients undergoing atrial fibrillation catheter ablation: rationale and design of the ELIMINATE-AF study. Clin Cardiol 2018;41:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Biase L, Lakkireddy D, Trivedi C, Deneke T, Martinek M, Mohanty S. et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm 2015;12:1162–8. [DOI] [PubMed] [Google Scholar]
- 14. van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M. et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–27. [DOI] [PubMed] [Google Scholar]
- 15. Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M. et al. Differences in activated clotting time and initial heparin dosage during atrial fibrillation ablation for patients with edoxaban compared with warfarin. J Cardiovasc Electrophysiol 2018;29:835–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified individual participant data and applicable supporting clinical study documents are available on request, depending on circumstances, at https://vivli.org. In cases in which clinical study data and supporting documents are provided pursuant to the sponsor’s policies and procedures, the sponsor will continue to protect the privacy of the clinical study participants. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo/.


