Abstract
We aimed to explore the underlying mechanism of peripheral myelin protein 22 (PMP22) in the development of chronic myeloid leukemia (CML). The level of PMP22 expression in CD34+ cells isolated from CML patients’ bone marrow samples (BMMCs) and peripheral blood samples (PBMCs) was determined by RT-PCR. In addition, PMP22-siRNA and scrambled control siRNA were transfected into human CML cell line K562 with Lipofectamine 2000 reagent. Cell viability and apoptosis were, respectively, determined by MTT assay and flow cytometry. Besides, the level of caspase 3 and Bcl-xL was then detected using Western blot. The level of PMP22 expression in CML patients’ CD34+ cells isolated from both PBMCs and BMMCs was significantly higher than the control group. PMP22 expression in K562 cells was successfully knocked down by siRNA. MTT analysis showed that knockdown of PMP22 inhibited the proliferation of CML cells. Flow cytometry showed that knockdown of PMP22 promoted the apoptosis of CML cells. Besides, Bcl-xL expression markedly decreased, while the expression of caspase 3 in CML cells significantly increased after knockdown of PMP22 expression. Our findings indicate that high expression of PMP22 may promote cell proliferation and inhibit cell apoptosis via upregulation of Bcl-xL or inhibition of caspase 3 activation, and thus may contribute to the development of CML. PMP22 may serve as a novel therapeutic target for the treatment of CML.
Key words: Chronic myeloid leukemia (CML), Peripheral myelin protein 22 (PMP22), Proliferation, Apoptosis, Bcl-xL, Caspase 3
INTRODUCTION
Chronic myeloid leukemia (CML) is a disease of hematopoietic stem cells with distinct biological and clinical features (1). It is characterized by the presence of a BCR-ABL fusion gene, which is the result of a reciprocal translocation t(9;22)(q34;q11) known as the Philadelphia (Ph) chromosome (2). CML always presents in chronic phase, in which an elevated white blood cell count is observed due to the clonal expansion of predominantly mature myeloid cells (3). Moreover, CML is likely to progress from chronic phase through accelerated phase to blast crisis because of the absence of an effective therapy (3). The estimated risk of transformation to the blastic phase increases to up to 20–25% per year in diagnosis of 2 years later (4). Therefore, elucidation of the molecular mechanisms of CML pathogenesis will facilitate the development of an effective therapy.
The availability of a molecular-targeted therapy has been shown to profoundly change the management of CML (5). A growing number of BCR-ABL kinase inhibitors, such as imatinib and dasatinib, has been shown to be effective therapeutic agents for treatment of chronic phase CML (6,7). Despite recent advances in the treatment of early stage disease, blastic phase of CML with rapid expansion of therapy-refractory and differentiation-arrested blasts is still a therapeutic challenge (8), and exploring more specific targets for the treatment of this disease is warranted. Recently, peripheral myelin protein 22 (PMP22) is recognized as a key player in a variety of prevalent cancers. PMP22 has been demonstrated to regulate cell spreading and proliferation in breast cancer cells (9) and can serve as an independent prognostic factor for overall survival in patients with breast cancer (10). PMP22 is also observed to be involved in the neoplastic transformation process in the development of pancreatic cancer (11). In addition, PMP22 has been identified to regulate growth and survival of leukemia stem cells by oncogene cooperativity analysis (12). Despite these findings, the molecular mechanism by which PMP22 contributes to the development of CML remains unclear.
In the current study, we first determined the level of PMP22 expression in CD34+ cells isolated from human CML bone marrow samples (BMMCs) and peripheral blood samples (PBMCs). Then knockdown of PMP22 expression by siRNA was used to investigate the effects of PMP22 suppression on the proliferation and apoptosis of CML cell line K562. The expression levels of Bcl-xL and caspase 3 were also investigated. Our study sought to elucidate the underlying mechanism of PMP22 in the development of CML and provide new insight in exploring molecular targets for the treatment of this disease.
MATERIALS AND METHODS
Patient Sample Collection and CD34+ Cell Isolation
Human CML bone marrow samples (BMMC) and peripheral blood samples (PMMC) were obtained under protocols in accordance with the guidelines of the Department of Health and Human Services. The protocol met all requirements of the Declaration of Helsinki and was approved by the institutional review board at City of Hope. CML patients were in chronic phase and had not received imatinib mesylate (IM) treatment. Separation of mononuclear cells was performed using Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA). CD34+ cells were isolated as previously described (13). In brief, sorting of CD34+ fractions was performed according to expression of the following cell surface markers: common myeloid progenitors (CMPs), Lin−CD34+CD38+CD123+CD45RA−; megakaryocyte–erythrocyte progenitors (MEPs), Lin−CD34+CD38+CD123−CD45RA−; granulocyte–macrophage progenitors (GMPs), Lin−CD34+CD38+CD123lowCD45RA+; and hematopoietic stem cells (HSCs), Lin−CD34+CD38−.
Cell Culture and Plasmid Transfection
Human CML cell line K562 was purchased from the KeyGEN Company (China), which was cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco) at 37°C in humidified incubator with 5% CO2. In some experiments, PMP22 levels were decreased in cells by transiently transfecting with 75 pmol PMP22-siRNA (L-010616-00-0005; Dharmacon, Lafayette, CO, USA), and cells transfected with 75 pmol scrambled control siRNA (D-001206-13-05; Dharmacon) were set as a negative control. In our study, 24 h before transfection, cells were plated into six-well plates and grown until they were 50–80% confluent. PMP22-siRNA and scrambled control siRNA were then transfected into cells with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Meanwhile, cells without transfection treatment were set as control groups. Afterward, cells were incubated with normal media for 4 h and were harvested after 48 h. The level of PMP22 expression at mRNA and protein level was then quantified using RT-PCR and Western blot analysis, respectively.
RT-PCR Analysis
As instructed by the manufacturer, total RNA was first isolated using TRIzol® reagent (Invitrogen, Burlington, ON, Canada), and a spectrophotometer (NanoDrop 2000; Thermo Scientific, USA) was used to determine the quality and concentrations of RNA. Reverse transcription (RT) for synthesizing cDNA was then carried out with the QuantiTect Reverse Transcription Kit (QIAGEN, Valencia, CA). Analysis of gene expression was performed with fluorescent quantitative RT-PCR using QuantiTect™ SYBR® Green RT-PCR kit (QIAGEN, Solna, Sweden). The primers specific for PMP22 were as follows: forward: 5′-GCCACCATGATCCTGTCGAT-3′; reverse: 5′-CCCTTGGTGAGGGTGAAGAGT-3′. The following PCR conditions were used: 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 1 min and a final dissociation stage. Each sample was performed in triplicate, and the cycle threshold (CT) values were analyzed using a Roche LightCycler480 real-time PCR system (Applied Biosystems, Foster City, CA, USA). Finally, the relative mRNA expression level of PMP22 was calculated using the 2−ΔΔCT method according to the expression of the corresponding β-actin.
MTT Assay
Cell viability was determined using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, the cells of each group were respectively seeded on 96-well plates at cell density of 2 × l04 cells/well and incubated at 37°C in a humidified atmosphere with 5% CO2. After 0 h, 72 h, and 7 days of transfection, 20 μl of MTT solution (Sigma-Aldrich) was added to each well. All determinations were performed in triplicate. The plates continued to incubate for 4 h. After terminating the reaction, 200 µl of dimethyl sulfoxide (DMSO) was added to sufficiently solubilize the formazan crystals for 10 min. Viable cell numbers were determined by tetrazolium salt conversion to its formazan dye, and absorbance at 550 nm was measured with an enzyme-labeled instrument (Bio-Rad, USA).
Apoptosis Assay
Cell apoptotic rate was detected by flow cytometry analysis (FCM) using annexin V-fluorescein isothiocyanate (AnnexinV-FITC) Apoptosis Detection Kits (KeyGEN Biotech, Nanjing, China). In brief, cells (1 × l05 cells) were seeded in 24-well plates and resuspended in 1× binding buffer. Afterward, AnnexinV-FITC and propidium iodide (PI) were added to incubate for 15 min. Finally, apoptosis analysis was done using a flow cytometer (BD FACSAria; Becton-Dickinson, San Jose, CA, USA). A dual-color flow cytometric method was used to count the annexin V-positive cells as apoptotic cells.
Western Blot Analysis
After treatment as indicated, cells were collected and lysed using radioimmunoprecipitation assay (RIPA) buffer (Roche, Basel, Switzerland). After protein concentrations were measured using Bradford (Bio-Rad, Madrid, Spain), 40 µg total cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to PVDF membranes. After being blocked, the membrane was probed with specific primary antibodies overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The following primary antibodies were used: anti-PMP22 and anti-caspases-3 were purchased from Abcam (Cambridgeshire, UK), and anti-Bcl-xL was obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-GAPDH and horseradish peroxidase-conjugated second antibody were purchased from Santa Cruz Biotechnology. Bands in the membrane were developed by chemiluminescence method using a chemiluminescence ECL kit (GE Healthcare Biosciences, Pittsburgh, PA, USA), and the relative protein expression levels were determined based on GAPDH expression as a loading control. To further quantify the results, blots were analyzed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA).
Statistics Analysis
The normal distribution of all collected data was first analyzed using one-sample K-S test. Enumeration data were presented as the mean ± SD and analyzed by Student t-test (for two groups) or post hoc Tukey test in one-way ANOVA (for more than three groups) to test the comparison between groups. Statistical analyses were done using SPSS 17.0 statistical software and a value of p < 0.05 was considered to be statistically significant.
RESULTS
Analysis of the PMP22 Expression in CML Patients
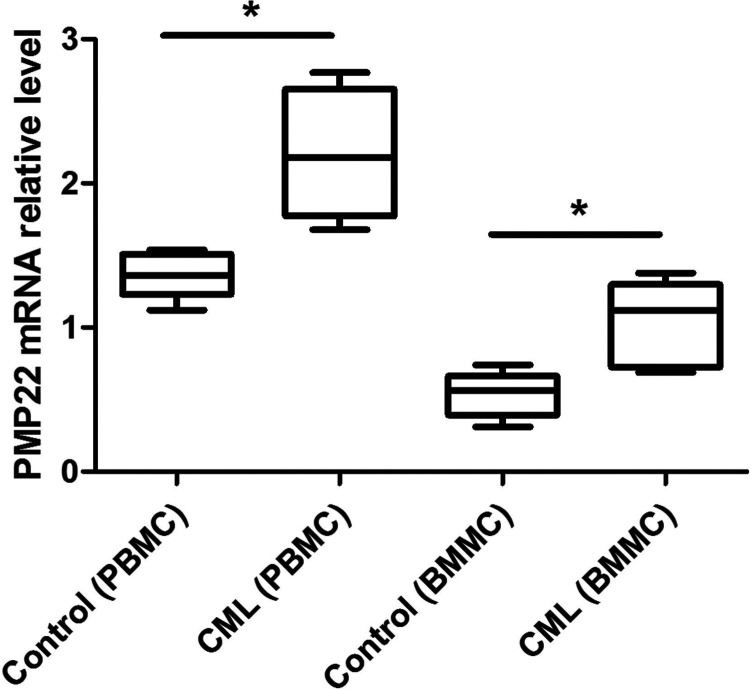
By analyzing the level of PMP22 expression in CD34+ cells isolated from PBMCs and BMMCs using RT-PCR analysis, we found that the level of PMP22 expression in CML patients’ CD34+ cells isolated from both PBMCs and BMMCs was significantly higher than the control group (p < 0.05) (Fig. 1).
Figure 1.
The expression of PMP22 expression in CD34+ cells isolated from CML bone marrow samples (BMMCs) and peripheral blood samples (PBMCs). Error bars indicate means ± SD. *Significant difference compared with blank group (p < 0.05).
PMP22 Expression Was Successfully Knocked Down by siRNA
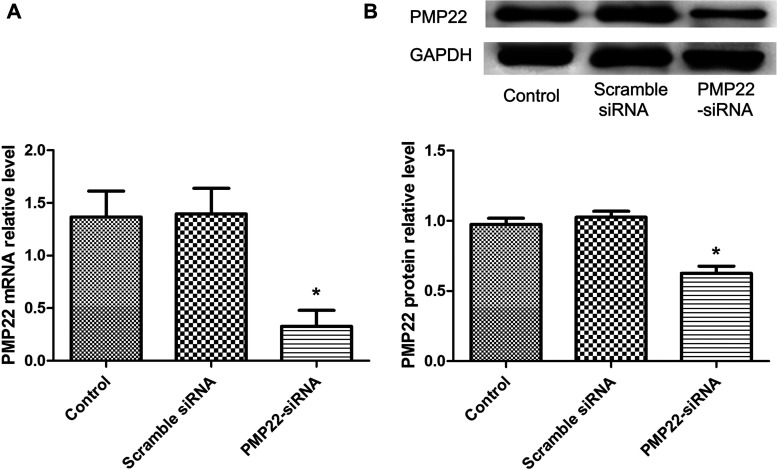
To verify whether PMP22 expression in CML cell line K562 was successfully knocked down by siRNA, RT-PCR and Western blot analyses were performed to determine (Fig. 2). The results showed that the expression of PMP22 was significantly decreased in the PMP22-siRNA group both in mRNA level and protein level compared with the control group (p < 0.05), while there was no significant difference between the scramble siRNA group and the control group (p > 0.05), indicating that PMP22 was successfully knocked down in our study.
Figure 2.
The expression level of PMP22 in CML cell line K562. (A) The expression level of PMP22 analyzed by RT-PCR. (B) The expression level of PMP22 analyzed by Western blot. The results showed that PMP22 expression was successfully knocked down by siRNA. Error bars indicate means ± SD. *Significant difference compared with blank group (p < 0.05).
Cell Viability Was Inhibited After Knockdown of PMP22 Expression
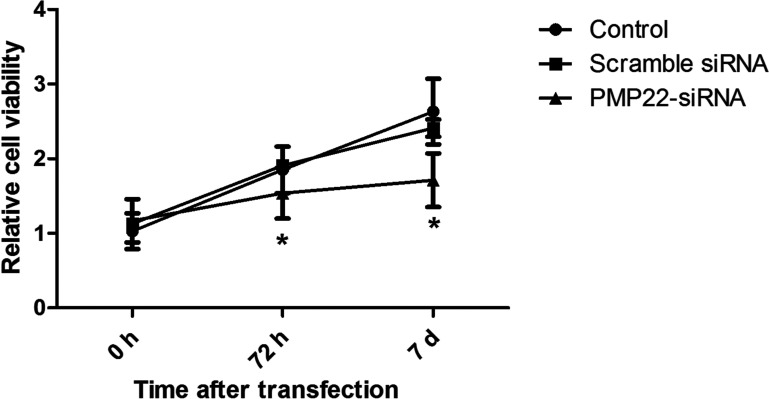
Figure 3 displays the cell viability in an experimental period of 7 days after transfection. The results showed that cell viability of the PMP22-siRNA group was significantly lower than the control group (p < 0.05), while no significant difference existed between the scramble siRNA group and the control group (p > 0.05), implying that knockdown of PMP22 expression could effectively inhibit the proliferation of CML cell line K562.
Figure 3.
MTT assay showed cell proliferation/viability of different groups. Error bars indicate means ± SD. *Significant difference compared with blank group (p < 0.05).
Cell Apoptosis Was Promoted After Knockdown of PMP22 Expression
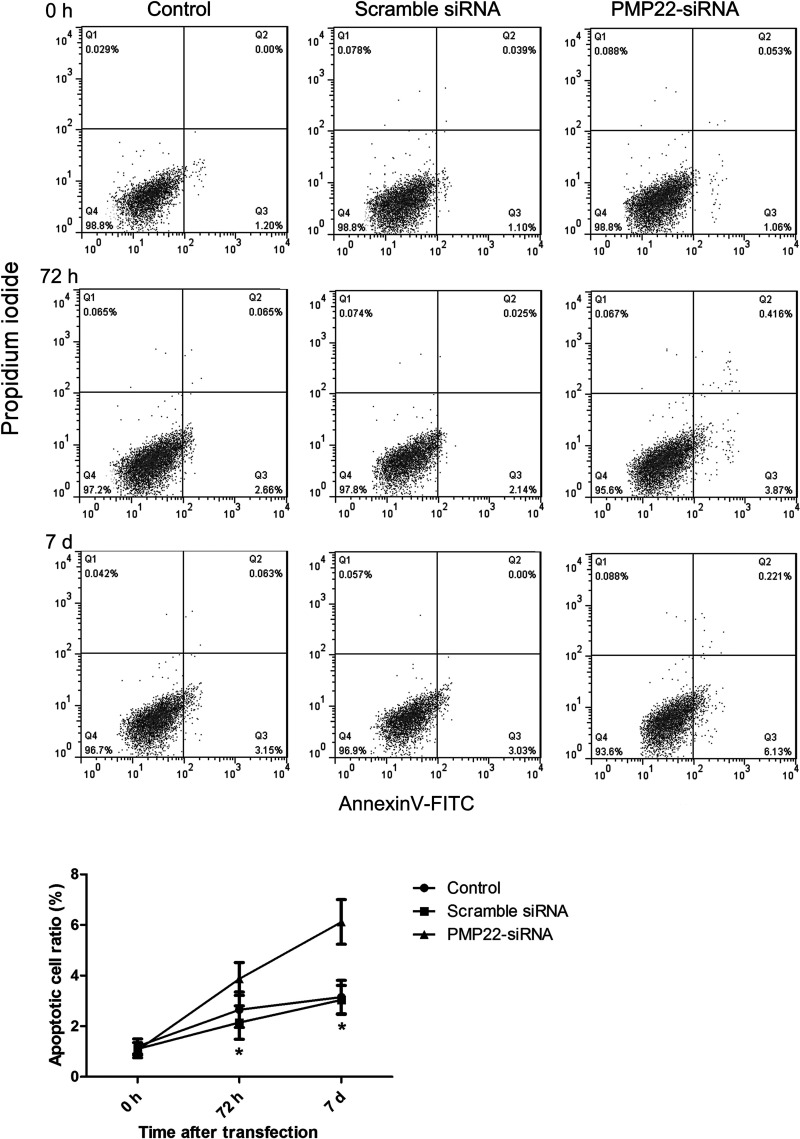
Flow cytometry analysis illustrated apoptotic cell ratio in an experimental period of 7 days after transfection. As shown in Figure 4, the apoptotic cell ratio of the PMP22-siRNA group significantly increased compared with the control group (p < 0.05), while transfection of scramble siRNA had no effect on the apoptotic cell ratio, confirming that knockdown of PMP22 expression could effectively promote the apoptosis of CML cell line K562.
Figure 4.
Flow cytometry displayed the cell apoptosis of different groups. Error bars indicate means ± SD. *Significant difference compared with blank group (p < 0.05).
Analysis of the Expression Levels of Apoptotic Proteins
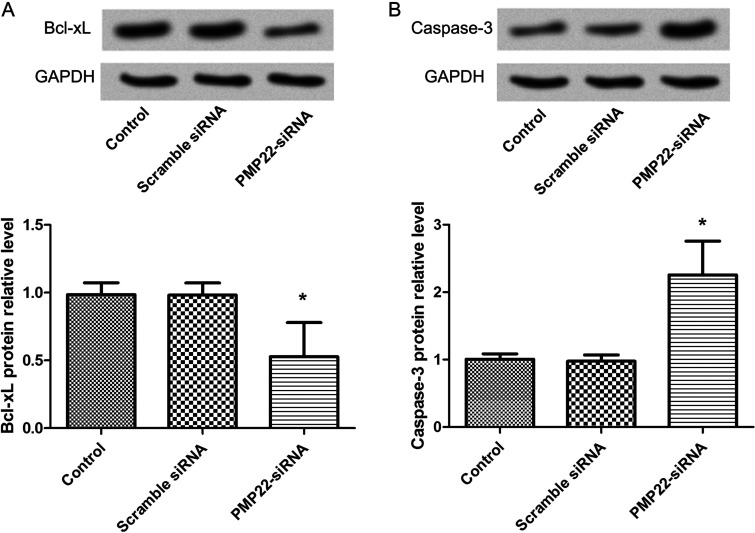
Western blot analysis showed decreased expression levels of antiapoptotic protein Bcl-xL and proapoptotic protein caspase 3 after cells were transfected with PMP22-siRNA (Fig. 5). The results showed that the expression of Bcl-xL expression of PMP22-siRNA was markedly decreased (p < 0.05) compared with the control group (Fig. 5A). However, expression of caspase 3 in the PMP22-siRNA group was significantly increased (p < 0.05) compared with the control group (Fig. 5B). There was no significant difference between the scramble siRNA group and the control group in the expression levels of Bcl-xL and caspase 3 (p > 0.05).
Figure 5.
Western blot analysis showed the expression levels of Bcl-xL (A) and caspase 3 (B). Error bars indicate means ± SD. *Significant difference compared with blank group (p < 0.05).
DISCUSSION
Elucidating the genetic events that contribute to the development of CML is an important step toward identifying effective and special targets for the management of CML. In the present study, we found that PMP22 was upregulated in CML patients’ CD34+ cells, which was in accordance with previous findings that PMP22 was frequently amplified in various cancers, such as breast cancer (10) and osteosarcoma (14). In addition, our results showed that knockdown of PMP22 expression significantly decreased the proliferation and induced apoptosis of CML cells. Besides, the expression of Bcl-xL markedly decreased, while the expression of caspase 3 in CML cells significantly increased after knockdown of PMP22 expression.
In breast cancer cells, PMP22 has been shown to be one mechanism for G3BP1-mediated cell growth regulation (9). Moreover, PMP22 is identified as differentially expressed genes between Fms-like tyrosine kinase 3 (Flt3) wild-type and Flt3-ITD subsets of acute promyelocytic leukemia, which is likely to be involved in growth regulation and in myelinization in the peripheral nervous system (15). In addition, PMP22 is found to interact with transforming growth factor-β (TGF-β) (16), and TGF-β has been proven to control cell proliferation (17). Also, a work of Blank and Karlsson indicated that TGF-β signaling can control a wide spectrum of biological processes, such as self-renewal and quiescence of hematopoietic stem cells (18). In our study, knockdown of PMP22 expression markly decreased the cell proliferation of CML K562 cells. In addition to the high expression of PMP22 in CML patients, we speculate that PMP22 may play a key role in promoting cell proliferation in the development of CML.
As another aspect of the present analysis, our results verified that knockdown of PMP22 expression could induce apoptosis of CML K562 cells. PMP22 is shown to target PERP (p53 apoptosis effector related to PMP22) and consequently activate p53 apoptosis signaling (19). Activation of p53 signaling can lead to cell apoptosis via activation of Bax, a Bcl-2 family member (19,20). Furthermore, high expression of PMP22 in premalignant and malignant pancreatic lesions is thought to be of importance for growth arrest, cell cycle stop and, consequently, apoptosis in fibroblasts (11). PMP22 was also observed to be highly expressed in CML patients in our study, and it is thus intriguing to speculate that PMP22 expression may induce cell apoptosis in the development of CML.
In studying PMP22’s effect on apoptosis, the expression levels of Bcl-xL and caspase 3 were determined to decrease after knockdown of PMP22 in our study. Bcl-xL, an antiapoptotic Bcl-2 family protein, can protect cancer cells from p53-mediated apoptosis and plays a central role in the regulation of the apoptotic pathway (21). Several Bcl-2 family antagonists, obatoclax mesylate (GX15-070) and ABT737, are also served as potential therapies for CML (22,23). Concurrent upregulation of Bcl-xL and Bcl2a1 is also reported to induce higher resistance to ABT-737 in chronic lymphocytic leukemia (24). It can therefore be speculated that PMP22 may induce cell apoptosis in the pathogenesis of CML, and cotargeting PMP22 with Bcl-xL may have feasibility and application prospects in cancer therapy. In addition, caspase 3, a widely accepted hallmark of programmed cell death, is a key mediator of mitochondrial events of apoptosis (25). Activation of caspase 3 may be a key mechanism to mediate berberine-induced apoptosis of human leukemia HL-60 cells (26). Caspase 3 activation is also implicated in the antitumor effects of celecoxib on K562 leukemia cells (27). Han et al. demonstrated that sanguinarine could induce apoptosis in human leukemia U937 cells via downregulation of Bcl-2 and activation of caspase 3 activation (28). In our study, Bcl-xL expression markedly decreased, while the expression of caspase 3 in CML cells significantly increased after knockdown of PMP22 expression. Although the apoptotic mechanism of PMP22 has not been fully discussed, we speculate that high expression of PMP22 may inhibit apoptosis in human CML cells via upregulation of Bcl-xL or inhibition of caspase 3 activation.
In conclusion, our findings indicate that high expression of PMP22 may promote cell proliferation and inhibit cell apoptosis via upregulation of Bcl-xL or inhibition of caspase 3 activation, thus contributing to the development of CML. PMP22 may serve as a novel therapeutic target for the treatment of CML. Our efforts provide a theoretical basis and new insights into the treatment of CML.
REFERENCES
- 1. Hehlmann R.; Hochhaus A.; Baccarani M. Chronic myeloid leukaemia. Lancet 370:342–350; 2007. [DOI] [PubMed] [Google Scholar]
- 2. Nowell P.; Hungerford D. A minute chromosome in human chronic granulocytic leukemia. In: Harper P. S., ed. Landmarks in medical genetics: Classic papers with commentaries. New York: Oxford University Press; 2004:103. [Google Scholar]
- 3. Radich J. P.; Dai H.; Mao M.; Oehler V.; Schelter J.; Druker B.; Sawyers C.; Shah N.; Stock W.; Willman C. L. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA 103:2794–2799; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quintás-Cardam,a A.; Cortes J. Management of patients with resistant or refractory chronic myelogenous leukemia. Oncology (Williston Park) 22:430; 2008. [PubMed] [Google Scholar]
- 5. Hehlmann R.; Berger U.; Hochhaus A. Chronic myeloid leukemia: A model for oncology. Ann. Hematol. 84:487–497; 2005. [DOI] [PubMed] [Google Scholar]
- 6. Deininger M.; Buchdunger E.; Druker B. J. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105:2640–2653; 2005. [DOI] [PubMed] [Google Scholar]
- 7. Kantarjian H.; Shah N. P.; Hochhaus A.; Cortes J.; Shah S.; Ayala M.; Moiraghi B.; Shen Z.; Mayer J.; Pasquini R. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 362:2260–2270; 2010. [DOI] [PubMed] [Google Scholar]
- 8. Zheng C.; Li L.; Haak M.; Brors B.; Frank O.; Giehl M.; Fabarius A.; Schatz M.; Weisser A.; Lorentz C. Gene expression profiling of CD34+ cells identifies a molecular signature of chronic myeloid leukemia blast crisis. Leukemia 20:1028–1034; 2006. [DOI] [PubMed] [Google Scholar]
- 9. Winslow S.; Leandersson K.; Larsson C. Regulation of PMP22 mRNA by G3BP1 affects cell proliferation in breast cancer cells. Mol. Cancer 12:156; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tong D.; Heinze G.; Pils D.; Wolf A.; Singer C. F.; Concin N.; Hofstetter G.; Schiebel I.; Rudas M.; Zeillinger R. Gene expression of PMP22 is an independent prognostic factor for disease-free and overall survival in breast cancer patients. BMC Cancer 10:682; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J.; Kleeff J.; Esposito I.; Kayed H.; Felix K.; Giese T.; Büchler M. W.; Friess H. Expression analysis of PMP22/Gas3 in premalignant and malignant pancreatic lesions. J. Histochem. Cytochem. 53:885–893; 2005. [DOI] [PubMed] [Google Scholar]
- 12. Ashton J. M.; Balys M.; Neering S. J.; Hassane D. C.; Cowley G.; Root D. E.; Miller P. G.; Ebert B. L.; McMurray H. R.; Land H. Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells. Cell Stem Cell 11:359–372; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu S.; Holtz M.; Gupta M.; Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood 103:3167–3174; 2004. [DOI] [PubMed] [Google Scholar]
- 14. van Dartel M.; Hulsebos T. J. Characterization of PMP22 expression in osteosarcoma. Cancer Genet. Cytogen. 152:113–118; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Marasca R.; Maffei R.; Zucchini P.; Castelli I.; Saviola A.; Martinelli S.; Ferrari A.; Fontana M.; Ravanetti S.; Torelli G. Gene expression profiling of acute promyelocytic leukaemia identifies two subtypes mainly associated with flt3 mutational status. Leukemia 20:103–114; 2006. [DOI] [PubMed] [Google Scholar]
- 16. Hagedorn L.; Suter U.; Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development 126:3781–3794; 1999. [DOI] [PubMed] [Google Scholar]
- 17. Huang S. S.; Huang J. S. TGF-β control of cell proliferation. J. Cell. Biochem. 96:447–462; 2005. [DOI] [PubMed] [Google Scholar]
- 18. Blank U.; Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood 125:3542–3550; 2015. [DOI] [PubMed] [Google Scholar]
- 19. Attardi L. D.; Reczek E. E.; Cosmas C.; Demicco E. G.; McCurrach M. E.; Lowe S. W.; Jacks T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Gene Dev. 14:704–718; 2000. [PMC free article] [PubMed] [Google Scholar]
- 20. Oltval Z. N.; Milliman C. L.; Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 74:609–619; 1993. [DOI] [PubMed] [Google Scholar]
- 21. Bharatham N.; Chi S.-W.; Yoon H. S. Molecular basis of Bcl-XL-p53 interaction: Insights from molecular dynamics simulations. PloS One 6:e26014; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Brien S. M.; Claxton D. F.; Crump M.; Faderl S.; Kipps T.; Keating M. J.; Viallet J.; Cheson B. D. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 113:299–305; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore V. D. G.; Brown J. R.; Certo M.; Love T. M.; Novina C. D.; Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 117:112; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vogler M.; Butterworth M.; Majid A.; Walewska R. J.; Sun X.-M.; Dyer M. J.; Cohen G. M. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 113:4403–4413; 2009. [DOI] [PubMed] [Google Scholar]
- 25. Lakhani S. A.; Masud A.; Kuida K.; Porter G. A.; Booth C. J.; Mehal W. Z.; Inayat I.; Flavell R. A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 311:847–851; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin C.-C.; Kao S.-T.; Chen G.-W.; Ho H.-C.; Chung J.-G. Apoptosis of human leukemia HL-60 cells and murine leukemia WEHI-3 cells induced by berberine through the activation of caspase-3. Anticancer Res. 26:227–242; 2006. [PubMed] [Google Scholar]
- 27. Zhang G. S.; Liu D. S.; Dai C. W.; Li R. J. Antitumor effects of celecoxib on K562 leukemia cells are mediated by cell-cycle arrest, caspase-3 activation, and downregulation of Cox-2 expression and are synergistic with hydroxyurea or imatinib. Am. J. Hematol. 81:242–255; 2006. [DOI] [PubMed] [Google Scholar]
- 28. Han M. H.; Yoo Y. H.; Choi Y. H. Sanguinarine-induced apoptosis in human leukemia U937 cells via Bcl-2 downregulation and caspase-3 activation. Chemotherapy 54:157–165; 2008. [DOI] [PubMed] [Google Scholar]