Abstract
The outbreak Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by a highly contagious and lethal beta coronavirus SARS-CoV-2, which has spread fast to encroach the entire globe and hence declare pandemic. Pregnancy alters body physiology and immune systems, can have worse effects of some respiratory infections and due to limited research and published data we still are in dilemma of appropriate management guidelines This article covers the updated guidelines for infection prevention and control (IPC), screening, sampling, antenatal visit schedules, risk scoring, triaging, supportive care, delivery, postpartum care and care of the newborn. This article aims to provide up-to-date information as per recent guidelines of various association which would serve as guidance in managing pregnant women and newborn with suspected or confirmed COVID-19. All the published papers till date, NCPRE, WHO Interim guidelines, RCOG, FOGS GCPRI, Medical Council of India, ICMR, MOFHW, CDC, ACOG guidelines are referred to compile this article to reach to a conclusion of evidence based management of pregnant ladies during COVID-19 pandemic. This article covers the not only infection prevention and control (IPC) guidelines, but also screening and sampling guidelines, antenatal visit schedules, risk scoring, triaging but also in-patient supportive care, delivery, postpartum care and care of the newborn. Data are very limited and hence very difficult to accurately define clinical management strategies and needs to be constantly updated.
Keywords: Breast feeding, COVID-19, newborn care, pandemic, pregnancy, SARI, SARS-CoV-2
Introduction
The outbreak Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by a highly contagious and lethal beta coronavirus SARS-CoV-2, started in Wuhan, China in December 2019 which has spread fast to become a global public health threat.[1,2]
WHO situation reports as provided on July 3, 2020 as total of 10 357 662 cases and 508 055 deaths. The epidemic has spread to more than 213 countries around the world.[3]
COVID-19 outbreak has alarmed the entire world and due to limited researches and available data various national and international bodies are constantly coming up with updated guidelines to facilitate obstetrician all over the world to get a vision in providing optimum care to the most vulnerable group of the society, the pregnant ladies and newborn with minimal complications.
Aim
This article aims to provide up-to-date information as per recent guidelines of various association which would serve as guidance in managing pregnant women and newborn with suspected or confirmed COVID-19. Although guidelines and recommendations are constantly being revised and updated by various association and bodies this article aims to enlighten readers with updated clinical management protocol which could serve as guidance in managing pregnant women and newborn with suspected or confirmed COVID-19 and by no means it is meant to replace clinical judgement or specialist consultation.
Methodology
A comprehensive electronic search was done through PubMed, Scopus, Medline, Cochrane database, and Google Scholar from December 01, 2019, to August 31, 2020, along with the reference list of all included studies. In this article all efforts have been made to compile the latest guidelines released by The International Federation of Gynaecology and Obstetrics (FIGO), The Royal College of Obstetricians (RCOG), The American College of Obstetricians and Gynaecologists (ACOG), Centre for Disease control and Prevention (CDC) and Society of feta Medicine (SFM). This article has also included practice guidelines released by low resource country like India through ICMR (Indian Council of Medical Research) and FOGSI GCPR (Federation of Obstetric and Gynaecological Societies of India Good Clinical Practice Recommendation), The National Centre for Photovoltaic Research and Education (NCPRE ) and Ministry of Health and Family Welfare (MOHFW) and thereby offering low resource countries of Asia and Africa to modify their management protocol as per situation in their country. All the published papers on this topic till date have also been referred so as to not miss any valuable and needed information in this time of crisis.
Result
This article covers the not only infection prevention and control (IPC) guidelines, but also screening and sampling guidelines, antenatal visit schedules, risk scoring, triaging but also in-patient supportive care, delivery, postpartum care and care of the newborn.
Discussion
Effect of COVID-19 Infection on Mother and foetus
Pregnancy is an immunocompromised condition and like any viral respiratory infections COVID-19 infection might have worse effect than general population.[4] This is particularly true in 3rd trimester of pregnancy having comorbidities such as diabetes, chronic lung disease, hypertension, obesity and advanced age or combination of elevated D-dimer and interleukin-6 levels.[5,6,7]
Majority of pregnant women are asymptomatic or present with influenza like illness.[8] There are contradictory evidences of the possibility of vertical transmission of COVID-19 infection from mother to baby making it difficult to reach to any definitive conclusion.[9,10] All the products of conception like amniotic fluid, umbilical cord, cord blood, neonatal blood or nasopharyngeal and throat swabs, placenta swabs, genital secretions or breast milk samples are till date found mostly negative of COVID-19 infected mothers.[6,8] No published data suggesting COVID-19 infection might increase chance of foetal loss or teratogenicity if mother acquired infection at time of organogenesis [11] hence recommending amniocentesis to diagnose foetal infection would do more harm than benefit.[9] Though Liang et al. quoted that infection if acquired in later gestational age may increase obstetric complications of premature rupture of membranes, preterm labour, foetal tachycardia and foetal distress but more data of larger population awaited to finally reach to any conclusion.[12]The rate of iatrogenic preterm birth and caesarean delivery is high; vertical transmission may be possible but has not been proved and prematurity can be iatrogenic due to maternal medical condition.[7]
Assessment and risk scoring in pregnant women (not in labour) with COVID-19 infection
The criteria for testing are same for antenatal women as for the general population and is as per ICMR guidelines to ensure uniformity in assessing and treating the patients.[13] [vi]
The WHO - Global surveillance for COVID-19((Interim guidance 20 March 2020) and Ministry of Health and Family Welfare has defined who are suspect, contacts and confirmed cases.[14,15]
Clinical presentation of COVID-19 in pregnancy
Incubation period ranges from 5 to 11 days of exposure.[16] Most common presentation would be influenza like illness like fever, fatigue, sore throat running nose, nasal congestion myalgia, dry cough and shortness of breath. As per Medical current Indian Council of Medical Research updates some might also present with abdominal pain, diarrhoea, nausea, vomiting, cough with expectoration haemoptysis and chest pain. Those with comorbid or immunocompromised may develop severe acute respiratory illness (SARI), hypoxia requiring ventilatory support.[17]
The disease severity is classified by WHO based on the clinical symptoms.[18]
Pregnant women admitted in Intensive care Unit need to be assessed using Quick sequential organ failure assessment score qSOFA score as an adjunct for decision making in management of these in patients.[6]
Test methods and facilities
Once suspected or admitted the CDC recommends testing for COVID-19 from samples mainly of nasopharyngeal swab [19] but samples of saliva, oropharyngeal or that from lower respiratory tract (sputum, endotracheal aspirate or bronchoalveolar lavage) can also be used for testing. Reverse-transcription polymerase chain reaction (RT-PCR) is the gold standard for diagnosis of active infection of COVID-19 which detects SARS-COV-2 nucleic acid responsible for this deadly infection. Many rapid and easy-to-use devices are being developed but they still need to be validated before use.[13,20] There are more than 114 ICMR approved public laboratories government has permitted testing in private laboratories from 22 March 2020.[13,21]
Other tests: 1. Rapid diagnostic tests based on antigen detection – detects the viral proteins expressed by the COVID 19 virus in a sample from respiratory tract.
2. Rapid diagnostic test based on host antibody detection which is not the reliable marker of active infection detects the antibodies produced in the blood of COVID–19 infected patients in response to immune reaction.[22,23,24]
Assessing the disease process
Peripheral white blood cells lymphocyte count, platelet count remains normal and then may reduce in later stages. C-reactive protein, liver enzymes and creatine phosphokinase may be increased. Radiographic investigation pertaining to COVID-19 e.g., Chest X-ray and CT scan are a part of initial assessment and shouldn't be delayed because of foetal concerns. Radiological pictures of viral pneumonia were found in many COVID-19 infected pregnant women.[25] Computed tomography (CT) scan of the chest without contrast is found to have greater sensitivity in diagnosing COVID-19 than that of RT-PCR (98% vs 71%) and can be used to confirm or rule out viral pneumonia in suspected cases and can be done in pregnant ladies too using abdominal shield to avoid radiation exposure to the foetus.[26]
Other investigations like Electrocardiography, Computed Tomography Pulmonary Angiogram, full sepsis screen should be used in indicated cases to assert the differential diagnosis.[25]
Arterial blood gas, serum lactate, Renal Function Test, Liver Function Test and cardiac enzyme assessment are also indicated to our septic shock, acute kidney injury or virus-related cardiac injury.[12,25]
Management of COVID-19 Pregnant
Supportive therapy
Titration of oxygen flow can be done to keep saturation level >94% among pregnant patients with inhaled oxygen (60–100% concentration at an initial rate of 4 L/min which can be increased up to 10–15 L/min. Some cases like ARDS or patients with SARI may warrant intubation, extra-corporal membrane oxygenation (ECMO), with lateral decubitus positioning to maintain oxygenation.[13,25,27,28] High flow Nasal Oxygen (HFNO) and Non-invasive ventilation (NIV) should be reserved for patients with hypoxemic respiratory failure.[29]
Fluid management can be started with bolus doses of 250–500 ml of crystalloid fluid with caution of fluid overload.[19,25] Adequate rest, nutritional support and electrolyte balance should be ensured.[6,13]
3) Symptomatic management with NSAIDS like paracetamol is safe and preferred.[13]
4) Antibiotics can be started if there is indication with raised white cell counts or suspicion of secondary bacterial pneumonia.[6,13,25]
Steroid i.e., betamethasone 12 mg IM is indicated in preterm delivery (especially before 30 weeks) as there is no documented evidence of harm in context of COVID-19.[13,25,28,29] However, the use of steroids needs to be individualized. Administration of steroids in COVID 19 pneumonia is known to delay the viral clearance but in China methylprednisolone (1–2 mg/kg bodyweight per day) has been used for small period of 3–5 days SARI patients to supress lung inflammation causing hypoxaemia, dyspnoea and ARDS in COVID-19 infected patients.[13,25,29]
6) Covid specific therapy- Combination therapy for oral capsule of antiproteases Lopinavir/Ritonavir (200 mg/50 mg per capsule) along with nebulization with α-interferon inhalation (5 million IU in 2 mL of sterile water) twice daily has been the preferred and safe drug regimen in pregnancy with doubtful efficacy.[6,19] CDC as updated on 16 June 2020 recommends use of Remdesivir in hospitalized patients with severe Covid19.[30]
Combination of Hydroxychloroquine and Azithromycin (500 mg once a day) for 10 days has been believed to give virological cure on day 6 of treatment in 100% of treated patients in one study. Other drugs experimented till date are Chloroquine at a dose of 500 mg twice a day for 7 days. Oseltamivir 75 mg twice daily along with hydroxychloroquine for five days is another regime of trial in COVID-19 patients.[18,19] The current CDC guidelines as updated on 16 June 2020 recommends use of Hydroxychloroquine only for prophylaxis and does not to be given routinely for treatment of COVID-19 patients.
For pregnant women with symptoms routine antenatal visits need to be postponed until 7 days of start of symptoms provided symptoms (aside from persistent cough) worsens. patients are advised to keep check on their daily counts of foetal movement. For women who are self-quarantined due to any possible reasons and not developing any symptoms of COVID-19, next appointment should be after 14 days but if any woman did not turn up for more than 3 weeks of their scheduled visit must be contacted.
Any women previously tested negative for COVID-19 but develops with symptoms again she would be treated as COVID-19 suspected case.
Antenatal ultrasound services for foetal growth surveillance not to be done unless actually indicated and needs to be deferred for 14 days following recovery from acute illness.[9]
Timing and mode of delivery
The Covid block must have a separate delivery room and operation theatres along with neonatal resuscitation corners located 2 metres away from the delivery tables with negative pressure isolation for delivering suspected or confirmed COVID-19 pregnant women neonatologist must be present to attend the baby.[6,12]
The use of tocolytic drugs in patients with preterm labour is as such contraindicated and only to be given for 48 hours for steroid cover to attain foetal lung maturity. Beta-mimetic agents should be avoided in case of pulmonary involvement.[4] Inj. Betamethasone 12 mg intramuscularly followed by repeat dose after 24 hrs should be given for foetal lung maturity if patient < 34 weeks and having mild symptoms.[25] Low molecular weight heparin needs to be started unless imminent delivery is suspected within next 12 hours in all infected or suspected COVID-19 pregnant patients to avoid thromboembolism.[14,16] Oxygen saturation must be maintained above 94%, titrating oxygen therapy accordingly.[4,31]
The decision for timing and mode of delivery needs to be individualized depending upon period of gestational keeping, foetal wellbeing and associated obstetric and medical co-morbidities.[6,18] Patients with mild disease and in stable condition who are well responding to treatment and assuring foetal condition can be continued till term.[27] In critical cases possible benefits of immediate induction or surgical intervention needs to be weighed against the possible risk guided by proper assessment of patient condition, gestational age, available infrastructure, risk of exposure to health care personnel and the couple's wishes to save the life of mother and baby both by reducing extra metabolic load.[16,32]
Pregnant women with COVID-19 infection for trial of normal vaginal delivery should only be intervened for caesarean for obstetric indication with close surveillance of foetus using continuous electronic foetal monitoring or ultrasonography is suggested.[4,5] The second stage of labour should be cut short specially in those with respiratory involvement to prevent hypoxaemia and maternal exhaustion.[4] Epidural anaesthesia is preferred over spinal during emergency caesarean section to avoid the use of general anaesthesia.[4,18]
Test for newborn and breastfeeding and newborn care
There were few case reports from China pointing at postnatal transmission due to close contact.[10,33] Hence testing protocol for COVID-19 is advised only to those neonates who are born to mothers who have contracted the infection between duration of 14 days prior to delivery till 28 days post-delivery. If first report comes negative, testing should be repeated after 2 days.[6,15] Nasopharyngeal and throat swabs are the routinely testing specimens but in case of mechanical ventilation tracheal aspirate is collected in viral media abiding by all standard protocols followed by disposal at concerned laboratories.[6,34] Rectal swabs of sick neonates with prolonged admission are also collected in institution where facilities are available.[32] There is no documentation of transmission via breast milk directly though infection transmission risk is attributed to close contact between neonate and mother.[6,10,15,30,31,32,33,34,35] As breast milk confers immunological protection, in present scenario, its benefits outweigh risks of transmission, hence breastfeeding is allowed after taking precautionary measures like hand hygiene, mask wearing, milk pump disinfection, etc., before and after every feed with strict maintenance of 2 meters distance between the mother and neonate in the same room.[34,36] Expressed breast milk should be used if the mother is infected or suspected with pending reports and temporary separation is permitted.[30,34,35] A recent review article quoted that COVID-19 infection in pregnancy leads to increased risk in pregnancy complications such as preterm birth, PPROM, and may possibly lead to maternal death in rare cases.[37] Ivermectin becoming very popular in treating COVID-19 patients has teratogenic effect should be avoided in pregnancy.[38]
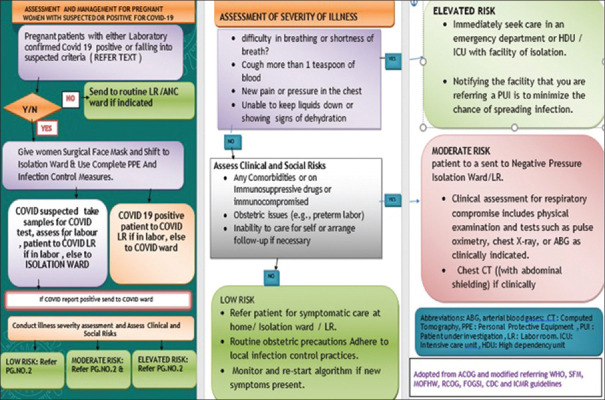
Assessment and management for pregnant women with suspected or positive COVID-19 is summarized in the following flowchart adopted from ACOG[39] and modified on the basis of RCOG, ICMR. WHO, &CDC guidelines [Figure 1].
Figure 1.
Assessment and management for pregnant women with suspected or positive COVID-19(flow chart)
Conclusion
The article has covered all the aspects of disease spread, prevention, testing and management of pregnant patients with mild to severe to critical disease but data are very limited and hence very difficult to accurately define clinical management strategies and needs to be constantly updated. More and more research work and data need to be published so as to revise the management protocol of COVID-19 positive pregnant women and newborn and to upgrade the patient care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I sincerely acknowledge and thank all the corona warriors with my slogan:
CORONA WARRIORS ARE INDISPENSABLE
A SINGLE LIFE IS NOT WORTH REPLACABLE
PERCOLATE IN YOU RIGHT DOFFING AND DONNING
BEAUTIFUL EARTH SHOULD NOT END UP MOURNING
References
- 1.Team NCPERE. Vital surveillances: The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China. China CDC. 2020;2:113–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-2019) situation report 163. July 1st, 2020. [Last accessed on 2020 Jul 3]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200327-sitrep-67-covid-19.pdf?sfvrsn=b65f68eb_4 .
- 4.Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. [Online] 2020. [Last accessed on 2020 Jul 03]. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-03-26-covid19-pregnancy-guidance.pdf . Cited on 2020 Mar 28.
- 5.Guan W-J, Ni Z-Y, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FOGSI GCPR, Good Clinical Practice recommendation on Pregnancy with Covid-19 Infection. [Online] 2020. [Last accessed on 2020 Jul 03]. Available from: https://www.fogsi.org/wp-content/uploads/covid19/fogsi_gcpr_on_pregnancy_with_COVID_19_version_1.pdf . Cited on 2020 Mar 28.
- 7.Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, Abdul-Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13329. doi: 10.1002/ijgo.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam CM, Wong SF, Leung TN, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–4. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, et al. Pregnant women with new coronavirus infection: A clinical characteristics and placental pathological analysis of three cases. Ultrasound Obstet Gynecol. 2020;55:724–5. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shek CC, Ng PC, Fung GP, Cheng FW, Chan PK, Peiris MJ, et al. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 12.Liang H, Acharya G. Novel corona virus disease (COVID-19) in pregnancy: What clinical recommendations to follow? Acta Obstet Gynecol Scand. 2020;99:439–42. doi: 10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- 13.Indian Council for Medical Research. COVID19 testing. [Online] [Last accessed on 2020 Jul 03]. Available from: https://icmr.nic.in/sites/ default/files/upload documents/20200320_covid19_ test_v3.pdf . Cited on 2020 Mar 28.
- 14.The WHOGlobal surveillance for COVID19 caused by human infection with COVID19 virus (Interim guidance 20 March 2020), Case definitions for surveillance. :1. [Google Scholar]
- 15.Government of India. Ministry of Health & Family Welfare, Directorate General of Health Services (EMR Division) [Last accessed on 2020 Apr 25];Revised Guidelines on Clinical Management of COVID-19. [Online] 2020 Mar 31; [Google Scholar]
- 16.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–82. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Global Surveillance for Human Infection with novel Coronavirus 2019. [Online] Available from: https://www.who.int/ publicationsdetail/globalsurveillanceforhumaninfectionwithnovelcoronavirus(2019ncov) Last cited on 2020 Mar 28.
- 18.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance, 13 March 2020. No. WHO/2019nCoV/clinical/2020.4. World Health Organization; 2020. [Google Scholar]
- 19.Center for Disease Control, USA. Coronavirus laboratory testing guidelines. [Online] Available from: https://www.cdc.gov/ coronavirus/2019nCoV/lab/guidelinesclinicalspecimens. html . Last cited on 2020 Mar 28.
- 20.The WHO – Advise on the use of point – of – care immunodiagnostic tests for COVID 19, scientific brief 8th April 2020 [Google Scholar]
- 21.ICMR Guidelines for private laboratories testing for COVID-19. [Online] Available from: https://icmr.nic.in/sites/default/files/whats_new/Noti (cation_ICMR_Guidelines_Private_Laboratories.pdf . Cited on 2020 Mar 28.
- 22.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. 10.1002/jmv.25727. doi: 10.1002/jmv.25727. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Gao Q, Wang T, Ke Y, Mo F, Jia R, et al. Evaluation of recombinant nucleocapsid and spice protein serological diagnosis of novel coronavirus disease 2019 (COVID-19) medxriv [Internet] 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.03.17.20036954v1 .
- 24.Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients medxriv [Internet] 2020. Available from: https://doi.org/10.1101/20200.03.13.20035428 . [DOI] [PMC free article] [PubMed]
- 25.Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. RCOG. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-04-17-coronavirus-covid-19-infection-in-pregnancy .pdf .
- 26.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coronavirus (COVID-19) and Pregnancy: What Maternal- Fetal Medicine Subspecialists Need to KNow. 2020;19 [Google Scholar]
- 28.Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect Dis [Internet] 2020;20:559–64. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical management of severe acute respiratory infection when COVID-19 is suspected [Internet] 2020. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected .
- 30.CDC. [Last accessed on 2020 Jul 3]. Available from: http://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcareguidance.html .
- 31.Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID- 19) Infection in Pregnancy. RCOG. Available from: http://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-24-coronavirus-covid-19-infection-in-pregnancy.pdf .
- 32.Poon LC, Yang H, Lee JC, Copel JA, Leung TY, Zhang Y, et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AAP. Available from: http://services.aap.org/en/pages/2019-novel-coronavirus-covid- 19-infections/#Clinical Guidance .
- 35.WHO. [Last accessed on 2020 Jul 3]. Available from: http://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf?sequence=1&isallowed=y .
- 36.Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID- 19) Infection in Pregnancy. RCOG. Available from: http://www.rcog.org.uk/globalassets/documents/guidelines/2020-04-09-coronavirus-covid-19-infection-in -pregnancy.pdf .
- 37.López M, Gonce A, Meler E, Plaza A, Hernández S, Martinez-Portilla RJ, et al. Coronavirus disease 2019 in pregnancy: A clinical management protocol and considerations for practice. Fetal Diagn Ther. 2020;47:519–28. doi: 10.1159/000508487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.India – Trial Site News [Internet] Ivermectin usage accelerates while the need for data is real: how about an ivermectin registry? 2020. May, Available from: https://www.trialsitenews.com/ivermectin-usage-accelerates-while-theneed-for-data-is-real-how-about-an-ivermectin-registry/ [Internet] Cited on 2020 Jul 19.
- 39.ACOG Publications, Obstetrics & Gynecology. 2020;135:989. doi: 10.1097/AOG.0000000000003763. doi: 10.1097/AOG.0000000000003763. [DOI] [PubMed] [Google Scholar]