Abstract
Purpose
The purpose of this study is to examine the differences in velopharyngeal dimensions as well as levator veli palatini (levator) muscle morphology, positioning, and symmetry of children with repaired cleft palate with velopharyngeal insufficiency (VPI), children with repaired cleft palate with complete velopharyngeal closure, and children with noncleft anatomy.
Method
Fifteen children ranging in age from 4 to 8 years were recruited for this study. Ten of the participants had a history of repaired cleft palate, half with documented VPI and the other half with velopharyngeal closure. Five participants with noncleft anatomy were matched for age from a normative database. The magnetic resonance imaging protocol, processing methods, and analysis are consistent with that used in previous literature.
Results
Regarding velopharyngeal dimensions, median values were statistically significantly different between groups for sagittal angle (p = .031) and effective velopharyngeal ratio (p = .013). With respect to the levator muscle, median values were statistically significant for average extravelar length (p = .018), thickness at midline (p = .021), and thickness between the left and right muscle bundles at the point of insertion into the velum (p = .037). Remaining measures were not statistically significant.
Conclusions
The levator muscle is significantly different among these three groups with respect to thickness at midline, extravelar length, and symmetry at the point of insertion into the velum. Sagittal angle and effective velopharyngeal ratio are also significantly different. Participants with repaired cleft palate and VPI displayed the greatest degree of asymmetry. Future research should control for surgical procedure type to determine the impact of surgery on the levator muscle and surrounding velopharyngeal anatomy.
The levator veli palatini (levator) muscle is widely accepted to play the most significant role in velar elevation to close off the passageway between the oral and nasal cavities for speech and swallowing (Bell-Berti, 1976; Dickson & Dickson, 1972; Hoopes et al., 1969; Moon et al., 1994; Perry, 2011). In adult individuals with noncleft anatomy, there is relatively consistent size, shape, and location of the levator muscle making up the middle one third of the soft palate, coursing without interruption via cohesive sling (Kuehn & Moon, 2005). However, when there is a cleft of the palate, the levator muscle bundles insert anteriorly, proximal to the posterior aspect of the hard palate, and need to be surgically altered to function properly. A primary palatoplasty is completed to achieve closure of the cleft in the hard and soft palate and create a physiological mechanism conducive to the development and production of normal speech.
Past research has found variation in morphology among adults with repaired cleft palate. Ha et al. (2007) found variation between four adult men with repaired cleft palate, including varying length and thickness of the levator muscle among the four participants. The distance between origin points, length, and thickness of the levator muscle bundles were smaller than those of noncleft anatomy reported by Ettema et al. (2002). Kotlarek et al. (2017) compared levator form via total volume, circumference, and diameter measures between adults with and without repaired cleft palate. Differences in total levator volume as well as circumference and diameter were found, specifically at the point of insertion of the levator muscle into the velum and at the velar midline. Perry et al. (2018) examined differences in velopharyngeal structures between adults with repaired cleft palate and normal resonance and adults without cleft palate, which revealed significant differences in measures of the hard palate, levator muscle, and velopharyngeal port. The authors found that, even in the absence of hypernasality, differences exist between adults with cleft and noncleft anatomy. It is currently unknown which of these variables are important to predicting velopharyngeal closure (VPC).
Significant differences between cleft and noncleft anatomy have also been observed in children. Kuehn et al. (2004) studied two children with cleft palate who underwent a Furlow double-opposing Z-plasty for primary repair of the palate. Postoperatively, both patients exhibited a cohesive midline and improved speech, but one patient still required further surgical intervention due to persisting velopharyngeal dysfunction. Nakamura et al. (2003) studied a group of seven children with repaired cleft palate and persistent velopharyngeal insufficiency (VPI) and found that the velar length was shorter and the pharyngeal length-to-depth ratio was significantly smaller than those with noncleft anatomy. Tian et al. (Tian, Li, et al., 2010; Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) studied similar groups to compare 19 participants with repaired cleft palate with and without VPI to a normative control group. They found that the group with VPI had a significantly shorter posterior velar length and longer uvular pharyngeal depth compared to those with VPC (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010). It has been hypothesized that these variables may be required for adequate VPC. When compared to the normative control group, significant differences were present for both cleft groups for measures of levator insertion width, hard palate length, pharyngeal depth (as measured in three ways, from the posterior hard palate to the posterior pharyngeal wall, basion, and the first cranial vertebra), and maxillary index (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010). Similar to the adult population with repaired cleft palate, differences also exist in children with repaired cleft palate despite velopharyngeal status.
Additional studies have also examined function of the velopharyngeal port during speech tasks. Through comparison of rest and sustained phonation tasks of 29 participants, it was found that the cleft group with VPI displayed significantly reduced mobility of the velum and lateral pharyngeal walls even though the levator muscle demonstrated sufficient function during elevation and contraction (Tian, Li, et al., 2010; Tian, Yin, Redett, et al., 2010). Perry et al. (2016) suggested that significant deviations from normative, noncleft velopharyngeal measures may attribute to aberrant function for normal resonance.
The levator muscle is widely considered a bilateral, symmetric muscle across the literature. Therefore, several investigations have used measurements along one side of the muscle to describe the form of the levator muscle. Although it is likely that the noncleft population exhibits bilateral muscle bundles of the same form and size, it is unclear whether surgical restoration of the soft palate during primary palatoplasty aims to restore the symmetrical nature of the levator muscle sling. Park et al. (2015) compared the levator muscle of 17 participants with 22q11.2 deletion syndrome to nine participants with nonsyndromic submucous cleft palate, indicating a thinner muscle with a greater degree of asymmetry in individuals from this syndromic population. It was proposed that the asymmetry and thinness observed in the syndromic population may lead to suboptimal results after a secondary palatal surgery that depends on levator muscle function (Park et al., 2015). To the best of our knowledge, symmetry of the levator muscle within individuals with repaired cleft palate has not been reported in the literature. It is plausible that, due to surgical intervention, symmetry of the levator muscle may not be established in children with repaired cleft palate.
The purpose of this study was to examine the two- and three-dimensional differences in the velopharyngeal port and levator muscle morphology of children with repaired cleft palate compared to children with noncleft anatomy. It was hypothesized that children with repaired cleft palate, and more so those with VPI, would exhibit greater asymmetry and anterior positioning of the levator muscle. Previous studies have failed to address the impact of levator muscle anatomy on three-dimensional measures, such as levator muscle volume and thickness, which has been found to be significantly different between adults with and without repaired cleft palate (Kotlarek et al., 2017). Furthermore, previous investigations regarding comparisons between children with and without repaired cleft palate have not addressed symmetry between right and left bundles of the levator muscle. Muscular symmetry is of specific interest due to the laterality of some forms of clefting and the effect of surgery on the restoration of the levator muscle.
Method
Participants
In accordance with the local institutional review boards, 15 English-speaking children ranging in age from 4 to 8 years (M age = 6.24 years, SD = 1.1 years) were recruited for this study. Ten of the participants had a history of repaired cleft palate, half with documented VPI (M age = 6.75 years, SD = 1.3 years) and the other half with adequate VPC (M age = 5.77 years, SD = 0.7 years). Participants with repaired cleft palate were recruited from three different craniofacial teams. Five participants with noncleft anatomy were matched for age from a normative database (M age = 6.21 years, SD = 1.3 years). Participants were not matched for sex or race due to the lack of sexual dimorphism regarding velopharyngeal variables in children within this age range (Perry et al., 2018). Participant details are reported in Table 1. Within the VPI group, two participants had bilateral complete cleft lip and palate, and three had unilateral left cleft lip and palate; the VPC group contained two participants with bilateral complete cleft lip and palate, one with unilateral left cleft lip and palate and two with cleft palate only. All of the participants with cleft palate underwent primary palatoplasty between the ages of 6 and 18 months. Surgical repair of the palate was completed by different surgeons using either a V-Y pushback (seven participants) or a Furlow double-opposing Z-plasty (three participants). None of the participants had received secondary palate repair at the time of the magnetic resonance imaging (MRI) study. For participants with repaired cleft palate, velopharyngeal status at the time of scanning (VPC or VPI group) was determined through information provided in the craniofacial team report. For the purpose of this study, the VPI group was defined as those participants who were referred for secondary surgical management by the craniofacial team following instrumental assessment using nasendoscopy. All participants within the normative control group had typical speech. For all participants, perceptual resonance evaluations were completed at the time of the MRI study by a speech-language pathologist with a minimum of 5 years of experience in craniofacial speech evaluations to confirm group inclusion.
Table 1.
Description of participant groups in this study.
| Group | n | M age (years) | SD (years) |
|---|---|---|---|
| Noncleft | 5 | 6.21 | 1.3 |
| VPC | 5 | 5.77 | 0.7 |
| VPI | 5 | 6.75 | 1.3 |
| Total | 15 | 6.24 | 1.1 |
Note. VPC = velopharyngeal closure; VPI = velopharyngeal insufficiency.
MRI Protocol and Analyses
Five different MRI scanners were used to scan the participants for this study because the participants were recruited in different geographic regions. MRI protocols were designed to produce similar images between scanners by establishing sequences across all scanners that yielded a similar in-plane isotropic resolution (0.8). Reliability between scanners has been reported in previous literature (Perry et al., 2018). The imaging protocol was consistent with that used in previous MRI investigations of the velopharyngeal muscles (Perry et al., 2013). All participants were scanned using a head coil while lying in the supine position. Children were prepared for the scan following a child-friendly protocol without the use of sedation (Kollara & Perry, 2014).
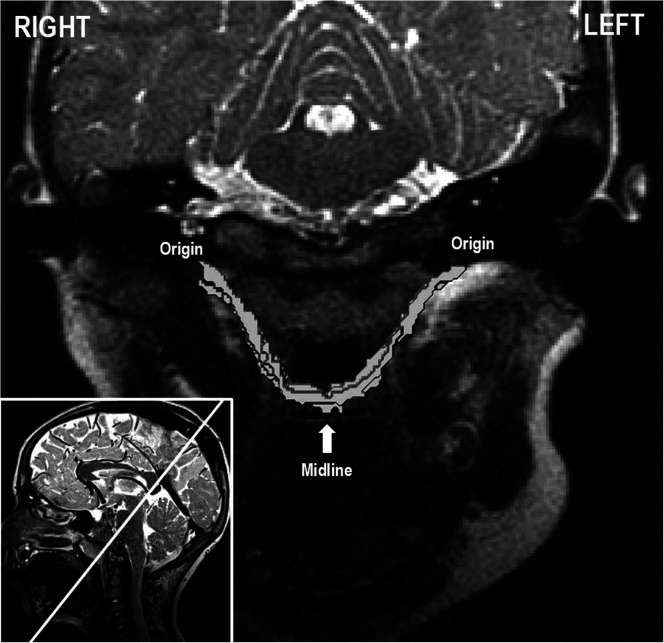
The MRI processing methods are consistent with that used in previous literature (Perry & Kuehn, 2007, 2009; Perry et al., 2013, 2011). Specifically, raw magnetic resonance images were transferred into Thermo Scientific Amira Software (Thermo Fisher Scientific), which includes a native Digital Imaging and Communications in Medicine support program to ensure that anatomical geometry was maintained. The entire data set was resampled from the three-dimensional anatomical scan to obtain the oblique coronal image for the full sling of the levator muscle to be visualized (see Figure 1). For volumetric measures, the levator muscle fibers were defined by manual segmentation of successive oblique coronal images, from which a voxel set was created and volume was calculated (Kotlarek et al., 2017). Two-dimensional measures of interest were taken from both the midsagittal and central-most oblique coronal image planes. In addition, thickness measurements were taken at six designated points along both sides of the length of the levator muscle belly, as described by Perry et al. (2013). All definitions of measures and terminology are detailed in Table 2. Averages between the left and right sides were calculated within the same participant. Symmetry measures were calculated using the absolute value of the difference between the right and left sides. Both average and symmetry measures were completed on the following levator measures: angle of origin, length, extravelar length, intravelar length, and muscle thickness at Points 1–5.
Figure 1.
In the lower left corner, a midsagittal image is shown with the white line depicting the oblique coronal image plane. This image plane reveals the levator muscle in its entirety from origin to insertion at midline. The oblique coronal image with the surface generation of the levator muscle volume is shown in the larger image.
Table 2.
List of measurements and corresponding definitions.
| Velopharyngeal variables | |
|---|---|
| Pharyngeal depth (PNS-PPW) | Linear distance (mm) between the posterior nasal spine and posterior pharyngeal wall (or adenoid) at the level of the palatal plane as seen on the midsagittal image |
| Velar thickness | Linear distance (mm) from the oral to the nasal surface of the velum at the thickest point from the midsagittal image |
| Velar length | Curvilinear distance (mm) from the posterior nasal spine to the uvular tip |
| Effective velar length | Linear distance (mm) from the posterior nasal spine to the middle of the levator muscle where it inserts into the body of the velum as seen on the midsagittal image |
| Muscle pharyngeal depth | Linear distance (mm) from the middle of the levator muscle where it inserts into the body of the velum to the posterior pharyngeal wall (or adenoid) parallel to the palatal plane as seen on the midsagittal image |
| Velopharyngeal ratio | Velar length divided by pharyngeal depth |
| Effective velopharyngeal ratio | Effective velar length divided by pharyngeal depth |
| Sagittal angle | Internal angle (degrees) between the plane of the levator muscle and the line coursing through the anterior tubercle of the 3rd and 4th cervical vertebrae as seen on the midsagittal image |
|
Levator muscle variables | |
| Origin to origin distance | Linear distance (mm) between the two points of origin for the right and left levator muscle bundles as seen on the oblique coronal image |
| Levator length | Curvilinear distance (mm) of the levator muscle from the base of the skull (origin) through the midline of the muscle bundle as seen on the oblique coronal image |
| Angle of origin | Angle (degrees) created by the line connecting the two temporal origins of the levator muscle and the line coursing through the levator muscle bundles as seen on the oblique coronal image |
| Extravelar segment length | Curvilinear distance (mm) of the levator muscle from base of the skull (origin) through the midline of the muscle bundle to the point where the muscle inserts into the body of the velum as seen on the oblique coronal image |
| Intravelar segment length | Curvilinear distance (mm) of the levator muscle from the point where the muscle inserts into the body of the velum to midline as seen on the oblique coronal image |
| Velar insertion distance | Linear distance (mm) between the locations where the levator bundles insert into the body of the velum as seen on the midsagittal image |
| Total volume | Total volume (mm3) of the levator muscle calculated from a voxel set of consecutive oblique coronal images |
| Muscle thickness | Side-to-side (medial–lateral for Points 1–3; superior–inferior for Points 4–6) diameter of the levator muscle as seen on the oblique coronal image |
Note. PNS = posterior nasal spine; PPW = posterior pharyngeal wall.
Statistical Treatment
Due to the small sample size and nonnormal distribution of data, nonparametric statistical analyses were adopted for comparing measures among the participant groups. All assumptions were adequately met for the Kruskal–Wallis H test, including a continuous dependent variable; one independent variable consisting of two or more categorical, independent groups; and independence of observations. Multiple Kruskal–Wallis H tests were conducted using IBM SPSS 24.0 (IBM Corp.) to determine if there were differences in the aforementioned variables among groups that differed in their velopharyngeal status: the “noncleft” (n = 5), “cleft with VPC” (n = 5), and “cleft with VPI” (n = 5) groups. Median values were also provided. Pairwise comparisons were conducted with Bonferroni correction for multiple comparisons, and adjusted p values were presented.
An intraclass correlation was used to assess inter- and intrarater reliability. Reliability was completed on 100% of participants using angle measures due to angles having the lowest reliability in previously reported MRI studies of the velopharyngeal mechanism. Interrater reliability ranged from .839 to .964, which was calculated using separate measurements competed by two researchers with experience in three-dimensional MRI data analyses. Intrarater reliability was completed on the same set of angle measures 2 weeks later. Intrarater reliability ranged from .824 to .997 for these selected measures.
Results
Multiple Kruskal–Wallis H tests were utilized to determine if there were differences in the variables among the three groups of participants. Participant age was not significantly different (p = .623) among the noncleft (M = 6.21 years, Mdn = 5.92 years), VPI (M = 6.75 years, Mdn = 7.23 years), and VPC (M = 5.77 years, Mdn = 5.89 years) groups. Facial height was also not significantly different (p = .093) among the noncleft (M = 91.08 mm, Mdn = 93.16 mm), VPI (M = 100.91 mm, Mdn = 99.39 mm), and VPC (M = 92.38 mm, Mdn = 88.92 mm) groups. Due to the small sample size and the nature of these nonparametric statistical methods, the influence of growth was not considered in further analysis. Additionally, age and facial height were not statistically significant among groups.
Regarding measures of the velopharynx, median values were significantly different across groups for sagittal angle, χ2(2) = 6.980, p = .031. Median sagittal angle measures increased from noncleft (50.2°) to cleft with VPC (57.8°) to cleft with VPI (69.7°) groups. Post hoc analysis revealed a significant difference in sagittal angle between the noncleft (mean rank = 4.60) and cleft with VPI (mean rank = 12.00; p = .027) groups but not between any other group combination. Median values were also significantly different for effective velopharyngeal ratio, χ2(2) = 8.720, p = .013, increasing from cleft with VPI (0.18) to noncleft (0.65) to cleft with VPC (0.72) groups. Post hoc analysis revealed a statistically significant difference in effective velopharyngeal ratio between the cleft with VPI (mean rank = 3.20) and noncleft (mean rank = 10.00, p = .049) groups as well as the cleft with VPI and cleft with VPC (mean rank = 10.80; p = .022) groups. Remaining measures of the velopharynx were not found to be significantly different among groups. Results regarding velopharyngeal dimensions are depicted in Table 3.
Table 3.
Results of the Kruskal–Wallis H test regarding velopharyngeal and levator muscle measures.
| Variable | Mdn |
H test |
Pairwise |
|||
|---|---|---|---|---|---|---|
| χ2 | p | Mean ranks | p | |||
| Velar length | VPI: 21.05 | 2.940 | .230 | |||
| VPC: 22.22 | ||||||
| Non: 25.79 | ||||||
| PNS-PPW | VPI: 20.70 | 2.420 | .298 | |||
| VPC: 14.21 | ||||||
| Non: 19.29 | ||||||
| VP ratio | VPI: 1.07 | 4.580 | .101 | |||
| VPC: 1.59 | ||||||
| Non: 1.34 | ||||||
| Effective velar length | VPI: 3.74 | 4.740 | .093 | |||
| VPC: 10.46 | ||||||
| Non: 12.48 | ||||||
| Muscle pharyngeal depth | VPI: 13.15 | 3.420 | .181 | |||
| VPC: 8.86 | ||||||
| Non: 6.04 | ||||||
| Effective VP ratio | VPI: .18 | 8.720 | .013* | VPI: 3.20 | Non: 10.00 | .049* |
| VPC: .72 | VPC: 10.80 | VPI: 3.20 | .022* | |||
| Non: .65 | Non: 10.00 | VPC: 10.80 | 1.00 | |||
| Velar thickness (midline) | VPI: 7.87 | .045 | .978 | |||
| VPC: 7.50 | ||||||
| Non: 6.88 | ||||||
| Sagittal angle | VPI: 69.7 | 6.980 | .031* | VPI: 12.00 | Non: 4.60 | .027* |
| VPC: 57.8 | VPC: 7.40 | VPI: 12.00 | .312 | |||
| Non: 50.2 | Non: 4.60 | VPC: 7.40 | .967 | |||
| Total levator volume | VPI: 1296.42 | .780 | .677 | |||
| VPC: 1489.13 | ||||||
| Non: 1741.80 | ||||||
| Velar insertion distance | VPI: 21.06 | .780 | .677 | |||
| VPC: 22.10 | ||||||
| Non: 17.34 | ||||||
| Origin–origin distance | VPI: 58.21 | .720 | .698 | |||
| VPC: 58.78 | ||||||
| Non: 57.30 | ||||||
| Angle of origin | VPI: 54.8 | .155 | .925 | |||
| VPC: 54.9 | ||||||
| Non: 54.1 | ||||||
| Levator length | VPI: 40.28 | .780 | .677 | |||
| VPC: 39.89 | ||||||
| Non: 41.61 | ||||||
| Extravelar length | VPI: 25.30 | 7.980 | .018* | VPI: 4.60 | Non: 7.00 | 1.000 |
| VPC: 31.85 | VPC: 12.40 | VPI: 4.60 | .017* | |||
| Non: 25.99 | Non: 7.00 | VPC: 12.40 | .169 | |||
| Intravelar length | VPI: 12.29 | .060 | .970 | |||
| VPC: 12.10 | ||||||
| Non: 10.98 | ||||||
| Levator thickness, 1 (origin) | VPI: 3.09 | .105 | .949 | |||
| VPC: 2.89 | ||||||
| Non: 2.86 | ||||||
| Levator thickness, 2 (between 1 and 3) | VPI: 2.55 | 1.680 | .432 | |||
| VPC: 2.83 | ||||||
| Non: 3.20 | ||||||
| Levator thickness, 3 (between 1 and 4) | VPI: 2.60 | 2.180 | .336 | |||
| VPC: 2.99 | ||||||
| Non: 3.34 | ||||||
| Levator thickness, 4 (velar insertion) | VPI: 3.95 | 1.680 | .432 | |||
| VPC: 2.88 | ||||||
| Non: 2.91 | ||||||
| Levator thickness, 5 (between 4 and 6) | VPI: 1.81 | 1.260 | .533 | |||
| VPC: 3.05 | ||||||
| Non: 2.72 | ||||||
| Levator thickness, 6 (midline) | VPI: 1.32 | 7.692 | .021* | VPI: 4.30 | Non: 12.10 | .017* |
| VPC: 3.16 | VPC: 7.60 | VPI: 4.30 | .727 | |||
| Non: 3.81 | Non: 12.10 | VPC: 7.60 | .333 | |||
Note. Pertinent pairwise comparisons are displayed. All values are in millimeters, with the exception of volume (mm3), angle (°), and ratio (no units) variables. VPI = velopharyngeal insufficiency; VPC = velopharyngeal closure; Non = noncleft. PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal
p < .05.
With respect to the levator muscle, median values were significantly different for extravelar length, χ2(2) = 7.980, p = .018. Median extravelar length increased from cleft with VPI (25.30 mm) to noncleft (25.98 mm) to cleft with VPC (31.85 mm) groups. Post hoc analysis revealed a statistically significant difference in extravelar length between the cleft with VPC (mean rank = 12.40) and cleft with VPI (mean rank = 4.60; p = .017) groups but not between any other group combination. Median values were also significantly different for levator muscle thickness at midline, χ2(2) = 7.692, p = .021, increasing from cleft with VPI (1.32 mm) to cleft with VPC (3.16 mm) to noncleft (3.81 mm) groups. Post hoc analysis revealed a statistically significant difference in levator muscle thickness at midline between the noncleft (mean rank = 12.10) and cleft with VPI (mean rank = 4.30, p = .017) groups but not between any other group combination. Median values were significantly different for the difference in thickness between the left and right levator muscle bundles at the point of insertion into the velum, χ2(2) = 6.620, p = .037, increasing from noncleft (0.11 mm) to cleft with VPI (1.25 mm) to cleft with VPC (1.31 mm) groups. Post hoc analysis revealed no significant pairwise comparisons for the difference in thickness of the right and left levator muscle bundles at the point of insertion into the velum; however, the differences between the noncleft (mean rank = 3.80) and cleft with VPI (mean rank = 10.00, p = .085) groups and between the noncleft (mean rank = 3.80) and cleft with VPC (mean rank = 10.20, p = .071) groups were approaching significance. Remaining measures of the levator muscle were not found to be significantly different among groups. Results regarding levator muscle dimensions are depicted in Table 3. Results of levator symmetry variables are depicted in Table 4.
Table 4.
Results of the Kruskal–Wallis H test regarding measures of symmetry and pertinent pairwise comparisons.
| Variable | Mdn |
H test |
Pairwise |
|||
|---|---|---|---|---|---|---|
| χ2 | p | Mean ranks | p | |||
| Angle of origin | VPI: 1.1 | .421 | .810 | |||
| VPC: 2.6 | ||||||
| Non: 1.0 | ||||||
| Levator length | VPI: 1.15 | 3.840 | .147 | |||
| VPC: 3.30 | ||||||
| Non: 1.01 | ||||||
| Extravelar length | VPI: 1.74 | 2.060 | .357 | |||
| VPC: 1.48 | ||||||
| Non: 1.04 | ||||||
| Intravelar length | VPI: 1.91 | 1.680 | .432 | |||
| VPC: 1.59 | ||||||
| Non: 1.22 | ||||||
| Levator thickness, 1 (origin) | VPI: .39 | 4.914 | .086 | |||
| VPC: .23 | ||||||
| Non: .12 | ||||||
| Levator thickness, 2 (between 1 and 3) | VPI: .54 | 3.500 | .174 | |||
| VPC: .50 | ||||||
| Non: .27 | ||||||
| Levator thickness, 3 (between 1 and 4) | VPI: .28 | 1.367 | .505 | |||
| VPC: .53 | ||||||
| Non: .31 | ||||||
| Levator thickness, 4 (velar insertion) | VPI: 1.25 | 6.620 | .037* | VPI: 10.00 | Non: 3.80 | .085 |
| VPC: 1.31 | VPC: 10.20 | VPI: 10.00 | 1.000 | |||
| Non: .11 | Non: 3.80 | VPC: 10.20 | .071 | |||
| Levator thickness, 5 (between 4 and 6) | VPI: .83 | 1.583 | .453 | |||
| VPC: .22 | ||||||
| Non: .17 | ||||||
Note. The symmetry variables below were calculated using the absolute value of left measure subtracted from the right measure. All values are in millimeters, with the exception of angle of origin (°). VPI = velopharyngeal insufficiency; VPC = velopharyngeal closure; Non = noncleft.
p < .05.
Discussion
This study examined whether differences exist in velopharyngeal dimensions and the symmetry, morphology, and position of the levator muscle among participants with repaired cleft palate and VPC, participants with repaired cleft palate and VPI, and noncleft controls. Significant differences among the three groups were found for variables of sagittal angle, effective velopharyngeal ratio, extravelar length, midline levator thickness, and the difference in thickness of the levator muscle bundles at the point of levator insertion into the body of the velum.
Comparison Between Cleft and Noncleft Groups
Tian and colleagues (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) found significant differences to be present for both cleft groups (VPI and VPC) for measures of levator insertion width, hard palate length, pharyngeal depth (as measured in three ways: from the posterior hard palate to the posterior pharyngeal wall, basion, and the first cranial vertebra), and maxillary index when compared to a normative control group (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010). This study found significant differences between cleft and noncleft (VPI or VPC) groups with respect to sagittal angle, levator muscle thickness at midline, and symmetry at the point of levator insertion into the velum. Levator insertion width (velar insertion distance) was not found to be different among groups in this study, which may be due to individual variability and an effect of the low sample size in both this study and the Tian et al. studies (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010; Tian & Redett, 2009).
Median values were significantly different among groups for sagittal angle, the cleft with VPI group having the largest sagittal angle. Post hoc analysis revealed significant differences in sagittal angle between the noncleft and cleft with VPI groups. Although this specific measure has not been employed to analyze children with cleft palate prior to this study, it has been reported in normative measures of the velopharynx in children (Perry et al., 2018). The more obtuse the sagittal angle measure, the more horizontal the levator muscle is positioned, which gives the muscle a disadvantageous pull on the body of the velum compared to a more vertically oriented muscle (Perry et al., 2013). In addition, sagittal angle could also be influenced by changes to the point of origin or insertion of the levator muscle, such as anteriorly positioned levator muscle bundles or cranial base variations. Given that the median sagittal angle of the VPI group was the greatest in combination with a reduced effective velar length, it would be probable that the levator muscle is positioned anteriorly within this group. Such anterior levator fibers result in an unfavorable biomechanical lever system that may result in a velopharyngeal gap.
Levator muscle thickness at midline was significant between groups. The group with VPI had the lowest median midline levator thickness. Two of these participants had midline separation of the levator muscle bundles, showing a thickness of zero for this measure. Post hoc analysis revealed significant differences between the noncleft and cleft with VPI groups, indicating midline levator muscle thickness may be indicative of velopharyngeal function. Many current surgical interventions aim to restore the levator muscle sling during primary palatoplasty with varying degrees of overlap. Future research should control for muscle overlap during surgery to determine if there is an optimal percentage of overlap to maintain muscle continuity and optimize function.
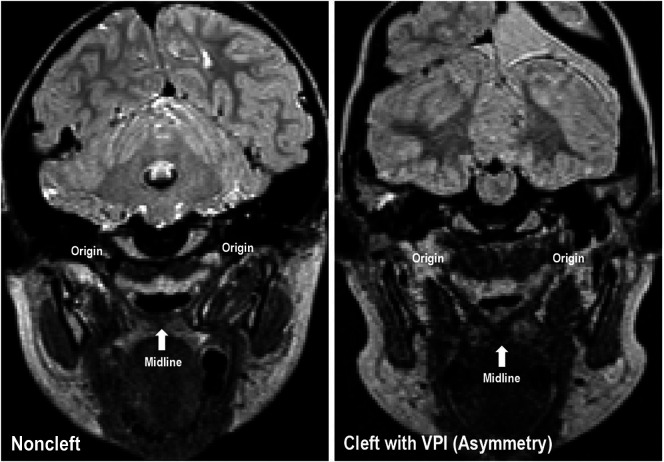
Asymmetry was observed at the point of levator insertion into the velum within the VPI and VPC groups, which can be visualized in Figure 2. Median values were significant for the difference in thickness between the left and right levator muscle bundles at the point of insertion into the velum, with the smallest amount of asymmetry observed in the noncleft group (0.11 mm). Asymmetry between the VPC and VPI groups was 0.06 mm different and likely irrelevant to velopharyngeal status. Although there were no significant pairwise comparisons, the comparisons between the noncleft group to both the VPC (p = .071) and VPI (p = .085) groups were approaching significance. This finding suggests that asymmetry of the levator muscle may be a structural change resulting from the cleft itself or palatal surgery and likely does not affect VPC. Future research employing a larger cohort of children with VPI and VPC should be completed. An addition, comparisons should be made to syndromic populations, such as 22q11.2 deletion syndrome, to determine if there is a greater degree of asymmetry relative to velopharyngeal status. Future research should also compare dynamic speech data via nasendoscopy or dynamic MRI to determine if this muscular asymmetry is functional.
Figure 2.
Oblique coronal images depicting the levator muscle sling of participants in the noncleft (left) and cleft with velopharyngeal insufficiency (VPI; right) participant groups are shown. Asymmetry of the muscle form can be observed in the participant with VPI.
Comparison Between VPI and VPC Cleft Groups
Tian et al. (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) studied similar groups to that of this study and found only two significant differences between the VPI and VPC groups. Specifically, the cleft group with VPI had a significantly shorter posterior velar length and longer uvular pharyngeal depth compared to those with VPC (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010). Tian and colleagues (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) found that velopharyngeal ratio and effective velopharyngeal ratio were not significantly different between these two groups, which led to the conclusion that the posterior velum may play an important role in velopharyngeal function for speech. In this study, differences were found in the VPI and VPC groups for variables of extravelar length and effective velopharyngeal ratio.
Extravelar length was significantly different across the three groups in this study. Significant differences were noted between the VPI and VPC groups, with the greatest extravelar length noted for the VPC group. To the best of our knowledge, this difference relative to velopharyngeal function has not been reported in previous literature. It is possible that this finding may be a result of the utilized surgical repair techniques or the low sample size employed in this study. Tian et al. (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) did not find a significant difference in the levator muscle between these groups; however, extravelar length was not measured separately from the entire levator muscle as it was in this study. It has previously been thought that the extravelar portion of the levator muscle plays an important role in velopharyngeal function, as it has the greatest potential for pulling the velum upward (Perry et al., 2014). Due to the lower variability observed within the extravelar segment (compared to the intravelar segment) of the levator muscle in the noncleft population, the extravelar segment may play an important role in velopharyngeal function (Perry et al. 2014). Further research is needed using a larger sample size to determine the impact of extravelar length on VPC in participants with repaired cleft palate.
Median values were also statistically significant for effective velopharyngeal ratio in this study. The VPI group displayed a drastically smaller (more disadvantageous) ratio than the noncleft or cleft with VPC groups. Post hoc analysis revealed significant differences in effective velopharyngeal ratio between the VPI group compared to the two other groups. This supports the hypothesis that effective velopharyngeal ratio is highly relevant to VPC. Additional investigations should examine if effective velopharyngeal ratio is able to predict VPC. Tian and colleagues (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010) reported the VPI group had a significantly shorter posterior velar length and longer uvular pharyngeal depth compared to those with VPC; however, effective velopharyngeal ratio was not significant. Although also not significant, a greater pharyngeal depth (posterior nasal spine to posterior pharyngeal wall) was noted for both the VPI and VPC groups, which may have contributed to a longer effective velar segment and more normalized effective velopharyngeal ratio in the Tian et al. studies (Tian, Yin, Li, et al., 2010; Tian, Yin, Redett, et al., 2010; Tian & Redett, 2009). Type of surgical intervention may be responsible for differences observed in this study.
Surgical Differences
Differences in surgical type as well as operating surgeon were not utilized as covariates within this study due to the low sample size. Participants were consecutively enrolled, which did not allow for limiting the population to a single surgeon. Four of the five participants within the VPI group underwent a V-Y pushback palatoplasty. Within the VPC group, three participants underwent a Furlow double-opposing Z-plasty while two received a V-Y pushback palatoplasty. Although it could be argued that a Z-plasty may lead to more asymmetry of the levator muscle, the participants who underwent a V-Y pushback showed greater difference in thickness at the point of insertion into the velum. In addition, two of the participants in this study (one VPC, one VPI) who showed midline dehiscence of the levator muscle bundles (midline thickness = 0 mm) underwent a V-Y pushback. Future research should include surgical variables within the statistical analysis to determine if certain interventions yield more advantageous postoperative anatomy.
Limitations
Generalization of these results is restricted based on the limited sample size employed by this study. Control for participant race, surgical type, cleft type, and operating surgeon were not considered in this study due to the limited sample size and should be controlled in future investigations.
Future Directions
Future research should compare children and adults with repaired cleft palate and differing velopharyngeal status using a larger sample size. Much research has been published comparing individuals with repaired cleft palate to their noncleft peers; however, research has shown that individuals with repaired cleft palate that achieve VPC and typical speech production have asymptomatic differences in levator and velopharyngeal variables (Perry et al., 2018). In addition, a larger sample size would allow for comparison across specific surgical techniques to determine how the surgical procedure impacts the velopharyngeal anatomy. Future research regarding postoperative anatomy and physiology of children with repaired cleft palate may enable surgery selection to be based on individual anatomy and reduce overall need for secondary surgical intervention for VPI.
Conclusion
The levator muscle is significantly different among these three groups with respect to symmetry at the point of insertion into the velum, sagittal angle, levator muscle thickness at midline, extravelar length, and effective velopharyngeal ratio. Participants with repaired cleft palate and VPI displayed the greatest degree of asymmetry. Continued evaluation of postsurgical anatomy and short- and long-term outcomes may contribute to a better understanding of surgical impact on velopharyngeal morphology. Future research should control for surgical procedure type to determine the impact of surgery on the levator muscle and surrounding velopharyngeal anatomy.
Acknowledgments
This study was made possible by Grant F31DE027878 (awarded to Kotlarek) from the National Institute of Dental and Craniofacial Research, Grant R21DC014570 (awarded to Blemker and Perry) from the National Institute on Deafness and Other Communication Disorders, and the Oral Maxillofacial Surgery Foundation research support grant (awarded to Perry and Jaskolka). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors have no personal or financial disclosures relevant to the content of this article.
Funding Statement
This study was made possible by Grant F31DE027878 (awarded to Kotlarek) from the National Institute of Dental and Craniofacial Research, Grant R21DC014570 (awarded to Blemker and Perry) from the National Institute on Deafness and Other Communication Disorders, and the Oral Maxillofacial Surgery Foundation research support grant (awarded to Perry and Jaskolka).
References
- Bell-Berti F. (1976). An electromyographic study of velopharyngeal function in speech. Journal of Speech and Hearing Research, 19(2), 225–240. https://doi.org/10.1044/jshr.1902.225 [DOI] [PubMed] [Google Scholar]
- Dickson D. R., & Dickson W. M. (1972). Velopharyngeal anatomy. Journal of Speech and Hearing Research, 15(2), 372–381. https://doi.org/10.1044/jshr.1502.372 [DOI] [PubMed] [Google Scholar]
- Ettema S., Kuehn D., Perlman A., & Alperin N. (2002). Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate–Craniofacial Journal, 39(2), 130–144. https://doi.org/10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2 [DOI] [PubMed] [Google Scholar]
- Ha S., Kuehn D., Cohen M., & Alperin N. (2007). Magnetic resonance imaging of the levator veli palatini muscle in speakers with a repaired cleft palate. Cleft Palate–Craniofacial Journal, 44(5), 494–505. https://doi.org/10.1597/06-220.1 [DOI] [PubMed] [Google Scholar]
- Hoopes J. E., Dellon A. L., Fabrikant J. I., & Soliman A. H. (1969). The locus of levator veli palatini function as a measure of velopharyngeal incompetence. Plastic and Reconstructive Surgery, 44(2), 155–160. https://doi.org/10.1097/00006534-196944020-00008 [PubMed] [Google Scholar]
- Kollara L., & Perry J. (2014). Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging. Cleft Palate–Craniofacial Journal, 51(6), 669–676. https://doi.org/10.1597/13-107 [DOI] [PubMed] [Google Scholar]
- Kotlarek K. J., Perry J. L., & Fang X. (2017). Morphology of the levator veli palatini muscle in adults with repaired cleft palate. Journal of Craniofacial Surgery, 28(3), 833–837. https://doi.org/10.1097/SCS.0000000000003373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn D. P., Ettema S. L., Goldwasser M. S., & Barkmeier J. C. (2004). Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate–Craniofacial Journal, 41(6), 584–592. https://doi.org/10.1597/03-060.1 [DOI] [PubMed] [Google Scholar]
- Kuehn D. P., & Moon J. B. (2005). Histologic study of intravelar structures in normal human adult specimens. Cleft Palate–Craniofacial Journal, 42, 481–489. https://doi.org/10.1597/04-125r.1 [DOI] [PubMed] [Google Scholar]
- Moon J. B., Smith A. E., Folkins J. W., Lemke J. H., & Gartlan M. (1994). Coordination of velopharyngeal muscle activity during positioning of the soft palate. Cleft Palate–Craniofacial Journal, 31(1), 45–55. https://doi.org/10.1597/1545-1569_1994_031_0045_covmad_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- Nakamura N., Ogata Y., Kunimitsu K., Suzuki A., Sasaguri M., & Ohishi M. (2003). Velopharyngeal morphology of patients with persistent velopharyngeal incompetence following repushback surgery for cleft palate. Cleft Palate–Craniofacial Journal, 40(6), 612–617. https://doi.org/10.1597/1545-1569_2003_040_0612_vmopwp_2.0.co_2 [DOI] [PubMed] [Google Scholar]
- Park M., Ahn S. H., Jeong J. H., & Baek R. M. (2015). Evaluation of the levator veli palatini muscle thickness in patients with velocardiofacial syndrome using magnetic resonance imaging. Journal of Plastic, Reconstructive & Aesthetic Surgery, 68(8), 1100–1105. https://doi.org/10.1016/j.bjps.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Perry J. L. (2011). Variations in velopharyngeal structures between upright and supine positions using upright magnetic resonance imaging. Cleft Palate–Craniofacial Journal, 48(2), 123–133. https://doi.org/10.1597/09-256 [DOI] [PubMed] [Google Scholar]
- Perry J. L., Kotlarek K. J., Sutton B. P., Kuehn D. P., Jaskolka M. S., Fang X., Point S. W., & Rauccio F. (2018). Variations in velopharyngeal structure in adults with repaired cleft palate. Cleft Palate–Craniofacial Journal, 55(10), 1409–1418. https://doi.org/10.1177/1055665617752803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., & Kuehn D. P. (2007). Three-dimensional computer reconstruction of the levator veli palatini muscle in situ using magnetic resonance imaging. Cleft Palate–Craniofacial Journal, 44(4), 421–423. https://doi.org/10.1597/06-137.1 [DOI] [PubMed] [Google Scholar]
- Perry J. L., & Kuehn D. P. (2009). Magnetic resonance imaging and computer reconstruction of the velopharyngeal mechanism. Journal of Craniofacial Surgery, 20(8), 1739–1746. https://doi.org/10.1097/SCS.0b013e3181b5cf46 [DOI] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., & Sutton B. (2013). Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate–Craniofacial Journal, 50(1), 64–75. https://doi.org/10.1597/11-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., Sutton B., Goldwasser M., & Jerez A. (2011). Craniometric and velopharyngeal assessment of infants with and without cleft palate. Journal of Craniofacial Surgery, 22(2), 499–503. https://doi.org/10.1097/SCS.0b013e3182087378 [DOI] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., Sutton B. P., & Gamage J. K. (2014). Sexual dimorphism of the levator veli palatini muscle: An imaging study. Cleft Palate–Craniofacial Journal, 51(5), 544–552. https://doi.org/10.1597/12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., Sutton B. P., Gamage J. K., & Fang X. (2016). Anthropometric analysis of the velopharynx and related craniometric dimensions in three adult populations using MRI. Cleft Palate–Craniofacial Journal, 53(1), e1–e13. https://doi.org/10.1597/14-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Li Y., Yin H., Zhao S., Li S., Wang Y., & Shi B. (2010). Magnetic resonance imaging assessment of velopharyngeal motion in Chinese children after primary palatal repair. Journal of Craniofacial Surgery, 21(2), 578–587. https://doi.org/10.1097/SCS.0b013e3181d08bee [DOI] [PubMed] [Google Scholar]
- Tian W., & Redett R. (2009). New velopharyngeal measurements at rest and during speech: Implications and applications. Journal of Craniofacial Surgery, 20(2), 532–539. https://doi.org/10.1097/SCS.0b013e31819b9fbe [DOI] [PubMed] [Google Scholar]
- Tian W., Yin H., Li Y., Zhao S., Zheng Q., & Shi B. (2010). Magnetic resonance imaging assessment of velopharyngeal structures in Chinese children after primary palatal repair. Journal of Craniofacial Surgery, 21(2), 568–577. https://doi.org/10.1097/SCS.0b013e3181d08bd1 [DOI] [PubMed] [Google Scholar]
- Tian W., Yin H., Redett R., Shi B., Shi J., Zhang R., & Zheng Q. (2010). Magnetic resonance imaging assessment of the velopharyngeal mechanism at rest and during speech in Chinese adults and children. Journal of Speech, Language, and Hearing Research, 53(6), 1595–1615. https://doi.org/10.1044/1092-4388(2010/09-0105) [DOI] [PubMed] [Google Scholar]