Abstract
COVID-19 is a global health threat with a huge number of confirmed cases and deaths all over the world. Human-to-human transmission via respiratory droplets and contact with aerosol-infected surfaces are the major routes of virus spread. Because SARS-CoV-2 can remain in the air and on surfaces from several hours to several days, disinfection of frequently touched surfaces and critical rooms, in addition to observing individual hygiene tips, is required to reduce the virus spreading. Here we report on an investigation into the use of gaseous ozone as a potentially effective sanitizing method against the new coronavirus.
Keywords: COVID-19, SARS-CoV-2, Contact transmission, Personal protective equipment, Infected surfaces, Ozone
Introduction
A novel human coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, in late 2019 and caused a pandemic, spreading worldwide and posing a huge global health threat. The total number of global deaths due to the COVID-19 (SARS-CoV-2-caused disease) at the end of September 2020 had risen to 991,224, with 37,730,945 infected [1]. To deal with this pandemic, in addition to research in medical fields, the main health measures recommended by World Health Organization (WHO) have focused on social distancing and lockdown, as well as application of a safety protocols, adoption of hygiene measures and use of personal protection equipment (PPE), such as masks, gloves, etc.
To adopt effective countermeasures to contain the pandemic and minimize the death toll, it is imperative to recognize all routes of possible virus transmission. Increasing recent evidences suggest that human-to-human transmission is primarily achieved through close contact of respiratory droplets, direct contact with infected individuals or by contact with contaminated objects and surfaces.
Aerosol transmission seems to be the most important route of SARS-CoV-2 infection: this virus is able to maintain its infectivity and virion integrity for up to 16 h in respirable-sized aerosols in closed rooms [2]. Moreover it has been demonstrated that viable virus can be isolated from air samples collected up to 4.8 m away from a COVID-19 patient in a hospital room [3].
While the primary spread of SARS-CoV-2 appears to be via aerosols and respiratory droplets, fomites may be also an important contributor in virus transmission, as demonstrated for other coronaviruses (as for NL63, 229E and MERS-CoV). Indeed, viruses are able to survive on surfaces for extended periods, sometimes up to months. Once contaminated from the environment, hands can then initiate a self-inoculation of mucous membranes of the nose, eyes or mouth. Fathizadeh et al. [4] showed that SARS-CoV-2 can persist on a variety of surfaces from hours to days, allowing the transmission via surface contact: up to 4 h on copper, up to 8 h on latex gloves, two days on surgical gowns, two to three days on steel, four days on glass, four to five days on wood, five days on metal, from four to nine days on plastic and from one to five days on paper. Riddel et al. [5] demonstrated that infectious SARS-CoV-2 can be recovered from four different and common non-porous surfaces – banknotes, stainless steel, glass, vinyl – for at least 28 days (at room temperature (RT) and 50% relative humidity (RH)). In addition, Chin et al. [6] demonstrated that the virus is more stable on smooth surfaces: a significant level of SARS-CoV-2 has been detected on the outer surface of surgical masks on day 7 after infection.
The antiviral and antimicrobial properties of ozone have been well documented, although the mechanisms of action are not well understood, and several macromolecular targets could be involved. In 2009 Hudson et al. [7] demonstrated the antiviral properties of gaseous ozone testing 12 different viruses (mostly human pathogens) in the laboratory and in the field, representing different virus families and structural features, on hard and porous surfaces and on aerosol. All tested viruses, DNA and RNA viruses with or without membranes, showed a similar inactivation kinetics on three hard surfaces (plastic, glass and stainless steel) and on cotton and fabric surfaces, suggesting that all or most viruses should be susceptible to ozone.
The aim of this preliminary evaluation was to investigate the efficacy of gaseous ozone on SARS-CoV-2 viability on different surfaces. This was in order to assess the feasibility of gaseous ozone as a potential effective sanitizing method to remove the virus from high-risk indoor rooms (such as hospital and nursing rooms), hard to reach and critical surfaces (where other disinfectants cannot be used) and medical equipment, for which pivotal precautions must be planned and taken.
Methods
Fifty-microlitre droplets (E-MEM 1% penicillin/streptomycin/glutamine added with 5 μL/mL trypsin) containing 100,000 SARS-CoV-2 copies, isolated from one COVID-19 patient hospitalized by Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia (Italy), were spotted on eight different surfaces: stainless steel, painted and not-painted aluminum, Plexiglas, glass, plastic, FFP2 mask and surgical gown. The size of all tested surfaces was 3 × 8 cm and all experimental steps were performed inside a closed Plexiglas box provided with a fan, humidity and temperature probes and an ozone gas probe, ensuring the complete control of all experimental parameters and the operator's safety. The contaminated surfaces, placed on a holed tray allowing gas flow, were fumigated with different concentrations of gaseous ozone (0.5 ppm, 1 ppm and 2 ppm) for 40 min and 60 min. Control samples were placed in the Plexiglas box for 40 min and 60 min with no ozone fumigation. All test and control samples were then collected. Surfaces were washed with 50 μL of medium and placed in 96-well cell culture microtitre plate. A four-fold dilution was performed and 30,000 VERO E6 cells in 50 μL of medium were added to each well in order to evaluate the virus viability (microtitration assay).
All experimental steps with SARS-CoV-2 were performed under conditions of biosafety level 3 containment.
Results and discussion
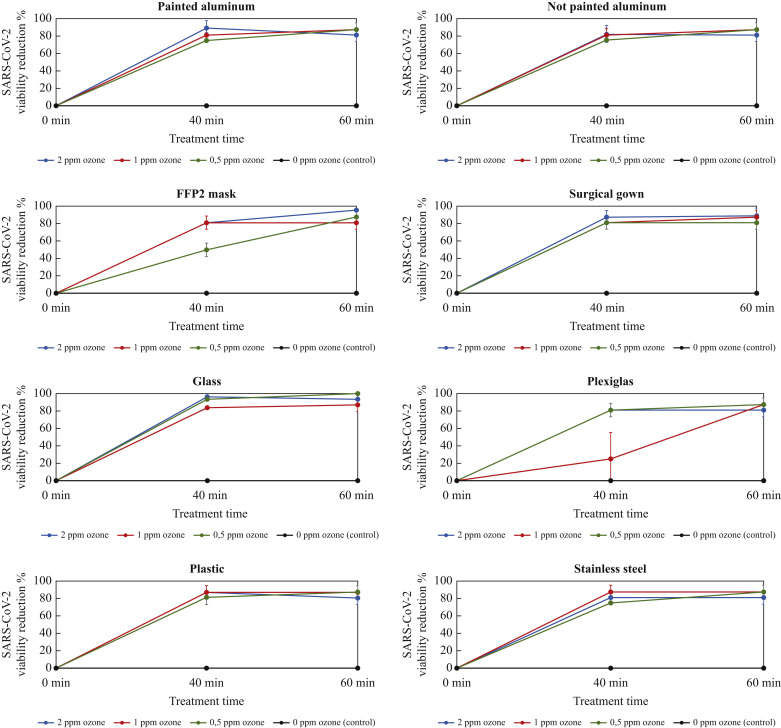
The results are summarized in Figure 1 . These show that gaseous ozone at the three tested concentrations of 0.5 ppm, 1 ppm and 2 ppm significantly reduced the SARS-CoV-2 titre after 40 min fumigation on all assessed surfaces, including FFP2 mask and surgical gown. These findings could have important implications in decreasing the risk of contamination for healthcare professionals associated with PPE management and in increasing their availability in hospitals and nursing rooms, helping to face the critical situation of extreme scarcity caused by the COVID-19 pandemic. Indeed, healthcare workers are in the front line of the COVID-19 outbreak response and are exposed to a high risk of SARS-CoV-2 infection daily. PPE (gowns, masks, gloves) is their main defence against viral contamination. While most PPE is designated for single use, during the COVID-19 pandemic re-use of PPE was considered, requiring effective and fast treatment conserving material properties. This could reduce consumption (increasing availability) and waste products (decreasing environmental impact).
Figure 1.
SARS-CoV-2 viability reduction percentage after fumigation with gaseous ozone 0.5 ppm, 1 ppm and 2 ppm on eight different surfaces: painted aluminum, not-painted aluminum, FFP2 mask, surgical gown, glass, Plexiglas, plastic and stainless steel. All experiments were performed at controlled and steady relative humidity (55%) and temperature (24°C).
The virucidal efficacy of ozone did not seem to be directly proportional to its concentration and was not dependent on surface type. Therefore, gaseous ozone could be a useful and widely accessible method to significantly reduce the infectious SARS-CoV-2 from almost all medical equipment and surfaces not susceptible to corrosion. It could help in reducing virus spreading, mostly in high-risk rooms/areas in healthcare facilities, and on hard-to-reach critical surfaces as a better alternative to disinfectants.
Ozone may react with viruses through direct molecular interaction and/or indirectly through reactive oxygen species (ROS), produced as a result of ozone decomposition. In addition, the reaction between ozone and its ROS with the constituents of the virus structure (including lipids, proteins and amino acids) could lead to the formation of other ROS, including reactive radicals, which further propagate oxidation through a chain reaction. As already assumed by Tizaoui [8], ozone, via an oxidative process, could attack the proteins and lipids of the SARS-CoV-2's spikes and envelope, destroying the virus integrity and inhibiting the mechanism by which it infects.
There are many advantages to using ozone as a surface and room sanitizer. Ozone is a much more potent oxidizer than other common disinfectants (such as chlorine and hypochlorite), with a wide biocidal effect, guaranteeing a meaningful reduction of airborne micro-organisms. As a gas, ozone can penetrate into every room corner, reaching all surfaces, and thus is much more efficient than manually applied liquid sprays and aerosols. As an unstable gas, ozone is generated on-site and on-demand and it is readily converted back to oxygen, leaving no harmful residuals (ozone is environmentally friendly).
Although gaseous ozone is effective and advantageous in the sanitizing processes, it presents risks for people safety and health if it is not properly handled. When inhaled, ozone can damage the lungs, causing chest pain, coughing, shortness of breath and throat irritation. Ozone may also worsen chronic respiratory diseases, such as asthma. Therefore, it is necessary to take adequate safety measures during its use. Healthcare buildings, such as hospitals and elderly care facilities, host vulnerable people making it especially important to plan ozone sanitizing. It is also considered one of the secondary pollutant components of photochemical smog, which produces effects on human health and property. These health hazards can be overcome in practice by removing gaseous ozone after treatment using a catalytic converter. Ozone is a strong oxidizing agent, reacting with organic compounds and inducing corrosion of certain materials. Sensitive materials such as natural rubber need to be removed or temporarily covered, to protect against corrosion.
The same experiments as in Figure 1 were replicated after drying the viral solution spotted on the eight different surfaces, leaving them under the direct laminar flow hood to dry before proceeding with gaseous ozone fumigation. In all samples, control and treated ones, no viable virus was found (data not shown). These results suggest that hydration and a certain percentage of humidity inside the droplet are mandatory to guarantee the SARS-CoV-2 viability on surfaces.
The persistence of viruses in the environment outside their hosts is essential to allow their spread. The environmental conditions (temperature, RH, sunlight, air flow, etc.), in addition to the characteristics of the virus itself (RNA/DNA virus with or without envelope, etc.) and the characteristics of the abiotic or biotic environmental surfaces it contaminates, are important factors that determine the infectivity retention and extent and speed of the virus spread. Many studies have shown that persistence of coronaviruses on surfaces and fomites is affected by temperature and RH. For example Aboubakr et al. and Biryukov et al. [9,10] demonstrated that coronaviruses, including SARS-CoV-2, survive longer at lower temperature and lower RH. Indeed, the fragile structure of viruses, particularly enveloped viruses, make them susceptible to heat, through the denaturation of the secondary structures of viral capsid proteins, altering the conformation of virion proteins involved in the attachment and replication within a host cell. Regarding humidity, our results suggest that it may have a double effect on SARS-CoV-2 viability: a higher humidity increases virus survival inside droplets, whilst it decreases the chances of survival if the virus is airborne. Once the droplet evaporates, the virus is not expected to survive.
The longer survival of SARS-CoV-2 at low temperature and low environmental RH may at least partly explain the observed peaks of COVID-19 cases during the cold and dry seasons in temperate regions, and is consistent with the seasonality of other respiratory viruses such as influenza virus.
In conclusion, alongside recommended WHO countermeasures (social distancing, wearing a mask, etc.), using gaseous ozone as a sanitizing method for high-risk indoor rooms and critical or hard to reach surfaces, especially in healthcare facilities, could help to reduce virus spread, keeping patients and healthcare professionals safe during the current pandemic. Further studies are required to confirm ozone efficacy in different conditions, potentially expanding possible applications.
Acknowledgements
The authors would like to thank the COVID-19 IRCCS San Matteo Pavia Task Force for the job done during the emergency period and for the support received for the laboratory experiments.
Conflict of interest statement
None declared.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
References
- 1.WHO–Health Emergency Dashboard . 2020. World health organization coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [last accessed September 2020] [Google Scholar]
- 2.Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020;26(9):2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lednicky J.A., Lauzardo M., Fan Z.H., Jutla a, Tilly T.B., Gangwar M. Viable SARS-CoV-2 in the air of hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fathizadeh H., Maroufi P., Momen-Heravi M., Dao S., Kose S., Ganbarov K. Protection and disinfection policies against SARS-CoV-2 (COVID 19) Infez Med. 2020;28(2):185–191. [PubMed] [Google Scholar]
- 5.Riddel S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol J. 2020;17:145–151. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin A., Chu J., Perera M., Hui K.P.Y., Yen H.L., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci Eng. 2009;31(3):216–223. [Google Scholar]
- 8.Tizaui C. Ozone: A potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone Sci Eng. 2020;42(5):378–385. [Google Scholar]
- 9.Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biryukov J., Boydston A., Dunning R.A., Yeager J.J., Woods S., Reese A.L. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5(4) doi: 10.1128/mSphere.00441-20. 2020 07 01. [DOI] [PMC free article] [PubMed] [Google Scholar]