Abstract
Cervical cancer is the second leading type of cancer in women living in less developed countries. The pathological and molecular mechanisms of cervical cancer are not comprehensively known. Though legumain has been found to be highly expressed in various types of solid tumors, its expression and biological function in cervical cancer remain unknown. In this study, we aimed to investigate legumain expression and functions in cervical cancer. We found that legumain was highly expressed in cervical cancer cells. When knocked down, legumain expression in HeLa and SiHa cells significantly reduced its migration and invasion abilities compared with control cells. Furthermore, legumain silencing suppressed the activation of matrix metalloproteases (MMP2 and MMP3) in cervical cancer cells. This study indicates that legumain might play an important role in cervical cancer cell migration and invasion. Legumain might be a potential therapeutic target for cervical cancer therapy.
Key words: Cervical cancer, Legumain, Migration, Invasion
INTRODUCTION
Cervical cancer is one of the most common gynecologic malignancies in developing countries (1,2). Nearly 99.7% of cervical cancer is caused by the human papillomavirus (HPV) infection (3). As reported, over 70% of females will suffer an HPV infection at least once in their lives. Fortunately, most of them evade a malignant lesion by a spontaneous recovery, and only 1–4% of the infections in this group will develop into a persistent infection (4). Considering the heterogeneity of cervical cancer, an increasing number of researchers have started to investigate the molecular mechanisms of cervical cancer that influence its prognosis. However, the molecular mechanism of cervical cancer progression, especially migration and invasion, is still far from clear (5).
Legumain, also called asparaginyl endopeptidase, is a unique member of the C13 family peptidases with strict specificity for asparagine bond cleavage (6–8). Legumain is synthesized as an inactive zymogen and goes through autocatalytic activation with sequential removal of C- and N-terminal propeptidase at different pH thresholds (9,10). Legumain has been shown to play crucial roles in immunity, inflammation, and tumor biology (11–17). In recent years, legumain overexpression was observed in both solid tumors and acute lymphoblastic leukemia with the ability to promote tumor cell angiogenesis and metastasis (18–23). Moreover, targeted inhibition of legumain through vaccine, antibody, or small molecular compound has shown significant suppression of tumor progression (24–26). Chen et al. have investigated the effects of legumain-activated prodrug CBZ-AAN-Dox in SiHa cervical cancer cells (27).
We now present evidence that legumain is highly expressed in cervical cancer cells. In addition, knockdown of legumain significantly reduced cervical cancer cell migration and invasion through regulation of activation of matrix metalloproteases (MMPs). Our findings suggest that legumain might serve as a therapeutic target for cervical cancer.
MATERIALS AND METHODS
Cell Lines
Cervical cancer cell line and breast carcinoma cell lines were cultured in DMEM (HyClone) supplemented with 10% FBS in 5% CO2 humidified atmosphere at 37°C.
Western Blotting
Western blot assays were performed using the following primary antibodies: goat anti-human legumain (AF2199; R&D); mouse anti-actin (Santa Cruz Biotechnology); rabbit anti-human MMP2 (1:500; Abcam); anti-MMP9 (1:1,000; Cell Signaling). Briefly, stimulated cells were lysed with RIPA buffer [50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100] containing protease inhibitors (Complete Mini; Roche); 25-µg samples of the lysates were separated on 8–10% SDS-PAGE gels and transferred to PVDF membranes. The membranes were incubated with primary antibodies overnight at 4°C. The primary antibody incubation was followed by incubation with an HRP-conjugated secondary antibody. The bound antibodies were detected using an ECL kit (PI32209; Pierce).
Lentivirus-Delivered shRNA Gene Knockdown
The shRNA sequences for human legumain gene knockdown were 5′-GATGGTGTTCTACATTGAA-3′ (KD-1) and 5′-AAACTGATGAACACCAATGAT-3′ (KD-2). The control shRNA sequence was 5′-GTAGCGCGGTGTATTATAC-3′. Independent stable clones were selected and evaluated by Western blotting.
Real-Time-PCR (RT-PCR) Analysis
Cellular RNA was isolated by TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA was synthesized from the purified mRNA using Moloney murine leukemia virus reverse transcription kit (Promega, Madison, WI, USA). Actin primer sets were used to produce a normalization control. RT-PCR was carried out in triplicate with the SYBR Green PCR Master Mix (Takara, Japan) in 7900HT Fast Real-Time PCR machine (Applied Biosystems, Carlsbad, CA, USA).
Wound Healing Assay
Cells grown in six-well plates were artificially injured by scratching across the plate with 200-µl pipette tips. The wound areas were photographed at 0 and 24 h and measured using a caliper. The wound closure percentages were calculated using the following formula: 1 − (current wound size/initial wound size) × 100.
Cell Invasion Assays
Cells (1 × 105) were plated in the top chamber of Matrigel-coated Transwell assay inserts (Millipore, Billerica, MA, USA) containing 8-µm pores in 200 ml of serum-free DMEM medium. The assays were conducted in triplicate. The inserts were then placed into the bottom chamber of a 24-well plate containing DMEM with 10% FBS as a chemoattractant. After 24 h, the top layer of the insert was scrubbed with a sterile cotton swab to remove any remaining cells. The invading cells on the bottom surface were stained with 0.1% crystal violet, counted, and imaged under microscopy. The number of cells in five random fields of each chamber was counted, and an average number of cells was calculated.
Statistics
Two-tailed Student’s t-tests were used to analyze differences between control and knockdown groups. One-way analysis of variance was initially performed to determine whether overall statistically significant change occurred before using two-tailed paired or unpaired Student’s t-tests. Multiple test-adjusted values of p < 0.05 were considered statistically significant.
RESULTS
Legumain Expression in Cervical Cancer Cells
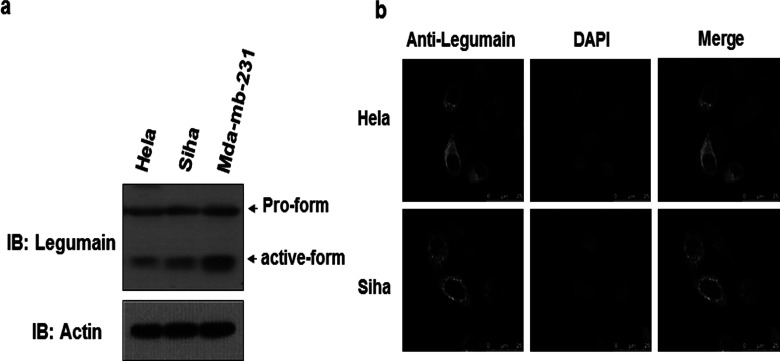
Though legumain has been found to be highly expressed in many solid tumors, for example breast cancer, its expression in cervical cancer has never been studied. We therefore investigated both legumain protein and mRNA levels in cervical cancer. Breast cancer cells MDA-MB-231 served as a positive control. Western blot analysis of cervical cancer cell (HeLa and SiHa) revealed that legumain was highly expressed in cervical cancer cells (Fig. 1a), indicating that legumain is highly expressed in cervical cancer cells. Moreover, confocal microscopy analysis of HeLa and SiHa cells stained with anti-legumain antibody and DAPI (nuclei) revealed that legumain was primarily distributed in the cytoplasm and membrane (Fig. 1b). Dot-like distribution was also found in the perinuclear area (Fig. 1b).
Figure 1.
Expression of legumain in cervical cancer cells. (a) Western blot of lysates of HeLa, SiHa, and Mda-mb-231 cells, probed with antibody to legumain and actin. (b) Confocal microscopy images of HeLa and SiHa cells stained with DAPI of blue fluorescence and anti-legumain of green florescence (scale bars: 25 µm).
Knockdown of Legumain in HeLa and SiHa Cells
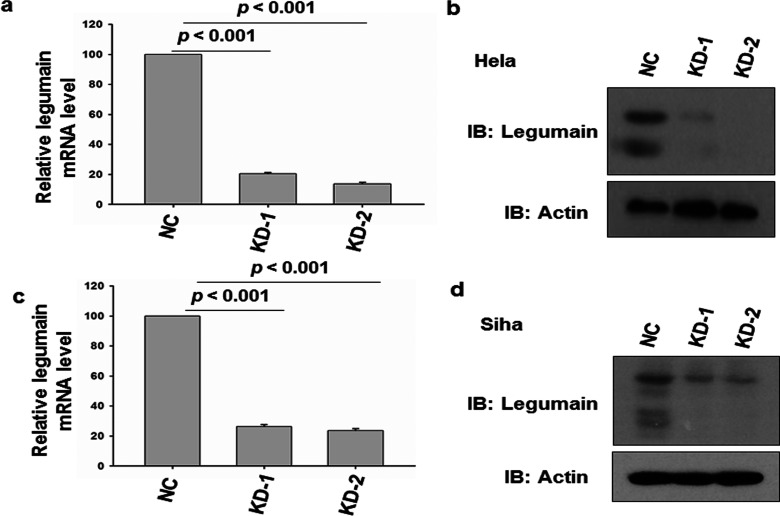
To investigate the role of legumain in cervical cancer progression, we further constructed two lentivirus-mediated legumain shRNA and infected HeLa cells. Stable legumain knockdown cells were selected and examined by RT-PCR and Western blot. Ninety-six hours after infection, both the RT-PCR and Western blot results confirmed that both shRNAs efficiently inhibited legumain expression in both HeLa and SiHa cells compared with negative control (Fig. 2).
Figure 2.
Knockdown of legumain in HeLa cells. (a) RT-PCR examination of HeLa cells with legumain knocked down and negative control. Actin served as internal control. (b) Western blot of lysates of HeLa cells with legumain knocked down and negative control, probed with antibody to legumain and actin. (c) RT-PCR examination of SiHa cells with legumain knocked down and negative control. Actin served as internal control. (d) Western blot of lysates of HeLa cells with legumain knocked down and negative control, probed with antibody to legumain and actin.
Silencing of Legumain Suppressed HeLa and SiHa Cell Migration and Invasion
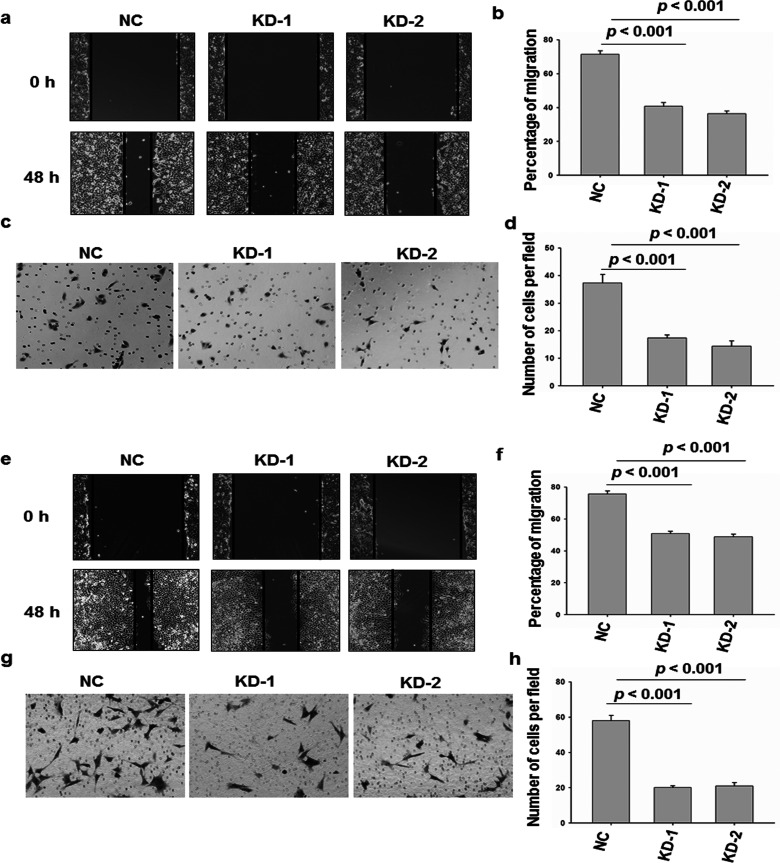
The effects of legumain on the migration and invasion of cervical cancer cells were analyzed. The scratch assay demonstrated that the knockdown of AEP significantly inhibited HeLa and SiHa cell migration (Fig. 3a, b, e, and f). The Transwell assay indicated that suppression of legumain reduced the invasion ability of HeLa and SiHa cells. Collectively, these results showed that legumain has a critical function in cervical cancer cell migration and invasion (Fig. 3c, d, g, and h).
Figure 3.
Migration and invasion assay of legumain knockdown HeLa cells. (a, b) Cell migration analysis of HeLa cells of legumain knockdown or control. (c, d) Transwell invasion analysis of the same cell described in (a). Images are representative of three independent experiments. (e, f) Cell migration analysis of HeLa cells of legumain knockdown or control. (g, h) Transwell invasion analysis of the same cell described in (e). Images are representative of three independent experiments. Values are mean ± SEM.
Legumain Knockdown Reduced MMP Activation
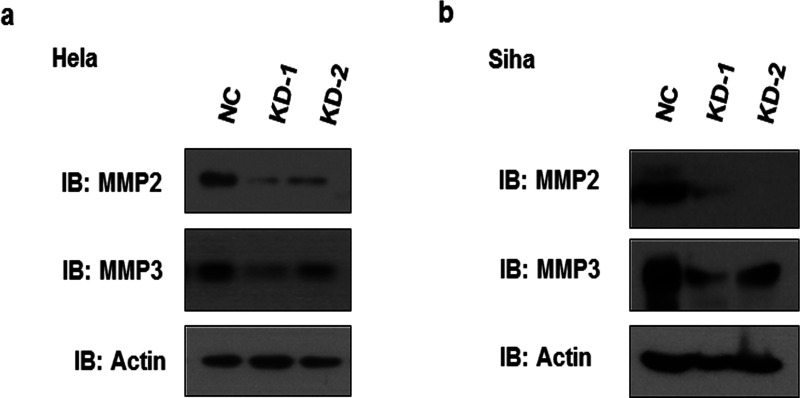
Degradation of extracellular matrix by proteases is an important process during cell migration and invasion, and we sought to determine whether MMPs are affected by legumain. Western blot assays showed that knockdown of legumain in HeLa and SiHa cells resulted in a significant reduction of MMP2 and MMP3 protein levels (Fig. 4), suggesting that MMP2 and MMP3 are the downstream mediators of legumain affecting cervical cancer cell migration and invasion.
Figure 4.
Legumain knockdown reduced MMP activation. (a) Western blot of lysates of HeLa cells with legumain knocked down and negative control, probed with antibody to legumain and actin. (b) Western blot of lysates of SiHa cells with legumain knocked down and negative control, probed with antibody to legumain and actin.
DISCUSSION
Legumain is a newly found lysosomal protease of the C13 family peptidases with strict specificity for asparagine bond cleavage (7). Several studies have verified that legumain was highly expressed in tumors, but not in normal tissues. High expression of legumain predicted poor prognosis and short survival time, in malignancies such as breast cancer, colorectal cancer, and prostate cancer (16–19), and legumain has become a new hot spot in tumor biology, prognosis, and targeted therapy. However, the expression of legumain in cervical cancer cells remains unclear. Our study has established that legumain is highly expressed in cervical cancer cells as it is in other types of cancer. The potential prognosis and therapeutic target need further investigation.
The function of legumain that has been reported includes processing and presentation of antigen (10), modulation of extracellular matrix, promoting angiogenesis factor release, and taking part in the function of tumor-associated macrophage (20,28). It was reported that legumain could promote extracellular matrix degradation and angiogenesis, resulting in invasion and metastasis of breast cancer in 2014 (28). Thus, legumain has been found to be closely associated with tumor metastasis. Consistently, we found that legumain is critical for cervical cancer cell migration and invasion though the mechanism of degradation of extracellular matrix.
In order to further clarify the mechanism that legumain uses in promoting cervical cancer cell invasion, we analyzed the protease MMPs that have an important role in tumor invasion and metastasis. Besides this, legumain has been found to bind with αvβ3 integrin and then modulate the infiltrating capacity of cancer cells and tumor angiogenesis (20). Legumain has also been reported to regulate the cathepsin family, which is also actively involved in cell migration and invasion (15).
In conclusion, this study has verified that legumain can promote invasion and metastasis in cervical cancer. Legumain might be a new diagnostic and therapeutic target in cervical cancer.
ACKNOWLEDGMENTS
We would like to thank Dr. Xipeng Wang (Tongji University) for the kind gifts of cervical cancer cells.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Vizcaino A. P.; Moreno V.; Bosch F. X.; Munoz N.; Barros-Dios X. M.; Borras J.; Parkin D. M. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int. J. Cancer 86:429–435; 2000. [DOI] [PubMed] [Google Scholar]
- 2. Berumen-Campos J. Human papilloma virus and cervical cancer. Gac. Med. Mex. 142:51–59; 2006. [PubMed] [Google Scholar]
- 3. Moreno V.; Bosch F. X.; Munoz N.; Meijer C. J.; Shah K. V.; Walboomers J. M.; Herrero R.; Franceschi S.; International Agency for Research on Cancer; Multicentric Cervical Cancer Study Group. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multi centric case-control study. Lancet 359:1085–1092; 2002. [DOI] [PubMed] [Google Scholar]
- 4. Walboomers J. M.; Jacobs M. V.; Manos M. M.; Bosch F. X.; Kummer J. A.; Shah K. V.; Snijders P. J; Peto J.; Meijer C. J.; Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12–19; 1999. [DOI] [PubMed] [Google Scholar]
- 5. Lars-Christian H.; Georgios R. Familial cancer history in patients with carcinoma of the cervix uteri. Eur. J. Obstet. Gyn. R. B. 101:54; 2002. [DOI] [PubMed] [Google Scholar]
- 6. Koblinski J. E.; Ahram M.; Sloane B. F. Unraveling the role of proteases in cancer. Clin. Chim. Acta 291(2):113–135; 2000. [DOI] [PubMed] [Google Scholar]
- 7. Chen J. M.; Dando P. M.; Rawlings N. D.; Brown M. A.; Young N. E.; Stevens R. A.; Hewitt E.; Watts C.; Barrett A. J. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J. Biol. Chem. 272(12):8090–8098; 1997. [DOI] [PubMed] [Google Scholar]
- 8. Dando P. M.; Fortunato M.; Smith L.; Knight C. G.; McKendrick J. E.; Barrett A. J. Pig kidney legumain: An asparaginyl endopeptidase with restricted specificity. Biochem. J. 339:743–749; 1999. [PMC free article] [PubMed] [Google Scholar]
- 9. Watts C.; Miller G.; Matthews S. P.; Reinheckel T.; Fleming S. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J. 25(5):1606–1617; 2011. [DOI] [PubMed] [Google Scholar]
- 10. Watts C.; Manoury B.; Hewitt E. W.; Morrice N.; Dando P. M.; Barrett A. J. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature 396(6712):695–699; 1998. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z.; Jang S. W.; Liu X.; Cheng D. M.; Peng J.; Yepes M.; Li X. J.; Matthews S.; Watts C.; Asano M.; Hara-Nishimura I.; Luo H. R.; Ye K. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol. Cell 29(6):665–678; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi S. J.; Reddy S. V.; Devlin R. D.; Menaa C.; Chung H. Boyce B. F.; Roodman G. D. Identification of human asparaginyl endopeptidase (legumain) as an inhibitor of osteoclast formation and bone resorption. J. Biol. Chem. 274(39):27747–27753; 1999. [DOI] [PubMed] [Google Scholar]
- 13. Clerin V.; Shih H. H.; Deng N.; Hebert G.; Resmini C.; Shields K. M.; Feldman J. L.; Winkler A.; Albert L.; Maganti V.; Wong A.; Paulsen J. E.; Keith J. C. Jr.; Vlasuk G. P.; Pittman D. D. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis 201(1):53–66; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Manoury B.; Sepulveda F. E.; Maschalidi S.; Colisson R.; Heslop L.; Ghirelli C.; Sakka E.; Lennon-Duménil A. M.; Amigorena S.; Cabanie L.; Manoury B. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity 31(5):737–748; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Smith A.; Mattock K. L.; Gough P. J.; Humphries J.; Burnand K.; Patel L.; Suckling K. E.; Cuello F.; Watts C.; Gautel M.; Avkiran M.; Smith A. Legumain and cathepsin-L expression in human unstable carotid plaque. Atherosclerosis 208(1):83–89; 2010. [DOI] [PubMed] [Google Scholar]
- 16. Sun X. F.; Murthy R. V.; Arbman G.; Gao J. F.; Roodman G. D. Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin. Canc. Res. 11(6):2293–2299; 2005. [DOI] [PubMed] [Google Scholar]
- 17. vonWasielewski R.; Gawenda J.; Traub F.; Luck H. J.; Kreipe H. Legumain expression as a prognostic factor in breast cancer patients. Breast Cancer Res. Treat. 102(1):1–6; 2007. [DOI] [PubMed] [Google Scholar]
- 18. Patel N.; Krishnan S.; Offman M. N.; Krol M.; Moss C. X.; Leighton C.; van Delft F. W.; Holland M.; Liu J.; Alexander S.; Dempsey C.; Ariffin H.; Essink M.; Eden T. O.; Watts C.; Bates P. A.; Saha V. A dyad of lymphoblastic lysosomal cysteine proteases degrades the antileukemic drug L-asparaginase. J. Clin. Invest. 119(7):196–1973; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrade V. A.; Guerra M.; Jardim C. A.; Melo F. M.; Silva W. A.; Ortega J. M.; Robert M.; Nathanson M. H.; Leite F. Nucleoplasmic calcium regulates cell proliferation through. J. Hepatol. 55(3):626–635; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu C.; Sun C. Z.; Huang H. N.; Janda K.; Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 63(11):2957–2964; 2003. [PubMed] [Google Scholar]
- 21. Chen J. M.; Fortunato M.; Stevens R. A. E.; Barrett A. J. Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol. Chem. 382(5):777–783; 2001. [DOI] [PubMed] [Google Scholar]
- 22. Briggs J. J.; Haugen M. H.; Johansen H. T.; Riker A. I.; Abrahamson M.; Fodstad Ø.; Maelandsmo G. M.; Solberg R. Cystatin E/M suppresses legumain activity and invasion of human melanoma. BMC Cancer 10:17; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo Y; Zhou H.; Krueger J.; Kaplan C.; Lee S. H.; Dolman C.; Markowitz D.; Wu W.; Liu C.; Reisfeld R. A.; Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Invest. 116(8):2132–2141; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James K. E.; Gotz M. G.; Caffrey C. R.; Hansell E.; Carter W.; Barrett A. J.; McKerrow J. H.; Powers J. C. Aza-peptide epoxides: Potent and selective inhibitors of Schistosoma mansoni and pig kidney legumains (Asparaginyl endopeptidases). Biol. Chem. 384(12):1613–1618; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Wu B. H.; Yin J.; Texier C.; Roussel M.; Tan K. S. W. Blastocystis legumain is localized on the cell surface, and specific inhibition of its activity implicates a pro-survival role for the enzyme. J. Biol. Chem. 285(3):1790–1798; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith R.; Johansen H. T.; Nilsen H.; Haugen M. H.; Pettersen S. J.; Maelandsmo G. M.; Abrahamson M.; Solberg R. Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie 94(12):2590–2599; 2012. [DOI] [PubMed] [Google Scholar]
- 27. Chen H.; Liu X.; Clayman E. S.; Shao F.; Xiao M.; Tian X.; Fu W.; Zhang C.; Ruan B.; Zhou P.; Liu Z.; Wang Y.; Rui W. Synthesis and evaluation of a CBZ-AAN-Dox prodrug and its in vitro effects on SiHa cervical cancer cells under hypoxic conditions. Chem. Biol. Drug Des. 86(4):589–598; 2015. [DOI] [PubMed] [Google Scholar]
- 28. Lin Y. Y.; Qiu Y. M.; Xu C.; Liu Q. L.; Peng B. W.; Gunnar K. F.; Chen X.; Lan B.; Wei C.; Lu D.; Zhang Y.; Guo Y.; Lu Z.; Jiang B.; Edgington T. S.; Guo F. Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis. J. Natl. Cancer Inst. 106(4):1–12; 2014. [DOI] [PubMed] [Google Scholar]