Abstract
Purpose
Auditory processing measures have been used in an attempt to understand the relationship between neurological mechanisms and autism spectrum disorder (ASD) symptomatology in school-age children. The focus of the current study was to understand neural auditory processing in 2- to 3-year-olds with ASD.
Method
Auditory processing measures (click auditory brainstem responses and speech-evoked frequency-following responses) were hypothesized to differ between typically developing children (n = 18) and children with ASD (n = 18). Auditory processing measures were hypothesized to relate to language development in children with ASD.
Results
The current study found limited differences in auditory processing measures between the two groups. No relationships were found between auditory processing measures and language development measures.
Conclusions
Future research is necessary to characterize auditory processing in toddlers with ASD. Longitudinal approaches should be considered when studying auditory processing in children with ASD in order to explore its developmental relationship with ASD symptomatology.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and restricted/repetitive behaviors and interests. Access to early intervention, the best predictor of improving functioning of children with ASD (National Research Council, 2001; Zwaigenbaum et al., 2015), is dependent on early identification. While behavioral testing is the current standard in the ASD diagnostic process (Lord et al., 2012), these measures are highly subjective. To overcome the limitations of behavioral testing, recent research has focused on the use of neurological measurements as a way to identify early biomarkers of ASD that may be present before behavioral symptoms (Bosl et al., 2011; Santos et al., 2017). However, the complex neurological basis of ASD is not well understood; it is likely that many hierarchical neurological systems, including both cortical and subcortical processes, underlie the heterogeneous presentation of ASD symptomatology. Neural auditory processing has been proposed as a system that may aid in understanding the neurological basis of ASD symptomatology (Otto-Meyer et al., 2017; Roth et al., 2012; Russo et al., 2008). Specifically, atypical neurological auditory processing may play a role in the behavioral presentation of ASD given the hallmark characteristic of social communication deficits in ASD.
Auditory Processing Differences in ASD
Neural auditory processing in children with ASD has been studied using a variety of methods that measure scalp-recorded auditory evoked potentials at the cortical level. Previous research has used electroencephalography to characterize cortical auditory processing. Overall, these studies have found that children with ASD have distinct auditory processing profiles when compared to their typically developing peers, characterized by impaired or slower processing, particularly in response to speech sounds (Dunn et al., 2008; Jansson-Verkasalo et al., 2003; Kuhl et al., 2005; Lepistö et al., 2008; O'Connor, 2012; Whitehouse & Bishop, 2008). While it is essential to understand higher order cortical auditory processing, lower level sensory encoding of auditory information plays a critical role in the neurological system. Before cortical regions are able to perceive and store auditory information, subcortical regions must efficiently and consistently encode the signal (Chandrasekaran & Kraus, 2010). Auditory brainstem responses (ABRs) to both nonspeech and speech sounds measure the precision and integrity of the brainstem and midbrain's encoding of auditory information.
ABRs have long been used in clinical settings as a noninvasive, objective method to assess auditory processing (Skoe & Kraus, 2010). Click-evoked ABRs produce a waveform characterized by five identifiable peaks (labeled I–V, respectively). Each peak corresponds to activity produced by specific neural generators as the signal travels from the brainstem to the auditory cortex. Although click-evoked ABRs are important to assess auditory functioning, ABRs in response to speech sounds are particularly important for the study of behaviorally relevant sounds because, unlike click-evoked responses, the integrity with which speech is processed in the brainstem represents a mix of afferent and efferent auditory activity (Skoe & Kraus, 2010). ABRs in response to speech sounds are composed of two distinct components: the onset response and a sustained frequency-following response (FFR). These two components represent how the brainstem and midbrain temporally and spectrally encode speech sounds (Chandrasekaran & Kraus, 2010; Skoe & Kraus, 2010). As with click-evoked ABRs, FFRs to speech sounds produce a waveform characterized by peaks that occur at a precise time in the signal and should occur at a corresponding time in the response. Generally, peaks manifest to reflect either a change in the stimulus (i.e., onset, offset, or transition) or the periodicity of the stimulus. In addition to looking at the FFR from a time domain by focusing on the peaks, one can look at the phase of individual frequencies within the response. The fundamental frequency is the lowest frequency of a periodic waveform, and the harmonics are integer multiples of it. Analyses of FFR focus on measures of response timing (peaks), magnitude (robustness of encoding of specific frequencies), and fidelity. The latter one is assessed by comparing FFR consistency within or across sessions, either to itself or another FFR or a stimulus. Response consistency refers to the analysis of within-session correlation of FFR trials, and it gives an index of how stable the FFR is from trial to trial (Krizman & Kraus, 2019). The neural encoding of speech sounds with all its richness of metrics is particularly relevant in characterizing the relationship between auditory processing and the development of language (Wible et al., 2005).
Previous research has compared click-evoked ABRs in typically developing children and children with ASD. Studies have found atypical click-evoked ABRs in school-age children with ASD as indicated by longer Wave V latencies (Rosenhall et al., 2003; Russo et al., 2009). Other studies, using speech-evoked FFRs, found that school-age children with ASD have lower levels of response consistency (Otto-Meyer et al., 2017), deficient pitch tracking (Russo et al., 2008), and longer wave latencies (Ramezani et al., 2019) when compared to their typically developing peers. A meta-analysis by Miron et al. (2017) demonstrated that studies of people with ASD below 18 years showed prolongation of Wave V latency, while studies of people with ASD above 18 years of age showed shortening of Wave V latency. The authors proposed that the early prolongation of Wave V may relate to the brain overgrowth that has been noted in children with ASD (Courchesne et al., 2011; Redcay & Courchesne, 2005). These findings indicate a developmental mechanism involved in the relationship between atypical auditory processing and ASD. Therefore, understanding the developmental mechanism requires examination of the relationship between ASD and auditory processing throughout all periods of development.
Of the studies that have characterized neural auditory processing in children with ASD, very few have included toddlers, and the results of these studies are inconsistent. A study by Santos et al. (2017) found no differences between children with ASD and children diagnosed with a language delay, ranging from 2 to 6 years old when comparing absolute and interpeak interval latency of click-evoked ABR measures; however, they did find significant differences in Wave I amplitude. Tas et al. (2007) found that children with ASD between 2 and 7 years of age differed only in click-evoked Wave III–V interpeak interval. Conversely, Roth et al. (2012) found that toddlers with ASD were significantly different across all measures of absolute and interpeak interval, except Wave III–V interpeak interval when compared to clinical norms of young adults. Finally, Miron et al. (2016) found that toddlers with ASD had significantly longer absolute latencies and interpeak intervals when compared to clinical norms of young adults. A summary of these previously reported ABR absolute latency findings are provided in Table 1.
Table 1.
Summary of previously reported click-evoked auditory brainstem response absolute latencies in children with autism spectrum disorder.
| Study | Comparison group | Use of sedation? | Ages (years) | Click Wave I latency | Click Wave III latency | Click Wave V latency |
|---|---|---|---|---|---|---|
| Miron et al. (2016) | Young adult clinical norms | Yes | 1.5–3.5 | SD | SD | SD |
| Roth et al. (2012) | Young adult clinical norms | Yes | 2–4 | SD | SD | SD |
| Santos et al. (2017) | Age-/sex-matched children with language delay | Yes | 2–6 | ND | ND | ND |
| Tas et al. (2007) | Typically developing peers | Yes | 2–7 | ND | ND | ND |
| Current study | Age-/sex-matched typically developing children | No | 2–4 | ND | ND | ND |
Note. SD = significant difference (p < .05); ND = no significant differences (p > .05).
To our current knowledge, only one study has studied speech-evoked FFRs in younger children with ASD. Chen et al. (2019) analyzed the longitudinal development of latency and amplitude components of speech-evoked FFRs for children between 3 and 6 years old with ASD. This study concluded that auditory processing development may differ in children with ASD when compared to their typically developing peers. To our knowledge, there is no study that has analyzed the frequency encoding and response consistency of speech-evoked FFRs in toddlers with ASD.
Due to a variation of findings, wide age ranges, inconsistent comparison groups, and age-inappropriate norms, additional research is necessary to characterize auditory processing in toddlers with ASD. Toddlers with ASD, a population with a high prevalence of sensory processing challenges, pose a unique challenge to the success of electrophysiological recordings, which has resulted in the use of sedation (Miron et al., 2016; Roth et al., 2012; Santos et al., 2017; Tas et al., 2007). However, sedation is costly and poses some risks, as repeated exposure to anesthesia has been linked to higher rates of learning disabilities (Padish-Clarin & Hawkins, 2015).
Relationships Between Auditory Processing and ASD Symptomatology
Very few studies have examined how ASD symptomatology related to communication deficits may relate to auditory processing. There has been reasonable amount of research linking auditory processing with language development in children without ASD. Lower language levels have been associated with longer speech-evoked FFR Wave V latencies in children with lower reading levels (Banai et al., 2009) and longer click-evoked Wave V latencies in typically developing young infants (Chonchaiya et al., 2012) and less consistent responses in populations of children with dyslexia (Hornickel & Kraus, 2013). In premature infants, Amin et al. (2014) found that longer click-evoked Wave I–V interpeak latencies measured at 8 months were associated with lower scores on the Preschool Language Scales–Fifth Edition (Zimmerman et al., 2011) at 3 years of age. By technique-driven necessity, stimuli used for FFR must be of short duration and thus of limited acoustic complexity compared to the whole of spoken language. Nevertheless, long-term experience with language, via corticofugal connections, is thought to shape the default auditory processing of speech signals such as a /da/ in the midbrain. Despite its length, /da/ still contains a rich array of the spectrotemporal complexity found in speech. Experience-induced plasticity and acoustical complexity, together, are believed to be the reasons that speech sounds, even of short duration, are more effective than the click stimulus most often used for ABR for uncovering relationships with complex behaviors such as language skills (White-Schwoch & Kraus, 2017).
Few studies have examined the relationship between language development and neural auditory processing in children with ASD. Russo et al. (2009) found no relationship between Wave V latencies and measures of language development in school-age children with ASD. However, the timing of FFR peaks V and A were delayed in the ASD group, which significantly lagged the controls in measures of receptive language ability. Chen et al. (2019) found a positive correlation between Wave A amplitude and measures of language development in preschool-age children with ASD. However, this relationship may be due to failure to correct for multiple comparisons. In summary, the FFR to /da/ has been a very fruitful probe of auditory processing in school-age children both with and without ASD. Given the strong relationship between language and auditory processing and the high incidence of communication and language difficulties in individuals on the ASD spectrum, we believe FFR timing may increase our understanding of the relationship between language development and auditory processing in toddlers with ASD.
Study Aims
The current study aims to (a) address the feasibility of recording ABRs and FFRs in toddlers with ASD without the use of sedation, (b) examine auditory processing differences in children with ASD when compared to their typically developing peers, and (c) examine the relationship between auditory processing and ASD symptomatology, specifically ASD severity, nonverbal cognition levels, and language developmental levels. We hypothesized that auditory processing measures in children with ASD would differ from their typically developing peers and that auditory processing measures would be related to measures of ASD severity, nonverbal cognition, and language development.
Method
Participants
Participants were recruited in the Chicagoland area from the Early Intervention Research Group registry and the Auditory Neuroscience Lab at Northwestern University. Participants included 40 toddlers with ASD, of whom 18 (M = 2.941 years, SD = 0.45, range: 2.187–3.995) completed the recording. All of the typically developing toddlers (n = 18, M = 3.058 years, SD = 0.35, range: 2.486–3.897) successfully completed the recording and served as the control group. The two groups were matched on age, t(17) = 1.475, p = .159, and gender (five girls in each group). ASD diagnoses were verified based on Autism Diagnostic Observation Schedule (ADOS) scores (Lord et al., 2012) completed by a research reliable ADOS assessor. All participants had normal hearing based on a review of audiology records. The methods for the current study were approved by the Northwestern University Institutional Review Board. Written informed consent was obtained for all individuals from a parent or guardian. All procedures have been carried out in accordance with the World Medical Association's Declaration of Helsinki for experiments involving humans.
Procedure
Auditory Processing Measures
Single-channel auditory evoked responses were recorded from three scalp electrodes (Cz active, forehead ground, and ipsilateral earlobe reference) using a Biologic Navigator Pro (Natus, Inc.). During the testing, children sat comfortably in a reclining chair and watched a movie at ≤ 40 dB SPL of their choice. The child's parent usually sat in the room to increase compliance and to notify the tester if any problems arose during recording. The click was presented first (the first two samples) in order to verify ear insert placement and general recording quality. The two /da/ samples followed and then, finally, the third click sample.
The click stimulus was a 100-μs square wave rarefaction click, presented at a rate of 31.1/s to the right ear at 98.5 dB ppe SPL via an ER-3A earphone with a pediatric-size foam tip. Responses were digitized at 40 kHz, filtered between 100 and 2000 Hz, and averaged with a time window of −0.8 to 9.8 ms re stimulus onset. Three samples of 2,000 sweeps each were collected. Sweeps exceeding ± 23 μV were online rejected.
The /da/ stimulus was a 40-ms five-formant synthetic consonant–vowel /da/—the synthesis parameters of which are described in detail elsewhere (Banai et al., 2009). The /da/ was presented at a rate of 10.9/s to the right ear at 80 dBA via an ER-3A earphone with a pediatric size foam tip. Responses were digitized at 12 kHz, filtered between 100 and 2000 Hz, and averaged with a time window of −15.8 to 69.45 ms re stimulus onset. Two samples of 3,000 sweeps each were collected. Sweeps exceeding ± 23 μV were online rejected.
Timing of peaks was measured for both click ABR (Waves I, III, and V) and speech-evoked FFR (Waves V, A, D, E, F, O). Interpeak latencies were measured for click ABR Waves I–V and III–V. We also measured amplitude of Waves I and V for both ABR and FFR in order to compare ratios as reported by Santos et al. (2017). Frequency-specific encoding of the fundamental frequency (75–175 Hz), first formant (175–750 Hz), and high-frequency (750–1200 Hz) components of the speech syllable were also assessed for the FFR. Response consistency, measured with Pearson's correlations between two response repetitions over the 19.5- to 44.2-ms portion of the response, was computed. To reduce the possibility of Type I errors, we limited neurophysiological dependent variables to those that have demonstrated consistent relationships with either ASD or language skills.
Behavioral Measures
Behavioral measures were collected for the children with ASD as a part of a larger, ongoing clinical trial. Behavioral measures were not collected for the typically developing children. Behavioral measures were completed prior to auditory processing measures. However, if the child was not able to complete auditory processing measures in the same visit, an additional visit was scheduled. All assessments were administered and scored by trained research assistants that had reached research fidelity of 80% or above on three consecutive administrations before assessing any of the current participants. Administration fidelity was monitored throughout the study by scoring randomized administrations.
The ADOS, a 30- to 45-min semistructured play-based observation, is a common assessment used to diagnose ASD. ASD severity (Lord et al., 2012) was measured as the ADOS comparison score ranging from 1 to 10, with higher scores indicating greater severity. The ADOS–Toddler Module was administered for children 30 months or younger. Comparison scores for the Toddler Module correspond to ranges of concern: little to no (1–3), mild to moderate (4–5), and moderate to severe (6–10; Esler et al., 2015). The ADOS Module 1 was administered for children 31 months or older. Comparison scores for the Module 1 correspond to level of autism-related symptoms: minimal to no evidence (1–2), low (3–4), moderate (5–7), and high (8–10). Nonverbal cognitive ability was measured using the Visual Reception Scale standard score of the Mullen Scales of Early Learning (Mullen, 1995). This measure yields a total standard score with a mean of 50 and a standard deviation of 10. Language skills were measured using the Preschool Language Scales–Fifth Edition (Zimmerman et al., 2011) and the MacArthur–Bates Communicative Development Inventories: Words and Gestures (MCDI; Fenson et al., 2007). The Preschool Language Scales–Fifth Edition is a commonly used clinical assessment of language used to measure children's overall expressive and receptive language abilities. The child is presented with different receptive and expressive tasks such as following simple directions and labeling pictures. This measure yields a total standard score with a mean of 100 and a standard deviation of 15 for both expressive communication and auditory comprehension subscales. The MCDI is a parent report measure used to assess vocabulary. The survey includes 396 early acquired vocabulary words from 19 semantic categories, such as animals, body parts, and household items. Parents select whether their child understands or says the word. Number of words produced was used as a measure of expressive vocabulary. The MCDI provides percentile rankings for words produced based on the child's age in months for both sexes combined. For 24 months, the 10th percentile is 77 words, the 50th percentile is 297 words, and the 90th percentile is 542 words. For 36 months, the 10th percentile is 263 words, the 50th percentile is 548 words, and the 90th percentile is 653 words.
Statistical Analyses
Study data were collected and managed using Research Electronic Data Capture (REDCap; Harris et al., 2008) hosted at the Northwestern University. REDCap is a secure, web-based application designed to support data capture for research studies, providing (a) an intuitive interface for validated data entry, (b) audit trails for tracking data manipulation and export procedures, (c) automated export procedures for seamless data downloads to common statistical packages, and (d) procedures for importing data from external sources. Shapiro–Wilk normality tests were used to assess the normality of demographic, behavioral, and auditory measures. Demographic measures for the ASD group were normally distributed. Auditory measures for the ASD were not normally distributed. Behavioral measures for the ASD group were not normally distributed. Auditory measures for the typically developing group were normally distributed. Therefore, independent t tests and chi-square analyses were used to compare demographic measures between children that were and were not able to complete the recording. Wilcoxon rank-sum nonparametric tests and chi-square analyses were used to compare behavioral measures between children that were and were not able to complete the recording. Wilcoxon rank-sum nonparametric tests and chi-square analyses were used to compare auditory processing measures in children with ASD and typically developing children. Effect sizes were computed using r = abs(Z)/√N (Rosenthal, 1994). Spearman correlations were performed to investigate the relationship between auditory processing measures and behavioral measures. Data analyses were performed using RStudio Version 1.1.453 (R Core Team, 2017).
Results
Feasibility
Of the typically developing participants, 18 out of 18 successfully completed the neural auditory processing recording. Of the participants with ASD, 18 out of 40 successfully completed the recording and all 18 produced usable data. Children were not able to complete the recording due to noncompliance during electrode application or excessive movement throughout data collection. To address the risk of sampling bias between those toddlers who were and were not able to successfully complete the recording, comparisons between groups in demographic and behavioral measures were performed. Independent t tests and chi-square analyses were performed to explore the difference between groups in demographics. Wilcoxon rank-sum tests were performed to explore the difference between groups in behavioral measures. There were no significant differences between the two groups on any demographic or behavioral measures, with the exception of the Mullen (W = 267.5, p = .049, r = .311). These results are reported in Tables 2 and 3.
Table 2.
Chi-square and t-test analyses of demographic information for children who were and were not able to successfully complete the recording.
| Variable | Successful | Unsuccessful | p |
|---|---|---|---|
| Gender | |||
| Male | 13 | 15 | .787 |
| Female | 5 | 7 | |
| Age (in months), M (SD) | 2.94 (0.45) | 3.00 (0.42) | .645 |
| Income range | |||
| Less than $100,000 | 8 | 13 | .974 |
| $100,000 or above | 5 | 7 | |
| Maternal education | |||
| Less than college graduate | 9 | 7 | .747 |
| College graduate or above | 9 | 15 | |
| Paternal education | |||
| Less than college graduate | 7 | 7 | 1 |
| College graduate or above | 12 | 12 | |
| Race | |||
| White | 11 | 11 | .619 |
| Other | 5 | 9 |
Table 3.
Wilcoxon rank-sum tests of behavioral measures for children who were and were not able to successfully complete the recording.
| Variable | All (n = 40) |
Successful (n = 18) |
Unsuccessful (n = 22) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mdn | M | SD | Range | Mdn | M | SD | Range | Mdn | M | SD | Range | p | |
| ADOS | 9.00 | 8.32 | 1.51 | 5–10 | 9.00 | 8.17 | 1.92 | 5–10 | 8.50 | 8.45 | 1.10 | 6–10 | 1.00 |
| Mullen | 24.00 | 28.12 | 10.99 | 20–75 | 20.00 | 30.50 | 9.06 | 20–45 | 31.00 | 26.18 | 12.22 | 20–75 | .049* |
| PLS EC | 57.00 | 74.58 | 12.25 | 55–119 | 57.00 | 75.39 | 14.52 | 55–119 | 55.50 | 73.91 | 10.34 | 57–92 | .826 |
| PLS AC | 71.50 | 62.55 | 17.94 | 50–121 | 71.50 | 63.17 | 18.64 | 50–121 | 71.00 | 62.05 | 17.75 | 50–118 | .849 |
| MCDI | 16.00 | 83.15 | 114.94 | 0–378 | 13.00 | 80.28 | 113.12 | 0–345 | 18.00 | 85.5 | 119.02 | 0–378 | .576 |
Note. ADOS = Autism Diagnostic Observation Scale comparison score; Mullen = Mullen Scales of Early Learning standard score; PLS EC = Preschool Language Scales Expressive Communication standard score; PLS AC = Preschool Language Scales Auditory Comprehension standard score; MCDI = MacArthur–Bates Communicative Development Inventories Total Number of Words Produced.
p < .05.
Auditory Processing Differences
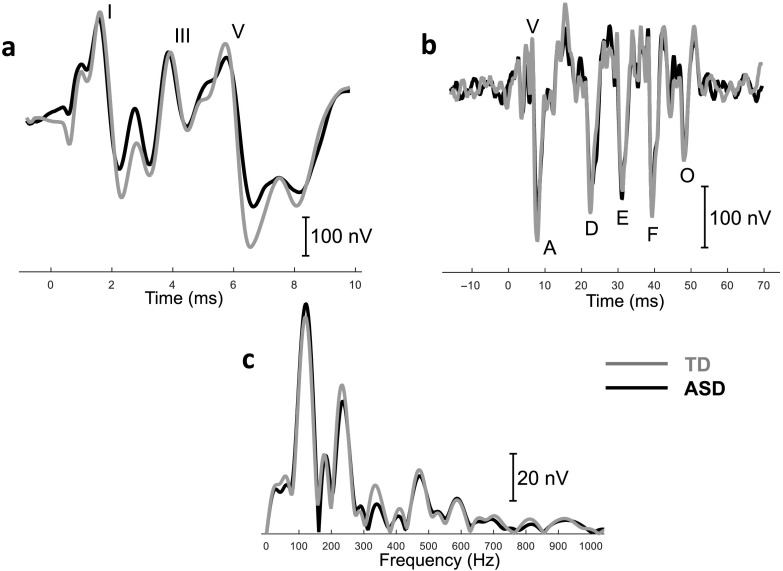
Because data were not normally distributed in the ASD group, auditory processing measures were compared between children with ASD and typically developing children using a nonparametric Wilcoxon rank-sum test. Of the 19 comparisons, differences in click Wave I–V interpeak latency, click Wave III–V interpeak latency, and /da/ Wave O latency across the two groups were statistically significant. We also followed the technique reported by Santos et al. (2017) and verified no differences between the groups in the incidence of a larger Peak I for either ABR or FFR. These results are reported in Table 4. Figures showing the average waveforms of ABRs and FFRs for both groups are shown in Figure 1.
Table 4.
Chi-square and Wilcoxon rank-sum tests comparing auditory processing measures comparing typically developing (TD) children and children with autism spectrum disorder (ASD).
| Variable | ASD |
TD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Mdn | M | SD | Mdn | W | Sig (2-sided) | Effect size (r) | |
| Click Wave I latency | 1.616 | 0.257 | 1.595 | 1.614 | 0.068 | 1.620 | 197 | .267 | .185 |
| Click Wave III latency | 3.938 | 0.244 | 3.885 | 3.914 | 0.142 | 3.930 | 158 | .911 | .019 |
| Click Wave V latency | 5.839 | 0.224 | 5.880 | 5.737 | 0.175 | 5.740 | 216 | .089 | .284 |
| Click Wave I–V interpeak latency | 4.223 | 0.228 | 4.240 | 4.120 | 0.169 | 4.120 | 224.5 | .049* | .328 |
| Click Wave III–V interpeak latency | 1.901 | 0.163 | 1.935 | 1.823 | 0.112 | 1.830 | 224.5 | .049* | .328 |
| Click Wave I amplitude | 0.285 | 0.145 | 0.270 | 0.302 | 0.104 | 0.315 | 140 | .501 | .112 |
| Click Wave V amplitude | 0.22 | 0.116 | 0.223 | 0.241 | 0.103 | 0.245 | 146 | .628 | .081 |
| /da/ Wave V latency | 6.635 | 0.300 | 6.620 | 6.524 | 0.205 | 6.530 | 201 | .220 | .204 |
| /da/ Wave A latency | 7.617 | 0.363 | 7.575 | 7.751 | 0.383 | 7.780 | 130 | .316 | .167 |
| /da/ Wave D latency | 22.422 | 0.571 | 22.280 | 22.371 | 0.389 | 22.325 | 166 | .912 | .019 |
| /da/ Wave E latency | 30.971 | 0.466 | 31.030 | 30.998 | 0.463 | 30.990 | 159.5 | .949 | .011 |
| /da/ Wave F latency | 39.456 | 0.544 | 39.360 | 39.284 | 0.316 | 39.280 | 197 | .272 | .183 |
| /da/ Wave O latency | 48.211 | 0.441 | 48.155 | 47.942 | 0.274 | 47.950 | 229 | .034* | .353 |
| /da/ Wave I amplitude | 0.067 | 0.041 | 0.058 | 0.053 | 0.066 | 0.059 | 168 | .863 | .029 |
| /da/ Wave V amplitude | 0.125 | 0.069 | 0.114 | 0.113 | 0.076 | 0.105 | 177 | .650 | .076 |
| /da/ Response consistency | 0.997 | 0.248 | 0.954 | 0.932 | 0.348 | 0.917 | 174 | .719 | .060 |
| /da/ F0 amplitude | 0.061 | 0.015 | 0.060 | 0.059 | 0.020 | 0.057 | 177 | .650 | .076 |
| /da/ F1 amplitude | 0.021 | 0.006 | 0.021 | 0.020 | 0.005 | 0.019 | 181 | .563 | .096 |
| /da/ HF amplitude |
0.006 |
0.002 |
0.006 |
0.007 |
0.002 |
0.007 |
150 |
.719 |
.060 |
|
ASD |
TD |
||||||||
|
n |
n |
χ2 |
Sig. (2-sided) |
||||||
| Click Wave I Amplitude > Wave V amplitude | 10 | 11 | 0.114 | .735 | |||||
| Click Wave V amplitude > Wave I amplitude | 8 | 7 | |||||||
| /da/ Wave I amplitude > Wave V amplitude | 3 | 6 | 1.333 | .248 | |||||
| /da/ Wave V amplitude > Wave I amplitude | 15 | 12 | |||||||
Note. Latencies are reported in milliseconds, amplitudes are reported in microvolts, and response consistency is reported as Z'. F0 = fundamental frequency; F1 = first formant; HF = high frequency.
p < .05.
Figure 1.
(a) Time domain of average click-evoked waveform for typically developing (TD) and autism spectrum disorder (ASD) groups. (b) Time domain of average speech-evoked waveform for TD and ASD groups. (c) Frequency domain of average speech-evoked waveform for TD and ASD groups.
Relationships Between Auditory Processing and Behavioral Measures
Spearman correlations were performed to analyze the relationship between a number of auditory processing and behavioral measures. Bonferroni-adjusted alpha level of .0005 was used in order to control for the number of comparisons. No significant relationships were found between any behavioral measure and measure of auditory processing that met the Bonferroni-corrected alpha level. All correlations between behavioral measures and measures of auditory processing are reported in Table 5.
Table 5.
Spearman correlations between auditory processing measures and behavioral measures.
| Variable | ADOS | Mullen | PLS EC | PLS AC | MCDI |
|---|---|---|---|---|---|
| Click Wave I latency | .233 | .263 | .138 | .035 | .035 |
| Click Wave III latency | .167 | −.023 | −.003 | −.069 | −.096 |
| Click Wave V latency | −.111 | .184 | .194 | .099 | .123 |
| Click Wave I–V interpeak latency | −.362 | .231 | .163 | −.020 | .158 |
| Click Wave III–V interpeak latency | −.504 | .293 | .211 | .073 | .187 |
| Click Wave I amplitude | −.291 | −.128 | .079 | .150 | −.081 |
| Click Wave V amplitude | .325 | −.139 | .076 | −.053 | −.481 |
| /da/ Wave V latency | .041 | .291 | .188 | .095 | .072 |
| /da/ Wave A latency | .033 | .402 | .150 | .110 | .285 |
| /da/ Wave D latency | .142 | .213 | .076 | .030 | .131 |
| /da/ Wave E latency | .083 | .324 | .140 | −.140 | −.011 |
| /da/ Wave F latency | .213 | .220 | .092 | .066 | .078 |
| /da/ Wave O latency | .664 | .158 | −.135 | −.065 | .092 |
| /da/ Wave I amplitude | .199 | .213 | −.137 | −.084 | .256 |
| /da/ Wave V amplitude | .117 | −.268 | −.300 | −.087 | −.135 |
| /da/ Response consistency | .100 | .035 | −.167 | −.167 | .317 |
| /da/ F0 amplitude | −.035 | −.382 | −.581 | −.494 | −.131 |
| /da/ F1 amplitude | −.039 | −.526 | −.265 | −.179 | −.082 |
| /da/ HF amplitude | −.021 | −.197 | −.033 | −.102 | .240 |
Note. ADOS = Autism Diagnostic Observation Scale comparison score; Mullen = Mullen Scales of Early Learning standard score; PLS EC = Preschool Language Scales Expressive Communication standard score; PLS AC = Preschool Language Scales Auditory Comprehension standard score; MCDI = MacArthur–Bates Communicative Development Inventories Total Number of Words Produced; F0 = fundamental frequency; F1 = first formant; HF = high frequency.
Discussion
Measures of auditory processing have been proposed as a potential biomarker for identifying ASD. The current study set out to address the feasibility of recording ABRs and FFRs in toddlers with autism without the use of sedation. Of the 40 participants with ASD, 18 were able to successfully complete the recording. The children who were not able to successfully complete the recording found the test too uncomfortable for quality data collection to proceed, either due to sensory issues or other demonstrated discomfort. There was a significant difference in Mullen scores between the two groups. It should be noted that Mullen scores were higher (indicating higher levels on nonverbal intelligence) in the group of children who were not able to successfully complete the recording. However, no differences in demographic measures, autism symptomatology, or language levels were observed between the successful and unsuccessful ASD groups. This suggests that toddlers at varying levels of developmental functioning may successfully complete recordings without the use of sedation. However, as less than 50% were able to complete a short recording, it also indicates the inherent difficulty in collecting these data from toddlers with ASD without sedation. Distinct clinical characteristics, such as sensory processing challenges and disruptive behaviors, may have influenced the child's ability to complete the recording. Future studies should include a specific sensory processing measure in order to address this possibility.
The current study explored the differences in auditory processing, as measured by click-evoked ABR and speech-evoked FFR, between typically developing toddlers and toddlers with ASD. Children with ASD were hypothesized to have atypical auditory processing when compared to age- and sex-matched typically developing peers. In the current study, three out of the 19 comparisons were significantly different. These include click Wave I–V interpeak latency, click Wave III–V interpeak latency, and /da/ Wave O latency. Due to the number of analyses that were performed, these results should be interpreted with caution. However, these results may suggest that an early atypical processing of sound is present in children with ASD in toddlerhood. Specifically, children with ASD may be less efficient in their processing of sound.
The current study further explored the relationship between ASD symptomatology, language measures, and nonverbal cognition with measures of auditory processing. Greater ASD severity, poorer cognitive levels, and lower language development levels were hypothesized to positively correlate with atypical auditory processing. However, no relationships were found. A lack of variation within language levels as well as a small sample size may be a factor in the lack of significant findings. Future studies should include a larger sample size of children with ASD with a wider range of language abilities. This may help to further characterize the relationship between ABRs and language development in children with ASD.
Differences in click-evoked ABRs were previously reported between toddlers with ASD and clinical norms (Miron et al., 2016; Roth et al., 2012). These studies found differences in overall latencies as well as interpeak latencies, which is consistent with the results of the current study. Differences in speech-evoked FFRs had previously been reported in children with ASD compared to typically developing children; however, the majority of these studies included school-age children (Otto-Meyer et al., 2017; Rosenhall et al., 2003; Russo et al., 2009). Only one study, to our current knowledge, has examined the development of speech-evoked FFRs in preschool children with ASD, and this study reported atypical development of speech-evoked FFRs (Chen et al., 2019). Further research is necessary in order to characterize the development of auditory processing in toddlers with ASD.
Research has suggested that click-evoked ABRs and speech-evoked FFRs are malleable throughout development and are influenced by life experiences. Moreover, each of the auditory processing measures (e.g., latency and amplitude) in response to both click and speech sounds has differential developmental trajectories (Skoe et al., 2015). On average, Wave V latencies, in response to both click and speech sounds, become shorter between infancy and 3–5 years old. Between 5 and 11 years old, Wave V latencies become longer and stabilize throughout adulthood. Additionally, amplitude measures in response to speech sounds increase between infancy and early childhood. Starting around 5–11 years old, amplitude measures progressively decrease, and this trend continues throughout adulthood. Previously reported atypical ABRs in older children with ASD may be a reflection of the neurological impact that ASD has on the sound encoding process over time. Future studies should include longitudinal investigations to address the development of atypical auditory processing in children with ASD. A recent study by Gopal et al. (2019) showed that auditory training may lead to changes in ABR latencies and amplitudes as well as FFR latencies in wide age range of young adults with ASD. These findings suggest that objective electrophysiological measures may be an important method to assess the efficacy of auditory training and the impact on auditory processing in children with ASD. Understanding the development of atypical auditory processing in toddlers with ASD may inform the development of auditory training methods that aim to target atypical auditory processing in children with ASD. This presents an additional avenue of further investigation in children with ASD.
Studying toddlers with ASD is essential to understanding the impact of the sound encoding process on language development. Previous research has suggested that lower language levels may be a result of deficiencies in the early stages of the sound encoding process (Banai et al., 2009; Chen et al., 2019; Chonchaiya et al., 2012). Although the current study found some deficiencies in the sound encoding process for children with ASD, there were no relationships between auditory processing measures and language development measures. Relationship between auditory processing measures and language development in children with ASD should be further explored. The extent to which the sound encoding process may impact early language development in toddlers with ASD should also be further evaluated.
Although there were no significant associations between language development and auditory processing measures in the current study, many other studies have found differences in auditory processing measures in other older populations with language learning difficulties (Banai et al., 2009; Chonchaiya et al., 2012; Hornickel & Kraus, 2013). It should be noted that electrophysiological measures, specifically the FFR, may provide an objective measure to characterize the role of perceptual processing of auditory information and the impact on language learning difficulties.
Findings from the current study are preliminary and should be interpreted as such. Limitations of the current study include a small sample size as well as a high attrition rate in the group of children with ASD. While attrition is high, this was expected, and this limitation is offset by the fact that this is the first study that has analyzed frequency encoding and response consistency measures of speech-evoked FFRs in toddlers with ASD. Studies that include large sample sizes of toddlers with ASD are necessary in order to further characterize the relationship between auditory processing and ASD symptomatology. It is also important to consider the heterogeneity inherent to ASD. The current study included toddlers with ASD with lower language levels. Atypical auditory processing may be present in a different subgroup of children with ASD. Finally, there is a chance that the two groups had a different reaction to the movie soundtrack that was playing softly in the background during testing. Because the soundtrack was not synchronized with the stimulus presentation, its effect should be minimal. However, we cannot rule out that a different influence of masking between the two groups may have obscured a finding. Auditory processing differences have been shown in older children with ASD, specifically high-functioning children with ASD (Ramezani et al., 2019). Future directions should focus on understanding the association between auditory processing differences and ASD core symptomatology (namely, social communication and restricted/repetitive behaviors), independent of language development levels. Taken together, these limitations suggest that future research across all age groups of children with ASD as well as across various presentations of ASD symptomatology is needed.
Overall, the understanding of auditory processing in children with ASD is still limited. Further research is necessary in order to evaluate the use of ABRs and FFRs as potential biomarkers for ASD. Current characterization of auditory profiles in toddlers with ASD is limited and varied. In order to fully characterize auditory profiles of children with ASD, it may be necessary to employ subcortical and cortical measures that predict ASD symptomatology. Additionally, future studies should employ a longitudinal, prospective approach to track auditory profiles of high-risk infant siblings of children with ASD. Doing so would advance our understanding of the early development of auditory profiles of children with ASD and address the potential utility of ABRs and FFRs as potential biomarkers for ASD. It is also essential that our understanding of neural auditory processing includes the wide heterogeneous presentation of ASD symptomatology across all ages and subgroups of children with ASD.
Acknowledgments
This research was supported in part by the National Institutes of Health (1R01DC014709 awarded to Megan Y. Roberts, UL1TR001422 awarded to Northwestern University Clinical and Translational Science Institute). The authors would like to thank Alana Glickman for help with data collection, as well as all participating families for their time and commitment.
Funding Statement
This research was supported in part by the National Institutes of Health (1R01DC014709 awarded to Megan Y. Roberts, UL1TR001422 awarded to Northwestern University Clinical and Translational Science Institute).
References
- Amin S. B., Vogler-Elias D., Orlando M., & Wang H. (2014). Auditory neural myelination is associated with early childhood language development in premature infants. Early Human Development, 90(10), 673–678. https://doi.org/10.1016/j.earlhumdev.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K., Hornickel J., Skoe E., Nicol T., Zecker S., & Kraus N. (2009). Reading and subcortical auditory function. Cerebral Cortex, 19(11), 2699–2707. https://doi.org/10.1093/cercor/bhp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl W., Tierney A., Tager-Flusberg H., & Nelson C. (2011). EEG complexity as a biomarker for autism spectrum disorder risk. BMC Medicine, 9(1), 18 https://doi.org/10.1186/1741-7015-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B., & Kraus N. (2010). The scalp-recorded brainstem response to speech: Neural origins and plasticity. Psychophysiology, 47(2), 236–246. https://doi.org/10.1111/j.1469-8986.2009.00928.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liang C., Wei Z., Cui Z., Kong X., Dong C., Lai Y., Peng Z., & Wan G. (2019). Atypical longitudinal development of speech-evoked auditory brainstem response in preschool children with autism spectrum disorders. Autism Research, 12(7), 1022–1031. https://doi.org/10.1002/aur.2110 [DOI] [PubMed] [Google Scholar]
- Chonchaiya W., Tardif T., Mai X., Xu L., Li M., Kaciroti N., Kileny P. R., Shao J., & Lozoff B. (2012). Developmental trends in auditory processing can provide early predictions of language acquisition in young infants. Developmental Science, 16(2), 159–172. https://doi.org/10.1111/desc.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Mouton P. R., Calhoun M. E., Semendeferi K., Ahrens-Barbeau C., Hallet M. J., Barnes C. C., & Pierce K. (2011). Neuron number and size in prefrontal cortex of children with autism. The Journal of the American Medical Association, 306(18), 2001–2010. https://doi.org/10.1001/jama.2011.1638 [DOI] [PubMed] [Google Scholar]
- Dunn M. A., Gomes H., & Gravel J. (2008). Mismatch negativity in children with autism and typical development. Journal of Autism and Developmental Disorders, 38(1), 52–71. https://doi.org/10.1007/s10803-007-0359-3 [DOI] [PubMed] [Google Scholar]
- Esler A. N., Bal V. H., Guthrie W., Wetherby A., Weismer S. E., & Lord C. (2015). The Autism Diagnostic Observation Schedule–Toddler Module: Standardized severity scores. Journal of Autism and Developmental Disorders, 45(9), 2704–2720. https://doi.org/10.1007/s10803-015-2432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L., Bates E., Dale P. S., Marchman V. A., Reznick J. S., & Thal D. J. (2007). MacArthur–Bates Communicative Development Inventories. Brookes. [Google Scholar]
- Gopal K. V., Schafer E. C., Mathews L., Nandy R., Beaudoin D., Schadt L., Brown A., Phillips B., & Caldwell J. (2019). Effects of auditory training on electrophysiological measures in individuals with autism spectrum disorder. Journal of the American Academy of Audiology, 31(2), 96–104. https://doi.org/10.3766/jaaa18063 [DOI] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., & Conde J. G. (2008). Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., & Kraus N. (2013). Unstable representation of sound: A biological marker of dyslexia. Journal of Neuroscience, 33(8), 3500–3504. https://doi.org/10.1523/JNEUROSCI.4205-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Verkasalo E., Ceponiene R., Kielinen M., Suominen K., Jäntti V., Linna S.-L., Moilanene I., & Näätänen R. (2003). Deficient auditory processing in children with Asperger syndrome, as indexed by event-related potentials. Neuroscience Letters, 338(3), 197–200. https://doi.org/10.1016/S0304-3940(02)01405-2 [DOI] [PubMed] [Google Scholar]
- Krizman J., & Kraus N. (2019). Analyzing the FFR: A tutorial for decoding the richness of auditory function. Hearing Research, 382, 107779 https://doi.org/10.1016/j.heares.2019.107779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P. K., Coffey-Corina S., Padden D., & Dawson G. (2005). Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science, 8(1), F1–F12. https://doi.org/10.1111/j.1467-7687.2004.00384.x [DOI] [PubMed] [Google Scholar]
- Lepistö T., Kajander M., Vanhala R., Alku P., Huotilainen M., Näätänen R., & Kujala T. (2008). The perception of invariant speech features in children with autism. Biological Psychology, 77(1), 25–31. https://doi.org/10.1016/j.biopsycho.2007.08.010 [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P. C., Risi S., Gotham K., & Bishop S. (2012). Autism Diagnostic Observation Schedule–Second Edition (ADOS-2). Western Psychological Services. [Google Scholar]
- Miron O., Ari-Even Roth D., Gabis L. V., Henkin Y., Shefer S., Dinstein I., & Geva R. (2016). Prolonged auditory brainstem responses in infants with autism. Autism Research, 9(6), 689–695. https://doi.org/10.1002/aur.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron O., Beam A. L., & Kohane I. S. (2017). Auditory brainstem response in infants and children with autism spectrum disorder: A meta-analysis of Wave V. Autism Research, 11(2), 355–363. https://doi.org/10.1002/aur.1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. M. (1995). Mullen Scales of Early Learning (pp. 58–64). AGS. [Google Scholar]
- National Research Council. (2001). Educating children with autism. Committee on Educational Interventions for Children With Autism. In Lord C. & McGee J. P. (Eds.), Division of behavioral and social sciences and education. National Academy Press; https://doi.org/10.17226/10017 [Google Scholar]
- O'Connor K. (2012). Auditory processing in autism spectrum disorder: A review. Neuroscience & Biobehavioral Reviews, 36(2), 836–854. https://doi.org/10.1016/j.neubiorev.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Otto-Meyer S., Krizman J., White-Schwoch T., & Kraus N. (2017). Children with autism spectrum disorder have unstable neural responses to sound. Experimental Brain Research, 236(3), 733–743. https://doi.org/10.1007/s00221-017-5164-4 [DOI] [PubMed] [Google Scholar]
- Padish-Clarin G., & Hawkins J. (2015). Retrospective analysis of decreasing the use of anesthesia in pediatric audiology: A preliminary study. American Journal of Audiology, 24(4), 557–562. https://doi.org/10.1044/2015_AJA-15-0043 [DOI] [PubMed] [Google Scholar]
- Ramezani M., Lotfi Y., Moossavi A., & Bakhshi E. (2019). Auditory brainstem response to speech in children with high functional autism spectrum disorder. Neurological Sciences, 40(1), 121–125. https://doi.org/10.1007/s10072-018-3594-9 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Redcay E., & Courchesne E. (2005). When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry, 58(1), 1–9. https://doi.org/10.1016/j.biopsych.2005.03.026 [DOI] [PubMed] [Google Scholar]
- Rosenhall U., Nordin V., Brantberg K., & Gillberg C. (2003). Autism and auditory brain stem responses. Ear and Hearing, 24(3), 206–214. https://doi.org/10.1097/01.AUD.0000069326.11466.7E [DOI] [PubMed] [Google Scholar]
- Rosenthal R. (1994). Parametric measures of effect size. In Cooper H. & Hedges L. V. (Eds.), The handbook of research synthesis (pp. 231–244). Russell Sage Foundation. [Google Scholar]
- Roth D. A.-E., Muchnik C., Shabtai E., Hildesheimer M., & Henkin Y. (2012). Evidence for atypical auditory brainstem responses in young children with suspected autism spectrum disorders. Developmental Medicine & Child Neurology, 54(1), 23–29. https://doi.org/10.1111/j.1469-8749.2011.04149.x [DOI] [PubMed] [Google Scholar]
- Russo N. M., Nicol T., Trommer B., Zecker S., & Kraus N. (2009). Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Developmental Science, 12(4), 557–567. https://doi.org/10.1111/j.1467-7687.2008.00790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N. M., Skoe E., Trommer B., Nicol T., Zecker S., Bradlow A., & Kraus N. (2008). Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clinical Neurophysiology, 119(8), 1720–1731. https://doi.org/10.1016/j.clinph.2008.01.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Marques C., Pinto A. N., Fernandes R., Coutinho M. B., & Almeida E Sousa C. (2017). Autism spectrum disorders and the amplitude of auditory brainstem response Wave I. Autism Research, 10(7), 1300–1305. https://doi.org/10.1002/aur.1771 [DOI] [PubMed] [Google Scholar]
- Skoe E., & Kraus N. (2010). Auditory brainstem response to complex sounds: A tutorial. Ear and Hearing, 31(3), 302 https://doi.org/10.1097/AUD.0b013e3181cdb272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E., Krizman J., Anderson S., & Kraus N. (2015). Stability and plasticity of auditory brainstem function across the lifespan. Cerebral Cortex, 25(6), 1415–1426. https://doi.org/10.1093/cercor/bht311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas A., Yagiz R., Tas M., Esme M., Uzun C., & Karasalihoglu A. R. (2007). Evaluation of hearing in children with autism by using TEOAE and ABR. Autism, 11(1), 73–79. https://doi.org/10.1177/1362361307070908 [DOI] [PubMed] [Google Scholar]
- Whitehouse A. J., & Bishop D. V. (2008). Do children with autism ‘switch off' to speech sounds? An investigation using event-related potentials. Developmental Science, 11(4), 516–524. https://doi.org/10.1111/j.1467-7687.2008.00697.x [DOI] [PubMed] [Google Scholar]
- White-Schwoch T., & Kraus N. (2017). The Janus face of auditory learning: How life in sound shapes everyday communication. In Kraus N., Anderson S., White-Schwoch T., Fay R. R., & Popper A. N. (Eds.), The frequency-following response: A window into human communication. Springer-Nature. [Google Scholar]
- Wible B., Nicol T., & Kraus N. (2005). Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain, 128(2), 417–423. https://doi.org/10.1093/brain/awh367 [DOI] [PubMed] [Google Scholar]
- Zimmerman I. L., Steiner V. G., & Pond R. E. (2011). Preschool Language Scales–Fifth Edition (PLS-5). Pearson. [Google Scholar]
- Zwaigenbaum L., Bauman M. L., Choueiri R., Kasari C., Carter A., Granpeesheh D., Mailloux Z., Roley S. S., Wagner S., Fein D., Pierce K., Buie T., Davis P. A., Newschaffer C., Robins D., Wetherby A., Stone W. L., Yirmiya N., Estes A., & Natowicz M. R. (2015). Early intervention for children with autism spectrum disorder under 3 years of age: Recommendations for practice and research. Pediatrics, 136(Suppl. 1), S60–S81. https://doi.org/10.1542/peds.2014-3667E [DOI] [PMC free article] [PubMed] [Google Scholar]