Abstract
Despite the efficacy of fluoropyrimidines and oxaliplatin-based chemotherapy for patients, this treatment leads to significant patient inconvenience, toxicity, and cost. This study aims to validate a nontoxic agent, curcumin, to the current chemotherapeutic regimen. In in vitro experiments, curcumin induced apoptosis in gastric cancer cell line BGC-823. Synergistic antitumor effects of curcumin were observed in combination with 5-fluorouracil (5-FU) and oxaliplatin. These effects were accompanied by downregulation of the expression of Bcl-2 protein and mRNA and upregulation of the expression of Bax and caspase 3, 8, and 9. In addition, the in vivo study showed that the combination of curcumin and 5-FU/oxaliplatin exhibited potent growth inhibition of BGC-823 xenograft tumors. Furthermore, compared with the control group, no significant difference was observed in the body weight of curcumin-treated nude mice. In conclusion, curcumin may act synergistically with the chemotherapeutic regimen FOLFOX in gastric cancer in vitro and in vivo by inducing apoptosis via Bcl/Bax–caspase 8,9–caspase 3 pathway.
Key words: Gastric cancer, Curcumin, FOLFOX, Apoptosis, Chemotherapy
INTRODUCTION
Postoperative adjuvant chemotherapy with 5-fluorouracil (5-FU) and leucovorin (LV) for patients with upper and lower gastrointestinal tract malignancies demonstrated an improvement in patient outcome (1,2) and became the standard of care in the early 1990s. Results with oxaliplatin combinations have been more successful. The Multicenter International Study of Oxaliplatin/5-FU in the Adjuvant Treatment of Colon Cancer (MOSAIC) showed that a significant improvement in 3-year DFS and a 23% reduction in relative risk of recurrence were observed for the oxaliplatin-containing regimen (3,4). Therefore, oxaliplatin in combination with 5-FU has become integrated into the standard systemic treatment for tumors of the gastrointestinal tract (5).
Despite the efficacy of fluoropyrimidines and oxaliplatin-based chemotherapy for patients, these treatments lead to significant patient inconvenience, toxicity, and cost. Particularly, oxaliplatin-induced cumulative dose-dependent neurotoxicity is clinically relevant (3,6). However, validation of a nontoxic agent to the current chemotherapeutic regimen not only enhances the treatment effect but also reduces the toxicity of these drugs.
Turmeric is a spice made from grinding the roots of the Curcuma longa plant. Its pharmacological safety is accepted, considering that it has been consumed as a coloring and flavoring additive for centuries in south and southeast tropical Asia, at doses up to 100 mg/day (7,8). Curcumin has been isolated and identified as a major active component derived from turmeric (9). Research over the last few decades has shown that curcumin blocks the transformation, proliferation, and invasion of tumor cells (10,11). In particular, it has been recently demonstrated that curcumin, in combination with FOLFOX, causes greater growth inhibition of colon cancer cells than each agent/regimen alone (12). However, research on whether curcumin alone or in combination with FOLFOX would affect gastric cancer cells is scarce.
The focus of this article is to explore the synergistic antitumor effect of curcumin and a chemotherapeutic regimen (FOLFOX) against gastric cancer in vitro and in vivo. The possible mechanism of the synergism focusing on the regulation of caspase-dependent apoptosis pathway, which is regulated by Bcl-2 protein family, will also be illustrated.
MATERIALS AND METHODS
Cell Line, Mice, and Materials
Human gastric cancer cell line BGC-823 was obtained from cell resource center of Shanghai Biological Sciences Institute (Chinese Academy of Sciences, Shanghai, China).Cells were maintained in RMPI-1640 (Beyotime) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin (Beyotime), and 100 µg/ml streptomycin (Beyotime). The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2.
Male BALB/c athymic nude mice (5 weeks old) were purchased from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). The protocol for the animal experiment was approved by the Institutional Animal Committee of Wenzhou University. All animals received care in accordance to the Guide for the Care and Use of Laboratory Animals [Permit No.: SYXK (zhe) 2010-0150].
5-FU, oxaliplatin, and curcumin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8 kit) and Caspase activity assay kits were purchased from Beyotime (Haimen, China). Annexin V-FITC Apoptosis Kit was purchased from Keygen Biotech (Nanjing, China).
Cell Proliferation Assay
Cells were plated in 96-well plates (Falcon; Becton Dickinson, Franklin Lakes, NJ, USA) at 104 cells per well. Twenty-four hours later, incubation was continued for another 36 h or 48 h in the absence (control) or presence of different testing agents as described in the legends to the figures. At the end of the 36-h or 48-h incubation period, a CCK-8 solution was added to each well (10 µl) and incubated for 1 h at 37°C. The intensity of the color developed was measured at a wavelength of 570 nm. The data expressed was the optical density (OD), which is the reflection of number of live cells. All assays were performed with five replicates.
Annexin V-FITC/PI Staining Experiment
Quantification of apoptosis cells was performed using Annexin V-FITC/propidium iodide (PI) apoptosis detection kit. After 36 h of incubation, cells (5 × 105) were suspended in 500 µl binding buffer. Subsequently, 5 µl Annexin V-FITC and 5 µl PI were added and incubated for 5–15 min at room temperature, with protection from light. Detection of viable cells (Annexin V-FITC negative, PI negative), apoptotic cells (Annexin V-FITC positive, PI negative), and (Annexin V-FITC positive, PI positive) was performed by using a FACSCalibur (Becton Dickinson).
Caspase Assays
The remaining cells (2 × 105/cells/hole) were inoculated in six-well plates and subsequently grouped the same way as described above for Annexin V-FITC/PI staining experiment. Six-well plates were put in an incubator for 6 h (caspase 8, caspase 9 tests) or 24 h (caspase 3 test), respectively. The cells of each hole were collected and lysed for 15 min on ice in 50-µl lysis buffer provided by the kit. After centrifugation (18,000 revolutions/min at 4°C for 10 min), the supernatants were collected. The protein concentration was measured using the Bradford method (13). The coupling of the substrates (Ac-DEVD-pNA, Ac-IETD-pNA, Ac-LEHD-pNA) were added to the supernatant (adjusted final concentration to 3 mg/ml) and then incubated at 37°C for 2 h. The absorbance was measured at a wavelength of 405 nm. Different concentrations of pNA provided by the kit were used to provide a standard curve under the same conditions. Finally, the unit activity of caspase 3, 8, and 9 was calculated according to instructions provided by the manufacturer.
In Vivo Therapeutic Efficacy in Established Tumors
We injected 1 × 107 BGC-823 cells subcutaneously into each anterior flank region of nude mice. Treatment was started when the injected cell mass reached a mean volume of 200 mm3. After the tumor established, the mice were randomized into four groups (n = 6 per group) as follows: curcumin alone, FOLFOX alone, curcumin plus FOLFOX, and control (saline). Treatment was started on the same day of initial randomization. Curcumin (10 mg/kg) was administered everyday intraperitoneally (IP) whereas 5-FU (33 mg/kg) was injected IP twice weekly. Oxaliplatin (10 mg/kg IP) was injected once during the treatment course (14). The mice were killed, and the tumors were excised after 14 days of treatment. The greatest tumor dimensions were measured serially with calipers, and tumor volume was calculated using the following formula: tumor volume (mm3) = (width2 × length)/2 (15).
Statistical Analysis
All quantitative assays were performed in triplicate. The results are expressed as means ± SD. The statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) or Dunnett’s T3 test. Statistical significance was accepted at p < 0.05.
RESULTS
Curcumin Enhanced the Effects of 5-Fluorouracil/Oxaliplatin in Inhibiting Gastric Cancer Cell Proliferation
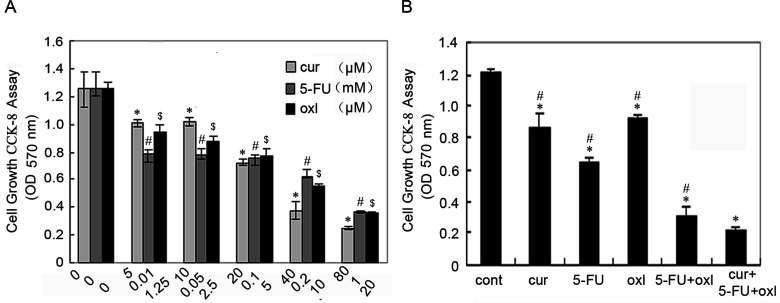
A dose–response study was performed with curcumin, 5-FU, and oxaliplatin in BGC-823 gastric cells. The three agents inhibited growth of BGC-823 in a dose-dependent manner (Fig. 1A). At the end of the 48-h incubation period, the half maximal (50%) inhibitory concentration (IC50) of curcumin, 5-FU, and oxaliplatin against BGC-823 cells were approximately 10 µM, 0.1 mM, and 5 µM, respectively (Fig. 1A); these concentrations were used in all subsequent experiments.
Figure 1.
Cell growth inhibition (CCK-8 assay) at 48 h in gastric cancer BGC-823 cells with the indicated group. Values represent the mean ± SEM of five observations. (A) Dose-dependent inhibition of growth of BGC-823 cells in response to curcumin, 5-FU, and oxaliplatin. *,#,$Statistically significant values compared to control (p < 0.01). (B) Cell growth inhibition of BGC-823 cells with curcumin (10 µM), 5-FU (0.1 mM), oxaliplatin (5 µM), FOLFOX [5-FU (0.1 mM) and oxaliplatin (5 µM)], or FOLFOX + curcumin. *Statistically significant values compared to control (p < 0.01); #statistically significant values compared to FOLFOX + curcumin (p < 0.01).
The next step of experiments was performed to determine whether curcumin would enhance the effects of the chemotherapeutic agents FOLFOX in inhibiting gastric cancer cell proliferation. As shown in Figure 1B, at the end of the 36-h incubation period, all agent groups were effective in significantly inhibiting the growth of BGC-823 cells, when compared with the control group. Curcumin together with FOLFOX caused further inhibition of growth, when compared with the curcumin, 5-FU, oxaliplatin, or FOLFOX group.
Assessment of Apoptosis on BGC-823 Cells
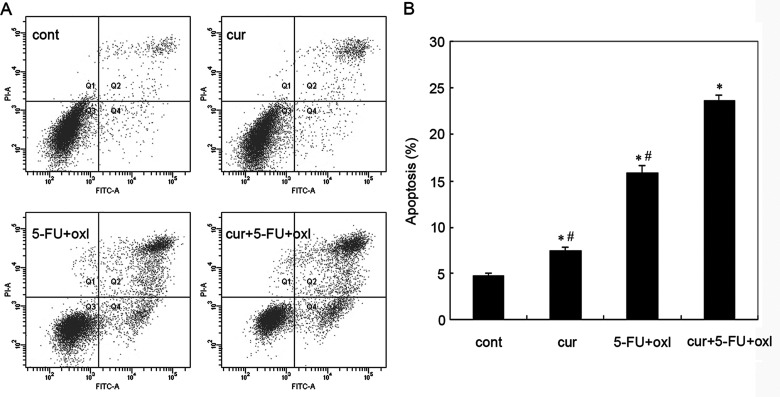
Apoptotic cell death was identified by flow cytometry analysis of Annexin V-FITC-stained apoptotic cells. The results demonstrated that the apoptosis of BGC-823 cells was observed after treatment with 10 µM curcumin, FOLFOX (0.1 mM 5-FU + 5 µM oxaliplatin), and the three-drug combinations, respectively. We also observed that combination of curcumin and FOLFOX caused a marked induction of apoptosis of BGC-823 cells, compared with curcumin or the FOLFOX group (Fig. 2). As shown in Figure 2B, the apoptotic populations of curcumin, FOLFOX, and the three-drug combination group were about 7.4%, 15.8% and 23.6%, respectively. Taken together, the results suggested that curcumin may act synergistically with the chemotherapeutic regimen of gastric cancer.
Figure 2.
Assessment of apoptosis of BGC-823 cells treated with curcumin (10 µM), FOLFOX [5-FU (0.1 mM) and oxaliplatin (5 µM)], or FOLFOX + curcumin. (A) Flow cytometric analysis of each group of BGC-823 cells stained with Annexin V-FITC and PI. Representative dot plots illustrating apoptotic status in BGC-823 cells. (B) The percentage of BGC-823 cell apoptosis. Data are expressed as mean% ± SD of each group of cells from three separate experiments. *Statistically significant values compared to control (p < 0.01); #statistically significant values compared to FOLFOX + curcumin (p < 0.01).
Apoptosis Induced by Curcumin and FOLFOX Was in Part Independent of Caspase Activation
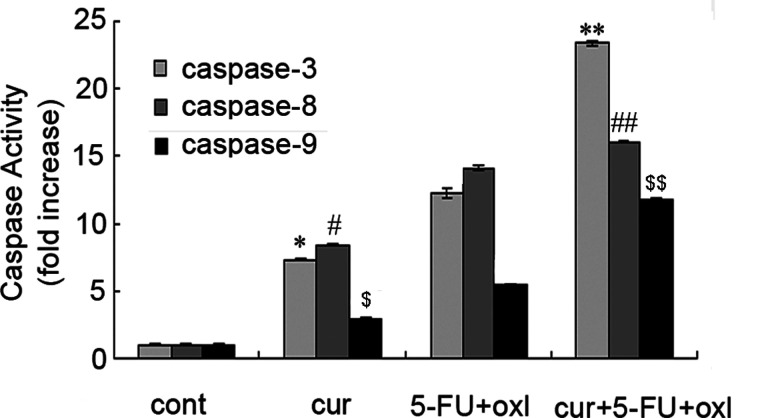
To investigate the apoptotic mechanisms in cells treated with curcumin and FOLFOX, caspases 3, 8, and 9 activity was assayed using the caspase activity assay kits. As shown in Figure 3, caspases 8 and 9 were activated after treated for 6 h, whereas caspase 3 for 24 h. In comparison with the control, caspase 3, 8, and 9 levels were found to be significantly (p < 0.01) higher in curcumin, FOLFOX, and the three-drug combination group. Compared to the three-drug combination group, caspase 3, 8, and 9 activity was found to be significantly (p < 0.01) lower in the FOLFOX group. As a result, it was observed that curcumin had an enhanced effect on the BGC-823 cells by increasing caspase 3, 8, and 9 activity, which are the precursors of apoptosis (Fig. 3).
Figure 3.
Apoptosis induced by curcumin and FOLFOX is in part independent of caspase activation. BGC-823 cells were incubated with curcumin (10 µM), FOLFOX [5-FU (0.1 mM) and oxaliplatin (5 µM)], or FOLFOX + curcumin for 6 or 24 h. The caspase 3/8/9 activity of cell extracts was measured by caspase 3/8/9 activity assay kit. A representative result of three experiments is presented. *,#,$p < 0.01 compared to control; **,##,$$p < 0.01 compared to FOLFOX.
In Vivo Antitumor Effects of Curcumin Alone or in Combination With FOLFOX
These effects were analyzed in a BGC-823 xenograft tumor model based on nude mice (immunosuppressed animals). Curcumin-treated and control mice did not show any evidence of significant side effects, and their body weights did not differ from each other. Also, there was no significant body weight loss between FOLFOX-treated and curcumin plus FOLFOX mice (Fig. 4A). The combination of curcumin and FOLFOX significantly inhibited the growth of xenograft tumors compared with the FOLFOX mice (Fig. 4B).
Figure 4.
The effect of curcumin and FOLFOX in a BGC-823 nude mice xenograft tumor model. (A) The body weights of the curcumin-treated mice were indistinguishable from those of saline controls, whereas FOLFOX treatment reduced body weight (p < 0.01). (B) Treatment was administered intraperitoneally when BGC-823 cell tumors (subcutaneously in the flank) reached an average volume of 200 mm3. Semiweekly assessment of tumor volume showed that administration of curcumin, FOLFOX, and curcumin plus FOLFOX groups inhibited BGC-823 tumor growth in nude mice. *p < 0.01 compared with another group. Each value represents the mean ± SE.
DISCUSSION
Gastric cancer is the second most common cause of cancer-related death worldwide (16). More than 50% of patients undergo surgery, but even after a curative resection, 60% of patients relapse locally or with distant metastases (17). 5-FU or 5-FU plus oxaliplatin (FOLFOX) remains the backbone of gastric cancer chemotherapeutics, but with extensive side effects and multidrug resistance in some cases (18). Therefore, validation of a nontoxic agent to the current chemotherapeutic regimen is vitally important. The numerous investigations and exhaustive studies conducted over the last few decades show that curcumin has strong therapeutic potential against a variety of cancers (19). It is also becoming increasingly evident that addition of nontoxic curcumin can improve the efficacy of colon cancer chemotherapy without any additional toxicity (12,20–22). Our current data further demonstrated that curcumin either alone or together with FOLFOX could be effective in eliminating gastric cancer cells in vitro and in vivo.
5-FU is a pyrimidine analog (23) transformed inside the cell into different cytotoxic metabolites, which are then incorporated into DNA and RNA, finally inducing cell cycle arrest and apoptosis. Oxaliplatin functions by forming both inter- and intrastrand cross links in DNA (24) to prevent DNA replication and transcription, resulting in apoptosis. The major mechanism of curcumin inhibiting cancer cell proliferation is also inducing apoptosis (25). The flow cytometry analysis results showed that combination of curcumin and FOLFOX caused a marked induction of apoptosis of BGC-823 cells, compared with curcumin or FOLFOX group.
Caspase and Bcl-2 families play an important role in the process of apoptosis. Caspases or cysteine-dependent aspartate-directed proteases are a family of cysteine proteases (26). It is noteworthy that the activation of caspase 3, 8, and 9 plays a central role in early apoptosis, regulated by various factors, including the Bcl-2 protein family (27). Moreover, the mitochondria-mediated apoptotic pathway, regulated by members of the Bcl-2 family (28), is dependent on the balance of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax. Previous studies have indicated that curcumin shifts the equilibrium of Bcl-2 family members toward apoptosis and elevates the expression of Bax and procaspases 3, 8, and 9 (29). In our previous study, we showed that curcumin/FOLFOX combination synergistically reduced the Bcl-2/Bax protein ratio, making them more susceptible to apoptosis (30). Additionally, the current study observed an obvious activation of caspase 3, 8, and 9 in curcumin/FOLFOX group. All these data indicated that the molecular mechanisms involved seem to be relevant to the Bcl/Bax–caspase 8,9–caspase 3 pathway.
In conclusion, our data suggest that curcumin by itself or in combination with the conventional gastrointestinal cancer chemotherapeutic regimen FOLFOX could be an effective therapeutic strategy to eliminate gastric cancer cells by inducing apoptosis, in which the Bcl/Bax–caspase 8,9–caspase 3 pathway may be involved through reducing the Bcl-2/Bax ratio and activating caspase 3, 8, and 9. Further studies are required to understand the molecular mechanism of synergism between curcumin and FOLFOX.
ACKNOWLEDGMENTS
This study was sponsored by a grant of Wenzhou City Science and Technology Plan projects (Y20140105, Y20140111), College Scientific Research Incubator Project of the First Affiliated Hospital of Wenzhou Medical University (FHY2014004).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. O’Connell M. J.; Mailliard J. A.; Kahn M. J.; Macdonald J. S.; Haller D. G.; Mayer R. J.; Wieand H. S. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J. Clin. Oncol. 15(1):246–250; 1997. [DOI] [PubMed] [Google Scholar]
- 2. Wolmark N.; Rockette H.; Fisher B.; Wickerham D. L.; Redmond C.; Fisher E. R.; Jones J.; Mamounas E. P.; Ore L.; Petrelli N. J. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J. Clin. Oncol. 11(10):1879–1887; 1993. [DOI] [PubMed] [Google Scholar]
- 3. André T.; Boni C.; Mounedji-Boudiaf L.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Zaninelli M.; Clingan P.; Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350(23):2343–2351; 2004. [DOI] [PubMed] [Google Scholar]
- 4. De Gramont A.; Boni C.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Bonetti A.; Clingan P.; Lorenzato C.; André T. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. Paper presented at: ASCO Annual Meeting Proceedings. J. Clin. Oncol. 20(Suppl. 18); 2007. [Google Scholar]
- 5. Hermann R.; Rave-Fränk M.; Pradier O. Combining radiation with oxaliplatin: A review of experimental results. Cancer Radiother. 12(1):61–67; 2008. [DOI] [PubMed] [Google Scholar]
- 6. André T.; Boni C.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Bonetti A.; Clingan P.; Bridgewater J.; Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 27(19):3109–3116; 2009. [DOI] [PubMed] [Google Scholar]
- 7. Bagchi A.; Ummul S.; Rao K.; Oduse Kayode A.; Idowu Micheal A.; Adegbite Adefolawe A.; Heravi H. M.; Ishak M. B.; Abdullah A. M. H.; Mahvi A. H. Extraction of curcumin. IOSR J. Environ. Sci. Toxicol. Food Technol. 1(3):1–16; 2012. [Google Scholar]
- 8. Ramsewak R.; DeWitt D.; Nair M. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine 7(4):303–308; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal B. B.; Kumar A.; Aggarwal M. S.; Shishodia S. Curcumin derived from turmeric (Curcuma longa): A spice for all seasons. In: Phytopharmaceuticals in cancer chemoprevention. Boca Raton, FL: CRC Press LLC; 2005:349–387. [Google Scholar]
- 10. Kunnumakkara A. B.; Anand P.; Aggarwal B. B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 269(2):199–225; 2008. [DOI] [PubMed] [Google Scholar]
- 11. Thangapazham R. L.; Sharma A.; Maheshwari R. K. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 8(3):E443–E449; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel B. B.; Sengupta R.; Qazi S.; Vachhani H.; Yu Y.; Rishi A. K.; Majumdar A. P. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer. 122(2):267–273; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Kruger N. J. The Bradford method for protein quantitation. In: Walker J. M., ed. The protein protocols handbook. Totowa, NJ: Humana Press; 2002:15–21. [Google Scholar]
- 14. Cusack J. C. Jr.; Liu R.; Xia L.; Chao T. H.; Pien C.; Niu W.; Palombella V. J.; Neuteboom S. T.; Palladino M. A. NPI-0052 enhances tumoricidal response to conventional cancer therapy in a colon cancer model. Clin. Cancer Res. 12(22):6758–6764; 2006. [DOI] [PubMed] [Google Scholar]
- 15. Naito S.; von Eschenbach A. C.; Giavazzi R.; Fidler I. J. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 46(8):4109–4115; 1986. [PubMed] [Google Scholar]
- 16. Jemal A.; Murray T.; Ward E.; Samuels A.; Tiwari R. C.; Ghafoor A.; Feuer E. J.; Thun M. J. Cancer statistics, 2005. CA Cancer J. Clin. 55(1):10–30; 2005. [DOI] [PubMed] [Google Scholar]
- 17. Ajani J. A. Evolving chemotherapy for advanced gastric cancer. Oncologist 10(Suppl. 3):49–58; 2005. [DOI] [PubMed] [Google Scholar]
- 18. Kim H. K.; Choi I. J.; Kim C. G.; Kim H. S.; Oshima A.; Michalowski A.; Green J. E. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One 6(2):e16694; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shishodia S.; Chaturvedi M. M.; Aggarwal B. B. Role of curcumin in cancer therapy. Curr. Probl. Cancer 31(4):243–305; 2007. [DOI] [PubMed] [Google Scholar]
- 20. Reddy S.; Rishi A. K.; Xu H.; Levi E.; Sarkar F. H.; Majumdar A. P. Mechanisms of curcumin-and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr. Cancer 55(2):185–194; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Koo J. Y.; Kim H. J.; Jung K.-O.; Park K.-Y. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J. Med. Food 7(2):117–121; 2004. [DOI] [PubMed] [Google Scholar]
- 22. Xu H.; Yu Y.; Marciniak D.; Rishi A. K.; Sarkar F. H.; Kucuk O.; Majumdar A. P. Epidermal growth factor receptor (EGFR)- related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol. Cancer Ther. 4(3):435–442; 2005. [DOI] [PubMed] [Google Scholar]
- 23. Johnson R. C.; Rogers P. 5-Fluorouracil as a selective agent for growth of leptospirae. J. Bacteriol. 87(2):422–426; 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham J.; Muhsin M.; Kirkpatrick P. Oxaliplatin. Nat. Rev. Drug Discov. 3(1):11–12; 2004. [DOI] [PubMed] [Google Scholar]
- 25. Karunagaran D.; Rashmi R.; Kumar T. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets 5(2):117–129; 2005. [DOI] [PubMed] [Google Scholar]
- 26. Earnshaw W. C.; Martins L. M.; Kaufmann S. H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68(1):383–424; 1999. [DOI] [PubMed] [Google Scholar]
- 27. Wyllie A. H. “Where, O death, is thy sting?” A brief review of apoptosis biology. Mol. Neurobiol. 42(1):4–9; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Breckenridge D. G.; Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr. Opin. Cell Biol. 16(6):647–652; 2004. [DOI] [PubMed] [Google Scholar]
- 29. Skommer J.; Wlodkowic D.; Pelkonen J. Cellular foundation of curcumin-induced apoptosis in follicular lymphoma cell lines. Exp. Hematol. 34(4):463–474; 2006. [DOI] [PubMed] [Google Scholar]
- 30. Zhou X.; You T.; Wang W.M.; Zheng Z. Q. The combination of cucumin and FOLFOX induced gastric cancer apoptosis and the mechanism. Chin. J. Integr. Med. 33(6):810–813; 2013. [PubMed] [Google Scholar]