Abstract
Since most poliovirus infections occur with no paralytic symptoms, the possibility of silent circulation complicates the confirmation of the end of poliovirus transmission. Based on empirical field experience and theoretical modeling results, the Global Polio Eradication Initiative identified three years without observing paralytic cases from wild polioviruses (WPVs) with good acute flaccid paralysis surveillance as an indication of sufficient confidence that poliovirus circulation stopped. The complexities of real populations and the imperfect nature of real surveillance systems subsequently demonstrated the importance of specific modeling for areas at high risk of undetected circulation, resulting in varying periods of time required to obtain the same level of confidence about no undetected circulation. Using a poliovirus transmission model that accounts for variability in transmissibility and neurovirulence for different poliovirus serotypes and characterizes country-specific factors (e.g., vaccination and surveillance activities, demographics) related to wild and vaccine-derived poliovirus transmission in Pakistan and Afghanistan, we consider the probability of undetected poliovirus circulation for those countries once apparent die out occurs (i.e., in the absence of any epidemiological signals). We find that gaps in poliovirus surveillance or reaching elimination with borderline sufficient population immunity could significantly increase the time to reach a high confidence about interruption of live poliovirus transmission, such that the path taken to achieve and maintain poliovirus elimination matters. Pakistan and Afghanistan will need to sustain high-quality surveillance for polioviruses after apparent interruption of transmission and recognize that as efforts to identify cases or circulating live polioviruses decreases, the risks of undetected circulation increase and significantly delay the global polio endgame.
Keywords: Disease outbreaks, Disease eradication, Infection transmission modeling, Poliomyelitis, Poliovirus, Disease surveillance, Environmental surveillance, Vaccination
1. INTRODUCTION
The World Health Assembly resolved to eradicate polio in 1988 (World Health Assembly, 1988), which led to the launch of the Global Polio Eradication Initiative (GPEI), and significant progress toward the global eradication of wild polioviruses (WPVs) using oral poliovirus vaccines (OPVs). As of 2018, only three countries (i.e., Pakistan, Afghanistan, and Nigeria) continue to sustain indigenous transmission of serotype 1 WPV (WPV1) (World Health Organization, 2018), with no known cases of paralytic poliomyelitis caused by naturally-occurring serotype 2 WPV (WPV2) since 1999 (Global Polio Eradication Initiative, 2017) or by serotype 3 (WPV3) since November 2012 (Kew et al., 2014). Following the global certification of WPV2 eradication in 2015 (Global Polio Eradication Initiative, 2015), in April 2016 the GPEI globally coordinated the switch from trivalent oral poliovirus vaccine (tOPV), containing all 3 attenuated poliovirus serotypes, to bivalent OPV (bOPV), containing only attenuated serotype 1 and 3 oral polioviruses (Hampton et al., 2016). As a result, serotype 2 monovalent OPV (mOPV2) vaccine released exclusively for emergency outbreak response remains the only available serotype 2-containing OPV.
Due to the risks of the live attenuated viruses in OPV, globally coordinated cessation of its use represents a critical step of the polio eradication endgame. Although relatively rare, the use of OPV can cause sporadic vaccine-associated paralytic poliomyelitis cases in vaccine recipients or their close contacts who become infected by virus shed by the vaccine recipient (Platt, Estivariz, & Sutter, 2014). In addition, the live attenuated viruses used in OPV can lose their attenuating mutations and become circulating vaccine-derived polioviruses (cVDPVs) as they continue to transmit in populations with low population immunity (World Health Organization, 2005, 2013). Unfortunately, cVDPVs behave similarly to homotypic WPVs with respect to transmissibility and neurovirulence and lead to outbreaks (Duintjer Tebbens, Pallansch, Kim, et al., 2013). In addition, while immunocompetent people generally clear OPV infections within weeks, some individuals with primary immunodeficiencies can develop infections and excrete immunodeficiency-associated vaccine-derived polioviruses for many years (Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, & Thompson, 2015). The coordinated cessation of the remaining bOPV use after global eradication of WPVs aims to stop the routine introduction of OPV viruses to eventually minimize the risks associated with all live polioviruses (LPVs) (i.e., WPV, OPV, OPV-related, and VDPV) in the polio eradication endgame (World Health Assembly, 2008; World Health Organization, 2013). After cessation of an OPV serotype, inactivated poliovirus vaccine (IPV) provides the only source of individual protection from paralysis caused by homotypic LPVs, although it does not reduce their ability to participate asymptomatically in fecal-oral poliovirus transmission by much (Anis et al., 2013; Kalkowska, Duintjer Tebbens, Grotto, et al., 2015).
Complete OPV cessation cannot occur without confidence that all WPV circulation has stopped, and therefore certification of the global eradication of WPV1 and WPV3 remains a critical requirement for OPV cessation. Based on empirical field experience and theoretical modeling results, the GPEI identified three years without observing any paralytic cases from WPVs in the context of good acute flaccid paralysis (AFP) surveillance (i.e., timely collection and sensitive testing of stool specimens from AFP patients) as an indication of sufficient confidence that poliovirus circulation stopped. Earlier models suggested that it takes approximately 3-4 years without reported WPV-caused paralytic cases to achieve 95% confidence about the interruption of transmission (Debanne & Rowland, 1998; Eichner & Dietz, 1996). A subsequent analysis (Kalkowska, Duintjer Tebbens, & Thompson, 2012) that used an infection transmission model of a small, simple hypothetical population (Eichner & Dietz, 1996) highlighted the importance of serotype-specific paralysis-to-infection ratios (PIRs), assumptions that impact population immunity (i.e., vaccination strategies), and population-specific seasonal fluctuations in poliovirus transmissibility. These factors impact the estimates of the probability and duration of undetected LPV circulation in real populations (Kalkowska et al., 2012). In the context of some hypothetical assumptions about vaccination strategies to achieve elimination in a highly simplified population structure, the date of occurrence of the last paralytic infection also influences the estimates of the risk of declaring WPV eradicated despite ongoing transmission (Houy, 2015). A recent analysis focused on specific populations that may represent conditions corresponding to the last global reservoirs of WPV transmission (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015). This study (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015) also included the impact of AFP surveillance and environmental surveillance (ES) on characterizing the confidence about no circulation as a function of time without detected events (i.e., paralytic cases or positive sewage samples). Recently, a non dynamic statistical model (Famulare, 2016) predicted shorter times required to reach high confidence of no undetected circulation for Nigeria (compared to the previously mentioned estimates for northwest Nigeria (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015)). Unfortunately, in 2016 Nigeria detected continued transmission of WPV1 and serotype 2 cVDPV (cVDPV2) viruses in the northeastern state of Borno in a subpopulation with compromised access following no detected circulation for 2 and 3 years for the two serotypes, respectively (Etsano et al., 2016; Nnadi et al., 2017). To date, the GPEI has considered 3 years of no reported polio AFP cases in the context of high quality surveillance as providing sufficient confidence to certify the eradication of the homotypic WPV.
Pakistan and Afghanistan represent an important epidemiological region in which WPV1 eradication and risk management after OPV2 cessation now represent simultaneous priorities. As time progresses since Nigeria detected its last WPV1 case in 2016, the likelihood increases that Pakistan and Afghanistan will become the last known global reservoirs of WPV1 transmission. We use a previously developed differential equation based model (Duintjer Tebbens et al., 2018) and stochastic approach (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015) to explore the confidence about no circulation of poliovirus transmission as a function of time without detected events for conditions relevant to Pakistan and Afghanistan. We extend the existing methods to specifically explore how the gaps in AFP surveillance and the expanding ES system in Pakistan and Afghanistan may affect the confidence about no circulation.
2. METHODS
Similar to prior work (Eichner & Dietz, 1996; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska et al., 2012), we estimate the probability of no circulation as a function of the time since the last detected event, and we base our analysis on prior comprehensive deterministic differential equation-based poliovirus infection transmission models (see appendix for model details) (Duintjer Tebbens et al., 2014; 2017; 2018; Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013; Duintjer Tebbens et al., 2005). We model LPV transmission within Pakistan and Afghanistan for various vaccination intensities and concentrate on AFP surveillance and ES quality (Duintjer Tebbens et al., 2018). The model characterizes individuals in the population using 8 immunity states further subdivided by a 5-stage process of waning of immunity to infection, and infections using a 6-stage infection process and a 20-stage poliovirus reversion process for both fecal-oral and oropharyngeal routes of transmission (Duintjer Tebbens et al., 2018; Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013; Duintjer Tebbens et al., 2005; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015). We divide the population into eleven age groups and combine them into 3 preferentially mixing age groups (Duintjer Tebbens et al., 2018).
We model Pakistan and Afghanistan as one closed epidemiological block but divide each country into a general population and an under-vaccinated subpopulation (i.e., the national population equals the sum of the general population plus the under-vaccinated subpopulation for each country). The model specifies demographic information, poliovirus transmissibility and seasonality, mixing among the four subpopulations, and the history of poliovirus vaccination (both OPV and inactivated poliovirus vaccine (IPV) use for routine immunization (RI) and supplemental immunization activities (SIAs) (World Health Organization, 2013)) as previously described (Table 1) (Duintjer Tebbens et al., 2018). For SIAs, we assume relatively high coverage in the general population in each country based on values fitted to reported dose histories of non-polio AFP cases. We multiply these by much lower relative SIA coverage values for the under-vaccinated subpopulations. We also account for the increased probability of missing an SIA dose among children missed by the prior SIA (Kalkowska, Duintjer Tebbens, & Thompson, 2014). We consider three immunization scenarios. The “current path” for serotypes 2 and 3 assumes no changes compared to the deterministic model (Duintjer Tebbens et al., 2018), which assumes continued immunization at the currently planned SIA frequency and estimated RI and SIA coverage for 2018 (Table 1). For serotype 1 we choose two alternative scenarios to the current path because WPV1 transmission does not die out in the deterministic model on the current path (Duintjer Tebbens et al., 2018) and doing so requires an increase in relative SIA coverage in the two under-vaccinated subpopulations. The “increased relative SIA coverage 0.20” and “increased relative SIA coverage 0.15” for serotype 1 increase relative SIA coverage in the under-vaccinated subpopulations by 0.20 and 0.15, respectively, from 2018 onward. The increase of relative SIA coverage by 0.15 represents the minimum value for which WPV1 dies out in the deterministic model, and we selected a slightly higher increase by 0.20 to demonstrate the impact of further increases above the minimum on the CNC values. We run the existing deterministic poliovirus transmission model up to a chosen (serotype-specific) starting date (see Table 1) and then transform it to a discrete, stochastic model (with an added poliovirus surveillance component, see appendix) (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015). We then run the stochastic model 1,000 times with a fixed time step of 0.125 days (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015). This results in 1,000 distinct realizations of times when paralytic cases can occur and monthly effective (i.e., weighted by the infectiousness of infected individuals, which depends on their prior immunity state) numbers of infectious individuals in each subpopulation, which we use to obtain estimates of confidence about no circulation (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015).
Table 1.
List of model inputs for the transmission model and the surveillance component
| Model input | Symbol | Value | Unit |
|---|---|---|---|
|
Deterministic and stochastic transmission model inputs: | |||
| Stochastic model start date (first day of given year) | |||
| Serotype 1 | 2018 | ||
| Serotype 2 | 2015 | ||
| Serotype 3 | 2011 | ||
| Paralysis-to-infection ratio for WPV and cVDPV | |||
| Serotype 1 | 1/200 | ||
| Serotype 2 | 1/2000 | ||
| Serotype 3 | 1/1000 | ||
| Average R0 (WPV1) considering the seasonal changes over the year | 11 | ||
| Annual bOPV SIA frequency from 2018 on | Cumulative fraction targeted by all SIAs in a year | ||
| Pakistan | 8.2 | ||
| Afghanistan | 6.6 | ||
| True SIA coverage from 2018 on (general population) | |||
| Pakistan | 0.80 | ||
| Afghanistan | 0.70 | ||
| Relative SIA coverage from 2018 on (under-vaccinated subpopulation) | |||
| Pakistan | 0.25 | ||
| Afghanistan | 0.20 | ||
| National RI coverage from 2018 on | |||
| Pakistan | 0.72 | ||
| Afghanistan | 0.57 | ||
| Relative RI coverage from 2018 on (under-vaccinated subpopulation) | |||
| Pakistan | 0.40 | ||
| Afghanistan | 0.60 | ||
| Surveillance model inputs: | |||
| Cluster length | cl | 90 | days |
| Probability of detecting a case by AFP surveillance in general population | p = (p1, p2, …, pi) | (0.75, 0.80, 0.85, 0.90, …, 0.90) | |
| Probability of detecting a case by AFP surveillance in under-vaccinated subpopulation, lower bound | p = (p1, p2, …, pi) | (0.10, 0.10, …, 0.10) | |
| Probability of detecting a case by AFP surveillance in under-vaccinated subpopulation, upper bound | p = (p1, p2, …, pi) | (0.50, 0.53, 0.57, 0.60, …, 0.60) | |
| Effective (i.e., infectiousness-weighted) number of infectious individuals | EI | Obtained from transmission model | infections |
| Number of people in the population | N | Obtained from transmission model | people |
| Catchment area population of the ith sampling site | Ni | Varies per site | people |
| Detection limit of ith ES sampling site | Varies per site | infections/people | |
Abbreviations: AFP, acute flaccid paralysis; ES, environmental surveillance; PV, poliovirus; R0, basic reproductive number; RI, routine immunization; SIA, supplementary immunization activity; WPV, wild poliovirus.
Similar to some prior work (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015), we focus on detected events (i.e., polio cases detected by AFP surveillance and/or positive sewage isolates indicating the presence of transmission detected by ES) and the detected-event-free periods, to account for the information provided by the different types of poliovirus surveillance. We describe the results using the following metrics (page 6 of (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015):
POE – “the probability of eradication defined as the fraction of stochastic iterations in which die-out occurs”
DEFP – “the detected-event-free period defined as the time in months since the last detected case (AFP) or positive isolate (environmental surveillance)”
CNC – “confidence about no circulation given the DEFP approximated as (1 - the number of DEFPs equal to t months with ongoing WPV circulation, divided by all DEFPs of t months)”
CNCx% – “the time when the confidence about no circulation exceeds x% (i.e., CNC95%, CNC99%)”
TUC – “the time of undetected circulation after the last detected-event (for those iterations in which extinction occurs)”
TUCx% – “the xth percentile of the TUC (i.e., TUC95%, TUC99%)”
To account for imperfect information from surveillance, we define a detection function (DF) as an indicator of overall surveillance quality. We compare the effect of imperfect surveillance to a reference case that assumes perfect surveillance. For AFP surveillance, the DF describes the probability pe of detecting the eth event (polio AFP case) in a cluster, where a cluster consists of a series of sequentially detected events in a subpopulation. We consider different levels of pe values for the general and under-vaccinated subpopulations, with a lower and upper bound in both under-vaccinated subpopulations to reflect a range of possible limited AFP surveillance access levels in those subpopulations (Table 1).
For ES, the DF describes the probability s of detecting the event of finding poliovirus in a sewage sample. We consider different approaches to 1) compute s (i.e., site-specific (SS) or system-wide (SW) approach), 2) allocate prevalence to sites in the SS approach (i.e., proportional prevalence (PP) or isolation-rate based prevalence (IP) allocation), and 3) distribute ES sites to the four subpopulations (i.e., national sites (NS), under-vaccinated subpopulation sites (US), or general population sites (GS) distribution).
The SS approach determines si for the ith sampling site from the detection limit (), defined as the effective (i.e., infectiousness-weighted) number of infected individuals (EI) per person required in the catchment area of the site to achieve a 50% probability of detecting the virus:
where Ni is the catchment area population of the ith sampling site and EIi is the site-specific prevalence. To determine the site-specific prevalence, we first calculate the national EI (EInat) for both countries by adding the EI from both subpopulations in each country. We use two approaches to assign EInat to the individual sites (i.e., PP and IP). The PP approach allocates EInat probabilistically to each site, with the probability proportional to the estimated catchment size of the sites. Areas not covered by any site also receive prevalence with some probability based on the total non-covered proportion. The IP approach first determines for each infection whether it happens in the catchment area of any ES site based on the total estimated catchment size of the country. If this occurs, we assign the infection to a specific site using a probability computed as the total WPV and cVDPV isolation rate during 2009-2017 at the given site divided by the total WPV and cVDPV isolation rate at all sites during 2009-2017. We determine value for each site by minimizing the difference between the observed total WPV and cVDPV isolation rates for each site and the modeled isolation rate using the above approach for 2009-2017 (see appendix).
Although we model Pakistan and Afghanistan as one epidemiological block divided into four subpopulations, our model does not identify these subpopulations with specific geographical locations because we assume that preferential mixing within ethnic groups may occur across geographies (Duintjer Tebbens et al., 2018). Therefore, we cannot unambiguously match actual ES sampling sites to the specified subpopulations. To convey the bounds of possible distributions of ES sites to the subpopulations, we determined three alternative sets of values for which: (1) the NS distribution assumes that all ES sampling sites listed for each country distribute evenly over the country (i.e., reflecting an ES system that targets any population equally), (2) the US distribution assumes that all ES sampling sites listed for each country distribute evenly only in the under-vaccinated subpopulation of the country (i.e., reflecting an ES system that specifically targets high-risk populations), and (3) the GS distribution assumes that all ES sampling sites listed for each country distribute evenly only in the general subpopulation of the country (reflecting an ES system that does not reach the high-risk populations) (see appendix Table A1 for the estimates).
In the SW approach, the DF directly describes the probability s of detecting poliovirus in any sampling site given the total catchment area from all ES sites and the prevalence in the country as:
where ∑aNi equals the number of people covered by all active sampling sites, and C represents a fitting coefficient. We chose the functional form so that it can flexibly describe a family of curves going from 0 with no ES coverage to 1 with full ES coverage at different speeds (see appendix), with the speed depending on the log of the prevalence and the fitting constant. We fit the system-wide value of C by iteratively minimizing the difference between the observed aggregated WPV and cVDPV isolation rates for both countries, and the modeled isolation rate using the above approach for 2009-2017. Similar to the previous approach, we considered the three distributions of ES sampling sites over the modeled subpopulations (see appendix).
Consequently, we considered the following nine ES options:
Site-specific approach with proportional prevalence allocation and national sites distribution: SS-PP-NS,
Site-specific approach with proportional prevalence allocation and under-vaccinated sites distribution: SS-PP-US,
Site-specific approach with proportional prevalence allocation and general sites distribution: SS-PP-GS,
Site-specific approach with isolation-rate based prevalence allocation and national sites distribution: SS-IP-NS,
Site-specific approach with isolation-rate based prevalence allocation and under-vaccinated sites distribution: SS-IP-US,
Site-specific approach with isolation-rate based prevalence allocation and general sites distribution: SS-IP-GS,
System-wide approach with national sites distribution: SW-NS,
System-wide allocation with under-vaccinated sites distribution: SW-US,
System-wide allocation with general sites distribution: SW-GS.
3. RESULTS
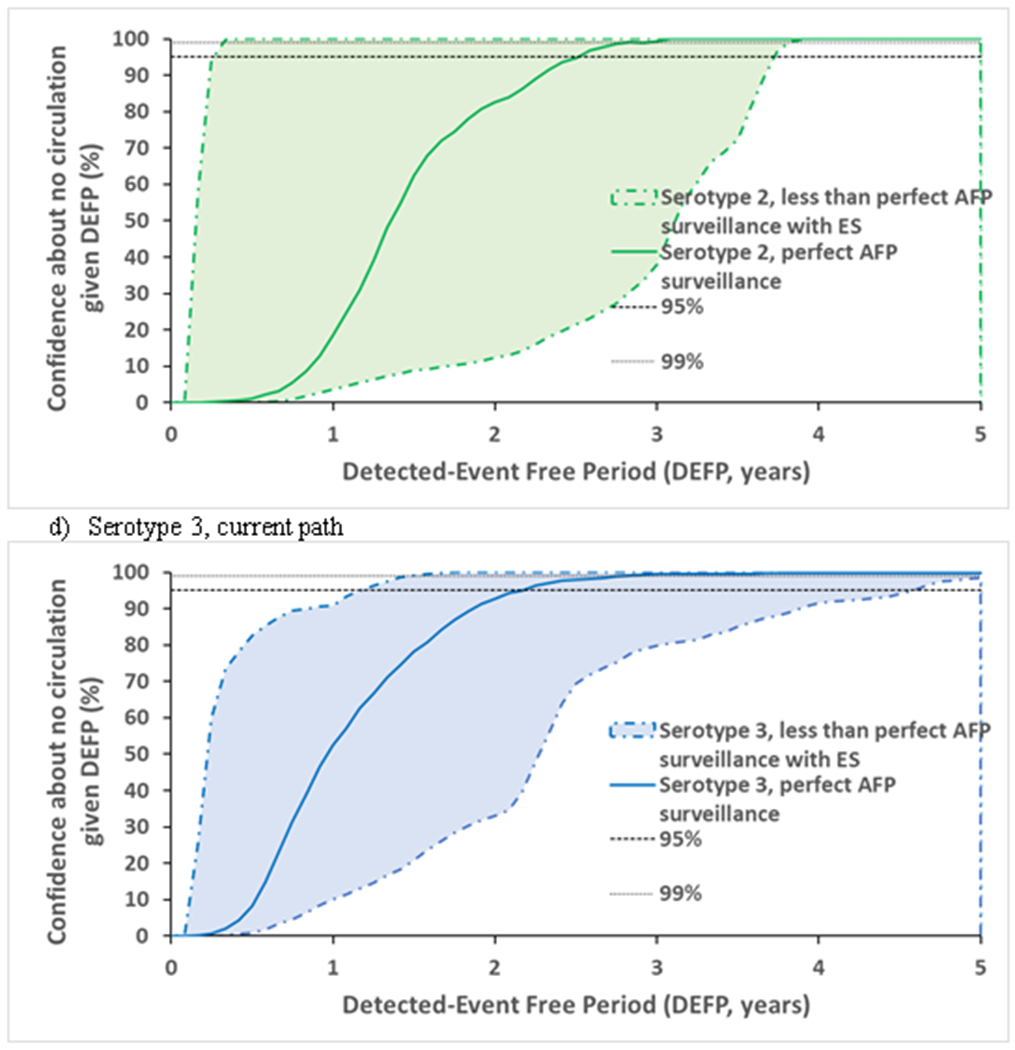
Figures 1 through 4 present the confidence about no circulation as a function of time without detected events, with black horizontal lines at the top showing the 99% (small dots) and 95% (larger dots) confidence levels for reference. In all of the figures, each line represents the result for the indicated serotype and scenario (i.e., red for serotype 1 with increased relative SIA coverage of 0.20, dark red for serotype 1 with increased relative SIA coverage of 0.15, green for serotype 2, and blue for serotype 3).
Figure 1.

Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event free period assuming perfect AFP surveillance without ES, and lines provided to indicate 95% and 99% for reference.
Figure 4.
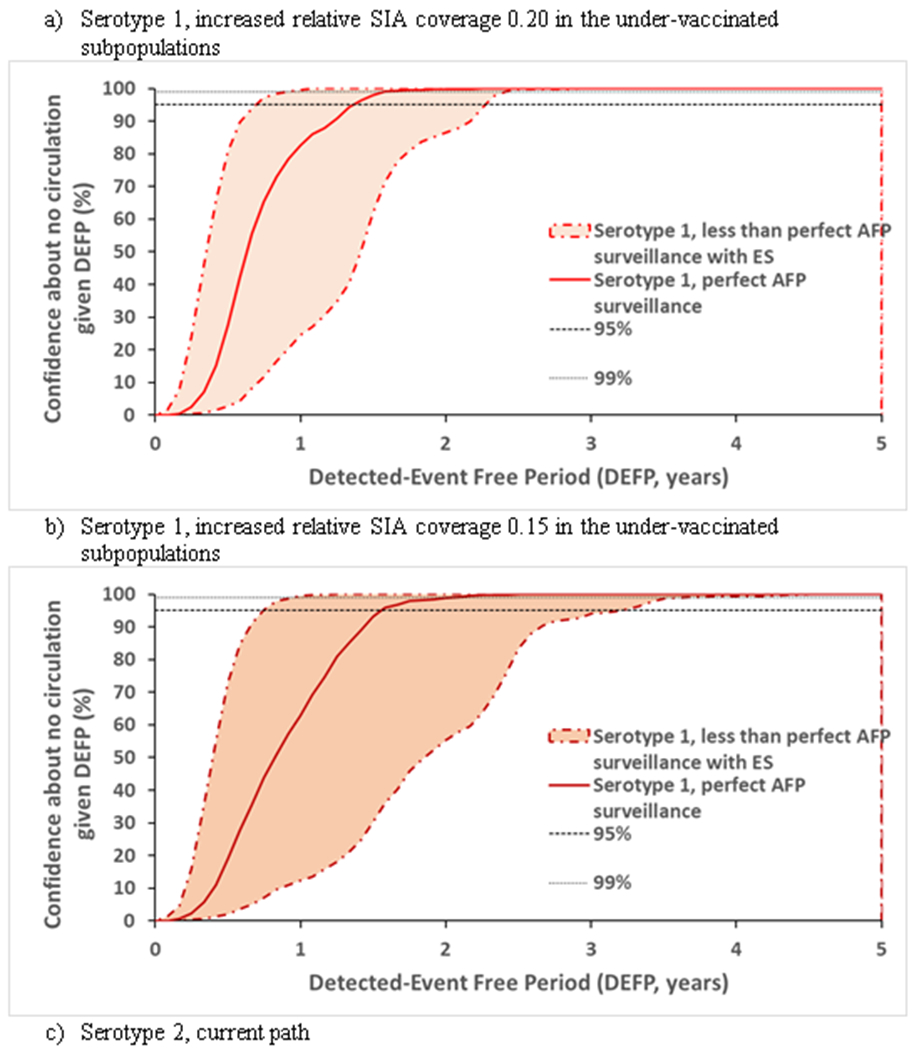
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event free period assuming a range of less than perfect AFP surveillance and a range of ES, with perfect AFP surveillance without ES, and lines provided to indicate 95% and 99% for reference
Figure 1 confirms that differences in the PIRs significantly affect the typical time between paralytic cases. Since cases occur more frequently per infection (Kalkowska et al., 2012) for serotypes with higher PIR values (i.e., serotype 2 < serotype 3 < serotype 1), relatively lower PIRs imply longer times required to reach confidence about no undetected circulation, all else being equal. The intensity of vaccination and resulting level of population immunity to transmission reached to interrupt transmission also affect the time between paralytic cases. As shown by the serotype 1 scenarios, a smaller increase in relative SIA coverage in the under-vaccinated subpopulations implies a need to wait longer to reach a specific level of confidence because it allows longer periods without any paralytic cases.
Figure 2 compares estimates of confidence about no circulation in the absence of any AFP surveillance for different ES approaches. We omitted the SS-PP-US approach because we did not observe a significant difference between that approach and the SS-PP-NS approach. Similarly, since most of the transmission takes place in the under-vaccinated subpopulations, we did not observe a significant difference between any of the GS methods and therefore we only presented the results of one of them (i.e., SW-GS). Figure 2 demonstrates a range of possible ES estimates of confidence about the absence of circulation based on our understanding of the ES system in Pakistan and Afghanistan and corresponding different approaches to model the system and allocate prevalence to the sampling sites. Figure 2 suggests that the SS approach performs better than the SW approach because 1) in the SS approach detection by the system on a given scheduled sampling day requires detection by just one (or more) of the active sampling sites and 2) the probability of detection in a site becomes very high for high prevalence, which can occur by chance for at least one site. The impact of ES also depends on the prevalence at the time of sampling consistent with varying prevalence levels for different serotypes, but also (within the same serotype) depending on sampling site location. All approaches perform best with sites located in the subpopulation in which poliovirus circulates the most at the time of sampling, with the SW approach appearing more sensitive to that effect.
Figure 2.

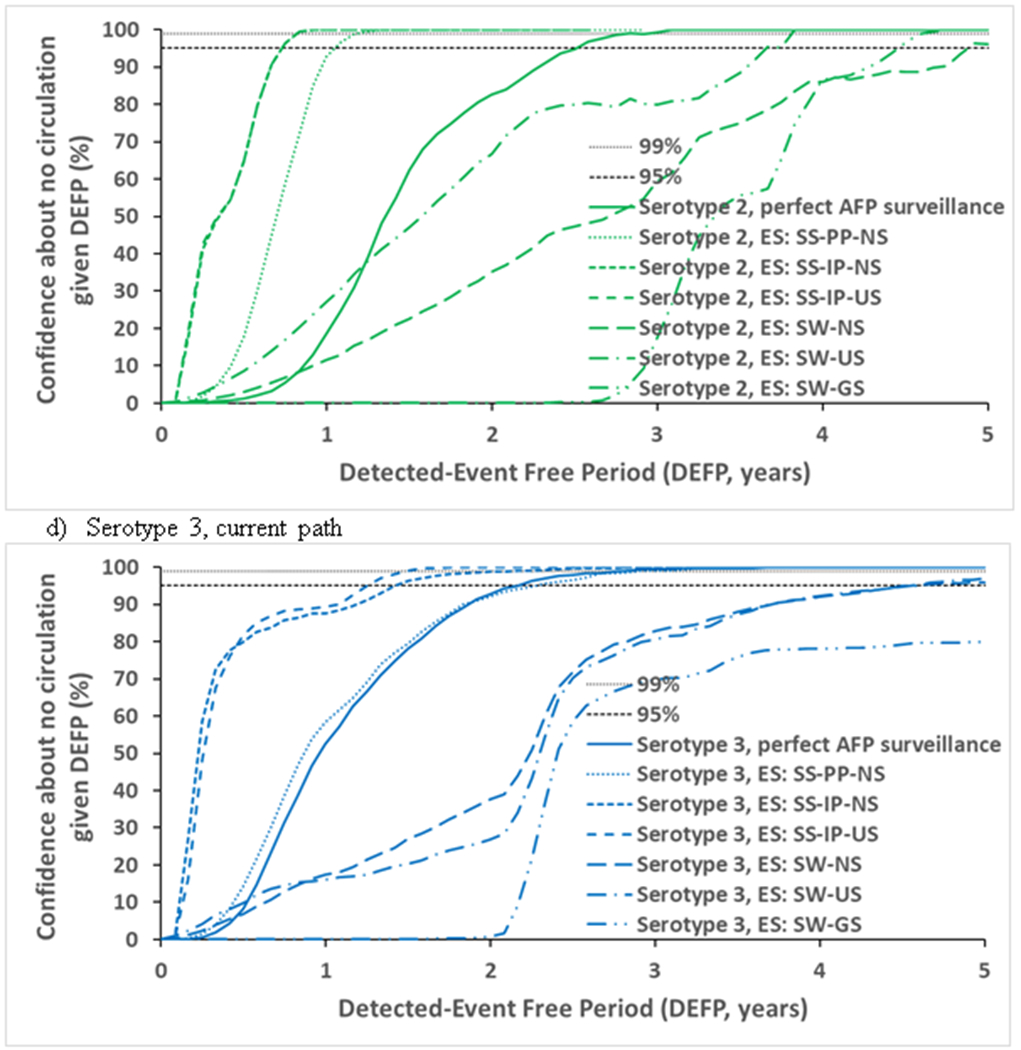
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event free period assuming different estimates of ES, with perfect AFP surveillance without ES, and lines provided to indicate 95% and 99% for reference.
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; ES, environmental surveillance; GS, general population sites distribution; IP, isolation-rate based prevalence; NS, national sites distribution; PP, proportional prevalence; SS, site-specific; SW, system-wide; US, under-vaccinated subpopulation sites distribution
Figure 3 compares less than perfect AFP surveillance of varied quality in the under-vaccinated subpopulations with the corresponding colors (as listed above), where each dotted line presents results using the lower bound estimates, each short-dashed line shows the results using the upper bound estimates for the probability of detecting a case by AFP surveillance in the under-vaccinated subpopulations (Table 1). Figure 3 shows that poor AFP surveillance quality in inaccessible areas may result in up to more than two years longer CNCs compared to perfect AFP.
Figure 3.


Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event free period assuming different estimates of less than perfect AFP surveillance, with perfect AFP surveillance without ES, and lines provided to indicate 95% and 99% for reference.
Figure 4 shows that the joint effect of the range of imperfect AFP surveillance and the spectrum of the investigated ES approaches leads to a space of possible outcomes. In Figure 4, each dotted-dashed line of the corresponding color marks the limits of the range of imperfect AFP surveillance combined with the range of possible ES approaches.
Table 2 reports the POE, CNC95%, CNC99%, TUC95% and TUC99% estimates assuming perfect surveillance only (top) and with our worst and best estimates of actual, imperfect surveillance quality (middle and bottom). The model suggests time periods of 1.4 to 2.6 years without paralytic cases caused by WPV or cVDPV required to achieve 95% confidence in the interruption of transmission in the context of perfect AFP surveillance (Table 2). Depending on the amount of poliovirus excreted into the sewage system and the quality of sampling sites, good quality ES used in addition to a good quality AFP surveillance could reduce the CNC95% by up to 28 months compared to perfect AFP surveillance, and by up to 42 months compared to worst performing one (Table 2). This interval increases for less than perfect AFP surveillance and decreases with the addition of sensitive ES.
Table 2.
Expected detected-event free period (DEFP) required for 95% and 99% confidence about no circulation (CNCx%) and time of undetected circulation between the last paralytic case and die-out (TUCx%) in Pakistan and Afghanistan assuming perfect and imperfect surveillance (based on 1,000 iterations)
| Virus | WPV1 (PIR = 1/200) | cVDPV2 (PIR = 1/2000) | WPV3 (PIR = 1/1000) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POE | 100% | 49% | 99% | |||||||||
| Metric | CNCx% | TUCx% | CNCx% | TUCx% | CNCx% | TUCx% | ||||||
| x% | 95% | 99% | 95% | 99% | 95% | 99% | 95% | 99% | 95% | 99% | 95% | 99% |
| DEFP values assuming perfect AFP surveillance without ES | ||||||||||||
| current path | - | - | - | - | 2.58 | 3.00 | 2.23 | 2.53 | 2.25 | 2.83 | 1.84 | 2.40 |
| increased relative SIA coverage 0.20 | 1.42 | 1.58 | 1.35 | 1.55 | - | - | - | - | - | - | - | - |
| increased relative SIA coverage 0.15 | 1.48 | 2.08 | 1.51 | 1.90 | - | - | - | - | - | - | - | - |
| DEFP values assuming worst performing surveillance (AFP with ES) | ||||||||||||
| current path | - | - | - | - | 3.75 | 3.83 | 3.19 | 3.34 | 4.67 | 6.42 | 3.39 | 5.70 |
| increased relative SIA coverage 0.20 | 2.33 | 2.42 | 2.28 | 2.40 | - | - | - | - | - | - | - | - |
| increased relative SIA coverage 0.15 | 3.25 | 3.67 | 3.14 | 3.55 | - | - | - | - | - | - | - | - |
| DEFP values assuming best performing surveillance (AFP with ES) | ||||||||||||
| current path | - | - | - | - | 0.25 | 0.33 | 0.22 | 0.25 | 1.17 | 1.50 | 0.70 | 1.36 |
| increased relative SIA coverage 0.20 | 0.67 | 0.92 | 0.59 | 0.87 | - | - | - | - | - | - | - | - |
| increased relative SIA coverage 0.15 | 0.75 | 0.92 | 0.62 | 0.82 | - | - | - | - | - | - | - | - |
Abbreviations: AFP, acute flaccid paralysis; CNCx%, DEFP at which the confidence about no circulation exceeds x%; cVDPV, circulating vaccine-derived poliovirus; DEFP, detected-event-free period; ES, environmental surveillance; PIR, paralysis-to-infection ratio; POE, probability of eradication; TUCx%, time at which the probability of undetected WPV circulation after the true last case becomes exceeds x%; WPV, wild poliovirus.
4. DISCUSSION
As suggested in our previous analysis (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015), we confirm that depending on the vaccination strategy and serotype, a 3-year period without detection may or may not suffice to reach 95% confidence about the absence of circulation, depending on the quality of the surveillance and population immunity. The example of Pakistan and Afghanistan shows the impact of imperfect information from AFP surveillance and varying ES systems, which underscores the need to focus on under-vaccinated areas to reach a high level of confidence about the absence of circulation.
The lower PIR of WPV3 compared to serotype 1, combined with gaps in surveillance quality may lead to significant increases in the length of DEFPs. However, with now more than 5 years since the last reported WPV3 case in Pakistan and Afghanistan (Kew et al., 2014), we estimate well over 95% confidence about no WPV3 circulation. If WPV1 transmission continues anywhere in the world, the increasing confidence in the absence of WPV3 circulation coupled with the continued burden of serotype 3 vaccine-associated paralytic poliomyelitis may raise the issue of a possible globally-coordinated switch from bOPV to serotype 1 monovalent OPV (instead of the planned strategy of simultaneous cessation of both OPV serotypes). Pakistan last detected a cVDPV2 case in 2016, with further serotype 2 VDPVs isolated from sewage as late as mid-2017, likely associated with the mOPV2 response vaccination campaign. Our results suggest that obtaining high confidence about no continuing cVDPV2 circulation may require 1-4 years with no detections, depending on the specific attributes of the surveillance system. For WPV1, our model suggests the need to further increase SIA quality (or alternatively frequency) to both achieve elimination and more rapidly achieve a high confidence in elimination.
Similar to our previous modeling work (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015), this analysis comes with several limitations. First, the use of a transmission threshold as the die-out criterion rather than absolute 0 total infected individuals represents a simplified construct of the complex dynamics of transmission die-out. Second, the nature and quality of information about ES influenced our assumptions about ES methods and model inputs, and as shown, the results remain sensitive to the approach to model ES and the assumptions about the distribution of sites. With respect to quality of information, the available data on watershed population of ES sampling sites remains incomplete and, in some cases inconsistent with expectations for a high-quality ES system (e.g., a surprisingly low watershed population of less than 100 for some sampling sites, see Table A1 in the appendix). These data limitations led us to assume country average watershed population in place of missing watershed estimates, and may mean that we underestimated the true watershed population for some sites. Third, in addition to the factors we modeled, the ability of ES to detect poliovirus also depends on other factors like the daily viral yield (Lodder et al., 2012), the methods for sample collection and virus concentration, and the ability to recover and identify poliovirus from concentrated ES samples, which represent finer levels of detail that we capture with our abstract model as part of site quality. Despite its limitations, we believe the analysis provides useful insights by exploring a range of possibilities.
The risk of undetected poliovirus circulation and the confidence about the absence of circulation in the absence of observed cases while actively looking will depend on population immunity and the quality of surveillance. As an addition to AFP surveillance, ES may help to reduce the time required to feel confident about the absence of undetected circulation under certain conditions. However, the efficiency of ES depends on its ability to detect transmission in areas with under-vaccinated subpopulations, which depends not only on security and access, but also on the nature of the sewage systems in those areas and system design choices (Kalkowska, Duintjer Tebbens, & Thompson, 2018). We emphasize that the sensitivity of ES remains 0 for people that do not excrete into the catchment areas of any ES sites and that the current overall population ES coverage for Pakistan and Afghanistan remains below 5%. We caution that the information from ES alone does not replace AFP surveillance and that countries should avoid a false sense of security about the absence of detections from ES alone.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge support for this work under Cooperative Agreement Number 5NU2RGH001913-02-00, funded by the US Centers for Disease Control and Prevention. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
REFERENCES
- Anis E, Kopel E, Singer S, Kaliner E, Moerman L, Moran-Gilad J, … Grotto I (2013). Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveillance, 18(38), pii=20586. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24084337 [DOI] [PubMed] [Google Scholar]
- Debanne SM, & Rowland DY (1998). Statistical certification of eradication of poliomyelitis in the Americas. Mathematical Biosciences, 150(1), 83–103. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, Wassilak SGF, Pallansch MA, Cochi SL, & Thompson KM (2014). The potential impact of expanding target age groups for polio immunization campaigns. BMC Infect Dis, 14, 45 Retrieved from http://www.biomedcentral.com/content/pdf/1471-2334-14-45.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, … Thompson KM. (2013a). Expert review on poliovirus immunity and transmission. Risk Analysis, 33(4), 544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, … Thompson KM. (2013b). Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Analysis, 33(4), 606–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Ehrhardt DT, Farag NH, Hadler SC, … Thompson KM. (2018). Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Analysis, 38(8), 1701–1717. doi: 10.1111/risa.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, & Thompson KM (2013). Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis, 23(4), 703–749. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, … Thompson KM. (2006). Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis, 26(6), 1471–1505. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, & Thompson KM (2005). A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. American Journal of Epidemiology, 162(4), 358–372. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, … Thompson KM. (2013). Review: Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Analysis, 23(4), 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, & Thompson KM (2015). Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infect Dis, 15, 379. doi: 10.1186/s12879-015-1115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2017). Costs and benefits of including inactivated in addition to oral poliovirus vaccine in outbreak response after cessation of oral poliovirus vaccine use. Medical Decision Making Policy & Practice, 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, & Dietz K (1996). Eradication of poliomyelitis: when can one be sure that polio virus transmission has been terminated? American Journal of Epidemiology, 143(8), 816–822. [DOI] [PubMed] [Google Scholar]
- Etsano A, Damisa E, Shuaib F, Nganda GW, Enemaku O, Usman S, … Wiesen E (2016). Environmental isolation of circulating vaccine-derived poliovirus after interruption of wild poliovirus transmission - Nigeria, 2016. Morbidity and Mortality Weekly Report, 65(30), 770–773. doi: 10.15585/mmwr.mm6530a4 [DOI] [PubMed] [Google Scholar]
- Famulare M (2016). Has wild poliovirus been eliminated from Nigeria? . PLoS One, 10, e0135765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Polio Eradication Initiative. (2015, September 20, 2015). Global eradication of wild poliovirus type 2 declared. Retrieved from http://www.polioeradication.org/mediaroom/newsstories/Global-eradication-of-wild-poliovirus-type-2-declared/tabid/526/news/1289/Default.aspx
- Global Polio Eradication Initiative. (2017). Eradication within reach. Annual report 2016. Retrieved from Geneva: http://polioeradication.org/wp-content/uploads/2017/08/AR2016_EN.pdf [Google Scholar]
- Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, … Immunization Systems Management Group of the Global Polio Eradication Initiative. (2016). Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide, 2016. Morbidity and Mortality Weekly Report, 65(35), 934–938. doi: 10.15585/mmwr.mm6535a3 [DOI] [PubMed] [Google Scholar]
- Horstmann DM, & Paul JR (1947). The incubation period in human poliomyelitis and its implications. Journal of the American Medical Association, 135(1), 11–14. [DOI] [PubMed] [Google Scholar]
- Houy N (2015). The probability of undetected wild poliovirus circulation: Can we do better? Journal of Theoretical Biology, 382, 272–278. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Grotto I, Shulman LM, Anis E, Wassilak SGF, … Thompson KM. (2015). Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. Journal of Infectious Diseases, 211(11), 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, & Thompson KM (2015). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Implications for surveillance and vaccination. BMC Infect Dis, 15(66), 1. doi: 10.1186/s12879-015-0791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2012). The probability of undetected wild poliovirus circulation after apparent global interruption of transmission. American Journal of Epidemiology, 175(9), 936–949. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2014). Modeling strategies to increase population immunity and prevent poliovirus transmission in the high-risk area of northwest Nigeria. Journal of Infectious Diseases, 210(Suppl 1), S412–S423. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2018). Environmental surveillance system characteristics and impacts on confidence about no undetected serotype 1 wild poliovirus circulation. Risk Analysis, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew OM, Cochi SL, Jafari HS, Wassilak SG, Mast EE, Diop OM, … Centers for Disease Control and Prevention (CDC). (2014). Possible eradication of wild poliovirus type 3--worldwide, 2012. Morbidity and Mortality Weekly Report, 63(45), 1031–1033. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25393222 [PMC free article] [PubMed] [Google Scholar]
- Lodder WJ, Buisman AM, Rutjes SA, Heijne JC, Teunis PF, & de Roda Husmana AM (2012). Feasibility of Quantitative Environmental Surveillance in Poliovirus Eradication Strategies. Applied and Environmental Microbiology, 78(11), 3800–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N, & Kew OM (2010). From emergence to eradication: the epidemiology of poliomyelitis deconstructed. American Journal of Epidemiology, 172(11), 1213–1229. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20978089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadi C, Damisa E, Esapa L, Braka F, Waziri N, Siddique A, … Adamu U (2017). Continued endemic wild poliovirus transmission in security-compromised areas - Nigeria, 2016. MMWR. Morbidity and mortality weekly report, 66(7), 190–193. doi: 10.15585/mmwr.mm6607a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel-T Innovative Solutions. Environmental Surveillance Maps. Retrieved from http://maps.novel-t.ch/#/catalog/all Retrieved November 9, 2017 http://maps.novel-t.ch/#/catalog/all
- Platt LR, Estivariz CF, & Sutter RW (2014). Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. Journal of Infectious Diseases, 210(Suppl 1), S380–389. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25316859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. (2017). World Population Prospects. The 2017 revision. Volume I: Comprehensive tables (ESA/P/WP/248). Retrieved from https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_Volume-I_Comprehensive-Tables.pdf
- World Health Assembly. (1988). Global eradication of poliomyelitis by the year 2000 (resolution 41.28). In. Geneva: World Health Organization. [Google Scholar]
- World Health Assembly. (2008). Poliomyelitis: mechanism for management of potential risks to eradication (resolution 61.1). In. Geneva: World Health Organization. [Google Scholar]
- World Health Organization. (2005). Polio Eradication Initiative. Cessation of Routine Oral Polio Vaccine (OPV) use after Global Polio Eradication Framework for National Policy Makers in OPV-using Countries (WHO/POL/05.02). Retrieved from Geneva, Switzerland: [Google Scholar]
- World Health Organization. (2013). Global Polio Eradication Initiative: Polio Eradication and Endgame Strategic Plan (2013-2018) (WHO/POLIO/13.02). Retrieved from Geneva: http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf [Google Scholar]
- World Health Organization. (2018). Polio this week as of 3 January 2018. Retrieved from http://polioeradication.org/polio-today/polio-now/this-week/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


