Abstract
Reports show that the stathmin gene may have a close relationship with tumor chemotherapeutic sensitivity. However, the effect of stathmin-1 on the chemosensitivity of gastric cancer to docetaxel has not been clearly determined. siRNA targeting stathmin-1 was introduced. The cell growth inhibition, expression of associated proteins, cell cycle, and apoptosis were evaluated by MTT, Western blot, and flow cytometric assays, respectively. The influence of silencing stathmin-1 was detected in situ and in vivo. SGC7901/docetaxel cells are the drug-resistant cells. After silencing stathmin-1, the resistance index (RI) reduced to 3.41, the expressions of STMN-1, MDR1, and ERCC1 were downregulated, but caspase 3 was upregulated. Stathmin-1 siRNA could improve the chemosensitivity of gastric cancer cells to docetaxel, making the percentage of cells at the sub-G1 stage increase and promote apoptosis. The growth of transplantation tumor was significantly suppressed. Therefore, stathmin-1 might be a potential target for enhancing the chemosensitivity of gastric cancer.
Key words: Stathmin-1 silencing, siRNA, Gastric cancer, Docetaxel, Chemosensitivity
INTRODUCTION
Gastric cancer is one of the most common malignant digestive tract tumors. Its onset and development is a multifactor and multistep process. Risk factors closely related to the occurrence of gastric cancer include diet, infections, precancerous diseases, genetic factors, and so on (1). At present, the treatment of gastric cancer mainly depends on surgery, but for the advanced, recurrent, and metastatic gastric cancers that are unlikely cured by surgery, chemotherapy is the main treatment method because it promises patients a longer and relatively good quality of life (2). However, in the clinical setting, tumor drug resistance to chemotherapeutics directly affects the prognosis of patients; therefore, the effects of chemotherapy are not satisfactory.
The drug resistance of cancer cells develops in a complicated way, and the mechanism is still unclear. Currently, it is believed that drug resistance is possibly related to the overexpression of relevant membrane proteins (3), the abnormal activation of signal transduction pathway (4), and the downregulated protein expression of runt-related transcription factor 3 (RUNX3) (5), P53 (6), and DNA mismatch repair gene (hMLH1) (7). Another important cause for lower drug sensitivity may be that the cell apoptosis induced by chemotherapeutic drugs is reduced (8). Tumors with multidrug resistance usually manifest as apoptosis resistance. Therefore, the relationship between apoptosis resistance and low tumor chemotherapy sensitivity arouses rising attention.
STMN-1 is an important microtubule regulatory protein. It participates in the formation and separation of spindle and polocyte, and cell division movement. In the malignant tumor, it interferes with cell mitosis and affects the proliferation and apoptosis of tumor cells. Recent studies have shown that STMN-1 is highly expressed in a variety of malignant tumor cells (9,10). The stathmin-1 mRNA expression level is directly related to the prognosis of patients. The higher the stathmin-1 expression level, the lower the survival rate of patients, and the greater the risk for tumor metastasis (11). In addition, it is found that the stathmin-1 expression level is closely associated with the efficacy of antimicrotubule drugs (12). Nevertheless, the effect of stathmin-1 gene on the chemotherapy sensitivity of gastric cancer remains unclear.
Chemotherapy resistance is considered to be the major reason for the failed chemotherapy of gastric cancer; therefore, it is of great clinical significance to improve the sensitivity of cancer cells to chemotherapeutic drugs. In the treatment of cancers, the molecular targeted therapy is used to inhibit the overexpression of oncogenes, cancer-related genes, and mutant genes by RNA interference so that they will be in the status of dormancy or low expression to realize an antitumor. RNA interference can effectively silence tumor-related genes, which makes it a better choice for cancer treatment than the traditional combination of chemotherapy and radiotherapy (13). This study used RNA interference to silence stathmin-1 gene in gastric cancer SGC7901 cells and then analyze the drug resistance index of the SGC7901 cell and the expressions of relevant proteins. Cell apoptosis and tumor inhibition rate were analyzed by flow cytometry and nude mice-transplanted tumor model, respectively. Association between stathmin-1 and the chemosensitivity of gastric cancer would be explored to find out the molecular target to improve the chemosensitivity of gastric cancer.
MATERIALS AND METHODS
Cells, Animals, and Reagents
Human gastric cancer SGC7901 cells and drug-resistant SGC7901/docetaxel cells were kindly donated by the Basic Medical College of Zhengzhou University. Thirty female nude mice 4 weeks old, weighing 10–15 g, were provided by the Animal Experimental Center of Zhengzhou University. RPMI-1640 culture medium and fetal bovine serum (FBS) were purchased from Zhejiang Tianhang Biotechnology Co., Ltd. (Hangzhou, China). Lipofectamine 2000 Transfection kit was purchased from Invitrogen Co. (Carlsbad, CA, USA). Docetaxel and MTT kits were provided by Sigma-Aldrich Co. (St. Louis, MO, USA). Annexin V/PI Apoptosis Detection kit and Protein Extraction kit were from Beyotime Biotechnology (Shanghai, China). STMN-1 (MDR1 or ERCC1 or caspase 3) mouse anti-human monoclonal antibody and rabbit anti-mouse antibody second antibody were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell Culture
SGC7901 cells and SGC7901/docetaxel cells were cultured in RPMI-1640 medium with 10% FBS under the condition of 37°C and 5% CO2. Pancreatin, 2.5 g/L, with 0.02% EDTA was used for cell passage, which was done every 3–5 days, and the medium was changed every 1–2 days.
siRNA Synthesis and Cell Transfection
The siRNAs targeted to stathmin-1 and scrambled siRNA were synthesized by Shanghai GenePharma Co., Ltd. according to the design principle of siRNA. The sequences of stathmin-1 siRNA include 5′-UAAAGAGAACCGAGAGGCA-3′, 5′-GAAACGAGAGCACGAGAAA-3′, and 5′-GAAGAGAAACUGACCCACA-3′. In the experiment, blank control group, scrambled siRNA group and stathmin-1 siRNA group were designed. SGC7901 and SGC7901/docetaxel cells in logarithmic growth phase were inoculated in a six-well culture plate. When the cell confluence reached 70–80%, siRNA–Lipofectamine 2000 mixture was prepared according to the siRNA production specification. After the cell culture media were removed, PBS was used to wash the cells. Then the siRNA–Lipofectamine 2000 mixture was added and cultured for 6 h. After the cell culture media were added, the cultivation continued, and cells at different stages were collected.
Detection of the Drug-Resistance Index
Transfected SGC7901 cell and SGC7901/docetaxel cell were collected and adjusted to the proper concentration and then put in 96-well plates with 5 × 103 cells in each well. When the cells grew adhering to the wall in an incubator with 5% CO2 at 37°C for 24 h, the docetaxel group was added with the drug-containing medium for 48 h with the final concentration of docetaxel as 0.5, 1, 5, 10, 20, and 50 µmol/L, respectively. Then, after the supernatant was carefully removed, 50 µl of MTT solution was added, and the incubation continued at 37°C for 4 h, and then 100 µl of working fluid was added to terminate the reaction. After leaving it at room temperature for 20 min until purple granules were dissolved, the absorbance (A) at the wavelength of 490 nm was measured by a microplate reader. The inhibition rate was calculated following the formulas: Inhibition rate = 1 − A drug group/A control group × 100%, Resistance index (RI) = IC50SGC7901/docetaxel/IC50SGC7901. Drug concentration at the cell growth inhibition rate of 50% (IC50) was calculated by IC50 calculation software.
Measurement of STMN-1, MDR1, ERCC1, and Caspase 3 Protein Expressions
After SGC7901 cells were treated with or without docetaxel (10 µmol/L) for 48 h, SGC7901 cells were digested by trypsin and collected. The total proteins were extracted following the specification of the extraction kit and measured by the spectrophotometer. SDS-PAGE electrophoresis was conducted with 40 µg protein loaded in each well. Then the proteins were transferred to the nitrocellulose membrane and sealed in 5% skim milk at 37°C for 1 h. Following this, STMN-1 (MDR1 or ERCC1 or caspase 3 or β-actin) mouse anti-human monoclonal antibodies were added and incubated overnight at 4°C. After washed by PBS, HRP-labeled rabbit anti-mouse IgG second antibody was added and hybridized at room temperature for 1 h, and then the membrane was washed, and ECL reagent was used for scotography. The relative expression of proteins was analyzed with ImageJ software, which was indicated as the ratio of the protein gray value to that of internal reference.
Apoptosis Detection Assay
SGC7901 cells in the blank control group and stathmin-1 siRNA group were treated, respectively, with or without docetaxel (10 µmol/L) for 48 h and then collected after centrifugation at 2,000 rpm at 4°C for 5 min. After the cells were washed twice with precooled PBS and suspended with 400 µl 1× binding buffer to reach an approximate concentration of 1 × 106 cells/ml, 5 µl Annexin V-FITC was added and gently mixed with the cell suspension for 15 min of incubation at 4°C under dark condition. Then 10 µl of PI staining solution was added and incubated at 4°C under dark condition for 5 min. The flow cytometer was used to detect the cell apoptosis within 1 h.
Cell Cycle Analysis
Control and stathmin-1 siRNA SGC7901 cells at 1 × 105 cell/well were incubated for 48 h with or without docetaxel (10 µmol/L). The cells were harvested by centrifugation and gently fixed with 70% ethanol at −20°C overnight. After fixation, the cells were stained with PI (1 mg/ml) and incubated for 30 min at room temperature in the dark. The cells were analyzed using a flow cytometer.
Establishment of Gastric Cancer Nude Mouse Transplantation Tumor Model
SGC7901 cells in logarithmic growth phase in the blank control group and stathmin-1 siRNA group were digested with 0.2% trypsin and fully dispersed. Then they were washed twice with normal saline by centrifugation. The survival cells were counted and adjusted to 5 × 106 cells/ml. Then the cells were suspended in normal saline. Adequate tumor cell suspension was extracted with 1 ml injector and inoculated into the subcutaneous tissue on the back of nude mice; 0.2 ml including 1 × 106 living cells were injected in every vaccination site. Drug administration was conducted after inoculation for 24 h by successive intraperitoneal administrations, with 40–50 mg/kg docetaxel for each mouse each time. The general conditions of mice were observed every day. When the tumor volume in the control group was up to 1 cm3, the mice were killed, and the tumor was taken and weighed. Then the tumor inhibition rate was calculated.
Statistical Analysis
All the data were collected and expressed as mean ± standard deviation. Statistical comparison between two groups was performed using the nonparametric Mann–Whitney U-test or Student’s t-test. The statistical significance was defined as p < 0.05.
RESULTS
Silencing Stathmin-1 Decreased the Resistance Index of SGC7901 Cell to Docetaxel
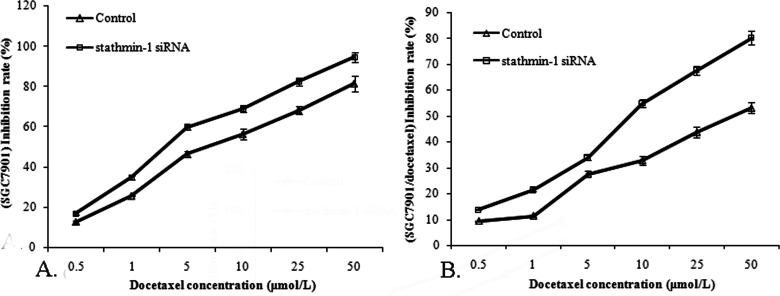
After the experimental groups were added with docetaxel of different concentrations (0.5, 1, 5, 10, 20, and 50 µmol/L), the absorbance (A) was measured, and the cell growth inhibition rate was calculated. Results (Fig. 1) showed that docetaxel of different concentrations had an obviously lower inhibition rate to SGC7901/docetaxel cells than to SGC7901 cells (p < 0.05). Moreover, docetaxel inhibited the growth of SGC7901 cells and SGC7901/docetaxel cells in a dose-dependent manner. The cell inhibition rate in the stathmin-1 siRNA group was obviously higher than that in the blank control group (p < 0.05). Before stathmin-1 gene was silenced, IC50 of SGC7901 cells was 7.55, IC50 of SGC7901/docetaxel cells was 40.17, and RI was 5.32. After stathmin-1 was silenced, IC50 of SGC7901 cells and SGC7901/docetaxel cells declined to 2.75 and 9.38, respectively, and RI was 3.41.
Figure 1.
Effect of stathmin-1 silencing on proliferative inhibition of docetaxel on SGC7901 and SGC7901/docetaxel cells. (A) Silencing stathmin-1 decreased the IC50 value of SGC7901 cells to docetaxel. (B) Silencing stathmin-1 decreased the IC50 value of SGC7901/docetaxel cells to docetaxel. Silencing stathmin-1 improved the sensitivity of SGC7901 and SGC7901/docetaxel cells to docetaxel.
Influence of Silencing Stathmin-1 on the Expression of Relevant Proteins
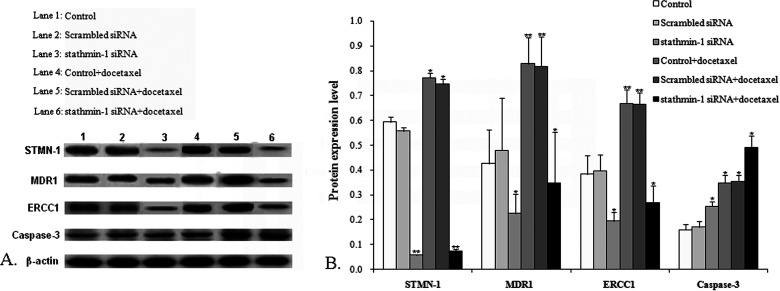
Cells in all the groups were collected after treatment with docetaxel for 48 h to determine the levels of STMN-1, MDR1, ERCC1, and caspase 3. β-Actin was taken as the internal reference; the ratio of each protein gray value to that of β-actin was regarded as the relative expression of protein. Results (Fig. 2) showed that compared with the control group, the protein expression of STMN-1, MDR1, and ERCC1 in the stathmin-1 siRNA group and the stathmin-1 siRNA + docetaxel group obviously decreased (p < 0.01 or p < 0.05) whereas it increased in the control + docetaxel group and scrambled siRNA + docetaxel group (p < 0.05 or p < 0.01). Caspase 3 expression in the stathmin-1 siRNA group, control + docetaxel group, scrambled siRNA + docetaxel group, and stathmin-1 siRNA + docetaxel group all significantly increased compared with the control group (p < 0.05).
Figure 2.
Determination of protein expression level. Western blot was used to determine the effect of silencing stathmin-1 on the protein expression of STMN-1, MDR1, ERCC1, and caspase 3. Compared with the control group, *p < 0.05, **p < 0.01.
Silencing Stathmin-1 Increased the Sensitivity of SGC7901 Cell to Docetaxel, Cell Apoptosis, and the Sub-G1 Accumulation
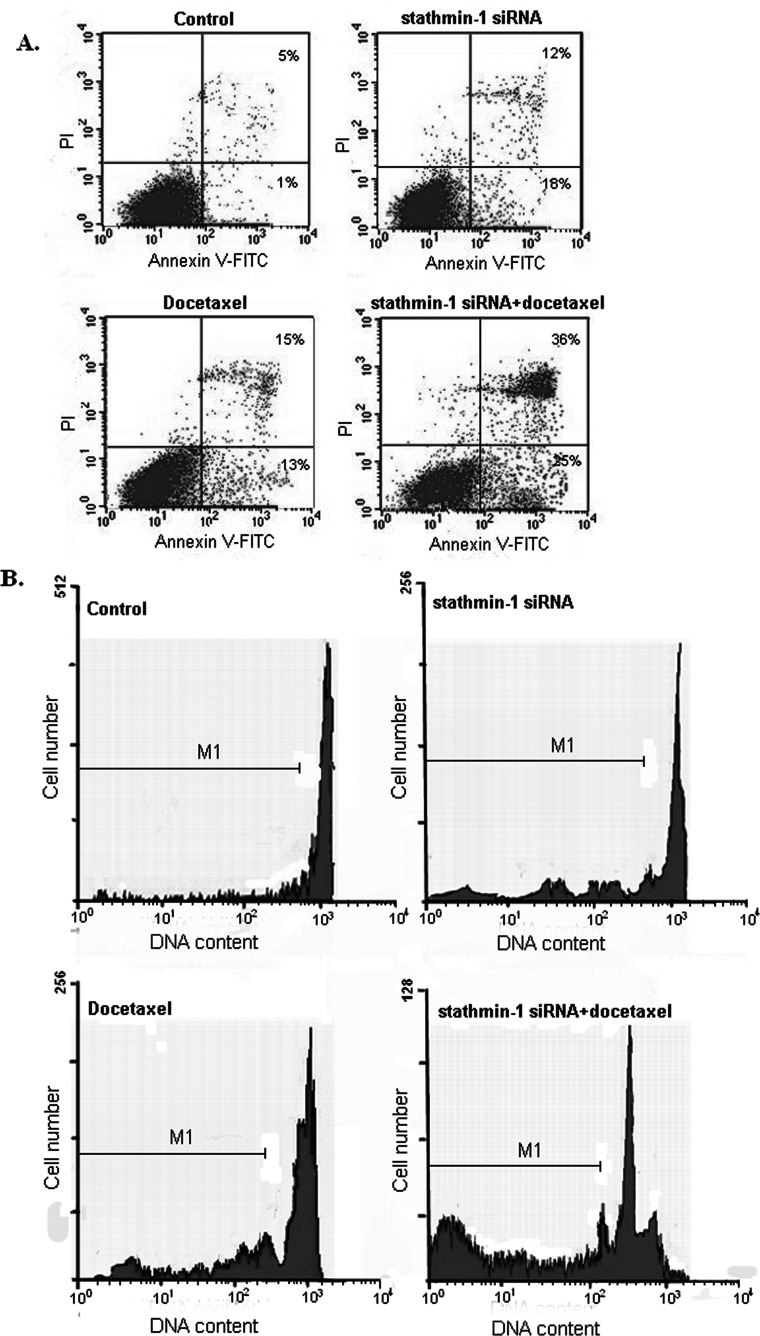
According to the flow cytometer detection, compared with the control group, the apoptosis rate in the stathmin-1 siRNA group, docetaxel group, and stathmin-1 siRNA + docetaxel group significantly increased (p < 0.01). Docetaxel and stathmin-1 siRNA both can enhance the apoptosis of SGC7901 cells. The apoptosis rate in the stathmin-1 siRNA + docetaxel combination group was significantly higher than that in the stathmin-1 siRNA group and the docetaxel group (p < 0.01) (Fig. 3A). The percentage of cells at the sub-G1 stage in the stathmin-1 siRNA + docetaxel combination group was significantly higher than that in the stathmin-1 siRNA group and docetaxel group (p < 0.01) (Fig. 3B). It indicates that docetaxel has synergistic effect with stathmin-1 siRNA in inducing apoptosis.
Figure 3.
Silencing stathmin-1 enhanced the sensitivity of SGC7901 cells to docetaxel and promoted the cells apoptosis. (A) Cell apoptosis was detected by Annexin V/PI staining technique. The percentage indicates the proportion of apoptotic cells. (B) Cell cycle analysis and M1 indicates the proportion of cells in the sub-G1 stage to total cells.
Silencing Stathmin-1 Increased the Tumor Inhibition Rate of Docetaxel
In the whole process of the experiment, nude mice of all groups performed well in growth, eating and drinking, and activity. The abnormal symptoms such as bloody stool or loss body mass were not observed. Transplanted tumors grew well and appeared to be large spherical tumor nodules or some small fascicular tumor nodules. Both docetaxel and stathmin-1 siRNA + docetaxel significantly inhibited the growth of transplanted tumors (p < 0.01) (Fig. 4), with the tumor inhibition rate of stathmin-1 siRNA + docetaxel (76.78 ± 3.12%) significantly higher than that of stathmin-1 siRNA (8.53 ± 0.95%) and control + docetaxel (34.54 ± 1.23%).
Figure 4.
Effect of silencing stathmin-1 on the tumor inhibition rate of docetaxel. Silencing stathmin-1 obviously increased the potency of docetaxel in suppressing tumor growth. Compared with the control group, **p < 0.01.
DISCUSSION
Currently, chemotherapy is still an important means to cure cancers. There are many factors affecting the effects of chemotherapy, such as the reduced drug accumulation in tumor cells, enhanced tumor cell detoxification, strengthened DNA repair capacity, and abnormal expression of apoptosis genes (14). Many proteins participate in the occurrence of the drug resistance of tumors. MDR1 is an ATP-dependent transfer protein, which can transport drugs like plant alkaloid, podophyllotoxin, and paclitaxel and other chemotherapeutic drugs inside cells to the outside so that the reduced chemotherapeutic drug accumulation can cause drug resistance (15). ERCC1 is involved in recognizing and excising DNA chain damage and is a key protein in the NER pathway. The overexpression of ERCC1 can rapidly repair DNA damage stagnated in the G2/M phase. Especially, ERCC1 can increase the scavenging of the DNA complex induced by platinum-based drugs, which results in drug resistance (16). The onset of tumors is not only related to the overproliferation of cancer cells but also closely related to limited apoptosis of cancer cells. Caspase 3 protein is the executor of apoptosis (17). The expression changes of MDR1, ERCC1, and caspase 3 protein under the action of docetaxel before and after silencing stathmin-1 gene were analyzed to clarify the sensitivity of SGC7901 cells to docetaxel. It turns out that after silencing the stathmin-1 gene, the expression of MDR1 and ERCC1 significantly decreased under the action of docetaxel, while the expression of caspase 3 significantly increased. That is to say, after silencing the stathmin-1 gene, the sensitivity of MDR1, ERCC1, and caspase 3 to docetaxel improved. In addition, flow cytometer detection showed that under the combined influence of stathmin-1 siRNA and docetaxel, the apoptosis rate of SGC7901 and the cell percentage at the sub-G1 stage were obviously higher than those in the control group and cells only added with stathmin-1 siRNA or docetaxel. This indicates that the combination of stathmin-1 siRNA and docetaxel can remarkably promote the apoptosis of SGC7901 cells and inhibit cell growth and proliferation. STMN-1, as a signal transduction factor, plays an important role in the mitogen-activated protein kinase signal pathway by regulating microtubule dynamics STMN-1 and involves in the process of cell cycle and cell growth (18). The STMN-1 expression level affects the efficacy of cancer chemotherapy drugs. Many in vitro studies demonstrate that the high expression of STMN-1 negatively regulates the reactivity of tumor cells to microtubule activity drugs (19–21). Ali et al. (22) indicate that STMN-1 could cause the reduction of susceptibility of tumor cells to paclitaxel drugs. The mechanism may be that STMN-1 blocks the microtubule polymerization and reduces the combination between microtubules and drugs.
Through establishing nude mice transplantation tumor models, it was found that, under the combined action of stathmin-1 siRNA and docetaxel, the transplanted tumor was obviously smaller than that treated by stathmin-1 siRNA or docetaxel. In conclusion, silencing stathmin-1 gene can improve the chemosensitivity of gastric cancer cells to docetaxel, and the combination of stathmin-1 siRNA and docetaxel can significantly improve the therapeutic effect of gastric cancer.
ACKNOWLEDGMENTS
Thanks are due to the Basic Medical College of Zhengzhou University for assistance with the experiments.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ang T. L.; Fock K. M. Clinical epidemiology of gastric cancer. Singapore Med. J. 55:621–628; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin Y.; Ueda J.; Kikuchi S.; Totsuka Y.; Wei W. Q.; Qiao Y. L.; Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J. Gastroenterol. 17:4421–4428; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdallah H. M.; Al-Abd A. M.; El-Dine R. S.; El-Halawany A. M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J. Adv. Res. 6:45–62; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonavida B. RKIP-mediated chemo-immunosensitization of resistant cancer cells via disruption of the NF-κB/Snail/YY1/RKIP resistance-driver loop. Crit. Rev. Oncog. 19:431–445; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y.; Lu Q.; Cai X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett. 587:3069–3075; 2013. [DOI] [PubMed] [Google Scholar]
- 6. Jacobsen C.; Honecker F. Cisplatin resistance in germ cell tumours: Models and mechanisms. Andrology 3:111–121; 2015. [DOI] [PubMed] [Google Scholar]
- 7. Arnold C. N.; Goel A.; Boland C. R. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int. J. Cancer 106:66–73; 2003. [DOI] [PubMed] [Google Scholar]
- 8. Giménez-Bonafé P.; Tortosa A.; Pérez-Tomás R. Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr. Cancer Drug Targets 9:320–340; 2009. [DOI] [PubMed] [Google Scholar]
- 9. Miceli C.; Tejada A.; Castaneda A.; Mistry S. J. Cell cycle inhibition therapy that targets stathmin in in vitro and in vivo models of breast cancer. Cancer Gene Ther. 20:298–307; 2013. [DOI] [PubMed] [Google Scholar]
- 10. Hemdan T.; Lindén M.; Lind S. B.; Namuduri A. V.; Sjöstedt E.; de Ståhl T. D.; Asplund A.; Malmström P. U.; Segersten U. The prognostic value and therapeutic target role of stathmin-1 in urinary bladder cancer. Br. J. Cancer 111:1180–1187; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei S. H.; Lin F.; Wang X.; Gao P.; Zhang H. Z. Prognostic significance of stathmin expression in correlation with metastasis and clinicopathological characteristics in human ovarian carcinoma. Acta Histochem. 110:59–65; 2008. [DOI] [PubMed] [Google Scholar]
- 12. Alli E.; Bash-Babula J.; Yang J. M.; Hait W. N. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 62:6864–6869; 2002. [PubMed] [Google Scholar]
- 13. Zheng W.; Cao C.; Liu Y.; Yu Q.; Zheng C.; Sun D.; Ren X.; Liu J. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater. 11:368–380; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Liu X.; Wang A.; Gao H.; Yuan Z.; Jiao Y. Expression and role of the inhibitor of apoptosis protein livin in chemotherapy sensitivity of ovarian carcinoma. Int. J. Oncol. 41:1021–1028; 2012. [DOI] [PubMed] [Google Scholar]
- 15. Qi S. N.; Song L. J.; Chen Y., Jing Y. X. Reversal of mdr1-mediated multidrug resistance in human leukemia cells by a new spin-labeled derivative of podophyllotoxin. Pharmazie 65:117–121; 2010. [PubMed] [Google Scholar]
- 16. Colombo P. E.; Fabbro M.; Theillet C.; Bibeau F.; Rouanet P.; Ray-Coquard I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit. Rev. Oncol. Hematol. 89:207–216; 2014. [DOI] [PubMed] [Google Scholar]
- 17. Grütter M. G. Caspases: Key players in programmed cell death. Curr. Opin. Struct. Biol. 10:649–655; 2000. [DOI] [PubMed] [Google Scholar]
- 18. Lin X.; Tang M.; Tao Y.; Li L.; Liu S.; Guo L.; Li Z.; Ma X.; Xu J.; Cao Y. Epstein-Barr virus-encoded LMP1 triggers regulation of the ERK-mediated Op18/stathmin signaling pathway in association with cell cycle. Cancer Sci. 103:993–999; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mistry S. J.; Atweh G. F. Therapeutic interactions between stathmin inhibition and chemotherapeutic agents in prostate cancer. Mol. Cancer Ther. 5:3248–3257; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Alli E.; Yang J. M.; Hait W. N. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene 26:1003–1012; 2007. [DOI] [PubMed] [Google Scholar]
- 21. Singer S.; Ehemann V.; Brauckhoff A.; Keith M.; Vreden S.; Schirmacher P.; Breuhahn K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology 46:759–768; 2007. [DOI] [PubMed] [Google Scholar]
- 22. Alli E.; Bash-Babula J.; Yang J. M.; Hait W. N. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 62:6864–6869; 2002. [PubMed] [Google Scholar]