Abstract
Objectives:
This study developed a musculoskeletal ultrasound (MSUS) protocol to evaluate rehabilitation outcomes in systemic sclerosis.
Materials & Methods:
Three MSUS methods (grey scale, Doppler, strain elastography) and two acquisition techniques (long versus short axis; transducer on skin versus floating on gel) were examined in the forearm before and after rehabilitation treatment. For grey-scale, tissue thickness measures, intra- and inter-rater reliability were calculated (ICCs), and paired t-tests examined differences among techniques.
Results:
Five people with diffuse cutaneous systemic sclerosis participated. The most valid and reliable grey-scale technique was with the transducer in long-axis, floating on gel. Doppler and strain elastography did not detect changes. Both dermal and subcutaneous thickness measurement error was small; intra- and inter-rater reliability was good to excellent. Preliminary data indicate that treatment may lead to dermal thinning.
Conclusion:
A replicable protocol was established and may be an adjunct to rehabilitation outcome measurement in systemic sclerosis.
Keywords: musculoskeletal ultrasound, scleroderma, rehabilitation, protocol
Systemic sclerosis (SSc) is a chronic autoimmune disease that results in excess collagen production and vascular changes1. Skin thickening is a key metric used by rheumatologists to track disease severity and progression. In rehabilitation, skin thickening is also important to occupational and physical therapists as they seek to improve functional consequences of skin thickening; notably, restriction of range of motion in fingers, hands, and arms; reduced strength; and associated pain. Therapists measure effectiveness of their treatments based on ability to improve movement, function, and pain, but do not typically measure skin involvement as an outcome in rehabilitation. Even when function improves, therapists often do not have methods to understand how their treatments work, limiting the ability to select targeted interventions and effectively monitor patients.
Clinical evaluation of skin involvement, particularly skin stiffness, is typically completed using the modified Rodnan Skin Score (mRSS), which involves applying pressure at various points on the body2. Although mRSS is the gold standard in rheumatology practice and research trials3, there is high measurement variability among raters4, and mRSS may not be sensitive in later-stage disease and in specific subgroups4, 5.
Musculoskeletal ultrasound (MSUS) is increasingly used in rheumatology; it is relatively inexpensive, non-invasive, and well-tolerated by patients6. Current applications of MSUS in rheumatology include diagnosis of inflammatory and non-inflammatory rheumatic disease and assessment of treatment response7–9. MSUS has the potential to add sensitivity and precision to measure changes in dermal and subcutaneous tissues10–12, which would be important in rehabilitation in which these tissues are manipulated with the goal of improving movement. Existing MSUS protocols for evaluating the skin are highly heterogeneous13, and there are currently no widely-adopted standardized protocols to measure skin involvement in SSc. Only one study could be found that used MSUS to examine the link between disease features and upper extremity function in SSc which focused only on the hand14.
To address critical gaps, our team developed a MSUS protocol to be used to detect changes in skin with rehabilitation treatments such as manual therapy or negative pressure treatment which involved treatment of the forearm and wrist. Our interdisciplinary team consisted of a rheumatologist, radiologist specialized in musculoskeletal disorders, rehabilitation sonographer, occupational therapy practitioners, and rehabilitation researchers. The study aimed to determine which MSUS image acquisition and analysis techniques were most valid and useful for examining morphology and pathophysiology of primary structures of interest in SSc upper extremity rehabilitation. We also explored the potential of MSUS for identifying change in these outcome measures.
Methods
A pre-post, multiple case series design was used to develop, refine, and evaluate a MSUS protocol for SSc patients receiving upper extremity rehabilitation. The study was approved by the University’s institutional review board. All participants provided written informed consent.
Participants
Five participants with SSc were recruited from the Scleroderma Center between August 2019 and January 2020. To be eligible, participants needed to be age ≥ 18 years, have a diagnosis of diffuse cutaneous SSc, be in an early disease stage (≤ 5 years from first non-Raynaud’s symptom), and be English-speaking. Exclusion criteria were any issues that would preclude meaningful participation in study procedures (e.g., concurrent medical issues, inability to tolerate treatment, inability to provide consent, and active hand ulcers on both extremities).
After eligibility was confirmed, each participant was scheduled for a research visit at the radiology clinic. At the visit, demographic data and common SSc clinical measures were obtained. Demographic information included age, sex, ethnicity, education, and employment status. Self-reported symptoms of pain, fatigue, and stiffness were rated on 0 – 10 numeric rating scales of severity, upper extremity disability was assessed using the QuickDASH15, and self-reported physical function was assessed by the PROMIS Physical Function scale 8b16. Objective hand and arm measures were collected by the therapist clinician on the study team (MB). Total active range of motion (TAM) was calculated by summing total active ROM for each finger and thumb using a goniometer; 1175 degrees is possible17. Grip strength was measured by Jamar dynamometer in pounds of pressure18; the mean value of three trials on the right upper extremity is reported. Skin hardness was assessed by durometer; a higher score means more severe skin hardness19. After baseline measures were obtained at the visit, all participants underwent a pre-treatment MSUS assessment, a negative pressure occupational therapy treatment, and a post-treatment MSUS assessment was repeated immediately following treatment. All treatments and measures were obtained on the right forearm (except for participant 4 who had an active hand ulcer on the right forearm, so the left forearm was used). The visit lasted between 1 – 2 hours.
Treatment
Tissue mobilization was conducted using LymphaTouch (LymphaTouch LLC, Helsinki, Finland), an electronic method of mild tissue mobilization that lifts or stretches the skin using negative pressure to promote manual lymph drainage20. The device has been previously tested as part of a broader occupational therapy intervention for SSc21. Although the mechanism of action for SSc rehabilitation is not known, this treatment is hypothesized to break up skin adhesions allowing freer movement and improving hand and arm function.
Each participant received treatment on dorsal and volar aspects of their forearm and wrist using a standardized protocol with the LymphaTouch device. Working from proximal to distal beginning at the lateral elbow, ten, 2-second pulses were provided along the length of the dorsal forearm covering approximately 8 areas using the 60 mm head with grey ring. Next, using either the 50 or 60 mm head, 10 pulses were used in 2 placements over the dorsal and volar hand. Lastly, a 35 mm head was used to apply 10 pulses to each finger volarly and dorsally. The same procedure was then completed distal to proximal from the dorsal wrist over dorsal forearm and finally from volar wrist to medial elbow. The pressure used was the maximum that the patient could tolerate, around 200–250 mmHg.
Sonographic Image Acquisition
Sonography was conducted using a GE Logiq 9 ultrasound (GE Healthcare, Milwaukee, WI) and a 12-mHz linear array transducer with frequency set at 15.0 and gain between 41–43. As much as possible, all environmental conditions were standardized across participants and treatment and evaluations were conducted in the same room. A radiologist with training in musculoskeletal techniques acquired all images. To maintain quality control, the transducers were checked by the radiologist and sonographer prior to imaging.
For the first two participants, a full regional scan of the upper extremity was conducted22. The scan began with examination of the anterior and posterior humeroradial and humeroulnar joints at the elbow in both longitudinal and transverse, with the participant positioned in both full elbow extension and in 90 degrees of flexion. Longitudinal and transverse scans on both ulnar and radial sides of the elbow, forearm and wrist were completed beginning on the dorsal side and followed by a full scan of the volar side. The study team used the images obtained from the comprehensive scans of these two participants to develop a standardized SSc protocol for the final three participants. Specifically, locations, images, and sonographic techniques were selected to best examine those tissues and sites primarily targeted by the rehabilitation treatment.
For the standardized SSc protocol, participants sat in a chair with the right upper extremity positioned palm down with the volar forearm and elbow supported on a table. The hand was positioned at the table’s edge, supported at the distal radial-ulnar joint so that the fingers dangled freely. The dorsal surface of the participant’s forearm was measured from the lateral epicondyle to the distal end of ulnar styloid, divided into thirds, and the midpoint of each third marked with an X using a surgical dermatological marker (Figure 1). Sonographic images and skin hardness were obtained at each of the three sites marked with an X.
Figure 1.
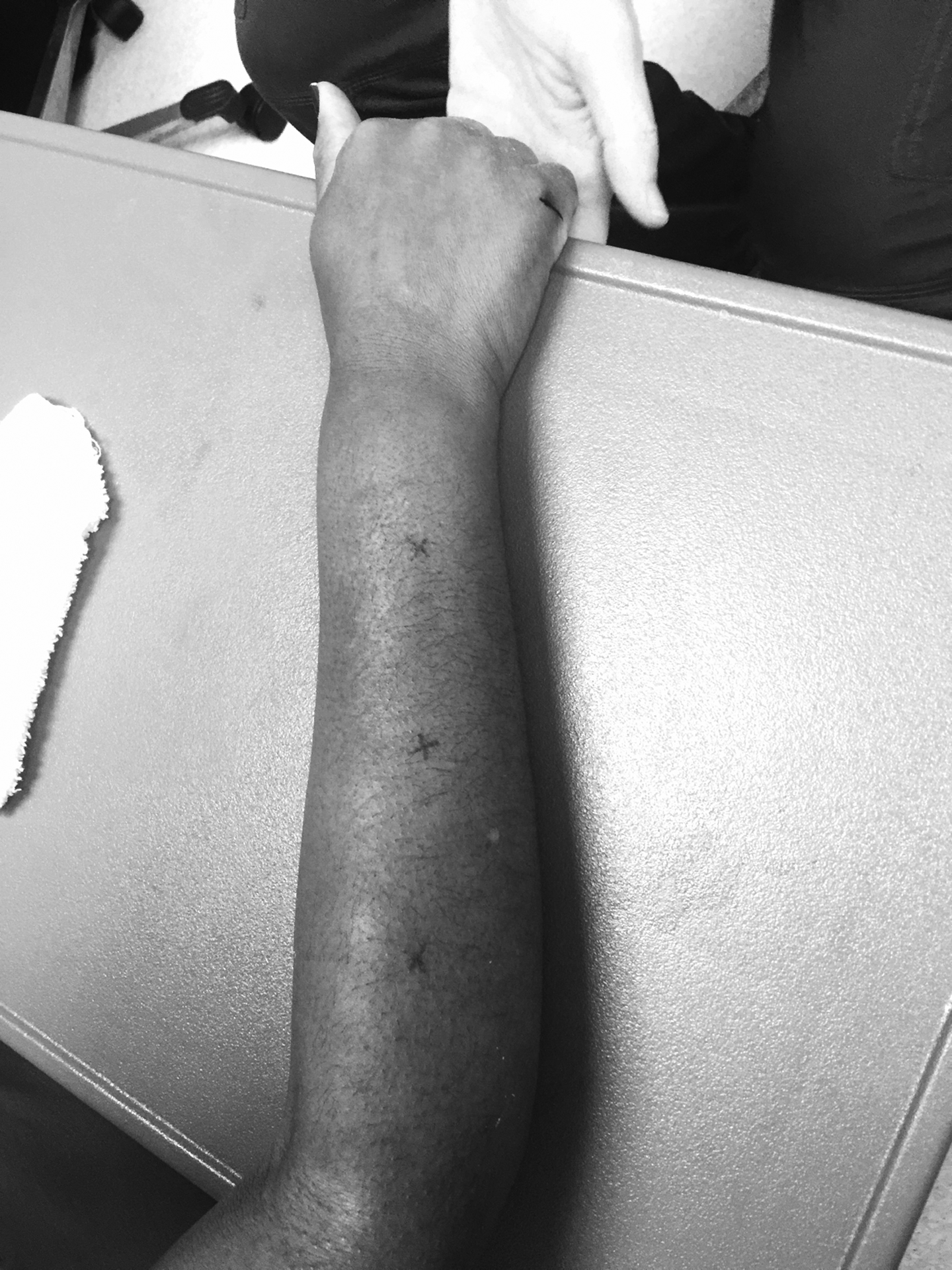
Extremity position and imaging marks
Image acquisition was conducted with the transducer in both transverse (short-axis) and longitudinal (long-axis) planes using grey-scale, color Doppler, and strain elastography at each of the three sites. Grey-scale images were obtained using two techniques to identify the most valid and useful acquisition protocol for examination of dermal and subcutaneous thickness (Figure 2). First, as noted in documented recommendations23, copious gel was used to allow the transducer to float without causing tissue compression. Second, the transducer was allowed to sit on the skin surface while the radiologist was cautious not to apply any additional pressure beyond weight of the transducer itself. Color Doppler was used to examine vascularity or blood flow changes within the subcutaneous soft tissue, muscles, or tendons at each treatment site; the number or intensity of colored pixels indicates amount of active blood perfusion24. Strain elastography images were obtained using a free-hand technique by the radiologist in which a gradual, uniform, and repetitive manual compression was applied through the transducer25; tissues’ elastic properties are reflected in colors assigned to each pixel with a scale ranging from red-softest tissue with high elasticity, to blue-hardest, minimal elasticity tissue structure26.
Figure 2.
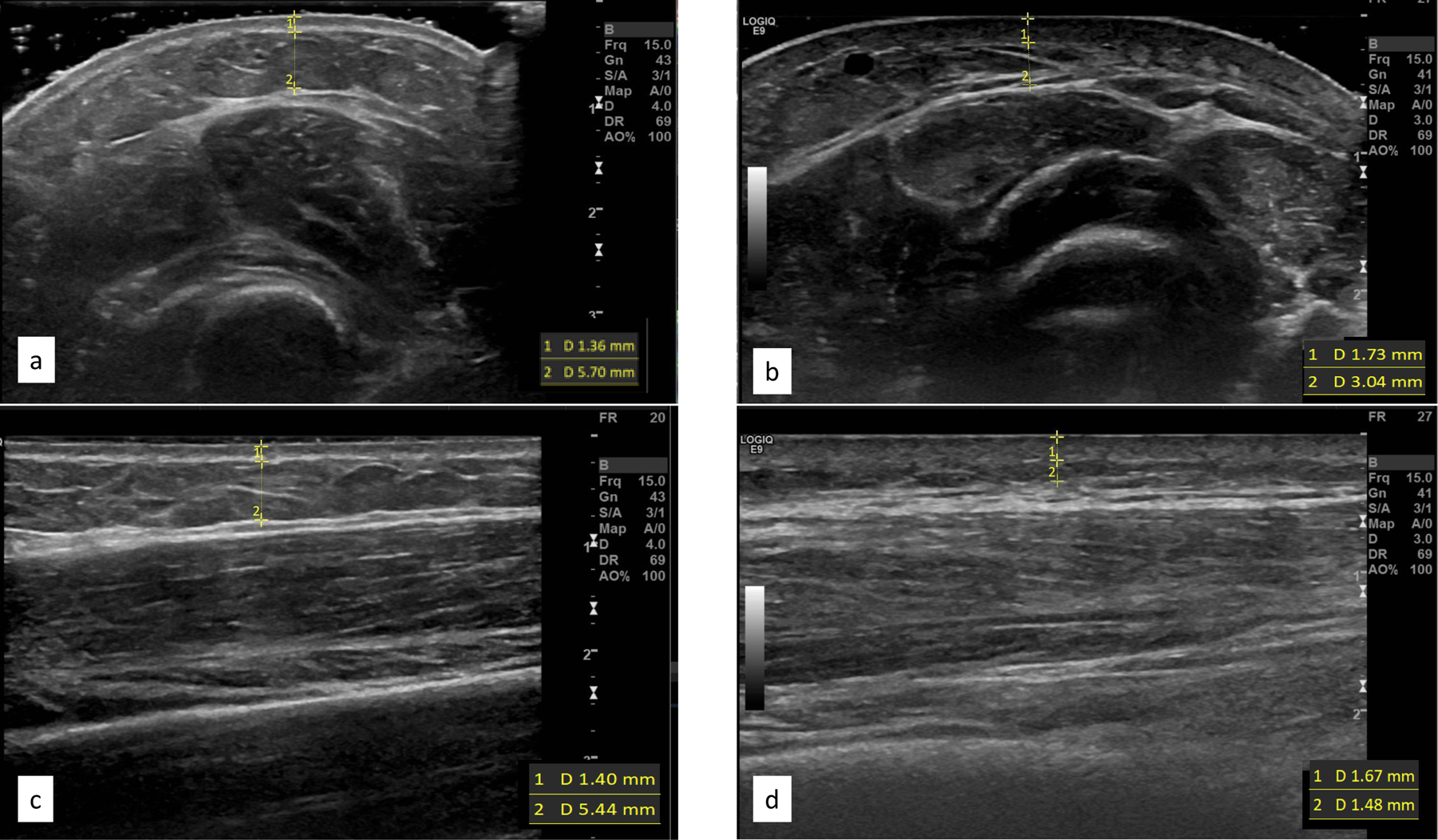
Grey-scale images of the forearm demonstrating floating of transducer in cross sectional plane (a) versus transducer on the surface in the same place (b), as images in the longitudinal planes floating and surface (c, d respectively). Borders and measures for dermal and subcutaneous images included
Sonographic Image Analysis
All images were numbered, de-identified, and transferred into ViewPoint 6 software (GE Healthcare) for analysis. Two researchers who did not obtain images nor provide treatments, one novice (DMK, Rater 1) and one expert who was a registered musculoskeletal sonographer with more than ten years of experience (SCR, Rater 2). Because images from the first two participants were used to develop a replicable protocol for the remaining participants, only images for Participants 3,4, and 5 were used in analysis. The raters reviewed all images from these last 3 participants and agreed upon the highest quality images to be analyzed. One grey-scale image was selected for each forearm location (site 1, 2, 3 from proximal to distal), time (pre, post), axis (short, long), and transducer placement (floating, surface); a total of 24 images per participant. In the case that a high-quality grey-scale image was not available, Doppler or elastography images were interrogated by the researchers to identify possible replacement images. The process was completed for Doppler and elastography images.
The same two researchers (SR, DMK) independently measured dermal and subcutaneous thickness on each grey-scale image (Figure 2). Dermal thickness was identified as the distance between epidermal entrance echo and the subcutaneous fat from the deep edge of the epidermis to the deep edge of the dermal layer27. Typical dermal layer measurements in normal skin are 1.6 mm, but in SSc it varies from 0.5 – 3 mm27. The subcutaneous layer was recognized as the distance between the deep edge of the dermal layer and superficial edge of the fascial layer of the muscle. Using digital calipers, measurements were taken in millimeters at a rater-selected point within the center one-third of each image where the individual structural boundaries were the most echogenic. The average of three measurements of each layer was recorded, and each rater completed three trials for all measures.
For Doppler and elastography analysis, pre- and post-intervention images were placed side-by-side for each site and axis. Because no appreciable color was noted on any Doppler images, no further analyses were completed. A two-step process was used for elastography images. First, color transitions from blue (hard) to red (soft) within the dermal and subcutaneous layers in the region of interest (ROI) were subjectively examined using the modified lesion scale28. The amount of change in tissue hardness from pre to post was scored using a 4-point Likert scale: 0-no difference noted, 1-slight difference (25–30% of the image color changed), 2-moderate difference (50% of the image color changed), 3-significant difference (≥75% of colors in the ROI changed). Second, if more than 25% of image-pairs were noted to have significant change in color (i.e., scoring 3 on the Likert-scale), an objective quantification of magnitude of color change and color intensity within both the dermal and subcutaneous layers within the ROI was interrogated using ImageJ.
Statistical Analyses
Descriptive statistics were calculated for demographic and clinical data. To evaluate measurement differences between raters, paired t-tests were used. Intraclass correlation coefficients (ICCs) were calculated within and between raters to examine intra- and inter-rater reliability. We considered ICC values less than 0.5 to be poor reliability, 0.5 and 0.75 to be of moderate reliability, 0.75 and 0.9 to be good reliability, and greater than 0.90 indicate excellent reliability29. Dermal and subcutaneous thickness measures were examined across all images, by subcategories of short and long axis, by transducer placement on the surface and floated with gel, and by the crossed conditions of short-axis surface, short-axis floating, long-axis surface, and long-axis floating. Using data from the most reliable rater, post-hoc analyses examined differences in short-long and floating-surface images. Finally using the technique identified as most reliable and valid, pre-post treatment changes in dermal and subcutaneous thicknesses were explored. All post-hoc comparisons used the Wilcoxon signed rank test due to the small sample size and a non-normal distribution in subcutaneous thickness values. Descriptive statistics were used for Doppler and elastography comparisons.
Results
Descriptive information for participants included in this case series study is in Table 1. Participants’ mean age was 48.2 years; four were female and one male. Mean baseline pain severity scores were 4 out of 10. Participants reported below average functional abilities on QuickDASH and PROMIS measures, had moderate limitations in total active ROM, and demonstrated below average grip strengths. Skin hardness from the durometer assessment averaged 40.6 units across the landmark sites of the dorsal surface of the forearm.
Table 1.
Participant Characteristics
| Participant | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age (years) | 71 | 66 | 47 | 31 | 26 |
| Sex | Female | Female | Female | Female | Male |
| Race | Caucasian | Caucasian | African American | African American | African American |
| Employment | Homemaker | Homemaker | Disabled | Full-time | Disabled |
| Education | Bachelors | Bachelors | Bachelors | High School | Some College |
| Symptoms | |||||
| Pain (Upper Extremity) | 2 | 0 | 3 | 8 | 6 |
| Fatigue | 8 | 3 | 4 | 5 | 6 |
| Stiffness | 8 | 5 | 4 | 7 | 8 |
| Durometer | 32.9 | 36.6 | 31.4 | 53.6 | 45.8 |
| QuickDASH | 45.5 | 27.3 | 34.0 | 88.8 | 61.3 |
| PROMIS Physical Function | 35.5 | 40.8 | 38.8 | 34.1 | 20.9 |
| TAM | 667 | 657 | 911 | 777 | 888 |
| Right Grip Strength (lbs/pressure) | 35.1 | 8.0 | 24.7 | 14.7 | 5.3 |
| Protocol Used | Initial | Initial | Refined | Refined | Refined |
Symptoms on a 0 – 10 scale with 10 being worst possible. For physical function, a lower score indicates worse function. QuickDASH is on a 0 – 100 scale; a higher score indicates worse function. TAM = Active Range of Motion; total score possible is 1175 degrees of movement.
Following initial review, 63 images were identified as having adequate quality for measurement of dermal and subcutaneous thickness across the three participants. Based on expert rater’s measures (Rater 2), the average dermal thickness was 1.74mm (SD 0.35mm) and average subcutaneous thickness was 3.45mm (SD 0.32mm) across all 63 images. Comparisons of means, measurement errors, and reliability statistics for dermal and subcutaneous thickness measurements between the two raters across all images and for each sub-categorization (i.e., short-axis, long-axis, surface, floating, short-surface, short-floating, long-surface, long-floating) are presented in Tables 2 and 3.
Table 2.
Dermal Thickness Averages, Measurement Errors, and Reliability by Image Categorization
| Rater 1 | Rater 2 | Rater 1 | Rater 2 | Inter-rater | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | SEM | Mean (SD) | SEM | p* | ICC** | ICC** | ICC*** | |
| All | 63 | 1.64 (0.42) | 0.05 | 1.74 (.35) | 0.04 | <0.01 | 0.94 | 0.98 | 0.86 |
| 95% CI | 0.91–0.96 | 0.97–0.99 | 0.75–0.92 | ||||||
| S. Axis | 31 | 1.70 (0.47) | 0.08 | 1.78 (0.36) | 0.06 | 0.16 | 0.92 | 0.98 | 0.83 |
| 95% CI | 0.86 –0.96 | 0.97 – 0.99 | 0.66 – 0.92 | ||||||
| L. Axis | 32 | 1.58 (0.37) | 0.06 | 1.69 (0.28) | 0.05 | <0.01 | 0.97 | 0.98 | 0.90 |
| 95% CI | 0.94 –0.98 | 0.96 – 0.99 | 0.70 – 0.96 | ||||||
| Floating | 31 | 1.75 (0.37) | 0.07 | 1.78 (0.30) | 0.05 | 0.61 | 0.88 | 0.97 | 0.75 |
| 95% CI | 0.78 – 0.94 | 0.95 – 0.99 | 0.48 – 0.88 | ||||||
| Surface | 32 | 1.53 (0.45) | 0.08 | 1.70 (0.35) | 0.06 | <0.01 | 0.97 | 0.98 | 0.92 |
| 95% CI | 0.95 – 0.99 | 0.97 – 0.99 | 0.51 – 0.97 | ||||||
| S. Axis x Floating | 15 | 1.84 (0.41) | 0.11 | 1.82 (0.34) | 0.09 | 0.80 | 0.78 | 0.98 | 0.63 |
| 95% CI | 0.48 – 0.92 | 0.95 – 0.99 | 0.17 – 0.88 | ||||||
| S. Axis x Surface | 16 | 1.56 (0.49) | 0.12 | 1.74 (0.39) | 0.10 | <0.01 | 0.98 | 0.99 | 0.93 |
| 95% CI | 0.95 – 0.99 | 0.97 – 1.0 | 0.30 – 0.98 | ||||||
| L. Axis x Floating | 16 | 1.66 (0.30) | 0.08 | 1.74 (0.26) | 0.06 | 0.08 | 0.97 | 0.97 | 0.89 |
| 95% CI | 0.93 – 0.99 | 0.93 – 0.99 | 0.68 – 0.96 | ||||||
| L. Axis x Surface | 16 | 1.51 (0.42) | 0.10 | 1.65 (0.32) | 0.08 | <0.01 | 0.96 | 0.98 | 0.90 |
| 95% CI | 0.92 – 0.99 | 0.96 – 0.99 | 0.57 – 0.97 | ||||||
S. Axis = Short Axis; L. Axis = Long Axis; SEM= Standard Error of the Mean, and 95% confidence interval (CI) is assumed.
p-value for paired t-test between rater 1 and rater 2 on same image; due to missing values, data not equally paired by site for short/long or floating/surface, so comparison between values within rater for these variables should not be made using these data.
two-way mixed effects model for consistency;
two-way random effects model for agreement
Table 3.
Subcutaneous Thickness Averages, Measurement Errors, and Reliability by Image Categorization
| Rater 1 | Rater 2 | Inter-rater | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | SEM | Mean (SD) | SEM | p* | ICC*** | |
| All | 63 | 3.03 (2.22) | 0.28 | 3.45 (2.54) | 0.32 | <0.01 | 0.97 |
| 95% CI | 0.92–0.99 | ||||||
| S. Axis | 31 | 3.21 (2.37) | 0.43 | 3.47 (2.68) | 0.48 | 0.02 | 0.98 |
| 95% CI | 0.96 – 0.99 | ||||||
| L. Axis | 32 | 2.84 (2.09) | 0.37 | 3.43 (2.44) | 0.43 | <0.01 | 0.96 |
| 95% CI | 0.80 – 0.99 | ||||||
| Floating | 31 | 3.34 (2.31) | 0.42 | 3.93 (2.79) | 0.50 | <0.01 | 0.97 |
| 95% CI | 0.85 – 0.98 | ||||||
| Surface | 32 | 2.72 (2.12) | 0.38 | 2.99 (2.23) | 0.39 | 0.01 | 0.98 |
| 95% CI | 0.95 – 0.99 | ||||||
| S. Axis x Floating | 15 | 3.80 (2.72) | 0.70 | 4.19 (3.09) | 0.80 | 0.03 | 0.98 |
| 95% CI | 0.94 – 1.0 | ||||||
| S. Axis x Surface | 16 | 2.67 (1.94) | 0.48 | 2.80 (2.12) | 0.53 | 0.35 | 0.98 |
| 95% CI | 0.95 – 0.99 | ||||||
| L. Axis x Floating | 16 | 2.92 (1.85) | 0.46 | 3.68 (2.55) | 0.64 | <0.01 | 0.94 |
| 95% CI | 0.60 – 0.98 | ||||||
| L. Axis x Surface | 16 | 2.77 (2.36) | 0.60 | 3.17 (2.38) | 0.60 | <0.01 | 0.98 |
| 95% CI | 0.90 – 0.99 | ||||||
S. Axis = Short Axis; L. Axis = Long Axis; SEM= Standard Error of the Mean, and 95% confidence interval (CI) is assumed. Rater 1 and Rater 2 reliability for each image categorization were all .99 with 95% CI 0.99– 1.0.
p-value for paired t-test between rater 1 and rater 2 on same image; due to missing values, data not equally paired by site for short/long or floating/surface, so comparison between values within rater for these variables should not be made using these data.
two-way mixed effects model for consistency;
two-way random effects model for agreement
Grey-Scale Measurement Reliability
Both raters, novice (Rater 1) and expert (Rater 2), obtained dermal thickness results with small overall measurement error, generally less than 0.10mm. Error was noted to be slightly higher in short-axis than long-axis and higher in surface than floating conditions. With exceptions of short-axis, floating, and short-axis by floating measures, the expert rater’s dermal thickness measures were statistically larger than the novice rater; however, these differences were all less than 0.20mm. The expert rater demonstrated excellent intra-rater reliability across all categories of measurement (i.e., all ICCs >0.95); whereas, the novice rater demonstrated good to excellent intra-rater reliability with exception of measurements of images obtained in short-axis with the transducer floating [ICC: 0.78, (0.48–0.92)]. Both raters were more reliable measuring in long-axis than in short-axis, and the expert rater was more reliable measuring floating than surface images. All inter-rater reliability measures for dermal thickness evaluation were good to excellent with the exception of the measurement short-axis by floating.
Similar to dermal measures, error in subcutaneous thickness measures was low, ranging from 0.28mm to 0.80mm, with slightly higher error in short-axis than long-axis and in floating than surface. Although the expert rater consistently measured significantly higher than novice rater, none of the measurement differences between raters exceeded the error, indicating that differences between raters were likely not clinically meaningfully. All intra-rater and inter-rater reliability outcomes for subcutaneous thickness were within excellent ranges (>0.90; Table 3).
Grey-Scale Technique Validation
Given the good to excellent reliability ratings for most measures, differences in values when the transducer was floating versus placed on the skin surface were examined. Dermal and subcutaneous thickness measures in both floating and surface by the expert rater were paired by location and time within participant. When taken in long-axis at the same location, dermal thickness measurements were statistically larger (p=0.09) when images were acquired with the transducer floating (1.77mm, SD 0.27mm) versus placed on the surface (1.70mm, SD 0.30mm). Similarly, subcutaneous thickness measures were statistically larger (p=0.049) with the transducer floating (3.40mm, SD 2.57mm) than on the surface (3.01, SD 2.36). No statistically significant differences were noted in either the dermal or subcutaneous thickness measures between the two image acquisition techniques in short-axis.
Doppler and Elastography Findings
No significant or interpretable signals were identified in any of the color Doppler images and consequently, no further analysis was completed. Analysis of strain elastography in short-axis images was difficult as the rounded shape of the forearm led to artifacts. These artifacts were realized as different shades of blue and green within the dermal layer clearly affected differentially by underlying bone as demonstrated in Figure 3a and 3b. Similar inconsistencies in color were noted in strain elastography images obtained with the transducer floating (Figure 3c), likely due to uneven wave compression resulting from not pushing on the skin surface. Strain imaging in long-axis with the transducer placed on the surface was the only valid technique without artifacts (Figure 3d). However, upon review, these strain images were considered to have low reliability as significant shifts in coloration were noted among repeated images obtained at the same location during the same time point (i.e., pre- or post-treatment). No further analysis of strain elastography images were conducted.
Figure 3.
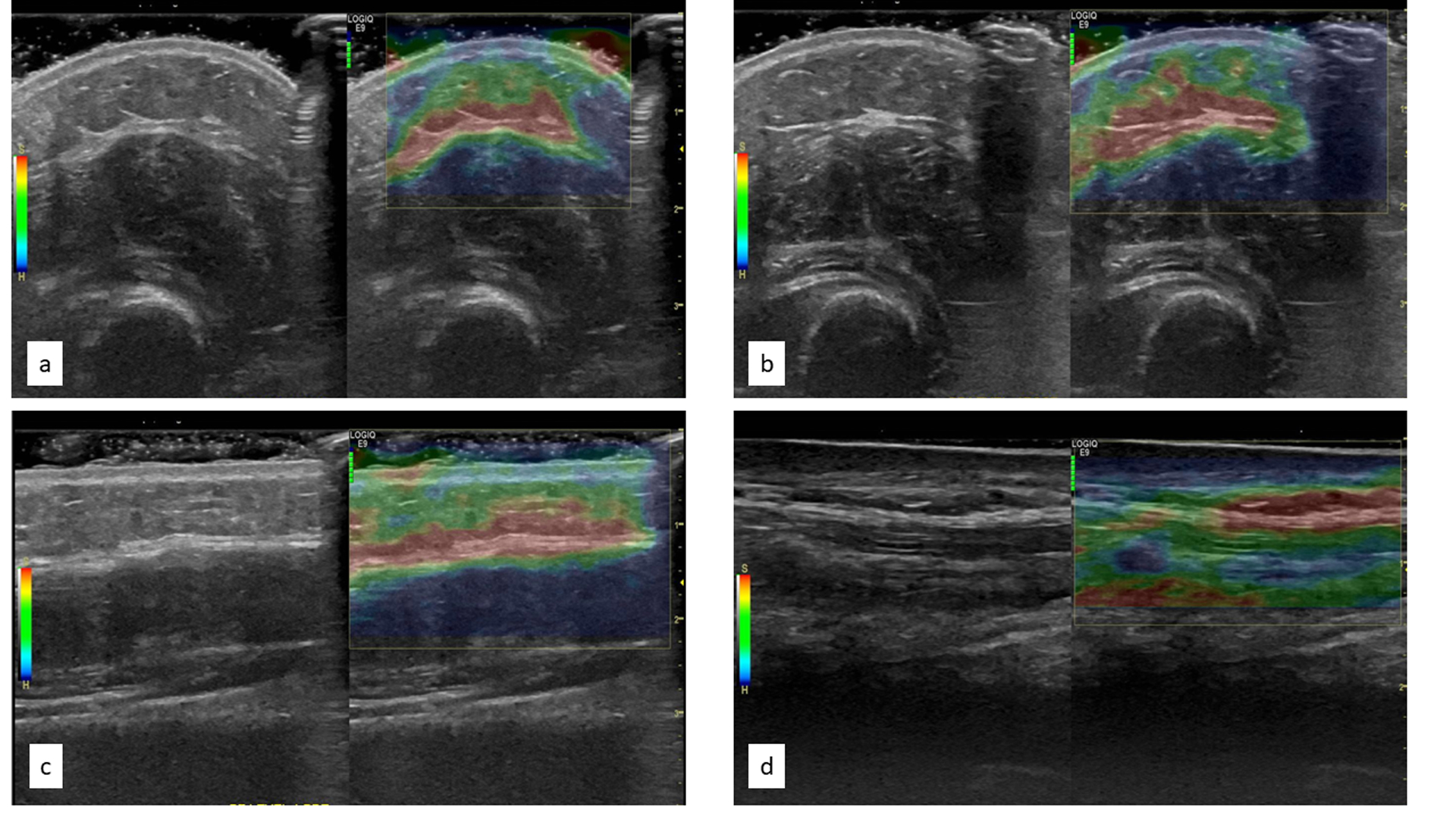
Pre-post treatment elastography images in short axis (a, b) and elastography image longitudinal floating versus surface (c, d respectively)
Exploration of Treatment Changes
Pre- and post-treatment values for dermal and subcutaneous thicknesses were identified by location of paired images acquired using the most reliable and valid technique (i.e., grey-scale, long-axis, floating). Data for six pre-post treatment pairs across the three participants are reported in Table 4. In these case series data, post-treatment dermal thickness values were consistently smaller than pre-treatment measures (p = 0.03). In four of six cases, decrease in dermal thicknesses exceeded the expert rater’s standard error of the mean (SEM) (0.06) for this imaging technique. No relationship existed among the pre-post measures of subcutaneous thickness (p = 0.35), with two cases decreasing by more than then SEM (0.64), one case increasing by more than this error, and three cases falling within the error range.
Table 4.
Case-wise Analysis of Changes in Dermal and Subcutaneous Thickness Pre and Post Treatment
| Participant | Arm Location | Dermal Thickness Pre-Treatment | Dermal Thickness Post-Treatment* | Subcutaneous Thickness Pre-Treatment | Subcutaneous Thickness Post-Treatment** |
|---|---|---|---|---|---|
| 3 | Proximal Forearm | 1.68 | 1.33 | 6.04 | 7.05 |
| 3 | Mid Forearm | 1.60 | 1.60 | 7.67 | 7.05 |
| 4 | Distal Forearm | 2.27 | 1.96 | 2.41 | 2.42 |
| 4 | Wrist | 1.47 | 1.40 | 1.92 | 0.65 |
| 5 | Proximal Forearm | 1.84 | 1.61 | 2.90 | 2.00 |
| 5 | Mid Forearm | 1.77 | 1.76 | 1.61 | 1.54 |
| Sample Mean (SD) | 1.77 (0.28) | 1.61 (0.23) | 3.76 (2.49) | 3.45 (2.85) | |
Wilcoxon signed ranks test indicates post-treatment dermal thickness values are lower (p = 0.03)
Wilcoxon signed ranks test indicates pre- and post-treatment values are similar (p = 0.35)
Discussion
This study aimed to determine a MSUS acquisition and analysis technique most appropriate for evaluating SSc rehabilitation outcomes, and to explore which measures were most sensitive to change after a one-session rehabilitation treatment. We have three main findings. First, grey-scale imaging with traducer floated on gel in the longitudinal axis was the most valid and reliable MSUS acquisition technique to measure dermal and subcutaneous thickness. Second, color Doppler and strain elastography were not useful for examining superficial forearm structures in our SSc patient sample. Third, there are preliminary indications that dermal thickness may be a useful outcome measure in upper extremity rehabilitation, which can be explored in prospective studies along with other indicators such as tendon mobility.
In this study, transducer placement either on the surface or floating resulted in equally reliable measures for the expert rater, but images acquired in long-axis were most reliable for obtaining measures dermal and subcutaneous thickness across both raters. Dermal and subcutaneous thickness measures were larger when the transducer was floating on gel compared to images acquired when the transducer was placed directly on the skin surface. These trends suggest that the weight of the transducer on the skin may compress dermal or subcutaneous layers, thereby decreasing the measurements. As such, floating the transducer is likely more valid for image acquisition. Moreover, demonstrating these trends in the long-axis images, but not in the short-axis, further support use of long-axis techniques.
Doppler US is useful for assessing vascularization and tissue perfusion of musculoskeletal structures, to evaluate inflammation, and monitor treatment response in joints and soft tissues24. Changes in Doppler may indicate neo-vascularization or increased blood flow, each could be indicative of a positive treatment outcome. One possible explanation for lack of Doppler findings from pre- to post-LymphaTouch treatment in this study is that the treatment may not be providing short-term changes in blood perfusion into the treated region. While color Doppler was not found to be useful, other Doppler techniques may be appropriate for evaluating SSc patients. For example, changes in blood perfusion may be detectable as a long-term outcome as opposed to following one-session treatment. Moreover, rather than investigating changes in perfusion within tissues of SSc patients, power or spectral Doppler may be more useful for evaluating changes in magnitude or rate of blood flow through specific vessels in or around a treated region. For the upper extremity, changes in these measures of blood flow in small finger vessels could be highly useful.
Strain elastography is an effective sonographic technique to evaluate differences in tissue density26; however, this technology is best used with homogenous tissue that allows for equal compression (e.g., breast tissue) and is less reliable in superficial musculoskeletal structures, as is evident in our findings. In particular, varying densities and inconsistent shapes of the forearm and limitations in sizing the ROI in the small superficial region results in a higher probability of image artifacts, which can lead to erroneous or inconclusive evidence26. Although we were unable to conduct rigorous review of elastography data across all participants, at least one participant had valid pre-post images which seemed to indicate a moderate shift in tissue stiffness. Given our data, strain elastography is likely not recommended for use in evaluating the forearm, hands, or fingers of SSc patients.
Improvements in point-of-care technology, increased access, and specialized education have broadened the ability for use of sonography by rheumatologists and rheumatology professionals for purposes beyond diagnostics30–32. For instance, rehabilitation treatments may be better targeted to specific subclinical SSc disease manifestations as detected by MSUS. Importantly, MSUS could help rheumatology professionals understand underlying mechanisms of action for their treatments. This type of information is critically important to the development and refinement of rehabilitation treatments. In addition, MSUS could offer more sensitive and accurate evaluation of disease changes in response to treatment, thereby improving outcome measurements that typically consist of self-reported function, assessment of movement, or therapist-observed disease features.
In addition to investigating other uses of Doppler and strain elastography, using dynamic sonographic techniques may also be worth further evaluation for use in examination of SSc treatment outcomes. Specifically, using sonographic imaging to capture tendon mobility may be a means of determining effective interventional methodology as identified by Korstanje and colleagues33. The operant theory of LymphaTouch is that the fibrotic plaques of SSc may be loosened by the negative pressure of the intervention, which may allow for increased movement of both skin and underlying structures. There is a need for further work to develop sonographic methods for measuring movement of the extensor digitorum communicus (EDC) and other tendons of the hand during the completion of functional tasks in patients with SSc to more fully evaluate treatment effects in this patient population.
Implications for therapy
Our findings indicate that MSUS may be a useful rehabilitation tool for measuring treatment effectiveness as it allows opportunity to see structural effects on dermal and subcutaneous levels in individuals with SSc. In our sample, average dermal thickness values measured by the expert rater in long-axis, floating were slightly higher than this normative data at 1.74 mm. The mean dermal thickness values were noted to be higher in pre-treatment measures (1.77mm) with a maximum individual value of 2.27 mm, with consistently lower dermal thickness measures on post-treatment images. These data provide preliminary support for dermal thinning immediately following LymphaTouch treatment, indicating that this may be a useful measure and treatment combination for rehabilitation clinicians working with individuals with SSc. This preliminary effect in our small-sample, case-series study should be further examined in a larger sample, over time, and compared with other functional outcomes to determine treatment impact. Studies should examine if this thinning is related to improvement in dermal pathology or momentary stretching of the skin following treatment, as well as to evaluate how long the effects of the treatment last. Identifying the duration of increased dermal pliability could provide understanding of the best therapeutic window for providing other focused intense therapeutic interventions to maximize overall outcomes.
Limitations
Only images from participants three through five were included in analysis and missing data points could have skewed analyses. Because the purpose of this study was to validate an imaging acquisition and analysis protocol, it was too preliminary to develop an a-priori plan for comparison of sonographic changes to other patient outcomes. Moreover, given that data were collected following one brief treatment session, significant changes in functional outcome measures were not expected. However, having developed a valid technique and identified preliminary effect size on change in morphologic measures, future interventional pilot studies can further explore and compare intervention effects between these physiologic measures and functional outcomes.
Conclusions
MSUS can provide reproducible results of skin and subcutaneous tissue changes particularly in SSc. Imaging recommendations from this study indicate the most valid and reliable MSUS technique is using grey-scale imaging with the transducer floated on gel in the longitudinal plane. The developed protocol should be tested with larger samples and can be used as a measure to evaluate rehabilitation treatments.
Funding
The project described was supported in part by a grant to Dr. Murphy from LymphaTouch LLC. Dr. Krause’s work was supported by an Advanced Rehabilitation Research Training grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (90AR5020-04). Dr. Khanna’s work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24-AR-063129).
Footnotes
Conflicts of Interest: There is no commercial benefit from any author for this manuscript.
References
- 1.Bolster M, Silver R. Clinical features of systemic sclerosis In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, (eds). Rheumatology. 5th ed. Philadelphia, PA: Mosby, Elsevier; 2011: 1373–1386. [Google Scholar]
- 2.Clements P, Lachenbruch P, Seibold J, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20: 1892–1896. [PubMed] [Google Scholar]
- 3.Merkel PA, Clements PJ, Reveille JD, Suarez-Almazor ME, Valentini G, Furst DE. Current status of outcome measure development for clinical trials in systemic sclerosis. Report from OMERACT 6. J Rheumatol. 2003;30(7):1630–1647. [PubMed] [Google Scholar]
- 4.Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumanovics G, Pentek M, Bae S, et al. Assessment of skin involvement in systemic sclerosis. Rheumatology. 2017;56(s5):53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane D, Balint PV, Sturrock R, Grassi W. Musculoskeletal ultrasound--a state of the art review in rheumatology. Part 1: Current controversies and issues in the development of musculoskeletal ultrasound in rheumatology. Rheumatology. 2004;43(7):823–828. [DOI] [PubMed] [Google Scholar]
- 7.Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: Implementation and practice in rheumatology. Arthritis Care Res. 2014;66(1):7–13. [DOI] [PubMed] [Google Scholar]
- 8.McAlindon T, Kissin E, Nazarian L, et al. American college of rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res. 2012;64(11):1625–1640. [DOI] [PubMed] [Google Scholar]
- 9.Hassan S Overview of musculoskeletal ultrasound for the clinical rheumatologist. Clin Exp Rheumatol. 2018;36:S3–S9. [PubMed] [Google Scholar]
- 10.Hesselstrand R, Carlestam J, Wildt M, Sandqvist G, Andréasson K. High frequency ultrasound of skin involvement in systemic sclerosis - a follow-up study. Arthritis Res Ther. 2015;17:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore TL, Lunt M, McManus B, Anderson ME, Herrick AL. Seventeen-point dermal ultrasound scoring system--a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology. 2003;42(12):1559–1563. [DOI] [PubMed] [Google Scholar]
- 12.Sulli A, Ruaro B, Smith V, et al. Subclinical dermal involvement is detectable by high frequency ultrasound even in patients with limited cutaneous systemic sclerosis. Arthritis Res Ther. 2017;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago T, Santiago M, Ruaro B, Salvador MJ, Cutolo M, da Silva JAP. Ultrasonography for the assessment of skin in systemic sclerosis: a systematic review. Arthritis Care Res. 2019;71(4):563–574. [DOI] [PubMed] [Google Scholar]
- 14.El Sawy N, Suliman I, Nouh M, Naguib A. Hand function in systemic sclerosis: A clinical and ultrasonographic study. Egypt Rheumatol. 2012;34(4):167–178. [Google Scholar]
- 15.Kennedy CA. Measurement properties of the QuickDASH (Disabilities of the Arm, Shoulder and Hand) outcome measure and cross-cultural adaptations of the QuickDASH: a systematic review. Qual Life Res. 2013;22(9):2509–2547. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epid. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams L, Greene L, Topoozian E. Range of Motion In: American Society of Hand Therapists; (ed). Clinical Assessment Recommendations. 1992; 55–70. [Google Scholar]
- 18.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg. 1984;9(2):222–226. [DOI] [PubMed] [Google Scholar]
- 19.Merkel PA, Silliman NP, Denton CP, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59(5):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iivarinen JT, Korhonen RK, Jurvelin JS. Modeling of interstitial fluid movement in soft tissue under negative pressure – relevance to treatment of tissue swelling. Comput Method Biomec. 2016(19):1089–1098. [DOI] [PubMed] [Google Scholar]
- 21.Murphy SL, Barber M, Homer K, Dodge C, Cutter G, Khanna D. Occupational therapy treatment to improve upper extremity function in individuals with early systemic sclerosis: a pilot study. Arthritis Care Res. 2018;70(11):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60(7):641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Möller I, Janta I, Backhaus M, et al. The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann Rheum Dis. 2017;76(12):1974–1979. [DOI] [PubMed] [Google Scholar]
- 24.Malliaras P, Chan O, Simran G, Martinez de Albornoz P, Morrissey D, Maffulli N. Doppler ultrasound signal in Achilles tendinopathy reduces immediately after activity. Int J Sports Med. 2012; 33(06):480–484. [DOI] [PubMed] [Google Scholar]
- 25.DiGeso E, Filippucci R, Girolimetti M, et al. Reliability of ultrasound measurements of dermal thickness at digits in systemic sclerosis: role of elastosonography. Clin Exp Rheumatol. 2011; 29:926–932. [PubMed] [Google Scholar]
- 26.Iagnocco A, Kaloudi O, Perella C, et al. Ultrasound elastography assessment of skin involvement in systemic sclerosis: lights and shadows. J Rheumatol. 2010;37;1688–1691 [DOI] [PubMed] [Google Scholar]
- 27.Kang T, Abignano G, Lettieri G, Wakefield R, Emery P, Del Galdo F. Skin imaging in systemic sclerosis. Eur J Rheumatol. 2014;1:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239(2):341–350. [DOI] [PubMed] [Google Scholar]
- 29.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. New Jersey: Prentice Hall; 2000. [Google Scholar]
- 30.Roll SC, Asai C, Tsai J. Clinical utilization of musculoskeletal sonography involving Non-physician rehabilitation providers: A scoping review. Eur J Phys Rehabil Med. 2016; 52:253– 262. [PMC free article] [PubMed] [Google Scholar]
- 31.Roll SC, McLaughlin-Gray J, Frank G, Wolkoff M. Exploring occupational therapists’ perceptions of the usefulness of musculoskeletal sonography in upper-extremity rehabilitation. Am J Occup Ther. 2015; 69(4):6904360010p1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takata SC. Broadening the perceptions of sonographic applications to promote client centered care, precision medicine, and holistic practices. J Diagn Med Sonogr. 2020; 36:1–2. [Google Scholar]
- 33.Korstanje J, Schreuders T, vanderSijdde J, Hovius S, Bosch J, Selle R Ultrasonographic assessment of long finger tendon excursion in zone v during passive and active tendon gliding exercises. J Hand Surg Am. 2010;35: 559–565. [DOI] [PubMed] [Google Scholar]


