Abstract
Pluripotent stem cells (PSCs) have the potential to provide homogeneous cell populations of T cells that can be grown at a clinical scale and genetically engineered to meet specific clinical needs. OP9-DLL4, a stromal line ectopically expressing the Notch ligand Delta-like 4 (DLL4) is used to support differentiation of PSCs to T-lymphocytes. This article outlines several protocols related to generation of T cells from human and non-human primate (NHP) PSCs, including initial hematopoietic differentiation of PSC on OP9 feeders or defined conditions, followed by coculture of the OP9-DLL4 cells with the PSC-derived hematopoietic progenitors (HPs), leading to efficient differentiation to T lymphocytes. In addition, we describe a protocol for robust T cell generation from hPSCs conditionally expressing ETS1. The presented protocols provide a platform for T cell production for disease modeling and evaluating their use for immunotherapy in large animal models.
Keywords: Human pluripotent stem cells, Human embryonic stem cells, Non-human primate pluripotent stem cells, Hemogenic endothelium, Hematopoietic progenitor, T cells, Hematopoietic differentiation
Background
T lymphocyte (T cells) play a key role in cell-mediated immune responses and are involved in monitoring and killing tumor cells. Throughout the last decades, several strategies have been developed to redirect, culture and/or enhance T lymphocytes against cancer ( Houot et al., 2015 ; June et al., 2018 ) and utilize them for T cell-based adoptive immunotherapies. Recent clinical trials have shown outstanding outcomes in relapsed and refractory lymphoma patients treated with chimeric antigen receptor (CAR)-T cells (Riviere and Sadelain, 2017).
Human pluripotent stem cells (hPSCs), including embryonic (hESCs) and induced (hiPSCs), provide a promising resource to produce T cells for adoptive cellular immunotherapies, which can be coupled with genetic engineering technologies to generate off-the-shelf supplies of CAR T cells. In addition, generating hPSCs from antigen (Ag)-specific cytotoxic T lymphocytes (CTLs) and redifferentiating them into functional CTLs could enable the scalable production of rejuvenated CTLs (Minagawa and Kaneko, 2014; Kaneko, 2016). Several reports have demonstrated T cell generation from hPSCs ( Nishimura et al., 2013 ; Vizcardo et al., 2013 ) and the feasibility of hiPSC based CAR T cell therapies ( Themeli et al., 2013 ). However, there is still a need to improve the efficacy of T cell generation and expansion from hPSCs. In addition, further advances in hPSC-based T cell therapies will require their preclinical evaluation in large animal models. Since macaques are physiologically and immunologically similar to humans, including possessing orthologous MHC genes ( Adams et al., 2001 ), and similarities in killer cell immunoglobulin-like receptors (KIR) with humans ( Bimber et al., 2008 ; Parham et al., 2010 ), nonhuman primates (NHPs) will be the most appropriate model to address the therapeutic efficacy, safety and immunogenicity of PSC-derived T cells.
Here, we describe an improved method for the derivation of T cells from human and NHP-PSCs with a higher efficiency and shorter time (as soon as 3 weeks) than existing protocols. Differentiation of T cells from hPSCs involves two major steps: induction of hematopoietic progenitor cells (HPs) from hPSCs and their subsequent differentiation into T cells. Our lab previously reported well-established protocols on the induction of hematopoietic lineages from hPSCs on OP9 feeders and in defined feeder- and serum-free conditions ( Vodyanik et al., 2005 ; Vodyanik and Slukvin, 2007; Uenishi et al., 2014 ). We showed that hemogenic progenitors from different stages of differentiation or different sources were cocultured on OP9-DLL4 to differentiate into T cells ( Kumar et al., 2019b ). We have also reported a protocol for the induction of hematopoietic lineages from NHP-PSCs ( D'Souza et al., 2016 ). T cells differentiation from both hPSCs or NHP-PSCs proceeds through a CD5+CD7+ progenitor stage that eventually transitions into CD8+CD4+ double-positive cells. Altogether, the protocol used for the PSC-derived T cells presents a platform for T cell production to evaluate their utility for adoptive immunotherapies and preclinical testing in large animal models.
Related Information
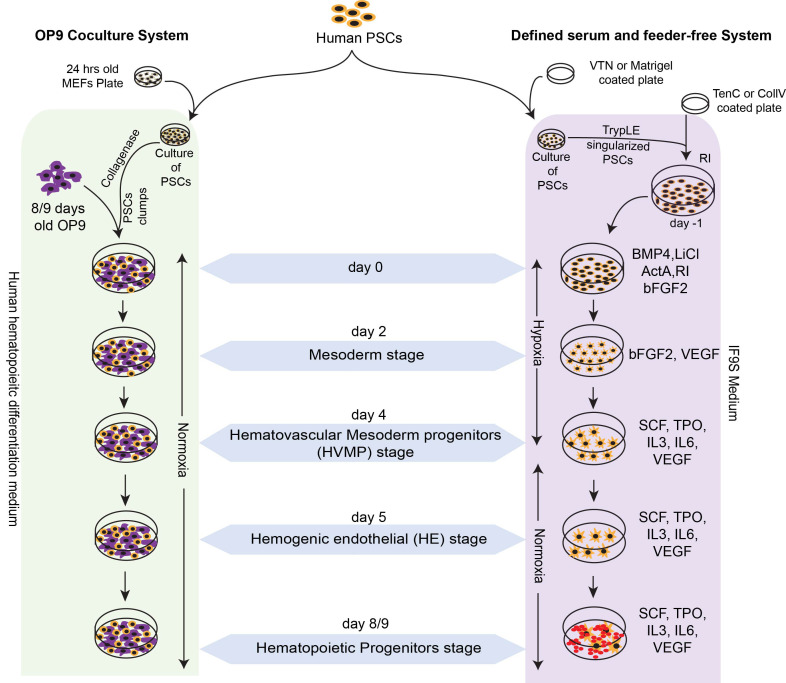
Hematopoietic differentiation from human PSCs in an OP9 co-culture system
Hematopoietic differentiation of hPSCs on mouse stromal OP9 feeders is performed in serum-containing medium without addition of any cytokines ( Vodyanik et al., 2005 ). In this system, hPSCs undergo stepwise progression toward APLNR+PDGFRα+ primitive posterior mesoderm with hemangioblast colony forming cells (HB-CFCs) that reflects primitive hematopoiesis, KDRhiPDGFRαlo/-VEC- hematovascular mesodermal progenitors with definitive hematopoietic potential, immature VE-cadherin (VEC)+CD43-CD73- HE, which specify into DLL4+ arterial hemogenic endothelium (HE) with definitive hematopoietic potential, and DLL4- non-arterial-type HE with mostly primitive hematopoietic potential; and CD34+CD43+ hematopoietic progenitors (HPs) that include CD43+CD235a+CD45+/- HPs, enriched in erythromegakryocytic progenitors and CD43+CD235a/41a- multipotent HPs with a lin-CD34+CD90+CD38-CD45RA- hematopoietic stem progenitor cells phenotype, and lymphomyeloid potential ( Vodyanik et al., 2006 ; Choi et al., 2009a and 2009b; Choi et al., 2012 ; Kumar et al., 2019a and 2019b; Uenishi et al., 2018 ) (Figure 1). CD43+ HPs generated in this coculture can be collected on days 8-9 of differentiation and subsequently cultured on OP9-DLL4 in lymphoid conditions to generate T cells ( Kumar et al., 2019b ). We also demonstrated that T cells can be generated directly from definitive hemogenic progenitors collected from earlier stages of development (hematovascular mesoderm or HE stage) and cultured in lymphoid conditions on OP9-DLL4 ( Kumar et al., 2019b ).
Figure 1. Schematic diagram shows the culture and hematopoietic differentiation of human pluripotent stem cells in serum free chemically defined feeder-free condition and on OP9 coculture system.
Use various MACS enriched progenitor from OP9 coculture system and floating cells from feeder free system for T lymphoid differentiation.
Hematopoietic differentiation from human PSCs in a chemically defined system
We have reported defined feeder- and serum-free conditions for generation of blood from hPSCs in chemically defined conditions. In this differentiation system, hPSCs follow stages of development similar to those described in hPSCs cocultured on OP9 feeders, including the formation of VE-Cadherin+CD73-CD235a/CD43- HE and CD34+CD43+ HPs with myeloid and T lymphoid potential ( Uenishi et al., 2014 ) (Figure 1). We typically use collagen IV coated plates for differentiation in defined conditions to reduce cost. However, more expensive matrix Tenascin C can be used instead of collagen IV to improve hematopoietic differentiation and promote development of HPs with T cell potential in defined conditions ( Uenishi et al., 2014 ).
Genetic engineering of Doxycycline-inducible iETS1 hPSCs
We have recently reported a protocol for the generation of conditional gene expression of ETS1 under tetracycline responsive element (TRE) promoter along with M2rtTA (reverse tetracycline transactivator) introduced into hPSCs using PiggyBac transposons ( Jung et al., 2016 ; Park et al., 2018a and 2018b). In one vector ETS1 is downstream from the TREtight promoter, along with the zeocin resistance gene driven by the EF1α promoter, subcloned between the ends of 2 ITRs of the transposon vector. For easy detection of transgene expression upon addition of doxycycline to the culture, ETS1 is linked with Venus through a 2A self-cleaving peptide sequence. The second vector has the M2rtTA promoter linked with a puromycin resistance gene through a 2A peptide sequence subcloned between the ends of 2 ITRs. Using 2 different antibiotic genes facilitates the selection of clones that incorporate both vectors in a single step. The use of a two-vector system allows flexibility to adjust the TRE/M2rtTA ratio to achieve robust doxycycline dependent gene expression in hPSCs while limiting transgene leakage. hPSCs are cultured and then transfected on matrigel plates in mTeSR1 medium. Single hPSC colonies can be picked up from low-density cultures of cell populations ( Park et al., 2018a and 2018b).
T cell production from iETS1 hPSCs
We have demonstrated that T cell production from hESCs can be increased through activation of the arterial program at the mesodermal stage of development by overexpression of the transcription factor ETS1. Hemogenic progenitors generated following induction of ETS1 were more than 100-fold enriched in T cell precursors as compared to control ( Park et al., 2018b ). Doxycycline treatment of differentiation cultures from days 2 to 6, enhances the generation of CD34+ HPs with lymphoid potential. HPs collected from day 9 of differentiation cultures in the presence of doxycycline can subsequently be differentiated into T cells in coculture with OP9-DLL4. Although T cell cultures from DOX- and DOX+ conditions generate a similar percentage of CD5+CD7+ and CD4+CD8+ T cells, total numbers of T lymphocytes produced per 104 CD43+ cells from DOX-treated cultures are dramatically (> 8-fold) greater.
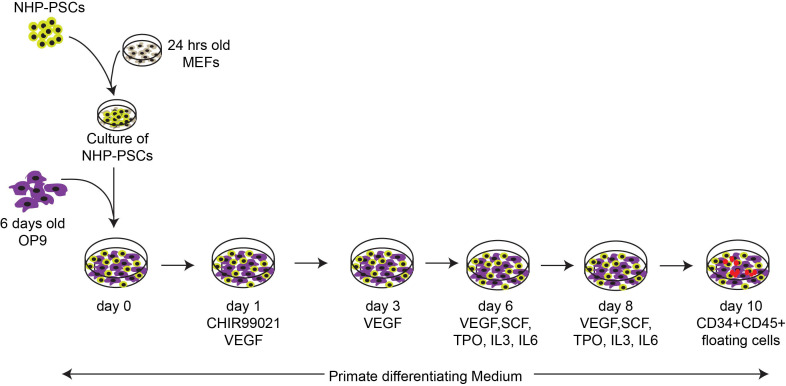
Hematopoietic differentiation of NHP-PSCs on OP9 coculture
Although the defined serum and feeder-free differentiation system described above works well with hPSCs, it does not support efficient generation of CD34+CD43+ HPs with T cell potential from NHP-PSCs. That is why we recommend to culture on OP9 feeders to induce efficient production of lymphoid progenitors from NHP-PSCs. OP9 coculture supports blood production from different NHP species, including ESCs and iPSCs derived from rhesus and cynomolgus macaques. However, in contrast to human PSCs, NHP-PCS/OP9 cocultures require addition of GSK3β inhibitor and VEGF to promote hemogenic mesoderm development and human hematopoietic cytokines to support blood development ( D'Souza et al., 2016 ) (Figure 2). Similar to human, NHP-PSC-derived HPs can be differentiated into T cells in coculture with OP9-DLL4 ( D'Souza et al., 2016 ).
Figure 2. Schematic representation of the established differentiation protocol for induction of mesoderm and blood formation for the NHP iPSCs.
Hematopoietic differentiation of NHP-PSCs was induced in coculture with OP9 in the presence of GSK3b inhibitor. Use floating CD34+CD45+ cells for T lymphoid differentiation.
Materials and Reagents
-
Cell lines
hESC WA01 and WA09 (WiscBank, WiCell, Madison, WI)
Transgene-free iPSCs, DF-19-9-7T and 4-3-7T33 (WiscBank, WiCell, Madison, WI)
Mouse embryonic fibroblasts (MEFs, WiCell, Madison, WI)
Non-human primate PSCs (NHP-iPSCs), RhF5-iPS 19.1, Cy.F 3L iPS, and Cy0669#1 iPS ( D'Souza et al., 2016 )
OP9 mouse bone marrow stromal cell line (Provided by Dr. Toru Nakano, Osaka University, Japan)
Lenti-XTM 293T Cell Line (Clontech, catalog number: 632180)
-
Reagents
α-MEM basal medium, powder (Life Technologies, catalog number: 12000-022)
DMEM/nutrient mixture F-12, powder (Life Technologies, catalog number: 12400-024)
DMEM powder (Life Technologies, catalog number: 12100-046)
Iscove's Modified Dulbecco's Medium (IMDEM), powder (Life Technologies, catalog number: 122-00036)
Ham's F-12 Nutrient Mixture (F12), powder (Life Technologies, catalog number: 217-00075)
Chemically Defined Lipid Concentrate (CDLC) (Life Technologies, catalog number: 119-050-31)
GlutaMax (Life Technologies, catalog number: 35050-061)
Non-essential Amino Acids (NEAA) (Life Technologies, catalog number: 11140-076)
DPBS powdered, without calcium and magnesium (Life Technologies, catalog number: 21600-044)
EDTA 0.5 M, pH 8.0 (Life Technologies, catalog number: 15575-038)
KnockOut Serun Replacement (KOSR) for hPSCs (Life Technologies, catalog number: 10828-023)
TrypLe Select (Life Technologies, catalog number: 12563-029)
Collagenase type IV (Life Technologies, catalog number: 17104-019)
Puromycin (Life Technologies, catalog number: A1113803)
Primate ES cell medium (REPROCELL, catalog number: RCHEMD001)
mTeSR1 defined feeder-free medium (StemCell Technologies, catalog number: 05850)
TeSR-E8 (E8) medium (Stem Cell Technologies, catalog number: 05990)
Vitronectin XFTM (VTN) (Stem Cell Technologies, catalog number: 07180)
Sodium Selenite (Millipore Sigma, catalog number: S5261)
Tenascin C (Millipore Sigma, catalog number: CC065)
Collagen IV (CollV) (Millipore Sigma, catalog number: C5533)
Acetic Acid (Millipore Sigma, catalog number: 537020)
Holo-transferrin (Millipore Sigma, catalog number: T0665)
Lithium Chloride (Millipore Sigma, catalog number: L9659)
7-Aminoactinomycin D (7-AAD; Millipore Sigma, catalog number: A9400)
Human insulin (Millipore Sigma, catalog number: 19278)
Monothioglycerol (MTG) (Millipore Sigma, catalog number: S5261)
HEPES (Millipore Sigma, catalog number: H4034)
Dextrose (Millipore Sigma, catalog number: G8270)
2-mercaptoethanol (Millipore Sigma, catalog number: M7522)
Gelatin from porcine skin, Type A (Millipore Sigma, catalog number: G-1890)
Hexadimethrine bromide, Polybrene (Millipore Sigma, catalog number: H9268)
Fetal bovine serum, defined (HyClone, catalog number: SH30070.03)
Trypsin 0.05%/EDTA 0.5 mM (HyClone, catalog number: SH30236.02)
Recombinant Human Flt3-Ligand (Peprotech, catalog number: 300-19)
Recombinant Human FGF basic (Peprotech, catalog number:100-18B)
Recombinant Human Stem Cell Factor (SCF) (Peprotech, catalog number: 300-07)
Recombinant Human Activin A (ActA) (Peprotech, catalog number: 120-14E)
Recombinant Human Interleukin 3 (IL-3) (Peprotech, catalog number: 200-03)
Recombinant Human Thrombopoietin (TPO) (Peprotech, catalog number: 300-18)
Recombinant Human Interleukin 6 (IL6) (Peprotech, catalog number: 200-06)
Recombinant Human Bone Morphogenic Protein 4 (BMP4) (Peprotech, catalog number: 120-05ET)
Recombinant Human Vascular Endothelial Growth Factor (VEGF) (Peprotech, catalog number: 100-20)
Recombinant Human Interleukin 7 (IL7) (Peprotech, catalog number: 200-07)
GSK-3β inhibitor (CHIR99021) (Tocris, catalog number: 4423)
Rock inhibitor (Tocris, catalog number: 1254)
Human ES cell qualified Matrigel (Corning, catalog number: 354277)
Calcium Chloride (CaCl2) (Alfa Aesar, catalog number: 33296)
Potassium Chloride (KCl) (Fisher ScientificTM, catalog number: BP366-1)
Sodium Chloride (NaCl) (Fisher ScientificTM, catalog number: S642-212)
Sodium Bicarbonate (Fisher ScientificTM, catalog number: S233-500)
Sodium azide, NaN3 (Fisher ScientificTM, catalog number: BP922-500)
BSA fraction V (Fisher ScientificTM, catalog number: BP1600-100)
Sodium Phosphate Dibasic Anhydrous (Na2HPO4) (Fisher ScientificTM, catalog number: BP332-1)
Polyvinyl alcohol (PVA) (MP Biomedicals, catalog number: 151-941-83)
Doxycycline (DOX) (MP Biomedicals, catalog number: 198955)
Antibodies (Table 1)
-
Materials
Cell strainer, 40 µm (Fisher ScientificTM, catalog number: 223663547)
Cell strainer, 70 µm (Fisher ScientificTM, catalog number: 22363548)
9″ Pasteur pipets, Flint glass (Fisher ScientificTM, catalog number: 1367820D)
Serological Pipettes (10 ml, VWR International, catalog number: 89130-898)
Serological Pipettes (95 ml, VWR International, catalog number: 89130-896)
Nalgene Disposable bottle top filter, Polyethersulfone membrane with 0.2 µm pore size (Fisher ScientificTM, catalog number: 595-4520)
0.5 ml microcentrifuge tube, autoclavable (Fisher ScientificTM, catalog number: 05-408-120)
Serological pipet, 1 ml Nonpyrogenic (Fisher ScientificTM, catalog number: 13-678-11B)
Borosilicate glass pipets, 5 ml (Fisher ScientificTM, catalog number: 1367827F)
5 ml Polystyrene round-bottom tube, 12 × 75mm, non-sterile (BD Bioscience, catalog number: 352008
MACS separation columns, LS (Miltenyi Biotec, catalog number: 130-042-401)
MACS Multistand (Miltenyi Biotec, catalog number: 130-042-303)
Pre-separation filters with 30 µm nylon mesh (Miltenyi Biotec, catalog number: 130-041-407)
Tissue culture dishes, polystyrene 100 × 20 mm (Thermo Scientific, catalog number: 130182)
Tissue culture 6-well plate, Polystyrene flat bottom (Thermo Scientific, catalog number: 130184)
15 ml Polypropylene Conical tubes (Thermo Scientific Nunc, catalog number: 339650)
50 ml Polypropylene Conical tubes (Thermo Scientific, catalog number: 339652)
Steriflip Filter Units, 50 ml Vacuum filtration system with 0.22 µm pore size membrane (Millipore Sigma, catalog number: SCGP00525)
Serological pipet, 5 ml Nonpyrogenic (VWR, catalog number: 89130-896)
Serological pipet, 10 ml Nonpyrogenic (VWR, catalog number: 89130-898)
Sterling Nitrile-xtra powder-free exam gloves (Kimberly-Clark, catalog number: 53139)
-
Medium and solutions
Human PSC growth Medium (see Recipes)
MEF growth medium (see Recipes)
NHP-iPSC Primate PSCs Medium (see Recipes)
Mouse OP9/OP9-DLL4 bone marrow stromal cell culture medium (see Recipes)
Human hematopoietic differentiation (OP9 coculture system) medium (see Recipes)
NHP differentiation medium (see Recipes)
IF4S Stock Medium (see Recipes)
5x PVA Stock Solution (see Recipes)
IF9S Medium (see Recipes)
T lymphoid differentiation medium (see Recipes)
HBS saline solution (2x) (see Recipes)
CaCl2 solution (2 M) (see Recipes)
Gelatin solution (0.1% (wt/vol) (see Recipes)
Magnetic cells sorting (MACS) buffer (see Recipes)
Flow cytometry buffer (see Recipes)
Reconstitution of cytokines (see Recipes)
Collagenase solution (1 mg/ml) (see Recipes)
Doxycycline (1 mg/ml) (see Recipes)
Table 1. List of antibodies used.
| Antigen | Fluorochrome | Clone | Company | Cat. No. | Species |
|---|---|---|---|---|---|
| CD3 | FITC | SK7 | BD Biosciences | 349201 | Human |
| CD3 | APC | S4.1 | Caltag-Invirtogen | MHCD0305 | Human |
| CD3 | PE | SP34 | BD-Bioscience | 552127 | NHP |
| CD4 | APC | RPA-T4 | BD Biosciences | 555449 | Human |
| CD4 | PE | L200 | BD Biosciences | 550630 | NHP |
| CD5 | PE | UCHT2 | BD Biosciences | 555353 | Human |
| CD5 | APC | UCHT2 | Biolegend | 300611 | NHP |
| CD7 | FITC | M-T701 | BD Biosciences | 555360 | Human |
| CD7 | PE | M-T701 | BD Bioscience | 555361 | NHP |
| CD8 | PE | HIT8a | BD Biosciences | 555635 | Human |
| CD8 | APC | SK1 | Biolegend | 344721 | NHP |
| CD43 | FITC | 1G10 | BD Biosciences | 555475 | Human |
| DLL4 | PE | 447506 | R&D Systems | FAB1506P | Human |
| TCRαβ | PE | T10B9.1A-BD Biosciences -‐31 | BD Biosciences | 555548 | Human |
| TCRαβ | APC | R73 | Biolegend | 201110 | NHP |
| CD34 | PE | 563 | BD Biosciences | 550761 | NHP |
| CD45 | FITC | MB4-6D6 | Miltenyl Biotech | 130-119-764 | NHP |
Equipments
MACSQuant analyzer (Miltenyl Biotech, catalog number: 130-096-343)
CellometerR Auto 2000 cell Viability Counter (Nexcelom Bioscience, model: 2000)
Hemocytometer, Reichert Bright-Line counting chamber (Fisher ScientificTM, catalog number: 02-671-5)
MACSmix Tube Rotator (Miltenyi Biotec, catalog number: 130-090-753)
Water bath (Fisher Scientific, catalog number: 16-462-10)
Thermo IEC Centra CL2 Centrifuge (Thermo Scientific, model: 4992)
Microcentrifuge (Eppendorf, model: 5418)
Centricon Plus-70 Centrifugal Filter (Millipore Sigma, catalog number: UFC701008)
Sterile biosafety cabinet (The Baker Company, model: SG603)
37 °C/5% CO2 incubator (Thermo Scientific, model: MCO-19A1)
Hypoxia incubator (Thermo Scientific, model: MCO-19M-PA)
Inverted microscope with objective lenses 4x, 10x and 20x (Olympus, model: IX71)
Object marker, Cell dotter for inverted microscope (Nikon, catalog number: MBW10020)
Balance (Denver Instrument, model: APX60)
Milli-Q water purification system (Millipore, Billerica, MA, USA)
Pipet-Aid, Filler/Dispensers (Drummond, model: 4-000-300)
Liquid waste disposal system for aspiration
Beckman Optima XL-A analytical ultracentrifuge (Beckman Coulter, catalog number: 369005)
Bright-Field microscope (Olympus, model: CKX31SF)
Software
FlowJoTM software (v10.6.1) (BD Bioscience, https://www.flowjo.com/)
GraphPad Prism 7 (GraphPad, www.graphpad.com)
Procedure
-
Coating of matrix
Prepare gelatin-coated 10 cm culture dish (10 ml/dish) or 6-well plate (2 ml/well) by adding sterile gelatin solution and allowing the gelatin solution to cover the entire plastic surface. Incubate the plasticware at least 3 h at 37 °C in an incubator. Gelatin coated dishes/ plates are used for culture for OP9/OP9-DLL4/MEFs.
Prepare VTN or Matrigel-coated 6-well plate by adding 2 ml of the coating solution to each well of the 6-well plate and incubate at 4 °C overnight. The coated plate can be stored in 4 °C for up to 1 month. VTN/Matrigel coated plates are used for culture of PSCs under feeder free conditions.
Prepare a TenC or CollV coating solution by adding 2ml of the coating solution to each well of a 6-well plate and incubate at 4 °C overnight. The coated plate can be stored in 4 °C for up to 1 month. TenC/CollV coated plates are used for hematopoietic differentiation of PSCs under feeder free conditions.
-
Culture of hPSCs/NHP-PSCs on feeder cells (MEFs)
Resuspend inactivated MEFs (provided by WiCell) at 2 x 105 cells/ml in pre-warmed (37 °C) MEF growth medium.
Add 2 ml/well of prewarmed MEF growth medium and then dispense MEF suspension on a gelatin-coated 6-well plate (1 ml/well) and distribute MEFs evenly with a back and forth movement of the plate.
Incubate MEF plates in a 5% CO2 incubator at 37 °C for at least 24 h before adding hPSCs or NHP-PSCs.
Aspirate growth medium from 1 well of the 6-well plate of hPSCs or NHP-PSCs.
Add 2 ml/well of collagenase IV solution (1 mg/ml) and incubate at 37 °C in CO2 incubator until the edges of the hPSCs or NHP-PSCs colonies begin to detach (approximately 7-10 min for hPSCs and 4-5 min for NHP-PSCs).
Aspirate collagenase, add 2 ml of growth medium and disperse the colonies into small cell aggregates by pipetting gently for couple of times until all cells are in suspension.
Transfer the cells to a 15 ml conical tube and centrifuge at 300 × g at room temperature for 3 min.
Aspirate the medium gently, resuspend the cells in 4-6 ml growth medium and break hPSC or NHP-PSCs colonies into small aggregates with a glass pipette.
Plate the cell suspension onto a confluent MEF grown 6-well plate from Step B3 (Splitting ratio for hPSCs is 1:6 and for NHP-PSCs is 1:4).
-
Culture of hPSCs or iETS1 hPSCs in feeder-free conditions
Aspirate VTN or Matrigel coating solution from each well and add 1 ml of TeSR-E8 (E8) in case of a VTN plate or mTeSR1 for a Matrigel plate.
To passage the PSCs, aspirate spent media from one well of PSCs culture plate and add EDTA-PBS solution (2 ml/well).
Incubate the plate for 3-4 min at 37 °C with 5% CO2.
Carefully aspirate EDTA-PBS solution and add 1 ml of E8 or mTeSR1.
Gently pipette to dislodge the cells and collect into a 15 ml conical tube.
Adjust the volume to 6 ml with E8 or mTeSR1.
Mix well and add cell suspension (1 ml/well of 6-well plate) to VTN or Matrigel-coated plate.
Thereafter change spent media to fresh E8 or mTeSR1 media daily.
-
Human DLL4 lentivirus production
Subculture 293T cells the day before transfection to 60-70% confluency in 9 ml of DMEM medium with 10% FBS per 100 mm dishes.
On day 0, perform Calcium phosphate (CaPO4) transfection of 293T cells in the morning. For CaPO4 transfection, for each dish, make a mixture containing 10 μg of expression lentiviral vector containing hDLL4 (pSIN4-EF1α-hDLL4-IRES-Puro), 10 μg of packaging vector, 7.5 μg of envelope vector, 62 μl of 2 M CaCl2 and make up the volume to 500 μl with deionized autoclaved water.
Add 500 μl of 2x HBS dropwise into this mixture while bubbling using another 1 ml pipette.
Bubble the mixture for an additional 5-10 s. Incubate the mixture for 15-20 min at room temperature.
Add the mixture to the 293T cells dropwise while continuously swirling the dish.
Place the dish into a 5% CO2 incubator at 37 °C for 6-8 h.
Aspirate the medium after 8 h of incubation.
Add 8 ml of fresh medium to the dishes and continue the culture for another 2 days.
On day 3, harvest the medium containing virus into 50 ml tubes.
Centrifuge the supernatant at 700 × g for 10 min at room temperature.
The supernatant containing the virus can be stored at -70 °C or concentrated using ethylene oxide-sterilized Centricon Plus-70 or Amicon Ultra-15 Centrifugal Filter Units.
To calculate the amount of Infectious Units (IFU) per ml of viral concentrate, transduce HeLa cells with the virus and select with puromycin (1 μg/ml).
-
Generation of human DLL4 expressing mouse OP9 stromal cells
Aspirate the gelatin solution from the dish. Do not allow dishes to dry.
Thaw OP9 cells quickly in a 37 °C water bath.
Pipette the cell suspension slowly and transfer the contents to the 15-ml tube containing 5 ml of medium.
Centrifuge the cells at 300 × g for 5 min at 4 °C and resuspend the cells in 10 ml of OP9 medium.
Transfer the resuspended cells into a 10-cm dish, and place the dish in a 37 °C incubator with 5% CO2.
For viral transduction, add polybrene (6 μg/ml) to the culture medium.
Add hDLL4 lentivirus to the cells at an MOI of 10 and incubate the culture dish at 37 °C with 5% CO2 for 24 h.
Repeat viral transduction one more time as described in E7-E8.
Select the transduced OP9-DLL4 cells by adding 20 ng/ml of puromycin into the culture medium.
To confirm the transduction of hDLL4 into OP9, resuspend the cells into 100 μl FACS buffer and stain with human DLL4 antibody for 20 min at room temperature (Table 1).
Wash the stained cells with FACS buffer and perform flow cytometry.
-
Maintenance of OP9/OP9-DLL4 cells
To passage the cells, aspirate OP9 growth medium and wash OP9/OP9-DLL4 cells twice with 10 ml of PBS.
Add 5 ml of trypsin/EDTA [0.05% (wt/vol)] solution and incubate for 10 min at 37 °C in a 5% CO2 incubator.
Add 5 ml of OP9 growth medium and collect cells by pipetting up and down until single cell suspension is formed.
Transfer cell suspension into a 15-ml conical tube and centrifuge for 5 min at 300 × g at room temperature.
Aspirate supernatant and resuspend the cells in 1 ml of OP9 growth medium.
Add 100 μl of the cell suspension to 10 ml of OP9 growth medium and plate cells onto 10-cm gelatin-coated culture dishes.
When cultures are confluent, split one dish for maintenance.
8-9 days old OP9 dishes should be used for hematopoietic differentiation for human ESCs and 5-6 days old OP9 dishes should be used for NHP-iPSCs hematopoietic differentiation.
3-4 days old OP9-DLL4 should be used for subsequent protocol, i.e., T lymphoid differentiation.
-
Hematopoietic differentiation
Human PSCs on OP9
From 1 well of a 6-well hPSC plate, aspirate hESC growth medium. Add 2 ml of collagenase IV solution (1 mg/ml) and incubate cells for 10 min at 37 °C (Figure 1).
Aspirate collagenase IV solution and add 2 ml per well of growth medium directly to the well and break up colonies into small cell aggregates by gently pipetting. Transfer cells into a 15-ml conical tube.
Centrifuge cells at 300 × g for 5 min at room temperature. Aspirate the medium gently without disturbing the pellet.
Resuspend the pelleted cells into 10 ml of differentiating medium.
For coculture, aspirate the OP9 growth medium from overgrown OP9 dishes.
Add 10 ml of cell suspension from Step 4 on OP9 dishes.
Evenly spread the cells on the OP9 Monolayer by moving the dish in a back/forth and right/left movement twice and keep at 37 °C in a 5% CO2 incubator.
On day 1 aspirate all of the medium and replace it with 10 ml of differentiation medium.
On day 4 and day 6, aspirate 5 ml of spent media and add 5 ml of fresh differentiating medium.
On day 9, aspirate all of the medium and add 5 ml of collagenase solution to each dish of coculture and incubate for 20 min at 37 °C in a CO2 incubator.
Remove the collagenase solution and keep it on ice in a 50-ml conical tube.
Add 5 ml of Trypsin/EDTA solution [0.05% (wt/vol)] to the dish and incubate for 10 min at 37 °C in a 5% CO2 incubator.
Add 5 ml per dish of MACS buffer, suspend cocultured cells by pipetting and transfer to the collection tube from Step 11.
Centrifuge the cell suspension at 300 × g for 5 min at room temperature.
Resuspend the pelleted cells with 5 ml of MACS buffer and centrifuge at 300 × g for 5 min at room temperature.
Resuspend cells in 0.5-1 ml of MACS buffer.
Stain the cells with anti-human CD43-FITC antibody.
Place the tube on the MACS mixer and incubate at 4 °C for 20-25 min.
Wash cells with ice-cold MACS buffer as described in Step 15.
Resuspend in 80 μl of MACS buffer and add anti- FITC magnetic beads.
Repeat Step 18.
Wash cells with ice-cold MACS buffer, as described in Step 15, and resuspend in 2 ml of MACS buffer.
Filter cells through a 30 μm pre-separation filter.
Assemble the MACS-LS separation unit according to the manufacturer's instructions.
Rinse the column with 2 ml of MACS buffer.
To purify CD43+ multipotent progenitors, apply the cell suspension from Step 22 into the LS column and allow cells to pass completely through the column into the collection tube.
Wash the column with 2 ml of MACS buffer and collect it in the same collection tube.
Discard the cell suspension in the collection tube.
Remove the column from the magnet and place it on an empty 15-ml tube.
Wash out CD43+ cells with 5 ml of MACS buffer using the plunger supplied with the column.
Centrifuge cells at 300 × g for 5 min at 4 °C.
Resuspend cells in 0.2 ml of MACS buffer.
The MACS enriched CD43+ multipotent hematopoietic progenitor cells are ready to be used for T lymphoid differentiation.
Human PSCs or iETS1-PSCs in a defined serum and feeder-free system
Prepare a single-cell suspension of human ESCs by using TrypLE select. Add 1ml/well of TrypLE solution to 80% confluent ESC plate and incubate for 4 min 37 °C with 5% CO2 (Figure 1).
Harvest the cells in 1ml TrypLE solution and collect it into a 15 ml conical tube with 9 ml of E8 medium.
Centrifuge 300 × g for 5 min at room temperature.
Resuspend the pelleted cells in 6 ml of fresh E8 media and count the cells.
Aspirate TenC or CollV coating solution from each well of the coated plate.
Add 1 ml of E8 media with 10 μM of rock inhibitor to each well of the coated plate.
Transfer the optimized number of cells from Step F4 to a new 15 ml conical tube and adjust the volume to 6 ml.
Add 1 ml of E8 containing ESCs into each well of Ten-C coated plates.
Incubate overnight at 37 °C in 5% CO2.
On day 0, aspirate E8 media and add 2 ml/well of differentiating medium (IF9S) containing Activin A (18 ng/ml), BMP4 (50 ng/ml), bFGF2 (50 ng/ml), LiCl (2 mM) and Rock inhibitor (1 μM).
Place the cells in a hypoxia (5% O2) incubator. During the next two days, do not remove the plate from the incubator.
On day 2, aspirate the spent differentiating medium and add 2 ml/well of fresh differentiating media with VEGF (50 ng/ml) and bFGF2 (50 ng/ml). Treat iETS1-PSCs differentiating plate with Doxycycline (2 μg/ml).
Place the cells back in the hypoxic incubator, i.e., 37°C with 5% CO2.
On day 4, aspirate the spent differentiating medium and add 2 ml/well of fresh differentiating medium with SCF (50 ng/ml), VEGF (50 ng/ml), TPO (50 ng/ml), IL6 (50 ng/ml), IL3 (10 ng/ml) and FLT3-L (10 ng/ml). Treat iETS1-PSCs differentiating plate with Doxycycline (2 μg/ml).
Place the cells in the normoxic incubator.
On day 6, add 1 ml/well of fresh differentiating medium with cytokines from Step 14.
On days 8-9, collect the floating multipotent HPs by passing through a 70 μm filter and use them for further Lymphoid differentiation.
Nonhuman Primate PSCs on OP9
From 1 well of a 6-well NHP-PSCs plate, aspirate NHP-PSC growth medium. Add 2 ml of collagenase IV solution (1 mg/ml) and incubate the cells for 5 min at 37 °C.
Aspirate collagenase IV solution and add 2 ml per well of growth medium directly to the well and break up colonies into small cell aggregates by gently pipetting. Transfer cells into a 15-ml conical tube.
Centrifuge cells at 300 × g for 5 min at room temperature. Aspirate the medium gently without disturbing the pellet.
Resuspend the pelleted cells into 10 ml of Primate differentiating medium.
Remove 6-7 days old OP9 dishes prepared for coculture from the incubator and aspirate OP9 growth medium (Figure 2).
Add the cells from Step 4 to the OP9 dish and move the dish back and forth to unformily spread the cells on the OP9 monolayer (day 0).
On day 1 of coculture, aspirate the medium and add 10 ml of primate differentiation medium with 4 μM of CHIR99021 and 50 ng/ml of VEGF.
On day 3, aspirate the medium and add fresh 10 ml of fresh primate differentiation media with 50 ng/ml of VEGF.
On day 6, hematopoietic cytokine cocktail containing 50 ng/ml of VEGF, 50 ng/ml of SCF, 20 ng/ml of TPO, 20 ng/ml of IL-3 and 20 ng/ml of IL-6 in primate differentiating medium is added to the coculture.
On day 8 repeat Step 9.
On day 10 of coculture, collect the floating multipotent HPs are collected by passing through a 70 μm filter and use them for further Lymphoid differentiation.
-
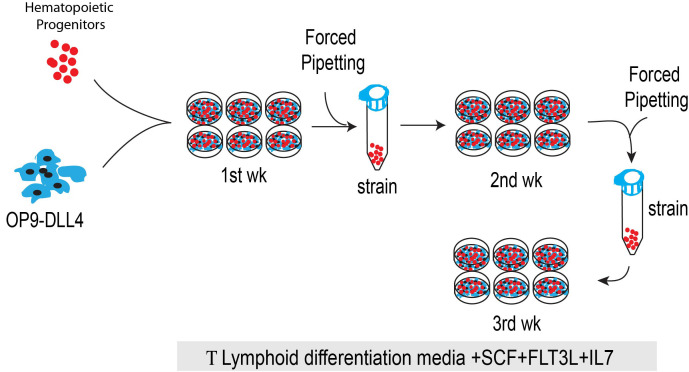
Lymphoid differentiation on OP9-DLL4 coculture system
Prepare the OP9-DLL4 containing 6-well plates by seeding 0.5 x 105 OP9-DLL4 cells per well (Figure 3).
Culture the cells at 37 °C with 5% CO2 for 3-4 days or until a monolayer of cells is formed.
Once the OP9-DLL4 plate is ready for coculture, seed the HPs cells from different sources (1 x 105-3 x 105 cells/well of 6-well plate) on the OP9-DLL4 with T cell media (OP9 medium containing 20% FBS with FLT3-L (5 ng/ml), IL-7 (5 ng/ml) and SCF (10 ng/ml)).
Culture the plates for 3-4 weeks at 37 °C with 5% CO2 in an incubator and passage weekly.
On day 3, add 1ml of fresh T cell differentiation media and prepare a new OP9-DLL4 plate for cell transfer on days 6-7.
On days 6-7, dislodge the progenitor cells by vigorously pipetting and collect the cells into a 15 ml conical tube (Figure 3).
Filter the cells using a 40 μm strainer.
Centrifuge the cells at 300 × g for 5 min at room temperature.
Aspirate the supernatant and resuspend the cells in 2 ml of T cell medium and transfer onto a fresh OP9-DLL4 monolayer (Figure 3).
On day 10, add 1 ml of fresh T cell differentiation media and prepare a new OP9-DLL4 plate for cell transfer on days 13-14.
On days 13-14, repeat Steps H6-H10.
On day 18, add 1ml of fresh T cell differentiation media and prepare a new OP9-DLL4 plate for cell transfer on days 20-21.
On days 20-21, repeat Steps H6-H10.
On day 24, add 1ml of fresh T cell differentiation media and prepare a new OP9-DLL4 plate for cell transfer.
On day 27, harvest the cells by vigorously pipetting and straining into a 15 ml conical tube.
Centrifuge the cells at 300 × g for 5 min at room temperature. Aspirate the supernatant and resuspend the cells in 1 ml of flow cytometry buffer.
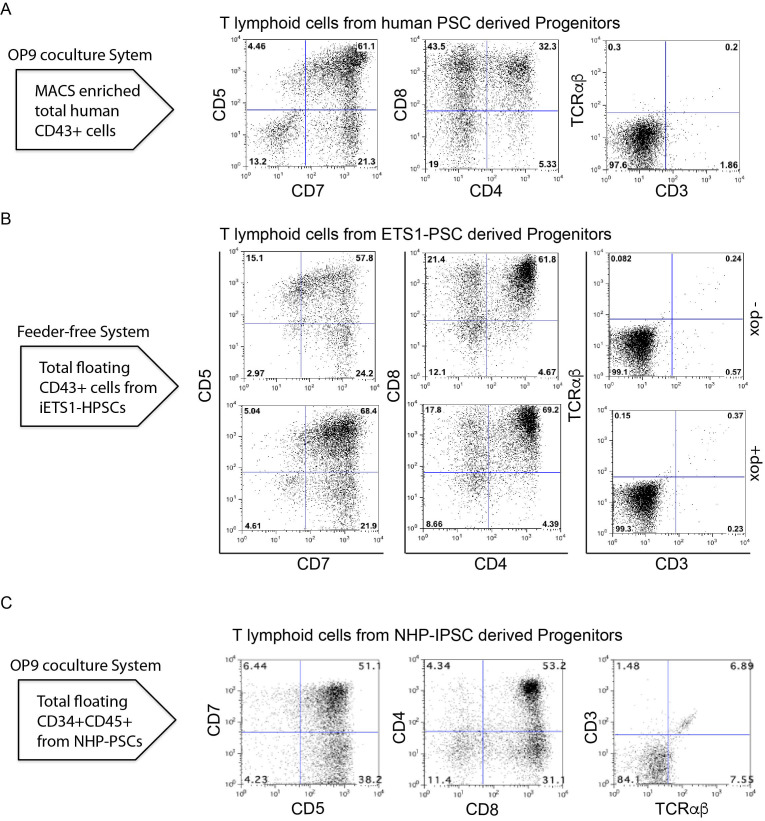
Evaluate the T cell differentiation by flow cytometry analysis of T cell markers (CD5, CD7, CD8, CD4, CD3, and TCRαβ) (Table 1) using MACSQuant Analyser 10 (Figure 4).
Figure 3. Schematic diagram shows the T lymphoid differentiation of HPs from different sources.
Collect and count the HPs from OP9/hPSC or OP9/NHP-PSCs or feeder-free system and culture on OP9-DLL4 to induce T cell differentiation.
Figure 4. T cell potential of HPs.
A. Flow cytometric profile of T cells generated after 3 weeks of culture of total CD43+ on OP9-DLL4 from the indicated human PSCs differentiated on OP9 for 8/9 days. B. Flow cytometric profile of T cells generated from iETS1-PSCs. iETS1-PSCs were differentiated with doxycycline or without doxycycline in chemically defined feeder free system for 9 days and floating cells were collected on day 9. C. Flow cytometric profile of T cells generated after 3 weeks of culture of CD34+CD45+ on OP9-DLL4 from NHP-PSCs.
Data analysis
The flow cytometry data was analyzed using FlowJo software (v10.6.1). Live cells were gated based on the 7AAD population to remove the dead cells.
The significance of differences between the mean values was determined by one-way ANOVA followed by Tukey post hoc test as appropriate using GraphPad Prism software (GraphPad, San Diego, CA).
Notes
hPSCs cultured on MEFs needs to be split once every 6-7 days whereas NHP-PSCs cultures need to be split once every 4 days.
Growth medium used to culture PSCs depends on the type of feeder/ matrix used for culture. For hPSCs grown on vitronectin use E8, while for those grown on Matrigel use mTeSR1. For hPSCs gown on MEFs use PSC growth medium, while for NHP-PSCs grown on MEFs use NHP-PSC medium.
After collagenase treatment, hPSCs/NHP-PSCs colonies are loosely attached and can be collected by gentle pipetting. Do not use excessive mechanical force or scraping, which can provoke spontaneous differentiation.
If spontaneous differentiation of hPSCs/ NHP iPSCs occurs, differentiated colonies should be eliminated during the maintenance; observe PSCs every day before changing the medium. Mark differentiated colonies with an objective marker under the inverted microscope and aspirate marked areas using a glass Pasteur pipette while feeding PSCs with fresh medium.
Matrigel is temperature sensitive and should be kept on ice during handling. It is recommended to avoid freeze/thaw cycles by preparing 200 μl aliquots for four 6-well plates and storing at -20 °C. Specific aliquot volumes vary by lot. Quickly dissolve each aliquot in 48 ml cold PBS and subsequently add 2 ml to each well of a 6-well plate. Incubate at least 1 h at room temperature or store overnight at 4 °C until ready to use. Matrigel-coated plates can be kept for several weeks at 4 °C.
Vitronectin is not temperature sensitive and can be thawed at room temperature. Dilute Vitronectin in 1x PBS to reach a final concentration of 10 μg/ml (2 ml Vitronectin/50 ml 1x PBS) and gently mix by pipetting. Coat 1 ml/well of 6-well tissue-culture plate with diluted Vitronectin. Incubate for at least 1 h at room temperature before use. If it is not used immediately, the coated culture plate must be sealed and can be stored up to 1 week at 4 °C. Stored, coated culture plates must be brought to room temperature 30 min before cells are passaged.
hPSCs can be maintained under feeder-free conditions. For the OP9 coculture system, we always prefer to use hPSCs or NHP-iPSCs maintained on MEFs. hPSCs maintained under feeder-free conditions are used for hematopoietic differentiation under chemically defined feeder-free conditions. We observe substantial differences in the efficiency of hematopoietic differentiation of hPSCs/NHP iPSCs maintained on MEFs or in feeder-free cultures in the OP9 coculture system.
We found that engineered iETS1 hPSCs retain undifferentiated morphology and efficient hematopoietic differentiation potential when maintained on Matrigel-coated plates. In contrast, cultures on vitronectin-coated plates, iETS1 cells do not exhibit uniform morphology and fail to differentiate efficiently.
It is essential to culture OP9/OP9-DLL4 on gelatin-coated plates to prevent spontaneous adipogenesis.
Human hematopoietic differentiation on OP9 coculture system prefers over-confluent OP9 (8-9 days) whereas NHP iPSCs hematopoietic differentiation on OP9 coculture system prefers 6-7 day old OP9 cultures.
Human hematopoietic differentiation in chemically defined conditions requires specific plating densities for optimal hematoendothelial differentiation. Human ESCs need to be plated around 7,500 cells/cm2. Harvesting, counting, and seeding of hPSCs on the Ten-C or CollV plate should be performed quickly as singularized hPSCs do not survive well until mixed with rock inhibitor and plated.
OP9-DLL4 cells should be split every 3-4 days for maintenance/expansion. Proper maintenance of OP9-DLL4 is most critical step for T cell differentiation. Our lab strictly uses the defined FBS without heat inactivation. Heat inactivation does not benefit culture but rather results in a higher adipogenic effect on OP9/OP9-DLL4.
We have found that different lots of HyClone-defined FBS provide relatively consistent effects on OP9-DLL4 maintenance and T cell differentiation without substantial adipogenesis. Results from other suppliers are more variable.
OP9-DLL4 cells should not get overconfluent as it reduces the T cell differentiation ability. Hence, it is always important to perform passages the cells before they get confluent, which is typically 3-4 days when cultured in an appropriate serum.
Cell density in initial HPs/OP9-DLL4 coculture may affect downstream differentiation. A very high number of HPs can cause rapid development of NK cells which could lead to rapid killing of OP9-DLL4 feeders and compromise T cell development.
Differentiating HPs on OP9-DLL4 may reach high density by the second week or earlier. If this occurs, split the cells onto fresh OP9-DLL4 plates. Around 1 x 105 T cells progenitors/well of 6-well plates are ideal for differentiation during the second or subsequent weeks. High density cultures may result in reduced T cell differentiation efficiency.
There is no strict rule of weekly passaging during T cell differentiation. If the differentiating cells appear highly dense, then it is advised to subculture the cells as soon as possible onto fresh OP9-DLL4.
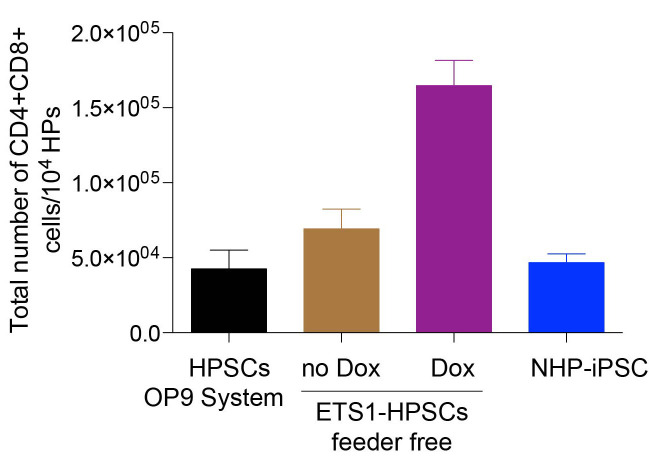
Total number of T cells generated from 1 x 104 CD43+ cells obtained from iETS1-hESCs in doxycycline treated and untreated conditions are much higher in comparison to other systems, cell types and sources (Figure 5).
Figure 5. Bar graph shows the total number of T cells generated from 104 HPs obtained from iETS1-hESCs in doxycycline treated and untreated conditions, wild-type NHP iPSCs and hPSCs.
Total number of T cells generated is higher from iETS1-PSC in comparison to total HPs generated from different methods and different sources.
Recipes
-
Human PSC growth Medium (Table 2)
Prepare stocks of DMEM/F-12 from powder according to the manufacturer’s instructions
Sterilize filter using a 0.22 μm membrane filter and store for up to 2 months at 2-8 °C
Use this basal media to prepare a complete 500 ml of hESC culture medium
-
MEF growth medium (Table 3)
Prepare stocks of DMEM from powder according to the manufacturer’s instructions
Sterilize filter using a 0.22 μm membrane filter and store for up to 2 months at 2-8 °C
Prepare 500 ml of MEF growth medium by using stocks of DMEM and store up to 3 weeks at 2-8 °C
-
NHP-PSC Medium (Table 4)
Use primate ESC medium from Reprocell to make 500 ml of NHP-PSCs medium
-
Mouse OP9/OP9-DLL4 bone marrow stromal cell culture medium (Table 5)
Prepare stocks of fresh α-MEM from powder according to the manufacturer's instructions
Sterilize by filtration using a 0.22-μm membrane filter and store for up to 2 months at 2-8 °C
Use this basal media to prepare a complete 500 ml of OP9/OP9-DLL4 culture medium
-
Human hematopoietic differentiation (OP9 coculture system) medium (Table 6)
Usexα-MEM basal medium to prepare 500 ml of human hematopoietic differentiation medium
-
NHP differentiation medium (Table 7)
Usexα-MEM basal medium to prepare 500 ml of primate differentiation medium
-
IF4S Stock Medium (Table 8)
Prepare 10x IF4S basal media by using the following components
-
5x PVA Stock Solution (Table 9)
Autoclave 1 L ddH2O with a stir bar. Once finished, place it on a heated magnetic stirrer
Slowly add PVA in 2.5 g increments
Make sure the PVA is dissolved before adding more and that it does not aggregate in the hot ddH2O
Once all of the PVA is added, continue stirring overnight at room temperature
Autoclave again the next day to sterilize
Store at room temperature up to 1 year
-
IF9S Medium (Table 10)
Prepare 500ml of IF9S medium for human hematopoietic differentiation media by using the IF4S medium
Add the following component without PVA into IF4S and sterilize by filtration using a 0.22 μm membrane filter
After filtration add 100 ml of 5x PVA
-
T lymphoid differentiation medium (Table 11)
Use α-MEMxbasal medium to prepare T lymphoid differentiation medium
To prepare 100 ml of T lymphoid differentiation medium, add the following component to α-MEM xbasal medium and sterilize by filtration using a 0.22 μm membrane filter
Alternatively, add SCF (10 ng/ml), IL-7 (5 ng/ml) and FLT3-L (5 ng/ml) to the OP9/OP9-DLL4 culture medium to make T lymphoid differentiation medium
-
HBS saline solution (2x)
Prepare 100 mlof 2x HBS solution by adding HEPES (50 mM), KCl (10 mM), dextrose (12 mM), NaCl (280 mM) and Na2HPO4 (1.5 mM)
Adjust the pH to exactly 7.05-7.08
Filter the solution through 0.2 μm filter
-
CaCl2 solution (2 M)
Prepare 50 ml of 2 M of CaCl2 solution by dissolving 14.702 g of CaCl2 in 50 ml of distilled water
Filter sterilize the solution using 0.2 μm filter
-
Gelatin solution [0.1% (wt/vol)]
Add 500 mg of gelatin to 500 ml of endotoxin-free reagent-grade distilled water
Solubilize and sterilize by autoclaving for 20 min at 121 °C
Store the solution at 4 °C for up to 6 months
-
Magnetic cells sorting (MACS) buffer (Table 12)
Prepare 500ml of MACS buffer as shown
Sterilize MACS buffer by filtration using a 0.22 µm membrane filter and keep at 2-8 °C for up to 6 months
-
Flow cytometry buffer (FACS) buffer (Table 13)
Prepare 500ml of FACS buffer as shown
Filtrate the buffer using a 0.22 µm membrane filter and store at 2-8 °C for up to 6 months
-
Reconstitution of cytokines
All the cytokines were reconstituted and stored in -80 °C according to manufacturer’s recommendations
Briefly, centrifuge the vials at maximum speed for 1 min to precipitate lyophilized pellets before opening vials
Dilute with 0.1% BSA/PBS solution for working concentration and store at -80 °C until needed for use
-
Collagenase solution (1 mg/ml)
Add 50 mg of collagenase to 50 ml of DMEM/F-12 basal medium and sterilize the solution by filtration using a 0.22 μm membrane filter
Keep the solution at 2-8 °C and use it for up to 1 week
-
Doxycycline (1 mg/ml)
Dissolve doxycycline to 1 mg/ml in ddH2O
Prepare 100 μl aliquotes in amber 1.5-ml microcentrifuge tubes for light protection and store up to 5 days at 4 °C or 2 months at 20 °C
Table 2. Human PSC growth medium.
| Composition | Volume (500 ml) | Final concentration |
|
DMEM/F-12 KO serum replacement NEAA solution (100x;10 mM) L-glutamine/2-mercaptoethanol (100 mM) Basic FGF-2 (100 μg/ml) |
390 ml 100 ml 5 ml 5 ml 10 μl |
20% 100 μM 1 mM 4 ng/ml |
Table 3. MEF growth medium.
| Composition | Volume (500 ml) | Final concentration |
|
DMEM FBS NEAA solution (100x,10mM) |
445 ml 50 ml 5 ml
|
10% 100 μM
|
Table 4. NHP-PSC medium.
| Composition | Volume (500 ml) | Final concentration |
|
Primate ES cell Medium Basic FGF2 |
500ml 20 μl |
4 ng/ml |
Table 5. OP9/OP9-DLL4 medium.
| Composition | Volume | Final concentration |
|
α-MEM Hyclone FBS |
400 ml 100 ml |
20% |
Table 6. OP9 coculture medium for differentiation of hPSCs.
| Composition | Volume | Final concentration |
|
α-MEM Hyclone FBS MTG Ascorbic acid |
400 ml 100 ml 500 μl 500 μl |
20% 100 μM 50 mg/ml |
Table 7. NHP-PSC differentiation medium.
| Composition | Volume | Final concentration |
|
α-MEM Hyclone FBS 2-mercaptoethanol |
450 ml 50 ml 1.76 μl |
10% 50 μM |
Table 8. IF4S stock medium.
| Composition | Volume | Stock concentration |
|
IMDM F12 ddH2O Sodium Bicarbonate Ascorbic acid MTG Sodium Selenite CDLC |
1 L x 5 1 L x 5 900 ml 21 g 640mg 400 μl 120 μl 20 ml |
5x 5x
21 g/L 640 mg/L 400 μl/L 140 μg/L 2x |
Table 9. PVA stock solution.
| Composition | Volume | Final concentration |
|
PVA ddH2O |
50 g 1 L |
50 g/L
|
Table 10. IF9S medium.
| Composition | Volume | Stock concentration |
|
IF4S (10x) ddH2O PVA GlutaMax (100x) NEAA(100x) Holo-transferrin Insulin |
50 ml 340 ml 100 ml 5 ml 5 ml 500 μl 1 ml |
10 g/L 1x 1x 10.6 mg/L 20 mg/L |
Table 11. T lymphoid differentiation medium.
| Composition | Volume | Final concentration |
|
α-MEM Hyclone FBS SCF-1 IL7 FLT3L |
80 ml 20 ml 10 μl 5 μl 5 μl |
20% 10 ng/ml 5 ng/ml 5 ng/ml |
Table 12. MACS buffer.
| Composition | Volume (500 ml) | Final concentration |
|
DPBS FBS EDTA (0.5M) |
473 ml 25 ml 2 ml |
5% 2 mM |
Table 13. FACS buffer.
| Composition | Volume (500 ml) | Final concentration |
|
DPBS FBS EDTA (0.5M) Sodium azide |
488 ml 10 ml 2 ml 0.25g |
2% 2 mM 0.05% |
Acknowledgments
We thank many members of the Slukvin Lab, especially Drs. Maxim Vidyanik and Kyung Dal Choi, who over the years have helped to develop and optimized human hematopoietic differentiation on the OP9 coculture system. We thank Dr. Toru Nakano (Osaka University, Osaka, Japan) for providing OP9 cells, Mitch Probasco (Morgridge Institute for Research) for cell sorting, and Mathew Raymond (Wisconsin National Primate Research Center) for editorial assistance as well as for cell sorting. This work was supported by funds from the National Institute of Health (R01 HL132891, RO1 HL142665, R24 OD021322, and P51 OD011106).
This protocol was adapted from previous work Vodyanik et al., 2006 ; Uenishi et al., 2014 ; D'Souza et al., 2016 Kumar et al., 2019b ; Park et al., 2018b .
Competing interests
The authors declare no conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Adams E. J., Cooper S. and Parham P.(2001). A novel, nonclassical MHC class I molecule specific to the common chimpanzee. J Immunol 167(7): 3858-3869. [DOI] [PubMed] [Google Scholar]
- 2. Bimber B. N., Moreland A. J., Wiseman R. W., Hughes A. L. and O'Connor D. H.(2008). Complete characterization of killer Ig-like receptor(KIR) haplotypes in Mauritian cynomolgus macaques: novel insights into nonhuman primate KIR gene content and organization. J Immunol 181(9): 6301-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi K. D., Vodyanik M. A. and Slukvin II(2009). Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors . J Clin Invest 119(9): 2818-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi K. D., Vodyanik M. A., Togarrati P. P., Suknuntha K., Kumar A., Samarjeet F., Probasco M. D., Tian S., Stewart R., Thomson J. A. and Slukvin II(2012). Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep 2(3): 553-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi K. D., Yu J., Smuga-Otto K., Salvagiotto G., Rehrauer W., Vodyanik M., Thomson J. and Slukvin I.(2009). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27(3): 559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Souza S. S., Maufort J., Kumar A., Zhang J., Smuga-Otto K., Thomson J. A. and Slukvin II(2016). GSK3beta inhibition promotes efficient myeloid and lymphoid hematopoiesis from non-human primate-induced pluripotent stem cells. Stem Cell Reports 6(2): 243-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houot R., Schultz L. M., Marabelle A. and Kohrt H.(2015). T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol Res 3(10): 1115-1122. [DOI] [PubMed] [Google Scholar]
- 8. June C. H., O'Connor R. S., Kawalekar O. U., Ghassemi S. and Milone M. C.(2018). CAR T cell immunotherapy for human cancer. Science 359(6382): 1361-1365. [DOI] [PubMed] [Google Scholar]
- 9. Jung H. S., Uenishi G., Kumar A., Park M. A., Raymond M., Fink D., McLeod E. and Slukvin I.(2016). A human VE-cadherin-tdTomato and CD43-green fluorescent protein dual reporter cell line for study endothelial to hematopoietic transition. Stem Cell Res 17(2): 401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneko S.(2016). In vitro generation of antigen-specific T cells from induced pluripotent stem cells of antigen-specific T cell origin . Methods Mol Biol 1393: 67-73. [DOI] [PubMed] [Google Scholar]
- 11. Kumar A., D'Souza S. S. and Thakur A. S.(2019). Understanding the journey of human hematopoietic stem cell development. Stem Cells Int 2019: 2141475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar A., Lee J. H., Suknuntha K., D'Souza S. S., Thakur A. S. and Slukvin II(2019). NOTCH activation at the hematovascular mesoderm stage facilitates efficient generation of T cells with high proliferation potential from human pluripotent stem cells. J Immunol 202(3): 770-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minagawa A. and Kaneko S.(2014). Rise of iPSCs as a cell source for adoptive immunotherapy. Hum Cell 27(2): 47-50. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura T., Kaneko S., Kawana-Tachikawa A., Tajima Y., Goto H., Zhu D., Nakayama-Hosoya K., Iriguchi S., Uemura Y., Shimizu T., Takayama N., Yamada D., Nishimura K., Ohtaka M., Watanabe N., Takahashi S., Iwamoto A., Koseki H., Nakanishi M., Eto K. and Nakauchi H.(2013). Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell 12(1): 114-126. [DOI] [PubMed] [Google Scholar]
- 15. Parham P., Abi-Rached L., Matevosyan L., Moesta A. K., Norman P. J., Older Aguilar A. M. and Guethlein L. A.(2010). Primate-specific regulation of natural killer cells. J Med Primatol 39(4): 194-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park M. A., Jung H. S. and Slukvin I.(2018). Genetic engineering of human pluripotent stem cells using PiggyBac transposon system. Curr Protoc Stem Cell Biol 47(1): e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park M. A., Kumar A., Jung H. S., Uenishi G., Moskvin O. V., Thomson J. A. and Slukvin II(2018). Activation of the arterial program drives development of definitive hemogenic endothelium with lymphoid potential. Cell Rep 23(8): 2467-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riviere I. and Sadelain M.(2017). Chimeric antigen receptors: a cell and gene therapy perspective. Mol Ther 25(5): 1117-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Themeli M., Kloss C. C., Ciriello G., Fedorov V. D., Perna F., Gonen M. and Sadelain M.(2013). Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol 31(10): 928-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uenishi G., Theisen D., Lee J. H., Kumar A., Raymond M., Vodyanik M., Swanson S., Stewart R., Thomson J. and Slukvin I.(2014). Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Reports 3(6): 1073-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uenishi G. I., Jung H. S., Kumar A., Park M. A., Hadland B., McLeod E., Raymond M., Moskvin O. V., Zimmerman C., Theisen D. J., Swanson S., Tamplin O., Zon L., Thomson J. A., Bernstein I. D. and Slukvin I. I.(2018). NOTCH signaling specifies arterial-type definitive hemogenic Endothelium from human pluripotent stem cells. Nature Commun 9(1):1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vizcardo R., Masuda K., Yamada D., Ikawa T., Shimizu K., Fujii S., Koseki H. and Kawamoto H.(2013). Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells . Cell Stem Cell 12(1): 31-36. [DOI] [PubMed] [Google Scholar]
- 23. Vodyanik M. A., Bork J. A., Thomson J. A. and Slukvin II(2005). Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential . Blood 105(2): 617-626. [DOI] [PubMed] [Google Scholar]
- 24. Vodyanik M. A. and Slukvin II(2007). Hematoendothelial differentiation of human embryonic stem cells. Curr Protoc Cell Biol Chapter 23: Unit 23 26. [DOI] [PubMed] [Google Scholar]
- 25. Vodyanik M. A., Thomson J. A. and Slukvin II(2006). Leukosialin(CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108(6): 2095-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]