Abstract
Background and aim:
The transcriptional co-activator Yes-associated protein-1 (YAP1) has been implicated as an oncogene and is overexpressed in different kinds of human cancers, especially hepatocellular carcinoma (HCC). However, the role of YAP1 has not been reported in residual/recurrent HCC after transarterial chemoembolization (TACE). Our aim is to determine whether YAP1 is overexpressed in the residual/recurrent HCC after TACE.
Methods:
A total of 105 tumor tissues from 71 patients including 30 cases of primary HCC without prior treatment, 35 cases of residual/recurrent HCC post TACE, and 6 cases of hepatoblastoma were included in the immunohistochemical study. YAP1 immunoreactivity was blindly scored as 0, 1+, 2+ or 3+ in density and percentages of positive cells.
Results:
About 33.3% (10/30) of primary HCC without prior treatment showed 2+ of YAP1 immunoreactivity. While 82.8% (29/35) of residual/recurrent HCCs after TACE treatment displayed 2–3+ of YAP1 immunoreactivity, which was significantly higher compared to primary HCC without prior treatment (P = 0.0002). YAP1 immunoreactivity was moderately to strongly positive (2–3+) in 100% of the hepatoblastoma, particularly in the embryonal components (3+ in 100% cases).
Conclusions:
YAP1 is significantly upregulated in the residual/recurrent HCCs post TACE treatment, suggesting that YAP1 may serve as a sensitive diagnostic marker and a treatment target for residual/recurrent HCC post TACE.
Keywords: Yes-associated protein-1 (YAP1), Residual/recurrent hepatocellular, carcinoma, Hepatoblastoma, Cancer stem cell (CSC), Immunohistochemistry, Transarterial chemoembolization (TACE)
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and occurs predominantly in patients with underlying chronic liver disease and cirrhosis. It is the sixth most common cancer and the third most frequent cause of cancer-related death worldwide.1 Considerable advances have been made in the treatment for HCC. Hepatic resection, liver transplantation (LT), and radio frequency ablation (RFA) are considered potentially curative for early-stage HCC. However, many patients were found to have advanced stage of disease at present. Numerous non-curative treatment modalities have been routinely used for advanced HCC, include transarterial chemoembolization (TACE), sorafenib, and systemic chemotherapy.2 TACE is, so far, the standard treatment for asymptomatic patients with a solitary or limited multifocal HCC without vascular invasion or extrahepatic spread and with well-preserved liver function.3,4 The advantage of TACE therapy rests in its ability to deliver high concentrations of cytotoxic agents to hypervascular liver tumors by selective disruption of feeding arteries and to minimize damage to the surrounding liver parenchyma.5,6 Combining chemotherapeutic drugs with embolic material results in a synergistic treatment effect; ischemic tumor necrosis and extended exposure of the tumor to the chemotherapeutic agent are the major treatment-related benefits of this approach. Moreover, the use of embolizing agents facilitates lower systemic drug levels, thereby reducing toxicity. Despite these advances and technical refinements, the long-term survival of patients managed with TACE remains dismal due to high rates of tumor recurrence as well as distant metastasis.
A progenitor cell population, sometimes called cancer stem cells (CSCs), able to seed new tumors with very low inoculum levels, has been proposed to be responsible for chemoresistance and the recurrence of HCC.7-9 Our previous work has shown that a progenitor/cholangiocyte marker, the cytokeratin (CK) 19, and a CSC marker, epithelial cell adhesion molecule (EpCAM), are significantly upregulated in the residual/recurrent HCC after TACE.10 These findings suggest that the existence of progenitor cell population, or CSCs, may be associated with residual/recurrent HCC after TACE. Therefore, a better understanding of the biology of the residual/recurrent tumors and finding the factors that strongly predict the recurrence post-TACE would have a major impact on the management of the disease.
Yes-associated protein-1 (YAP1) is a transcriptional co-activator, which can suppress apoptosis, induce epithelial-to-mesenchymal transition (EMT) and growth factor-independent proliferation, and oncogenesis.11 YAP1 is an important downstream effector of the Hippo pathway, which is one of the key signaling pathways regulating cell proliferation and apoptosis associated with normal development, stem cell self-renewal, and differentiation, mainly through phosphorylation and subsequent retention of YAP1 in the cytoplasm.12 YAP has been implicated as an oncogene and is overexpressed in different kinds of human cancers including HCC, gastric cancer, colorectal cancer, non-small-cell lung cancer and small-cell lung cancer.13 However, the expression of YAP1 has not been studied in residual/recurrent HCC after TACE.
We hypothesized that YAP1 may be overexpressed in residual/recurrent HCC after TACE. To test this hypothesis, we compared the immunoreactivity of YAP1 in a total of 105 tumor tissues from 71 patients including 30 cases of primary HCC without prior treatment, 35 cases of residual/recurrent HCC post TACE, and 6 cases of hepatoblastoma.
2. Materials and methods
2.1. Patient selection
A total of 105 tumor tissues from 71 patients including 30 cases of primary HCC without prior treatment, 35 cases of residual/recurrent HCC post TACE, and 6 cases of hepatoblastoma were included in the immunohistochemical study. Institutional review board approval was obtained at Saint Louis University for this study. The patients’ clinical information and etiology of the disease are shown in Table 1.
Table 1.
Characteristics of study samples and univariate analysis stratified by HCC treatment status.
| Characteristics | Total (N = 65) | HCC treated with TACE (N = 35) | HCC without treatment (N = 30) | P-value |
|---|---|---|---|---|
| Age (years) | 59.4 ± 6.3 | 59.7 ± 6.9 | 59.1 ± 5.6 | 0.7430a |
| Sex | ||||
| Male | 46 (70.8) | 24 (68.6) | 22 (73.3) | 0.6739b |
| Female | 19 (29.2) | 11 (31.4) | 8 (26.7) | |
| Cause of HCC | ||||
| HBV | 12 (18.5) | 6 (17.1) | 6 (20.0) | 0.9096c |
| HCV | 37 (56.9) | 20 (57.2) | 17 (56.7) | |
| Alcohol | 4 (6.1) | 3 (8.6) | 1 (3.3) | |
| Cryptogenic | 12 (18.5) | 6 (17.1) | 6 (20.0) |
Data are shown as N (%) or means ± standard deviation (SD).
P-value from independent sample t-test
P-value from chi-square test
P-value from Fisher’s exact test.
Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus.
2.2. Immunohistochemistry
Immunostaining for YAP1 was performed in all 105 tumor tissues. Tissue sections (4 μm) were cut from paraffin-embedded tissue blocks and stained with antibodies against YAP1 (ab56701; Abcam, Cambridge, UK). Immunohistochemical staining for YAP1 was performed on the Ventana Benchmark Ultra (Ventana, Tucson, AZ, USA). Pretreatment was performed with an on-board antigen retrieval method. The immunization intensity was evaluated with a previously reported scoring system.14 The intensity of YAP1 nuclear staining was evaluated as follows: 0, negative; 1 +, weak; 2 +, moderate; and 3 +, strong. The intensity was based on comparison with staining of external positive controls or internal positive controls (reactive ductules). The percentage of positive cells was recorded for each case.
2.3. Statistical analysis
Descriptive characteristics of study participants were calculated using Chi square and Fisher’s exact tests for categorical variables and independent sample t-test for continuous variables. Analysis was stratified by HCC treatment status (TACE treatment vs. no treatment). The analysis was conducted in SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). All tests were two-sided and were deemed statistically significant when P < 0.05.
3. Results
3.1. Patients’ demographics
Descriptive statistics of study sample are presented in Table 1. Among the total of 65 patients with HCC, 53.8% were treated with TACE versus 46.2% without any treatments. All of them had a diagnosis of cirrhosis. The mean age of patients was 59.4 ± 6.3 years. The majority of patients were male (70.8%) and had a diagnosis of HCV (56.9%) as the etiology for cirrhosis.
In the univariate analysis, stratifying the analysis by HCC treatment status, patient characteristics were somewhat uniformly distributed between those who were treated with TACE vs. those without treatment (Table 1). Therefore, no statistically significant associations between age, sex, and cause of HCC, and the HCC treatment status (TACE vs. no treatment) were noted, P = 0.7430, 0.6739, and 0.9096, respectively.
3.2. YAP1 is moderately expressed in ductules and some HCCs while strongly expressed in hepatoblastoma
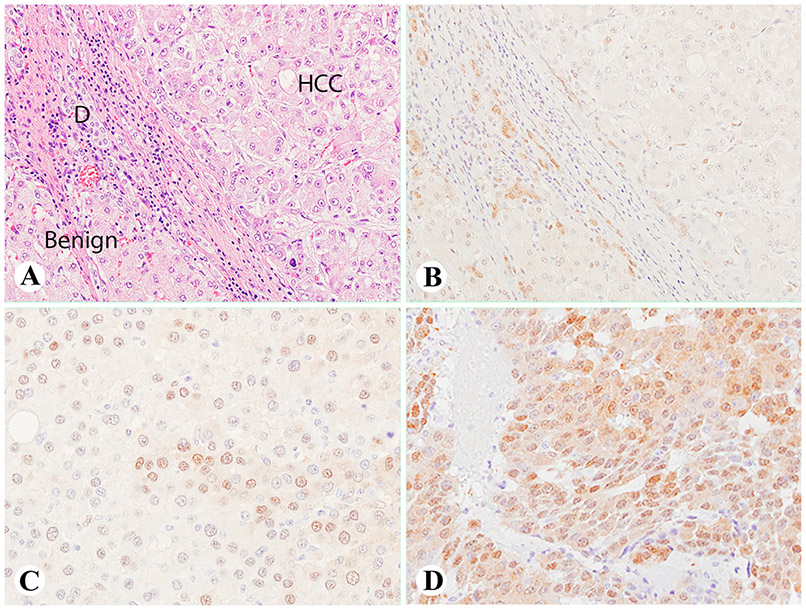
We found that in some HCC cases, YAP1 immunoreactivity was only positive in the ductules surrounding the cirrhotic nodules, but negative in the HCC cells and normal hepatocytes (Fig. 1A and B). And some HCC cases showed focally and moderately positive YAP1 in the HCC cells (Fig. 1C). While in all the hepatoblastoma cases, YAP1 was strongly and diffusely positive in the tumor cells (Fig. 1D).
Fig. 1. YAP1 immunoreactivity in benign cirrhotic liver, HCCs and hepatoblastoma.
(A, B) The HCC case showing positive YAP1 in the ductules surrounding the cirrhotic nodules (A, H&E staining), but negative in the HCC cells and normal hepatocytes. (C) Another HCC case showing focally and moderately positive YAP1 in the HCC cells. (D) A hepatoblastoma case showing moderately to strongly positive YAP1 in the tumor cells. (A–D, 400×). D denotes ductule in Fig. 1A. Abbreviations: YAP1, Yes-associated protein-1; HCC, hepatocellular carcinoma; H&E, hematoxylin-eosin.
3.3. TACE causes HCC cell necrosis and increases YAP1 immunoreactivity in the tumor cells near the necrotic areas
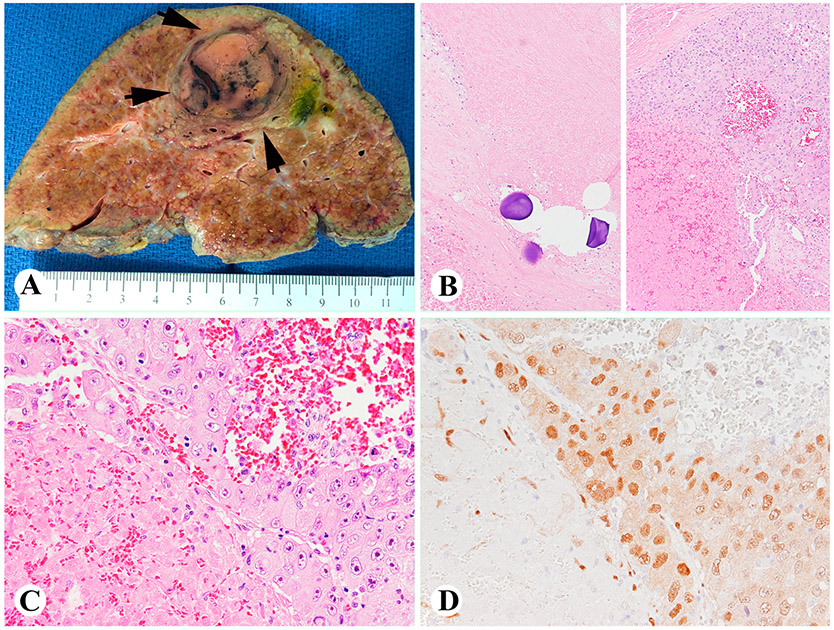
As shown in Fig. 2A and B, TACE caused massive HCC cell necrosis, but some residual/recurrent tumors were found adjacent to the necrotic areas. Interestingly, YAP1 immunoreactivity was strongly and diffusely positive in the viable residual/recurrent tumor cells (Fig. 2C and D).
Fig. 2. TACE causes HCC necrosis and increases YAP1 immunoreactivity in the tumor cells near the necrotic area.
(A) A gross cross section of TACE treated HCC (arrows). (B, C) H&E staining showing TACE beads causes HCC necrosis (B, left) and residual/recurrent tumor adjacent to the necrotic area (B, right, 100×; C, 400×); (D) Viable HCC cells of (C) showing strong and diffuse YAP1 immunoreactivity (400×). Abbreviations: TACE, transarterial chemoembolization; YAP1, Yes-associated protein-1; HCC, hepatocellular carcinoma; H&E, hematoxylin-eosin.
3.4. YAP1 is significantly overexpressed in residual/recurrent HCCs post TACE
We then analyzed the immunoreactivity of YAP1 in 105 tumors from 71 patients including 30 cases of primary HCC without prior treatment, 35 cases of residual/recurrent HCC post TACE, and 6 cases of hepatoblastoma. Among the 30 primary HCCs without prior treatment, YAP1 immunoreactivity was negative in 9/30 (30%), weakly positive (1+) in 11/30 (36.7%) and moderately to strongly positive (2+) in 10/30 (33.3%) of the cases. While, among the 35 cases with residual/recurrent HCCs post TACE, YAP1 reactivity was negative in 3/35 (8.6%), weakly positive (1+) in 3/35 (8.6%) and moderately to strongly and diffusely positive (2–3+) in 29/35 (82.8%) of the cases. The frequency of YAP1-positive HCC nodules was significantly higher in recurrent/residual HCCs after TACE treatment than in primary HCC without prior treatment (P = 0.0002) (Table 2). Further, there was a statistically significant difference of YAP1 staining between HCC without prior treatment and residual/recurrent HCC after TACE treatment from the same explanted livers of 16 patients; the majority of patients with residual/recurrent HCC after TACE treatment had either focal (45.7%) or diffuse (45.7%) YAP staining which was in contrast to HCC patients without treatment in which the majority of patients had either no (30.0%) or focal (56.7%) YAP staining (P = 0.0071).
Table 2.
Expression of YAP1 in residual/recurrent HCC treated with TACE vs. HCC without treatment.
| Characteristics | Total (N = 65) | HCC treated with TACE (N = 35) | HCC without treatment (N = 30) | P-value |
|---|---|---|---|---|
| YAP1 activity | ||||
| 0 | 12 (18.5) | 3 (8.6) | 9 (30.0) | 0.0002a |
| 1+ | 14 (21.5) | 3 (8.6) | 11 (36.7) | |
| 2–3+ | 39 (60.0) | 29 (82.8) | 10 (33.3) | |
| YAP1 staining | ||||
| None | 12 (18.5) | 3 (8.6) | 9 (30.0) | 0.0071a |
| Focal | 33 (50.8) | 16 (45.7) | 17 (56.7) | |
| Diffuse | 20 (30.8) | 16 (45.7) | 4 (13.3) |
Data are shown as N (%). Percentages may not total 100 because of rounding.
P-value from Fisher’s exact test.
Abbreviations: YAP1, yes-associated protein-1; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization.
Interestingly, among the 6 hepatoblastoma cases, YAP1 immunoreactivity was moderately to strongly positive (2–3+) in 100% of the cases, particularly in the embryonal components (3+ in 100% cases).
4. Discussion
Our study indicated that: (i) YAP1 is not expressed in normal hepatocytes, it is expressed in the ductules surrounding the nodules of cirrhosis patients and in focally some HCC patients, and strongly expressed in hepatoblastoma, (ii) YAP1 expression is strong and diffuse in the majority of residual/recurrent HCC post TACE.
TACE can induce ischemic tumor necrosis and extended exposure of the tumor to the chemotherapeutic agent and remains a standard approach for unresectable tumors with preserved liver function. However, the high rates of tumor recurrence and residual tumors after TACE treatment is still a big problem. The existence of a progenitor cell population or CSCs, has been proposed to be one mechanism accounting for the chemotherapy resistance and recurrence of HCC.7,8 We investigated whether the TACE treatment induces the expression of YAP1, a transcription cofactor, which stimulates cell proliferation, suppresses apoptosis and promotes stem cell renewal, regeneration, and oncogenesis.11 In the present study, YAP1 is moderately positive in the ductules surrounding the cirrhotic nodules and some cases of HCC cells, but negative in normal hepatocytes and some cases of HCC cells, while hepatoblastoma shows strongly and diffusely positive YAP1 in the tumor cells. Further study shows that YAP1 is significantly overexpressed in residual/recurrent HCC post TACE than in primary HCCs without prior treatment (82.8% versus 33.3% of moderately to strongly positive (2+ or 3+)).
YAP1 is a major downstream target of the Hippo-signaling pathway, which regulates tissue homeostasis, organ size, stem cell self-renewal and tumorigenesis.15-17 Phosphorylation and subsequent restriction of YAP1 transcriptional activity is the principal mechanism of growth and tumor suppression by the Hippo pathway. YAP1 activation has been described in hepatic and biliary regeneration and also detected in multiple tumor types, including HCC.18-20 Furthermore, a study showed that YAP1 expression was more frequently noted in the HCCs and the combined hepatocellular-cholangiocarcinoma (cHC-CC) with positive cancer stem cell marker (EpCAM), or progenitor/cholangiocyte marker (cytokeratin (CK) 19), and the overall survival rate was significantly lower in HCCs and cHC-CC with YAP1 expression compared to those without YAP1 expression.21 YAP1 is also reported to enriched and activated in the biliary compartment within the normal liver and injured liver.22 Studies have found that a population of atypical ductal cells, usually referred to as “oval cells”, emerges from the bile ducts, has been referred to as facultative stem cells or as a reserve stem cell compartment and is thought to participate in liver repair under conditions of extreme stress or chronic injury.23,24 The mechanisms of YAP signaling in HCC involve multitude of cancer-associated pathways including hepatocyte proliferation, deregulated endoplasmic reticulum/unfolded protein response, suppression of apoptosis, and chromosomal instability. Recent studies also showed that the activated YAP can target monocyte chemotactic protein 1 (Mcp1), which triggers the accumulation of tumor-infiltrating macrophages that impair immune clearance of transformed hepatocytes and promote HCC development.25 Consistently, in the present study, YAP1 is moderately positive in the ductules surrounding the cirrhotic nodules and some HCC cells but negative in normal hepatocytes and some cases of HCC cells. These results suggest that YAP1 is highly expressed in the cells with progenitor/cholangio-differentiation potentials.
Hepatoblastoma is an uncommon malignant liver cancer occurring in infants and childhood and composed of tissue resembling fetal liver cells, mature liver cells, or bile duct cells. Accumulating evidence suggests that hepatoblastoma is derived from stem cells.26 It has been reported that β-catenin and YAP1 interact physically and are activated in most human hepatoblastoma tissue; overexpression of activated forms of these proteins in mouse livers leads to rapid tumor development.27 In accordance with these findings, our current analysis showed that among the 6 hepatoblastoma cases, YAP1 immunoreactivity was moderately to strongly positive (2–3+) in 100% of the cases, particularly in the embryonal components (3+ in 100% cases). The strong expression of YAP1 in hepatoblastoma further implies that YAP1 is associated with progenitor cell population or CSCs.
The progenitor cell population or CSCs can seed new tumors with very low inoculum levels and has been proposed to be responsible for chemoresistance and the recurrence of HCC.7,8 Here, we find that among the 35 cases with residual/recurrent HCCs post TACE, YAP1 reactivity was moderately to strongly and diffusely positive (2–3+) in 29/35 (82.8%) of the cases. While among the 30 primary HCC patients without prior treatment, YAP1 immunoreactivity was moderately positive (2+) in 10/30 (33.3%) of the cases. In addition, patients with residual/recurrent HCC after TACE treatment had more diffuse YAP staining while HCC patients without treatment had more focal staining. YAP1 exhibits significantly overexpression in residual/recurrent HCCs post TACE than in primary HCCs without prior treatment, which strongly suggesting that TACE caused increased expression of YAP1 contributes to gain the cancer stem cell potential of the liver or ductal cells, which account for the residual/recurrent HCCs after TACE treatment.
5. Conclusions
We here reported the clinic pathological evidence that YAP1 is more frequently expressed in residual/recurrent HCC after TACE treatment, compared to primary HCC without prior treatment. Our findings suggest that YAP1 expression may contribute to the gain of stem cell differentiation in HCC after TACE treatment, and YAP1 could be a sensitive diagnostic marker and a potential therapeutic target for the residual/recurrent HCC after TACE treatment.
Acknowledgements
This study was supported by the USA National Institutes of Health grant R01 CA187027 (to N. Kang).
Footnotes
Declaration of competing interest
All authors declare that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. [DOI] [PubMed] [Google Scholar]
- 6.Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505–516. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z, Ren J, O’Neil M, et al. Impact of stem cell marker expression on recurrence of TACE-treated hepatocellular carcinoma post liver transplantation. BMC Cancer. 2012;12:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zen C, Zen Y, Mitry RR, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17:943–954. [DOI] [PubMed] [Google Scholar]
- 9.Schulte LA, López-Gil JC, Sainz B Jr, Hermann PC. The cancer stem cell in hepatocellular carcinoma. Cancers (Basel). 2020;12:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai JP, Conley A, Knudsen BS, Guindi M. Hypoxia after transarterial chemoembolization may trigger a progenitor cell phenotype in hepatocellular carcinoma. Histopathology. 2015;67:442–450. [DOI] [PubMed] [Google Scholar]
- 11.Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19:454–464. [DOI] [PubMed] [Google Scholar]
- 12.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010;14:855–868. [DOI] [PubMed] [Google Scholar]
- 13.Shibata M, Ham K, Hoque MO. A time for YAP1: tumorigenesis, immunosuppression and targeted therapy. Int J Cancer. 2018;143:2133–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. [DOI] [PubMed] [Google Scholar]
- 16.Kango-Singh M, Singh A. Regulation of organ size: insights from the Drosophila Hippo signaling pathway. Dev Dyn. 2009;238:1627–1637. [DOI] [PubMed] [Google Scholar]
- 17.Lian I, Kim J, Okazawa H, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24: 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM. Liver regeneration requires Yap1-TGFbeta-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018;69:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas-Paz L, Sun T, Pikiolek M, et al. YAP, but not RSPO-LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell. 2019;25:39–53(e10). [DOI] [PubMed] [Google Scholar]
- 20.Patel SH, Camargo FD, Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim GJ, Kim H, Park YN. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PloS One. 2013;8, e75449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manmadhan S, Ehmer U. Hippo signaling in the liver - a long and ever-expanding story. Front Cell Dev Biol. 2019;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruck P, Xiao JC. Stem-like cells in hepatoblastoma. Med Pediatr Oncol. 2002;39:504–507. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Wu L, Tu J, et al. miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. EBioMedicine. 2018;35:142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]