Abstract
Background:
Stomach cancer incidence shows substantial racial-ethnic disparity in the United States (US), with Korean Americans experiencing by far the highest incidence. We examined stomach cancer incidence trends in Korean Americans by tumor subsite, histology and stage, and compared them with incidence rates in racial-ethnic groups with the second highest rate (Japanese Americans) and the lowest rate (non-Hispanic whites; NHWs) as well as populations in South Korea and Japan.
Methods:
We calculated age-adjusted incidence rates by racial-ethnic groups and sex, by tumor characteristics, using the 1988–2012 California Cancer Registry data. Data on South Korea and Japan were obtained from the literature and other resources.
Results:
Between 1988 and 2012 in California, Korean Americans had about 5 times greater incidence than NHWs and twice that of Japanese Americans. Tumor characteristics differed by ethnic group and gender. The incidence in Korean Americans has declined during recent years, for both cardia- and non-cardia sites and for both intestinal- and diffuse-type histology. Although Korean Americans were diagnosed at an earlier stage than other Californians, the proportion with localized disease (43%) was much smaller than in South Korea (57%), where population-based screening is available.
Conclusions:
Stomach cancer incidence declined in the highest risk ethnic groups. However, the persistent disparity between Korean Americans and other racial/ethnic groups warrants additional strategies for prevention and earlier diagnosis.
Impact:
Analysis of California Cancer Registry data identified a racial-ethnic subgroup with stomach cancer disparity who may benefit from targeted prevention and screening efforts.
Keywords: stomach cancer, incidence, race-ethnicity, disparity, Korean Americans
Introduction
Stomach cancer is an often fatal disease with <30% 5-year relative survival and has considerable impact on quality of life on surviving patients (1). Although stomach cancer incidence has decreased markedly over the past several decades in the United States (US), from 11.7 per 100,000 in 1975 to 7.0 per 100,000 in 2012 (1), a substantial disparity in stomach cancer burden is seen across racial ethnic groups (2–4). Korean American men and women continue to have by far the highest stomach cancer incidence of all racial/ethnic groups (2–4), having a 4 to 5-fold higher incidence compared to non-Hispanic whites (NHW) in 2003–2008 (5). Unlike the significant decrease in stomach cancer incidence in all other racial/ethnic groups, including Asian American subgroups such as Chinese, Japanese, and Vietnamese for which stomach cancer is among the top 5 cancers, the incidence in Korean Americans did not significantly decrease between 1990 and 2008 (3). This disparity parallels the high incidence in South Korea. South Korea has the highest stomach cancer rate worldwide (about 10 times of the incidence in US whites), followed by Japan (6). Although the incidence in Japan has decreased substantially in recent decades (5, 7), the incidence in South Korea has decreased little (8, 9).
Etiology of stomach cancer is known to differ by tumor subsite (cardia vs. non-cardia) and histology (intestinal vs. diffuse-type) (10). Cardia gastric cancer is associated with obesity and reflux disease, whereas non-cardia gastric cancer is associated with Helicobacter pylori (H. pylori) infection, especially in countries with lower stomach cancer incidence (10). Intestinal type is primarily associated with H. pylori infection coupled with environmental factors such as salt and other dietary factors, whereas diffuse type is thought to be related to H. pylori and genetic factors (11–14). These subsite- and histology-based stomach cancer subtypes have shown different incidence trends and racial disparity. For example, in contrast to the substantial decrease in overall stomach cancer incidence in all ethnic groups in the US, the incidence of cardia cancer increased among men between the 1970’s and 1980’s (15, 16) and the incidence of diffuse-type stomach cancer increased between 1973 and 2000 in men and women, whites and non-whites (15, 17). Incidence rates of most subtypes were higher in non-whites than in whites, with the exception of higher incidence of cardia subtype in whites (15, 16). Given the differential etiology and epidemiology across subtypes and race groups (10), it is important to examine the incidence trend and ethnic disparity by tumor subtype as well as stage at diagnosis to plan efficient prevention strategies. However, such information has not been described separately for Asian American subgroups.
To examine more recent data and reasons of stomach cancer disparity in Korean Americans, which may warrant focused prevention and screening efforts, we investigated the time trends of overall and subtype-specific stomach cancer incidence in Korean Americans. In addition, since migrant studies may provide insight into environmental factors related to etiology, we also provide incidence rates for NHWs, Japanese Americans, and populations in South Korea and Japan for comparison. Population prevalence of known risk factors is discussed within these population subgroups to gain additional insights into the stomach cancer disparity.
Materials and methods
Cancer Incidence Data
US data:
We used the population-based California Cancer Registry (CCR) data. Approximately one third of all Korean Americans in the US reside in the state of California (18). Cases included all California residents diagnosed during 1988–2012 with primary invasive stomach cancer (International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes C16.0-C16.9, histology codes 8000–8999) (2). Among the 40,038 invasive stomach cancer patients included in this analysis, there were 32,549 NHWs, 2,455 Korean Americans, and 2,007 Japanese Americans.
Stomach cancer patients were further classified according to stage, anatomic subsite and histologic type. Tumor stage was grouped into localized, regional and distant according to the Surveillance, Epidemiology, and End Results Program (SEER) Summary Stage 2000 definition (19). Histology codes were categorized into intestinal type and diffuse type (20) based on ICD-O-3 histology codes (intestinal-type: 8010, 8140, 8144, 8211; diffuse-type: 8490, 8142, 8145). This histologic type classification has been used in previous investigations (2, 15, 17). We considered changes in histology coding between ICD-O-2 and ICD-O-3: ‘8255’ (mixed type; ICD-O-3) was coded as ‘8490’ (i.e. diffuse type) prior to 2001 (15). Results remained similar when considering ‘8255’ as diffuse type due to the small number of cases with ‘8255’. Tumor subsite was categorized into cardia (C16.0), non-cardia (C16.1 - C16.6) and unknown, which includes overlapping or unspecified (C16.8, C16.9). Overlapping subsite code (C16.8) indicates not only an overlap between cardia and non-cardia sites but also an overlap between two or more non-cardia subsites. Because the overlapping subsite code was noted for only 7–10% of cases across race/ethnic groups and does not necessarily indicate cardia and non-cardia overlap, we decided to combine overlapping and unspecified sites together. The main findings did not change when separating overlapping (C16.8) and unspecified (C16.9) subsites.
South Korea data:
We used 1999–2012 cancer incidence data collected by the Korea Central Cancer Registry (KCCR) (8). Since 1999, the KCCR has covered the entire population of South Korea with a completeness of 97.7% in 2012 (8). Subsite-specific incidence data were obtained from 2003–2008 data reported in Cancer Incidence in Five Continents (CI-5), Vol. X (5). Stage distribution was reconstituted from 2006–2010 data (21).
Japan data:
We used 1975–2010 national estimates of cancer incidence data published by the Cancer Information Service based on 25 population-based cancer registries in Japan (7, 22). Subsite-specific incidence data were obtained from the 2003–2008 Miyagi Prefecture data reported in CI-5, Vol. X (5). Miyagi Prefecture had an incidence rate close to the median of all registries in Japan contributing the incidence rates to CI-5 and had a proportion of ‘overlapping or unspecified sites’ comparable to those from US registries. Stage distribution for Japan was reconstituted using 2003–2005 data presented in Japan Cancer Statistics 2014 (23).
Annual Population Estimates
We used the annual population estimates for NHWs in California provided in the 1990–2012 National Cancer Institute (NCI) SEER*Stat software package. We extended the 1990–2000 linear growth trends by sex and age to obtain the NHW population estimates for 1988–1989.
Annual population estimates for Asian subgroups were based on the Asian ethnic-specific population counts from the 1990, 2000, and 2010 population censuses for California. Due to the multi-racial scheme used in the 2000 and 2010 census, population counts for each Asian subgroup by age and sex were represented by the simple average between the count for one race alone and the count for one race alone or two or more races for each sex-age-ethnicity-specific combination in each census year respectively. Annual population estimates for Asian subgroups in California during 1988–2012 were obtained by linear interpolation of sex-age-specific population counts between census years for 1990–2000 and 2000–2010, and extrapolation of the linear trend in the adjacent decade for 1988–1989 and 2011–2012 respectively.
Statistical Methods
Annual age-adjusted incidence rates (AAIRs) per 100,000 population were calculated by direct standardization to the world standard population (24, 25) for the time periods of 1988–1990, 1991–1995, 1996–2000, 2001–2005, and 2006–2012, by sex and race/ethnicity. We also calculated AAIRs for each period specifically by tumor stage, histologic type and anatomic subsite.
Joinpoint Regression Program Version 4.1.0 (National Cancer Institute) (26) was used to describe changes in incidence trends and estimate the average annual percent change (AAPC) in AAIRs during 1988–2012 by race/ethnicity and sex. AAPC is a summary measure of a trend over a fixed period of time, which uses a single number to describe the average trend over a period of multiple years (27).
In this paper, we refer to Koreans and Japanese living in California as Korean Americans and Japanese Americans, respectively, and the populations in South Korea and Japan as South Koreans and Japanese, respectively.
Results
Comparison of overall stomach cancer incidence and time trends across racial-ethnic groups
Between 1988 and 2012 in California, Korean Americans had the highest stomach cancer incidence of any racial/ethnic group both among men and women, with an AAIR that was ~5 times higher than that of NHWs and about twice that of Japanese Americans (Figure 1 and Supplementary Table 1). The AAIRs for Korean Americans and Japanese Americans are much lower than the rates in South Korea and in Japan. In 2006–2012, the Korean American AAIRs were only 40% (for men) and 50% (for women) of the rates in South Korea. The same proportion for Japanese Americans in comparison to Japanese was 17% for men and 30% for women, respectively (Figure 1). Nearly all (~98%) of Korean American stomach cancer patients included in this study were born in Korea, whereas ~50% of Japanese American patients were born in the US. In all population subgroups, the rates were much higher in men than in women. Among men, incidence rates in all subgroups in the US significantly and steadily declined between 1988 and 2012. The rates in Japanese men also substantially declined during this time period. However, the rates in South Korean men declined only slightly, and the decline was not apparent until 2010. Among women, incidence rates declined continuously in Japanese Americans and NHWs between 1988 and 2012. The rates in Korean American women also declined during this time period, although the decline was not clearly observed until the most recent years. As in South Korean men, the decline South Korean women were observed only during the most recent years.
Figure 1.
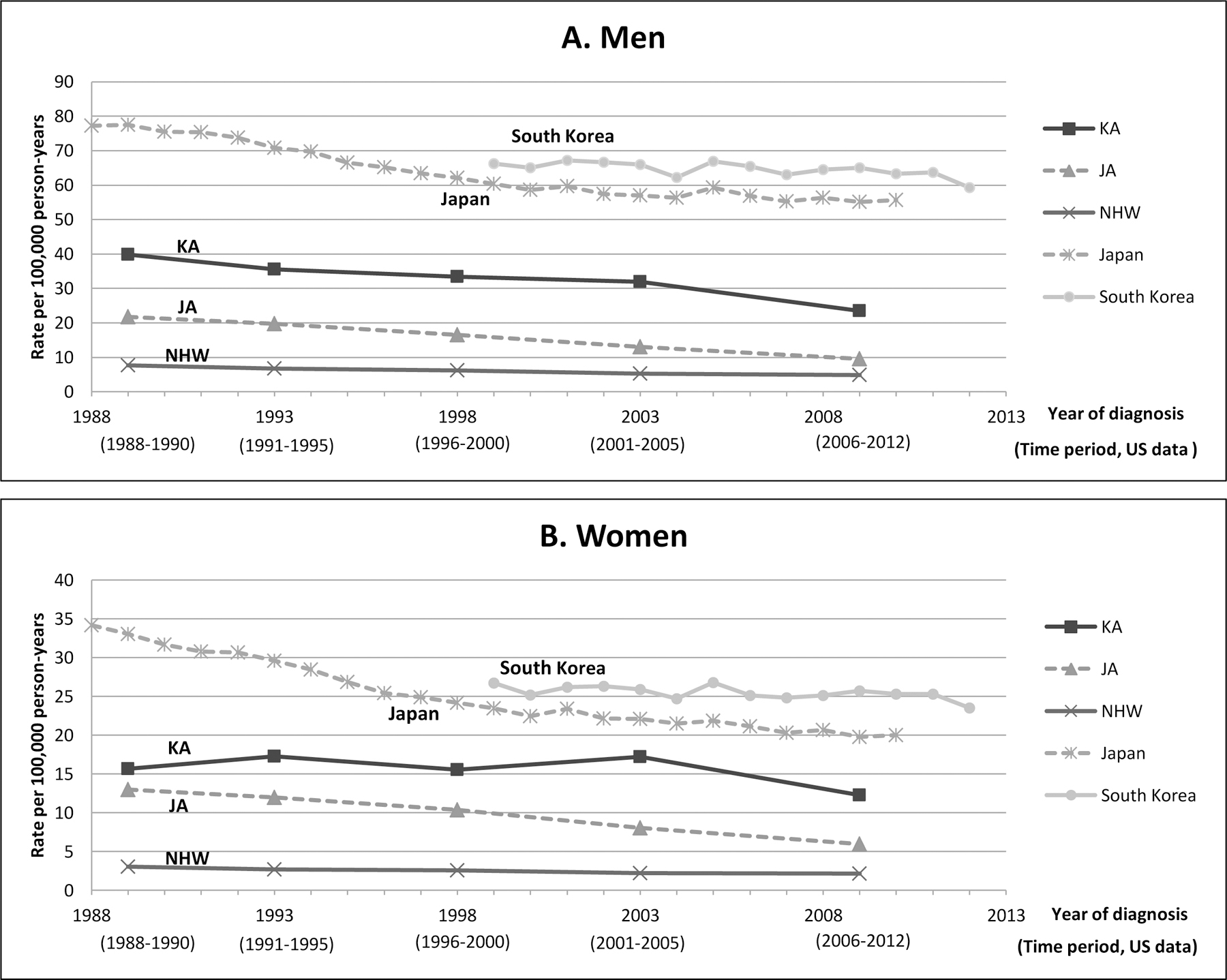
Stomach cancer age-adjusted incidence rates (AAIR) by sex and time period among non-Hispanic whites (NHW), Korean Americans and Japanese Americans in California, South Koreans, and Japanese, 1988–2012. Data for South Korean and Japanese AAIRs were obtained from publically available incidence data (7, 8, 22). A. Men; B. Women.
Abbreviation: JA, Japanese American; KA, Korean American; NHW, Non-Hispanic White
Comparison of tumor characteristics and time trends by tumor characteristics across racial-ethnic groups
By tumor subsite:
Tumor subsite distributions varied widely across ethnic groups and by sex, and this pattern was consistent over the 25 year period (Figure 2). The proportion of cardia stomach cancer was highest among NHWs (40–49% in men; 16–24% in women) and lowest among Korean Americans (2–7% in men; 3–8% in women) and South Koreans (4% in men; 3% in women). The cardia proportions in Japanese Americans were slightly higher than (for men) or similar to (for women) the proportions in Japanese. Over the 25 years, the proportion of cardia cancer has increased, especially among NHWs. Reflecting the substantial ethnic difference in subsite distribution, incidence of cardia cancer was higher in NHWs and Japanese Americans than in Korean Americans among men (Supplementary Figure 1 and Supplementary Table 2). The incidence rates of cardia cancer generally decreased in all subgroups during this period, except for NHW women. Incidence rates of other parts of the stomach (e.g. non-cardia, overlapping/unspecified) also decreased in all population subgroups, although the decrease in Korean American women was less clear and appeared to be limited to the most recent years.
Figure 2.
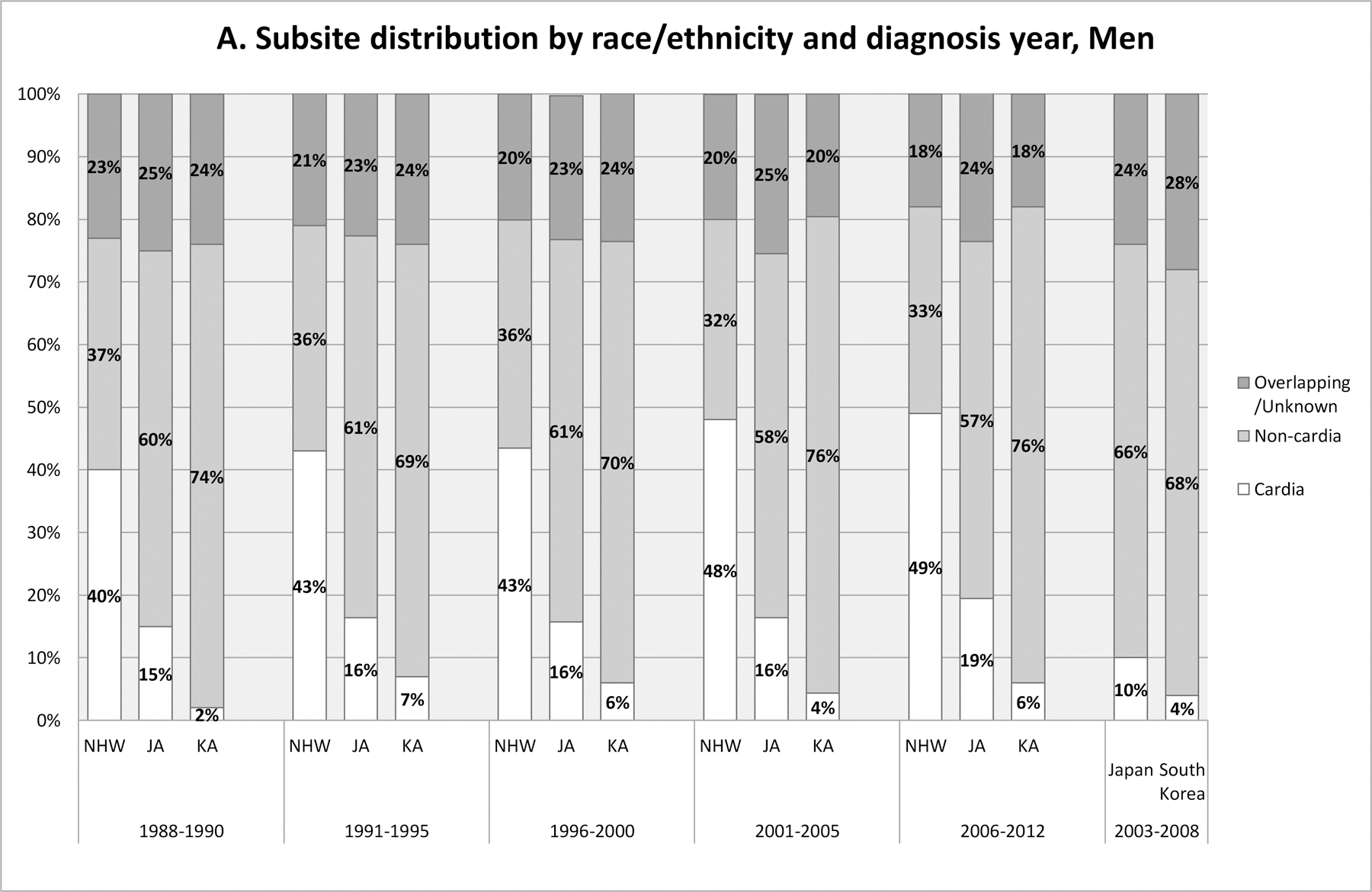
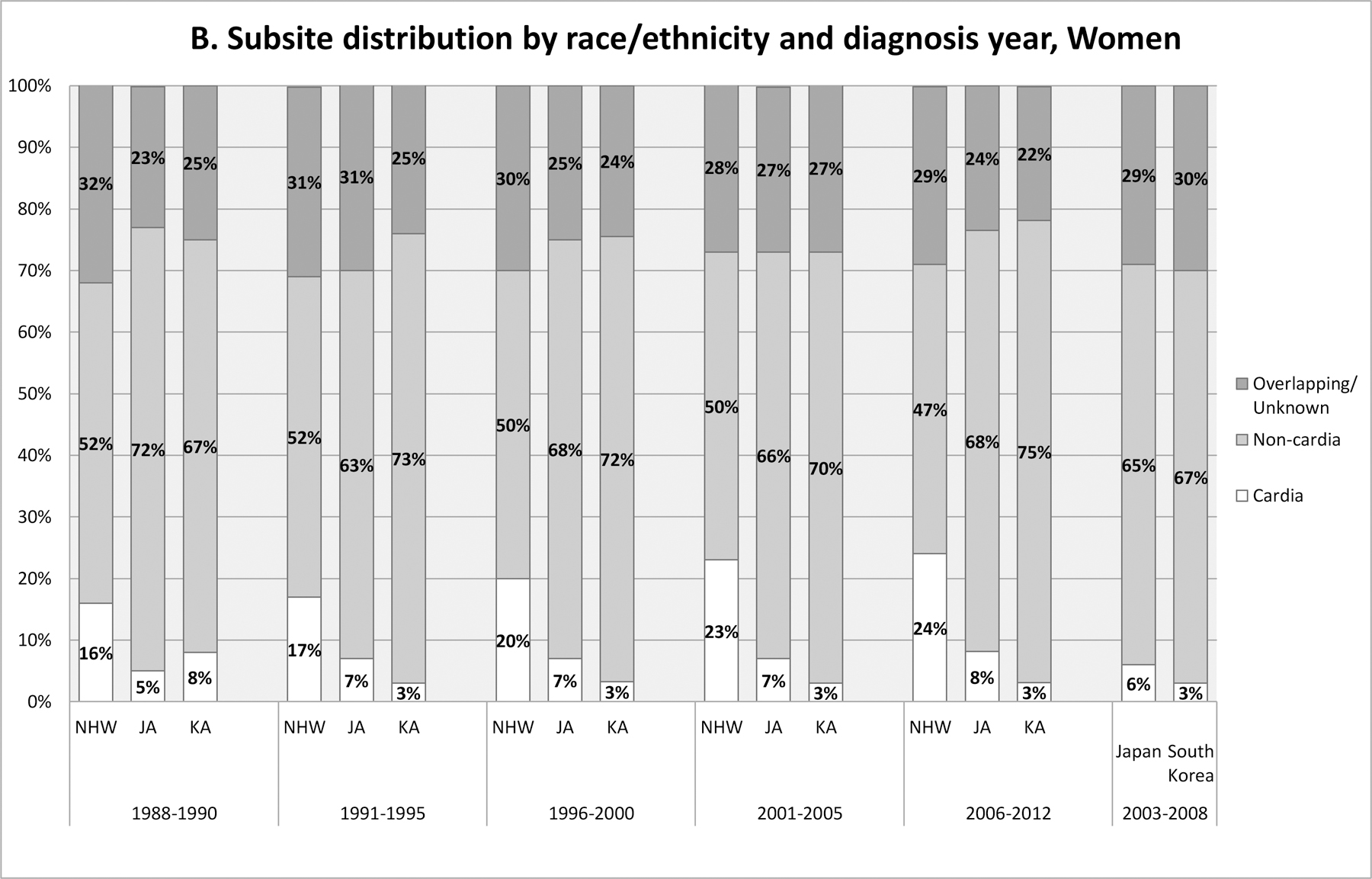
Subsite distribution by race/ethnicity and diagnosis year time period in California (1988–2012) in comparison with that in South Korea and in Japan (2003–2008). A: Men; B: Women. Source for data for Japan and South Korea: CI5 Vol. X (5). Japan data presented are from Japan, Miyagi Prefecture (5).
Abbreviation: JA, Japanese American; KA, Korean American; NHW, Non-Hispanic White
By stage at diagnosis:
The proportion of localized stage disease increased over the past 25 years in all ethnic groups, with the largest increase being observed between late 1980’s and early 1990’s among Korean- and Japanese Americans (Figure 3). There was no sex difference in stage distribution within each ethnic subgroup (data not shown). Of all ethnic groups, Korean Americans consistently had the highest proportion of localized disease throughout the study period. In 2006–2012, 43% of Korean American patients were diagnosed with a localized disease, which is much higher than those in other ethnic groups (~30%). The proportion of localized disease in South Korea and in Japan was even higher (57% and 55%, respectively). When examining the incidence trends by stage (Supplementary Figure 2 and Supplementary Table 3), incidence of localized disease in Korean American men and Japanese American men substantially increased during the first half of the 25 years, consistent with the stage conversion during this time period. A similar pattern was observed for women, although the magnitude of change was smaller than in men. More advanced stage disease decreased over the 25 years in all ethnic groups both in men and women.
Figure 3.
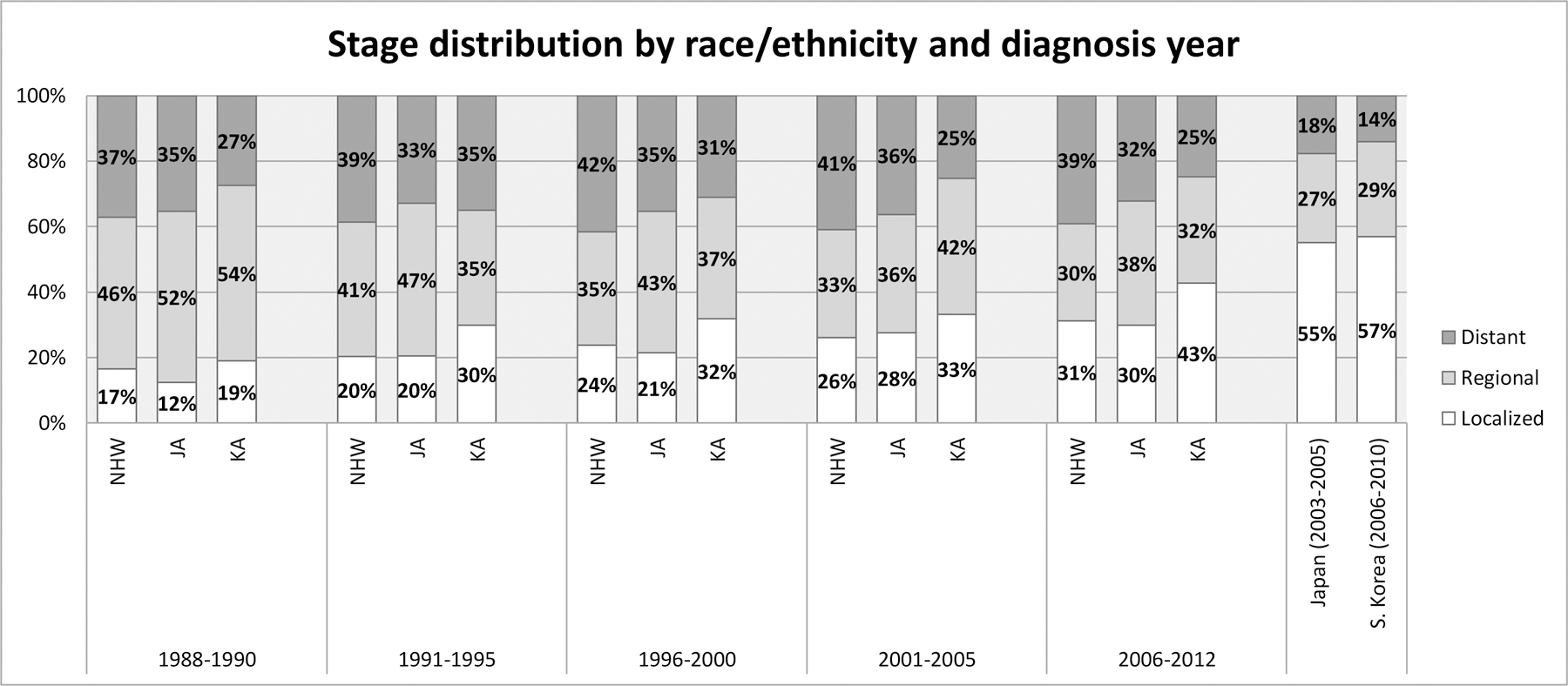
Stage distribution by race/ethnicity and diagnosis year time period in California (1988–2012), South Korea (2006–2010), and Japan (2003–2005)*.
* Unknown stage tumors were not included in calculating stage distribution. Proportion of cases with unknown stage was similar across racial/ethnic groups in California (10%−12%); this was also similar to the unknown % in South Korea (11%) and Japan (8%). Stage distribution for South Korea was reconstituted from Table 3 in Jung et al. 2013 (21). Stage distribution for Japan was reconstituted using data presented in Japan Cancer Statistics 2014 (23).
Abbreviation: JA, Japanese American; KA, Korean American; NHW, Non-Hispanic White; S. Korea, South Korea.
By histology type:
When examining intestinal- and diffuse-type using the ICD-O-3 histology codes as described in the methods section, males had a higher proportion of intestinal type than females; the difference across ethnic groups within each sex was smaller (Supplementary Table 4). When examining the incidence trends, incidence of intestinal type substantially decreased across all ethnic groups regardless of sex (Figure 4 and Supplementary Table 4). Incidence of diffuse type decreased in general in all ethnic groups among men and women during the more recent years.
Figure 4.
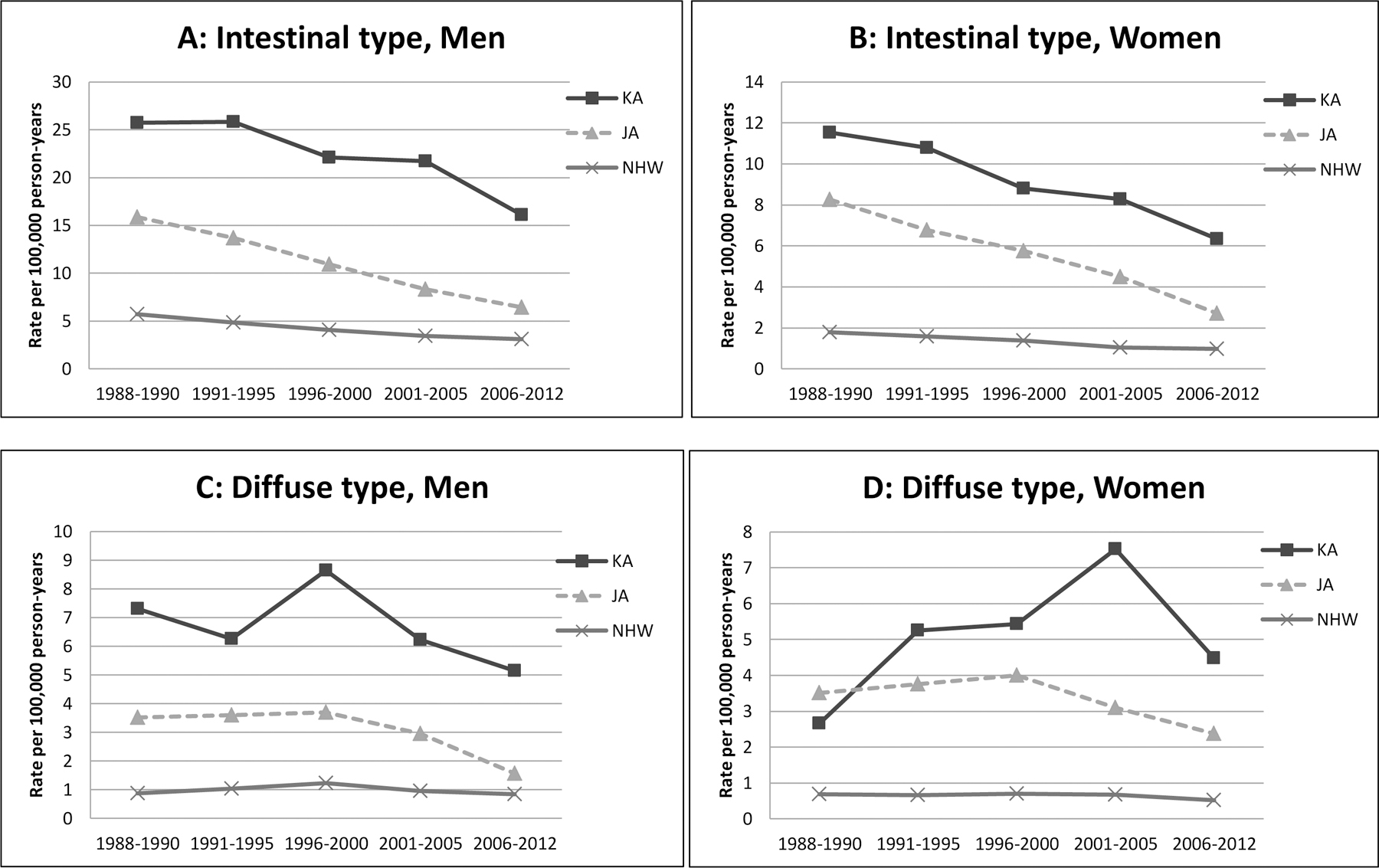
Stomach cancer incidence rate by histologic type, time period, race/ethnicity and sex in California, 1988–2012. A: Intestinal type, men; B: Intestinal type, women; C: Diffuse type, men; D: Diffuse type, women.
Abbreviation: JA, Japanese American; KA, Korean American; NHW, Non-Hispanic White
Discussion
To our knowledge, this is the first population-based investigation of stomach cancer disparity in Korean Americans examining tumor characteristics. Our results demonstrate a persistent stomach cancer disparity in Korean Americans, and demonstrate for the first time that the incidence in Korean Americans has started to decline in recent years. Tumor characteristics with respect to subsite and histology showed difference by ethnicity and sex: the proportion of cardia stomach cancer was highest among NHW men and lowest among Korean American and South Korean men and women, and the intestinal type histology was more frequent among men than in women for all racial-ethnic groups. Although Korean Americans had an earlier stage at diagnosis than others in California, the proportion with localized disease was still much smaller than in South Korea.
Two recent reports based on 1990–2008 SEER data (3) and 1988–2007 data from Los Angeles County (9) showed that stomach cancer rates were not decreasing in Korean American men and women. Our updated data from California suggest that Korean American rates have started to decline both in men and women. Despite this encouraging trend, our data show that Korean Americans still experience the highest incidence, consistent with previous reports of higher stomach cancer incidence in Korean Americans compared to all other ethnic groups in the US, including Hispanics and other Asian Americans (2–4, 9). The high incidence rates among Korean Americans mirror those of South Koreans, who have the highest incidence in the world (6, 28) and about twice the incidence of Korean Americans (9, 29). It is noteworthy that stomach cancer incidence in Korean Americans declined much earlier than in South Koreans. This is in line with the well documented observation that Asian immigrants experience lifestyle changes and divergence in cancer incidence patterns after migration (30, 31). Interestingly, Japanese Americans have a much lower stomach cancer incidence compared to Korean Americans, even though the incidence in Japan is high (5). This likely reflects the difference in the immigration history: more recent immigration of Korean Americans compared to Japanese Americans. The majority of Korean Americans immigrated after 1965 (32, 33), and only ~25% of Korean Americans in California in 2014 (and ~15% of adult Korean Americans) were born in the US (34). In contrast, Japanese American immigration was highest between the1900’s and mid 1920’s and then continued in a relatively smaller volume since the 1950’s (35), and 71% of California Japanese Americans are U.S. born second, third or even fourth generation immigrants (34). Lifestyle changes among immigrants are larger in subsequent generations than in the first generation (30, 31). At present, we do not have sufficient power to examine stomach cancer incidence by birth place for these Asian American subgroups. Additional efforts in monitoring stomach cancer disparity are warranted given the continuing immigration from these two countries.
Understanding cancer risk factors in ethnic subgroups and comparing the risk factor prevalence between immigrants and their population of origin and the US population is helpful in identifying prevention strategies. The strongest risk factor for stomach cancer is H. pylori infection. Analysis of pre-neoplastic gastric lesions from a large national pathology database (36) showed that H. pylori prevalence in Korean Americans was about 2 times that of NHWs (19% vs. 10%). Similarly, analysis of blood samples from large nationwide surveys in the US (37) and South Korea (38) found H. pylori seroprevalence (detecting H. pylori immunoglobulin G) among South Koreans to be about 3 times higher than that of US NHWs (67% vs. 21%), which is in line with the difference in the stomach cancer rates. However, when considering H. pylori prevalence in other ethnic groups in the US, H. pylori prevalence itself does not completely explain the stomach cancer disparity in Korean Americans. For example, while Chinese Americans have similar H. pylori prevalence to Korean Americans (36), their stomach cancer incidence is only ~30% that of Korean Americans (3). Likewise, US non-Hispanic blacks and Mexican Americans have similar seroprevalence rates (52% and 64%, respectively) to South Koreans (37, 38) but experience stomach cancer rates that are only 15–30% of the incidence in South Koreans (3, 5). Increased pathogenicity of East Asian strains of H. pylori has been proposed as an additional contributing factor (39–41). The interplay between H. pylori and environmental factors, and possibly host genetic factors, may also play a role (11, 12, 42, 43).
Salted food intake, particularly salted fish intake, is an established stomach cancer risk factor (44–47). In Korea, the mean daily sodium intake was 4546mg (5212mg in men and 3868mg in women) (48). This is higher than that in US adults (4043mg in men; 2884mg in women) (49). Even within Korean American immigrants, birthplace (US-born vs. Korea-born) and length of residence in the US are associated with decreased levels of salt intake (32) and consumption of a traditional Korean diet (50) which typically includes diverse types of salted fish. Thus, the ecological comparison of levels of salt intake, and possibly salted fish intake, is compatible with the differences in the stomach cancer incidence across these populations. A synergistic effect between salt intake and H. pylori has been proposed by available, although limited, data from epidemiologic and laboratory studies (14, 51, 52). Therefore, prevention efforts to reduce salted food intake and H. pylori infection in high-risk populations are warranted.
Smoking and heavy alcohol drinking are additional stomach cancer risk factors (11, 53–55). In 2001, the prevalence of current smoking in South Korean, California Korean American, and California NHW men was 61%, 38%, and 20%, respectively, which corresponds with the differences in stomach cancer incidence (34, 56). The role of heavy drinking on the observed disparity is less clear. South Korean men consume nearly double the amount of alcohol and four times the amount of liquor than men in the US (per capita) (57), consistent with the incidence difference between South Korean men and Korean American men. However, the prevalence of heavy drinking in Korean American men was similar to that of NHW men (34) though there may be ethnic differences in the amount and pattern of alcohol consumption and burden of heavy drinking on stomach cancer (58). Among women, smoking and heavy drinking were inconsistent with the stomach disparity in that South Korean and Korean American women have lower prevalence of smoking and drinking than NHW women (34, 56, 59).
The ethnic difference in subsite distribution was consistent with earlier reports showing that stomach cancer in the cardia has been particularly high among white men (15) and scarce in South Koreans (60, 61). The reasons for this pattern are not clearly understood but may be related to the higher prevalence of erosive reflux disease in men than in women and in whites than in other ethnic groups (62). Obesity and reflux disease are key risk factors of cardia cancer in the US (10). H. pylori infection is not associated with cardia cancer in western countries, and only weakly associated with cardia cancer in high-risk populations (10, 63). An earlier study reported a continued increase in cardia cancer up to 2005 among men of ‘other races’, the majority of whom are Asians (15). Our results, together with an updated report focused on Hispanics in California (2), suggest a promising decrease in cardia cancer among many ethnic groups in men and also, at least since the late 1990’s, in women.
The observed histology distribution is similar to that from one previous study in the US, which reported a higher proportion of diffuse type for women (~25%) than for men (~15%) (15). The incidence of diffuse type stomach cancer increased up to the year 2000 (17), but since then decreased up to 2005 in all ethnic groups (i.e. whites, blacks, ‘others’) regardless of sex (15). Our results extends earlier findings and show that diffuse-type incidence has decreased in NHWs, Korean Americans, and Japanese Americans in recent years. H. pylori is involved in carcinogenesis of both intestinal-type and diffuse-type with similar relative risks (64), although there are differences in their downstream or alternative events in each carcinogenic pathway (12, 13). Thus, it is likely that decreasing H. pylori prevalence to some extent contributed to the decreasing trend of diffuse-type stomach cancer. It is unclear why the decreasing trend of diffuse type was much more substantial in men than women and warrants further research. The trends of ‘other’ histological types are difficult to interpret because it includes a diverse of types of rare forms of stomach cancer such as other types of adenocarcinoma, sarcoma, carcinoid tumor, squamous cell carcinoma.
Recent data suggest that endoscopy-based screening leads to earlier diagnosis (65) and improves gastric cancer survival (66). In South Korea, stomach cancer screening is offered as part of a national cancer screening program to persons aged ≥40 years with 2 year intervals using upper endoscopy (predominantly) or upper gastrointestinal series (67). In addition, endoscopy screening is widely available through opportunistic cancer screenings. As a result, 80% of the screen-target population ( age ≥40 years) has ever had stomach cancer screening, primarily through upper endoscopy (67), and the majority are following the program guidelines with regards to the procedure and screening interval. Unfortunately, in the US, well-defined guidelines for stomach cancer screening are currently lacking (68), although guidelines from the American Society for Gastrointestinal Endoscopy pointed out the importance of race and ethnicity considerations and suggest that endoscopic stomach cancer screening be considered for first-generation immigrants from high-risk regions (69). The earlier stage at diagnosis in Korean Americans compared to other ethnic groups in the US suggests that aggressive diagnostic work-up and/or opportunistic screening practices may be occurring in some part of the community medical practice. However, the unfavorable stage distribution in Korean Americans compared to South Koreans or Japanese suggests that additional strategies of prevention and early diagnosis are warranted to reduce stomach cancer disparity. It has been shown that nearly all early stage stomach cancer would progress with time and lead to death if untreated (70). Endoscopy can also detect precancerous changes, creating prevention opportunities such as lifestyle changes and/or H. pylori eradication as well as opportunities for aggressive endoscopy surveillance for disease progression.
Thus, in addition to designing strategies to reduce prevalence of stomach cancer risk factors in high risk populations, the medical community and policy makers should consider offering endoscopy screening in the absence of symptoms to Korean Americans and other high risk Asian Americans such as Japanese and Vietnamese Americans (4) to detect stomach cancer at an early stage or to prevent the disease. Lung cancer screening in the US provides a precedent for such targeted screening. Lung cancer screening through low-dose computed tomography is being recommended for selected asymptomatic high risk populations with heavy smoking history, and the cost is covered by the Centers for Medicare and Medicaid Services (CMS) and private insurance companies (71). Following a similar approach, it is timely to consider insurance/CMS coverage of stomach cancer screening for high-risk populations such as Korean Americans and other high risk Asian Americans. A recently proposed ethnicity-based targeted screening algorithm comprising initial endoscopy screening of high-risk individuals such as immigrants from high-incidence regions followed by subsequent treatment and periodic surveillance depending on H. pylori infection or other pre-malignant changes, merits further discussion (68).
The strengths of the current study include the use of population-based CCR data and the fact that this is the first investigation of Korean American stomach cancer disparity considering tumor characteristics and comparing with South Korean incidence rates. The limitations include potential misclassification of race/ethnicity of cancer cases, primarily derived from medical records, and potential errors in population estimates (3), as in any studies based on US cancer registries. However, these errors are likely to be minimal and non-differential by tumor characteristics (72), thus unlikely to explain the 5-fold higher incidence in Korean Americans compared to NHWs and the difference in tumor characteristics by race/ethnicity and sex. Our data on intestinal- and diffuse-type classification were reconstituted from histology codes; however, we adopted classification methods used in previous investigations (2, 15, 17). Although comparisons of cardia stomach cancer were based on small sample sizes, the ethnic difference in tumor subsite distribution was sufficiently large. Finally, we were not able to examine stomach cancer incidence in second generation Korean Americans because the majority (~98%) of Korean American stomach cancer patients were first generation immigrants.
In conclusion, our results show persistent stomach cancer disparity in Korean Americans and substantial ethnic difference in tumor characteristics. Comparisons of incidence rates and stage distribution between Korean Americans and South Koreans along with ecological comparisons of risk factors indicate that additional prevention and screening strategies need to be implemented targeting high risk immigrant populations such as Korean Americans.
Supplementary Material
Acknowledgments
Financial support: This study was supported by grant CDPH 12–10211 from the California Department of Public Health to D. Deapen. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862–04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Conflicts of Interest: None
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. 2015 [cited 2016 3/1/2016]. [Google Scholar]
- 2.Chang ET, Gomez SL, Fish K, Schupp CW, Parsonnet J, DeRouen MC, et al. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21(5):709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. Journal of the National Cancer Institute. 2013;105(15):1096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCracken M, Olsen M, Chen MS Jr., Jemal A, Thun M, Cokkinides V, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57(4):190–205. [DOI] [PubMed] [Google Scholar]
- 5.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, et al. Cancer Incidence in Five Continents, Vol. X IARC Scientific Publication No. 164. . Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. [March 2015] Available from: http://globocan.iarc.fr. [Google Scholar]
- 7.Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44(4):388–96. [DOI] [PubMed] [Google Scholar]
- 8.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47(2):127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Park J, Nam BH, Ki M. Stomach cancer incidence rates among Americans, Asian Americans and Native Asians from 1988 to 2011. Epidemiol Health. 2015;37:e2015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugge M, Fassan M, Graham DY. Epidemiology of Gastric Cancer In: Strong VE, editor. Gastric Cancer. Switzerland: Springer International Publishing Switzerland; 2015. p. 23–34. [Google Scholar]
- 12.Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2(2):88–94. [DOI] [PubMed] [Google Scholar]
- 13.Sipponen P Gastric cancer: pathogenesis, risks, and prevention. J Gastroenterol. 2002;37 Suppl 13:39–44. [DOI] [PubMed] [Google Scholar]
- 14.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens Part B: Biological agents Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- 15.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18(7):1945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60(12):1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128(7):765–70. [DOI] [PubMed] [Google Scholar]
- 18.Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian population: 2010. 2010 Census Briefs. Issued March 2012. 2010 [May 2015]. [Google Scholar]
- 19.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA. SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01–4969 Bethesda, MD: 2001. Available from: http://seer.cancer.gov/tools/ssm/SSSM2000-122012.pdf#search=summary+stage+2000. [Google Scholar]
- 20.Lauren P The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 21.Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of korean adult cancer patients by stage at diagnosis, 2006–2010: national cancer registry study. Cancer Res Treat. 2013;45(3):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Statistics in Japan: National estimates of cancer incidence based on cancer registries in Japan (1975–2011): Cancer Information Service, National Cancer Center, Japan; [cited 2015]. Available from: http://ganjoho.jp/en/professional/statistics/table_download.html. [Google Scholar]
- 23.The Editorial Board of the Cancer Statistics in Japan. Cancer statistics in Japan 2014: Foundation for Promotion of Cancer Research (FPCR); 2015. [cited 2015 December 2015]. Available from: http://ganjoho.jp/en/professional/statistics/brochure/2014_en.html#a3.
- 24.Segi M Cancer mortality for selected sites in 24 countries (1950–1957). Sendai, Japan: Tohoku University School of Medicine; 1960. [Google Scholar]
- 25.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: A technical report. Berline: Springer-Verlag; 1966. [Google Scholar]
- 26.Joinpoint Regression Program, Version 4.1.0 - April 2014: Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute; [April/25/2014]. Available from: http://surveillance.cancer.gov/joinpoint. [Google Scholar]
- 27.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–44. [DOI] [PubMed] [Google Scholar]
- 29.Gomez SL, Le GM, Clarke CA, Glaser SL, France AM, West DW. Cancer incidence patterns in Koreans in the US and in Kangwha, South Korea. Cancer Causes Control. 2003;14(2):167–74. [DOI] [PubMed] [Google Scholar]
- 30.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4(7):519–27. [DOI] [PubMed] [Google Scholar]
- 31.Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14(3):431–9. [PubMed] [Google Scholar]
- 32.Park SY, Murphy SP, Sharma S, Kolonel LN. Dietary intakes and health-related behaviours of Korean American women born in the USA and Korea: the Multiethnic Cohort Study. Public Health Nutr. 2005;8(7):904–11. [DOI] [PubMed] [Google Scholar]
- 33.Zong J, Batalova J. Korean immigrants in the United States: Migration policy institute; 2014. [September 2015]. Available from: http://www.migrationpolicy.org/article/korean-immigrants-united-states.
- 34.The California Health Interview Survey (CHIS) [May 2015]. Available from: http://healthpolicy.ucla.edu/chis/Pages/default.aspx.
- 35.United States. Department of Homeland Security. Yearbook of Immigration Statistics: 2014. Washington, D.C.: U.S. Department of Homeland Security, Office of Immigration Statistics, 2016. [Google Scholar]
- 36.Choi CE, Sonnenberg A, Turner K, Genta RM. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig Dis Sci. 2015;60(7):2070–6. [DOI] [PubMed] [Google Scholar]
- 37.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong QJ, Zhan SH, Wang LL, Xin YN, Jiang M, Xuan SY. Relatedness of Helicobacter pylori populations to gastric carcinogenesis. World journal of gastroenterology : WJG. 2012;18(45):6571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97(5):1106–12. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka Y Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn HJ, Lee DS. Helicobacter pylori in gastric carcinogenesis. World J Gastrointest Oncol. 2015;7(12):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocco A, Nardone G. Diet, H pylori infection and gastric cancer: evidence and controversies. World journal of gastroenterology : WJG. 2007;13(21):2901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10(2):75–83. [DOI] [PubMed] [Google Scholar]
- 45.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–4. [DOI] [PubMed] [Google Scholar]
- 46.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11(3):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn YO. Diet and stomach cancer in Korea. Int J Cancer. 1997;Suppl 10:7–9. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Oh K. Methodological issues in estimating sodium intake in the Korea National Health and Nutrition Examination Survey. Epidemiol Health. 2014;36:e2014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoy MK, Goldman JD, Murayi T, Rhodes DG, Moshfegh AJ. Sodium Intake of the U.S. Population: What We Eat In America, NHANES 2007–2008. Food Surveys Research Group Dietary Data Brief No. 8. October 2011. [2/1/2016]. Available from: http://ars.usda.gov/Services/docs.htm?docid=19476.
- 50.Yang EJ, Kerver JM, Song WO. Dietary patterns of Korean Americans described by factor analysis. J Am Coll Nutr. 2005;24(2):115–21. [DOI] [PubMed] [Google Scholar]
- 51.Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World journal of gastroenterology : WJG. 2009;15(18):2204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM Jr., et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81(6):2258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28–36. [DOI] [PubMed] [Google Scholar]
- 54.Ma SH, Jung W, Weiderpass E, Jang J, Hwang Y, Ahn C, et al. Impact of alcohol drinking on gastric cancer development according to Helicobacter pylori infection status. Br J Cancer. 2015;113(9):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norat T, Chan D, Vingeliene S, Aune D, Abar L, Vieira AR, et al. The Associations between Food, Nutrition and Physical Activity and the Risk of Stomach Cancer London: World Cancer Research Fund International; 2015. [7/5/2016]. Available from: http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/stomach-cancer. [Google Scholar]
- 56.Korea National Health and Nutrition Examination Survey (KNHANES) [March 2015]. Available from: https://knhanes.cdc.go.kr/knhanes/eng/index.do.
- 57.World Health Organization. Global status report on alcohol and health 2014. World Health Organization: Geneva, Switzerland: 2014. [Google Scholar]
- 58.Ryu SY, Crespi CM, Maxwell AE. Drinking patterns among Korean adults: results of the 2009 Korean community health survey. J Prev Med Public Health. 2013;46(4):183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol Health. 2014;36:e2014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shim JH, Song KY, Jeon HM, Park CH, Jacks LM, Gonen M, et al. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol. 2014;21(7):2332–9. [DOI] [PubMed] [Google Scholar]
- 61.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Annals of surgery. 2010;251(4):640–6. [DOI] [PubMed] [Google Scholar]
- 62.Abraham A, Lipka S, Hajar R, Krishnamachari B, Virdi R, Jacob B, et al. Erosive Esophagitis in the Obese: The Effect of Ethnicity and Gender on Its Association. Gastroenterol Res Pract. 2016;2016:7897390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22(3):375–87. [DOI] [PubMed] [Google Scholar]
- 64.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112(3):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8(11):e79088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004–2013. Cancer Res Treat. 2016;48(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84(1):18–28. [DOI] [PubMed] [Google Scholar]
- 69.Wang A, Shaukat A, Acosta RD, Bruining DH, Chandrasekhara V, Chathadi KV, et al. Race and ethnicity considerations in GI endoscopy. Gastrointest Endosc. 2015;82(4):593–9. [DOI] [PubMed] [Google Scholar]
- 70.Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000;47(5):618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ersek JL, Eberth JM, McDonnell KK, Strayer SM, Sercy E, Cartmell KB, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–31. [DOI] [PubMed] [Google Scholar]
- 72.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control. 2006;17(6):771–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


