Abstract
In the process of tumor cell apoptosis induced by specific regents, calreticulin (CRT) was transferred from endoplasmic reticulum (ER) onto the cell membrane. These tumor cells, when used as the cellular vaccine to immunize experimental animals, could initiate effective antitumor immunoresponse against homologous tumor cells. This is referred to as immunogenic cell death. Lidamycin (LDM) is an enediyne antibiotic, which has extremely potent cytotoxicity to cancer cells. In this study, the mouse melanoma B16-F1 cancer cells were used to investigate the ability of LDM in promoting immunogenic cell death. Our data showed that LDM could induce apoptosis of B16-F1 cancer cells, accompanied by CRT translocation onto the cell membrane. These LDM-treated B16-F1 cells could be recognized and phagocytosed more efficiently by macrophage and dendritic cells. When the LDM-treated apoptotic B16-F1 cells were used as a whole-cell tumor vaccine to immune mice, the mice obtained resistance against rechallenged B16-F1 living cells. At the same time, the specific antitumor immune response was observed in these vaccinated mice. The splenocytes from the mice vaccinated with LDM-treated B16-F1 cells showed significantly enhanced NK lymphocyte activities and also faster growth rate and increased secretion of IFN-γ when encountering the cellular antigens from B16-F1 cells. All these results suggested that LDM could promote immunogenic cell death in B16-F1 cells, and these LDM-treated B16-F1 cells could be used as a sort of cell vaccine to initiate effective antitumor immunoresponse in mice.
Key words: Lidamycin (LDM), Immunogenic cell death, Calreticulin, Melanoma, Immunotherapy, Mouse
INTRODUCTION
Cancer immunogenic cell death could be induced by special stimuli, such as ultraviolet light and antitumor anthracyclins. One of the most important events in this process is that calreticulin (CRT) was relocated from endoplasmic reticulum (ER) onto the cell surface (1–3). CRT is a highly conservative Ca2+-binding protein, which mostly presents in the ER lumen and is involved in lectin binding, activation of store-operated Ca2+ influx, and regulation of cell adhesion, etc (4,5). Recently, it has been reported that during the apoptotic progress of tumor cells induced by special drugs, CRT translocated from ER to the cell surface quickly. These CRT-coated tumor cells could be recognized and phagocytosed by the antigen-presenting cells, such as DCs (dendritic cells). Within the DCs, the tumor-specific antigens were processed and presented to the lymphocytes, which then resulted in the specific antitumor immune response (6–8). These researches indicate that the drugs, which can induce the translocation of CRT onto the cell surface, have potential value in antitumor immunotherapy.
Lidamycin (LDM) is a member of the enediyne antibiotic family and is produced by Streptomyces globisporus C-1027 strain (9,10). LDM contains an enediyne chromophore responsible for its bioactivity and a noncovalently bound apoprotein, which forms a hydrophobic pocket for protecting the chromophore (11,12). LDM has shown extremely potent antitumor activities such as apoptosis induction, cell cycle arrest, antiangiogenesis, and marked growth inhibition of transplantable tumors in mice (13–17).
Up to now, it is not clear whether LDM can induce cancer immunogenic cell death. In order to investigate the value of LDM in antitumor immunity, in this study the specific antitumor immune response mediated by LDM-treated mouse melanoma B16-F1 cells was evaluated. Results demonstrated that LDM could induce apoptosis and CRT membrane translocation in the B16-F1 cells. When the LDM-treated apoptotic B16-F1 cells were used as cell vaccine to immune mice, the specific immune response against the homologous tumor cells was observed.
MATERIALS AND METHODS
Experimental Animals and Cell Line
BALB/c mice (female, 7–8 weeks old) were obtained from Wuhan Institute of Biologic Products. All the animals were housed under specific pathogen-free condition. Mouse melanoma B16-F1 cells were purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China).
Materials
Mouse IL-4, GM-CSF, and IL-2 were purchased from Peprotech (Rocky Hill, NJ, USA). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and CFDA-SE were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-β-actin and anti-cytochrome-C antibodies were products of Santa Cruz (Dallas, TX, USA). Rabbit-anti-human CRT polyclonal antibody was purchased from Stressgen (Victoria, BC, Canada). Goat anti-rabbit IgG-HRP was a product of Jackson (Philadelphia, PA, USA). LDM was prepared in the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences. BENS (bisethyl-norspermine) was kindly provided by Prof. Robert A. Casero at Johns Hopkins University. ELISA kits for TNF-α and IFN-γ were purchased from Boster (Wuhan, Hubei, China). Annexin V-FITC/PI apoptosis detection kit was a product of Invitrogen (San Diego, CA, USA). LDH detection kit was a product of Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell Proliferation Assay
MTT assay was used to determine cell proliferation. The B16-F1 cells were plated in a 96-well plate with 2 × 103/well, incubated in 37°C for 24 h, and then exposed to different concentrations of LDM for 24 h, 48 h, and 72 h. MTT solution (final concentration as 0.2 g/L) was added to each well and incubated for another 4 h at 37°C. The supernatant was removed, and 200 µl of DMSO was added to each well. The absorbance at 570 nm was measured by a microplate reader. Growth inhibition was calculated as a percentage of the nontreated controls.
Cell Cycle and Apoptosis Analysis
B16-F1 cells were exposed to LDM-containing medium (2.5, 5, 10 ng/ml) for 48 h. The cells were harvested and fixed in 80% ethanol at −20°C for 1 h, washed with PBS, and then incubated with the staining solution (50 µg/ml propidium iodide, 0.1% Triton X-100, 5 µg/ml RNase A, and 5 mM EDTA in PBS, pH 7.4) for 30 min. The cell cycle analysis was performed by flow cytometry.
Annexin V-FITC/PI kit was used to detect apoptotic cells. Briefly, B16-F1 cells were plated at a density of 5 × 105 cells/well in a six-well plate. After 24 h, the cells were exposed to LDM (1 ng/ml) for 48 h, and then the cells were collected and treated according to the manual supplied by the manufacturer. The samples were then analyzed by flow cytometry.
Detection of Epimembranal CRT Protein
B16-F1 cells were plated in 24-well plates for 24 h and then treated with 5 ng/ml LDM for 48 h. Then the cells were collected and washed once with PBS. The cells were incubated with rabbit anti-CRT polyclonal antibody (1:1,000) at room temperature for 2 h, followed by washing and incubation with the rhodamine-conjugated polyclonal antibody (1:1,000) at room temperature for 1 h (avoiding light). After washing by PBS, the cells were analyzed by flow cytometry to identify CRT on the cell surface.
Western Blot Analysis
The cells were lysed for 20 min on ice in the lysis buffer (10 mmol/L HEPES, pH 7.2, 210 mmol/L d-mannitol, 70 mmol/L sucrose, 5 mmol/L sodium succinate, 0.2 mmol/L EGTA, 100 mg/L digitonin). The cell lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C to remove the mitochondria. BCA kit was used to determine the protein concentration in the supernatant (cytosol). Then the proteins samples were applied on a 12% SDS-PAGE and electrobloted onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% milk before incubating with primary antibodies (diluted 1:1,000). After washing by Tris-buffered saline with 0.5% Tween-20 five times, the membranes were incubated with HRP-conjugated goat anti-mouse/rabbit IgG antibody (diluted 1:4,000) at room temperature for 1 h and developed using ECL.
Phagocytotic Assay
Bone marrow (BM) cells were collected from the tibias and femurs of the BALB/c mice using culture medium. Following centrifugation, the BM cells were resuspended in red cell lysis solution (0.15 mol/L NH4Cl, 10 mmol/L KHCO3, and 1 mmol/L EDTA) for 1 min to remove the red blood cells. The BM cells were collected by centrifugation and cultured in a medium supplemented with 10 ng/ml recombinant mouse GM-CSF and 5 ng/ml recombinant mouse IL-4 in six-well plates (1 × 106 cells/well). After 7 days, the nonadherent and loosely adherent cells were harvested as dendritic cells (DCs) and used as the effector cells for the phagocytosis assay. The macrophages (MØ) from the abdominal cavity of BALB/c mice were also collected and used as effector cells. The B16-F1 cells were labeled with green dyes CFDA-SE (5 µmol/L) for 30 min and used as target cells. The effector and target cells were cocultured at 37°C for 4 h at 1:5 E/T (effector/target) ratio. Then PE-coupled anti-CD11c polyclonal antibody was added to the cell mixture for 30 min at room temperature (avoiding light) to label the effector cells. The cells were washed with PBS and analyzed by flow cytometry. The phagocytotic efficiency was represented by the ratio of the cell number with double-positive fluorescence over the total cell number.
Animal Experiments
The 6- to 8-week-old female BALB/c mice were randomly divided into three groups: the mice in group 1 (n = 20) were inoculated with the B16-F1 cells treated with LDM; the mice in group 2 (n = 20) were inoculated with the B16-F1 cells treated with BENS; the mice in group 3 (n = 20) were inoculated with PBS. In groups 1 and 2, the B16-F1 cells were treated with LDM or BENS to induce apoptosis and then used as the whole-cell vaccine to inoculate the mice. The whole-cell vaccine or PBS was injected into each mouse subcutaneously once a week for 4 weeks. Ten days after the last immunization, 10 mice in each group were sacrificed, and the spleen and serum samples were collected. The remaining mice were subcutaneously injected with the living B16-F1 cells at the back of each mouse. Then the mice were monitored every day after injection. Appearance of black-blue spots in the inoculated area was regarded as the tumor beginning to generate.
Detection of TNF-α and IFN-γ by ELISA
TNF-α and IFN-γ concentrations in the serum from experimental mice or cell culture medium were determined by ELISA assay kit according to the manufacturer’s protocol.
Determination of Natural Killer (NK) Cell Activity
The splenocytes from the vaccinated mice were isolated and suspended in the complete RPMI-1640 medium with 10% fetal calf serum and used as the effector cells to assay their NK cell activity. B16-F1 cells were used as the target cells in this experiment. The effector cells (5 × 105 cells/well) were stimulated with IL-2 (20 µg/ml) before use and incubated with the target cells (1 × 104 cells/well) at E/T ratios of 50:1 in a total volume of 200 µl RPMI-1640 medium. The released lactate dehydrogenase (LDH) was measured by LDH detection kit according to the manufacturer’s instructions after 4 h of incubation at 37°C in 5% CO2. The percentage of specific killing was calculated as follows: specific killing (%) = (experimental release − spontaneous release)/(total release − spontaneous release).
Statistical Analysis
All data were presented as mean ± standard deviation (SD). Statistical analysis between groups was assessed by Student’s two-tailed t-test. The tests were performed using SPSS software. A value of p < 0.05 was regarded as statistically significant.
RESULTS
Lidamycin Inhibited Growth of B16-F1 Cells by Inducing Apoptosis
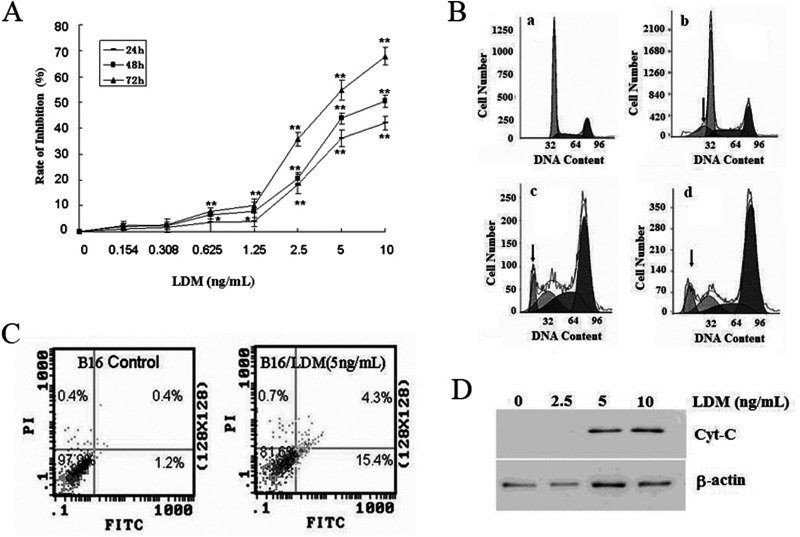
The antiproliferation effect of LDM was assayed by the MTT method. The results showed that LDM could inhibit the proliferation of B16-F1 obviously in a dose- and time-dependent manner. When B16-F1 cancer cells were treated by LDM (5 ng/ml) for 24, 48, and 72 h, the inhibitory ratios were 36.09%, 43.74%, and 54.58%, respectively (Fig. 1A).
Figure 1.
LDM inhibits the growth of B16-F1 cells by inducing apoptosis. (A) MTT method was used to assay the antiproliferation effects of LDM, which inhibits the proliferation of B16-F1 in a dose- and time-dependent manner. Compared with untreated cells in the same time group, *p < 0.05, **p < 0.01. (B) LDM induces B16-F1 cell cycle arrest and stimulate apoptosis detected by flow cytometry. (a) B16-F1 control cells; (b, c, d) B16-F1 cells were treated with concentrations of 2.5 ng/ml, 5 ng/ml, and 10 ng/ml LDM. The arrow indicates the position of sub-G1. (C) Flow cytometry was performed to show apoptosis of B16-F1 cells treated with 5 ng/ml LDM by Annexin V/PI assay. (D) Western blot assay was used to determine the level of Cyt-C in the cytosol of apoptosis B16-F1 cells treated with LDM.
In order to elucidate the mechanism of LDM for its antiproliferative activity, the B16-F1 cells were treated with LDM (2.5, 5, 10 ng/ml) for 48 h, and then the cell cycle distribution was analyzed by flow cytometry. The results showed that the cell ratio in G2/M phase was increased, and the cell ratio in the G0/G1 phase was decreased effectively by LDM treatment in a dose-dependent manner. At the same time, more and more cells appeared in the sub-G1 peak as the increase in LDM concentration, suggesting that LDM could induce cell cycle arrest and stimulate apoptosis (Fig. 1B and Table 1).
Table 1.
The Effect of LDM on Cell Cycle in B16-F1 Cells
| LDM (ng/ml) | Sub-G1 | G0/G1 | S | G2 |
|---|---|---|---|---|
| 0 | 0.32 ± 0.65 | 61.5 ± 2.14 | 18.4 ± 2.88 | 20.1 ± 0.84 |
| 2.5 | 4.6 ± 2.11* | 49.7 ± 1.59* | 25.4 ± 0.45* | 24.9 ± 0.37† |
| 5 | 12.3 ± 1.13* | 21.8 ± 3.65* | 30.3 ± 4.61* | 47.9 ± 3.40* |
| 10 | 7.2 ± 3.19* | 17.3 ± 2.46* | 17.8 ± 1.38 | 64.9 ± 0.73* |
Values are mean ± SD (n = 3).
*p < 0.01, †p < 0.05 compared with the untreated group.
To further verify the function of LDM in inducing apoptosis, Annexin V/PI assay was performed by flow cytometry. The result also indicated that LDM could induce apoptosis of B16-F1 cells effectively. Exposure of B16-F1 cell to LDM (5 ng/ml) for 48 h resulted in a greater percentage of apoptotic cells (19.7%) than that of the control cells (1.6%) (Fig. 1C).
The release of cytochrome-C (Cyt-C) from the mitochondria into the cytosol is a key step for endogenous apoptosis. To further examine the effect of LDM on Cyt-C release, the B16-F1 cells were treated with LDM for 48 h, and then Western blot analysis was performed to determine Cyt-C in the cytosol. As shown in Figure 1D, LDM at the concentration of 5 and 10 ng/ml could increase the Cyt-C level in the cytosol. This result suggests that LDM induces apoptosis in B16-F1 cells through the mitochondria pathway.
Lidamycin Induced Membrane Translocation of CRT in B16-F1 and Enhanced Phagocytosis In Vitro
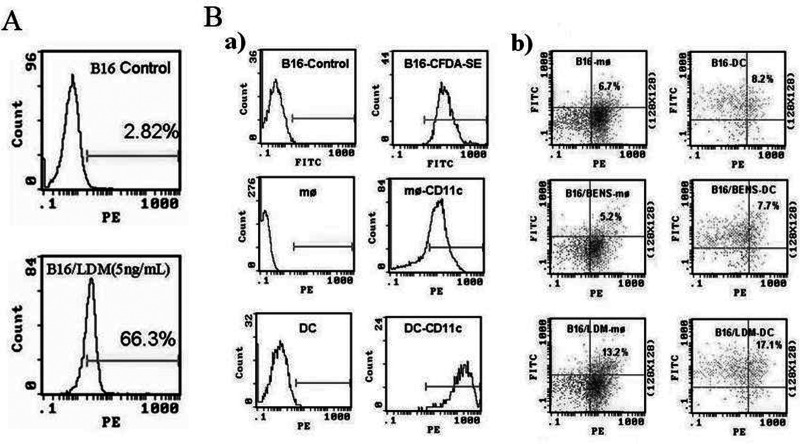
To validate whether LDM treatment could affect CRT subcellular localization in B16-F1 cells along with the apoptosis, the flow cytometry assay was used to observe the expression of CRT on the cell surface of B16-F1 cells. Results showed that LDM (5 ng/ml) treatment for 48 h induced an obvious membrane translocation of CRT. The percentage of membrane CRT-positive cells increased from 2.82% to 66.3% (Fig. 2A).
Figure 2.
Treatment with LDM could enhance phagocytosis of apoptosis B16-F1 cells by inducing membrane translocation of CRT on the cell surface. (A) LDM-induced membrane translocation of CRT on the cell surface of B16-F1 cells was observed by flow cytometry. Cells were incubated with rabbit anti-CRT polyclonal antibody, followed by incubation with rhodamine-conjugated polyclonal antibody. (B) LDM treatment enhanced the phagocytosis of B16-F1 apoptotic cells by DCs or macrophages (MØ) in vitro. (a) Labeled target and effector cells with green dye CFDA-SE and PE-CD11c, respectively. (b) The percentage of macrophages and DCs that have taken up target cells.
In view of the established role of CRT as an “eat me” signal (1), the possible implication of LDM treatment in enhancing phagocytosis was investigated. The labeled macrophages (MØ) or DCs from BALB/c mice (the effector cells) and the labeled B16-F1 cells (the target cells) were cocultured for 4 h in a 1:5 effector/target ratio and then applied to flow cytometry assay. The results showed that, compared with the B16-F1 cells treated with BENS, which can induce apoptosis but without CRT membrane translocation, more than twofold higher phagocytotic efficient was observed when LDM-treated B16-F1 cells were used as the target cells, indicating that CRT membrane translocation in B16-F1 cancer cells induced by LDM enhanced the macrophage- and DC-mediated phagocytosis (Fig. 2B).
The Mice Vaccinated With LDM-Treated B16-F1 Cells Obtained Resistance Against Rechallenged B16-F1 Living Cells
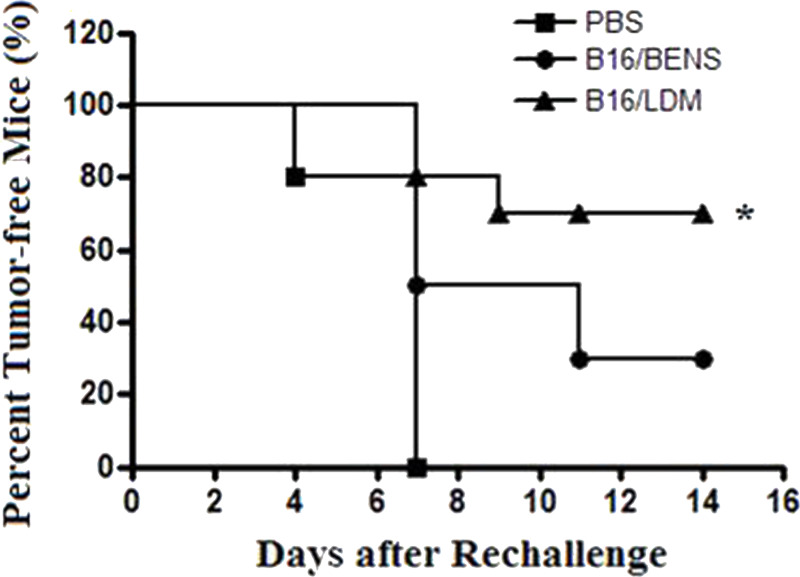
Since LDM treatment resulted in obvious CRT translocation from the cell interior onto the cell surface in B16-F1 cells, it is possible that these cells could be used as a whole-cell vaccine to stimulate specific antitumor immune effect in the experimental animals. In this experiment, the apoptotic B16-F1 cells induced by LDM and BENS were prepared and used as the vaccines to immunize animals. BENS is an antitumor polyamine analog and used here as a control drug, which can induce apoptosis but without CRT membrane translocation (18,19). After vaccination, the living B16-F1 cells were subcutaneously injected into the back of each mouse, and then the mice were monitored everyday for tumor generation. The results showed that, at the 14th day after rechallenged by the living B16-F1 cells, there was a much lower percentage of tumor generation in the mice group vaccinated with LDM-treated cells (30%) compared to that in the mice groups vaccinated with BENS-treated cells (70%) or PBS (100%) (Fig. 3).
Figure 3.
Immunizing mice with apoptotic B16-F1 cells induced by LDM resulted in a specific antitumor effect against the homogeneous tumor. BALB/c mice were randomly divided into three groups: (1) PBS; (2) B16 treated with BENS; (3) B16 treated with LDM. Ten days after the last immunization, living B16-F1 cells were subcutaneously injected into the back of each mouse. Then the mice were monitored every 2 days after injection. Appearance of black-blue spots in the inoculated area was regarded as the tumor beginning to generate (*p < 0.05 compared to the other two groups).
Apoptotic B16-F1 Cells Treated With LDM Induced a Specific Antitumor Immunological Effect
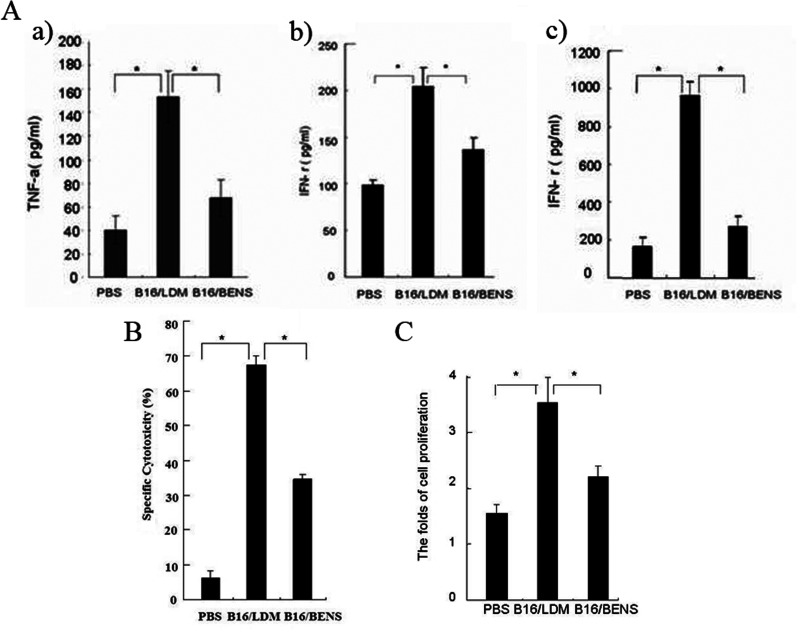
TNF-α and IFN-γ are cytokines that are critical for innate and adaptive immunity and are produced by the lymphocytes once antigen-specific immunity develops. In this study, the TNF-α and IFN-γ levels in the serum of the vaccinated mice were determined by ELISA. The data showed that the serum TNF-α and IFN-γ levels in the mice group vaccinated with LDM-treated cells were significantly higher than that in the other two groups (Fig. 4Aa, b). In order to validate whether immunizing mice with LDM-induced apoptotic B16-F1 cells could promote the release of TNF-α by immune cells, the splenocytes from the vaccinated mice were cocultured with B16-F1 cells, and then IFN-γ level in the culture medium was determined by ELISA assay. The results showed that IFN-γ content secreted by the splenocytes from the mice vaccinated with LDM-treated B16-F1 cells was significantly higher compared with the other groups (Fig. 4A c), indicating that vaccination with LDM-treated B16-F1 cells can promote the synthesis and secretion of cytokine IFN-γ from mice spleen cells.
Figure 4.
Apoptotic B16-F1 cells treated with LDM stimulate immune activation in mice. (A) ELISA was used to determine concentrations of TNF-α and IFN-γ. Blood was collected from the experimental mice and incubated at 37°C for 30 min to obtain serum. The splenocytes were isolated and suspended in the complete RPMI-1640 medium with 10% fetal calf serum. (a) serum TNF-α levels; (b) serum IFN-γ levels; (c) IFN-γ secreted by mouse splenocytes. (B) Vaccination with LDM-treated apoptotic B16-F1 cells stimulated activity of NK cells in the mice spleens detected by LDH assay. The splenocytes from the vaccinated mice were used as the target cells incubated with B16-F1 cells at E/T ratio of 50:1. (C) The B16-F1 cellular antigen stimulated proliferation of the splenocytes from the mice vaccinated with LDM-treated B16-F1 cells detected by MTT assay. Each bar presents the mean ± SD (n = 10), *p < 0.01.
To further validate whether the apoptotic B16-F1 cells induced by LDM could stimulate immune activation, the NK cells were isolated from the spleens of vaccinated mice and stimulated by IL-2 (20 µg/ml) before being used as the expanded effector cells (E). The B16-F1 cells were used as the target cells (T) in this experiment to co-incubate with the effector cells at E/T ratios of 50:1 for 4 h. The cytotoxicity of NK cells was determined using the LDH assay. The results showed that the NK cells from the mice vaccinated with the LDM-treated B16-F1 cells had significantly higher cytotoxicity compared with the other mice groups (Fig. 4B).
Since activated lymphocytes would proliferate rapidly when encountering the same antigen stimulation again, in this experiment the splenocytes from the vaccinated mice were cocultured with the cell lysates from B16-F1 cells (used as the antigen) for 72 h, and then the proliferation rate of the splenocytes was determined by MTT assay. As shown in Figure 4C, the proliferation rate of the splenocytes from the mice inoculated with LDM-treated B16-F1 cells was much higher than that in the other groups.
DISCUSSION
LDM is a potential chemotherapeutic agent, which can induce apoptosis or mitotic cell death in many cancer cells by inducing DNA damage responses to double-strand DNA breaks, altering cell cycle progression, and inducing chromosomal aberrations/telomere dysfunction (20–24). In terms of IC50 values, the cytotoxicity of LDM was 10,000-fold more potent than that of mitomycin and doxorubicin (25). But so far there is no research to investigate the immune activity of apoptotic tumor cells induced by LDM treatment. It has been reported that, during cancer immunogenic cell death, one of the most important events is the translocation of CRT from ER lumen onto the cell surface, which determines the immunogenicity of apoptotic tumor cells. When these CRT-coated apoptotic cells were used as the whole-cell antigen to inoculate mice, the specific antitumor effect against homogeneous tumor cells was observed in the experimental animals (12,26–28).
In this research, we discovered that LDM was extremely toxic to mouse B16-F1 melanoma cells. LDM treatment could induce apoptosis of B16-F1 and, at the same time, promote CRT translocation onto the cell surface. These LDM-induced apoptotic cells can be phagocytosed more efficiently by DCs and macrophages, and more than twofold higher phagocytotic efficiency was observed compared to B16-F1 cells treated with BENS. The reason BENS was chosen in this experiment was that BENS could induce apoptosis of B16-F1 cells but have no effect on the subcellular localization of CRT (19). When the mice were vaccinated with LDM-treated B16-F1 cells as the whole cell vaccine and then rechallenged by the living B16-F1 cells, a much higher percent of tumor-free mice was observed in the mice group vaccinated with LDM-treated B16-F1 cells than that in the mice group vaccinated with BENS-treated B16-F1 cells, indicating that vaccination with LDM-treated B16-F1 cells can induce a protective effect against attack of living B16-F1 cancer cells in the mice.
We further validated the effects of LDM-treated B16-F1 cells in inducing specific antitumor immune activity in the mice. The results showed that the splenocytes from the mice vaccinated with LDM-treated B16-F1 cells exhibited a more potent cytotoxic effect on the B16-F1 cells, the higher proliferation rate when incubated with antigens from B16-F1 cells, and increased TNF-α/IFN-γ secretion than those in the mice vaccinated with BENS-treated B16-F1 cells.
Taken together, our data suggest that LDM can induce immunogenic apoptosis in the mouse melanoma B16-F1 cells. When used as the whole-cell vaccine to immune mice, these LDM-treated cells can initiate the antitumor immune response. The possible mechanism underlying the LDM-mediated antitumor immunity is that LDM can induce membrane translocation of CRT in B16-F1 cells and then enhance phagocytosis of these CRT-coated tumor cells by the antigen-presenting immunocytes in which the tumor-specific antigens are processed and presented.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (No. 30973445) and Research Foundation for Advanced Talents, China Three Gorges University (No. KJ2014B068).
REFERENCES
- 1. Obeid M.; Tesniere A.; Ghiringhelli F.; Fimia G. M.; Apetoh L.; Perfettini J. L.; Castedo M.; Mignot G.; Panaretakis T.; Casares N.; Métivier D.; Larochette N.; van Endert P.; Ciccosanti F.; Piacentini M.; Zitvogel L.; Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13:54–61; 2007. [DOI] [PubMed] [Google Scholar]
- 2. Obeid M.; Panaretakis T.; Joza N.; Tufi R.; Tesniere A.; van Endert P.; Zitvogel L.; Kroemer G. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC lightinduced apoptosis. Cell Death Differ. 14:1848–1850; 2007. [DOI] [PubMed] [Google Scholar]
- 3. Cao C. Y.; Han Y.; Ren Y. S.; Wang Y. L. Mitroxantrone-mediated apoptotic B16-F1 cells induce specific anti-tumor immune response. Cell. Mol. Immunol. 6:469–475; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostwald T. J.; MacLennan D. H. Isolation of a high affinity calcium binding protein from sarcoplasmic reticulum. J. Biol. Chem. 249:974–979; 1974. [PubMed] [Google Scholar]
- 5. Michalak M.; Robert Parker J. M.; Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32:269–278; 2002. [DOI] [PubMed] [Google Scholar]
- 6. Gardai S. J.; McPhillips K. A.; Frasch S. C.; Janssen W. J.; Starefeldt A.; Murphy-Ullrich J. E.; Bratton D. L.; Oldenborg P. A.; Michalak M.; Henson P. M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334; 2005. [DOI] [PubMed] [Google Scholar]
- 7. Lauber K.; Blumenthal S. G.; Waibel M.; Wesselborg S. Clearance of apoptotic cells: Getting rid of the corpses. Mol. Cell 14:277–287; 2004. [DOI] [PubMed] [Google Scholar]
- 8. Kroemer G.; Galluzzi L.; Kepp O.; Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31:51–72; 2013. [DOI] [PubMed] [Google Scholar]
- 9. Hu J. L.; Xue Y. C.; Xie M. Y.; Zhang R.; Otani T.; Minami Y.; Yamada Y.; Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J. Antibiot. 41:1575–1579; 1988. [DOI] [PubMed] [Google Scholar]
- 10. Otani T.; Minami Y.; Marunaka T.; Zhang R.; Xie M. Y. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J. Antibiot. 41:1580–1585; 1988. [DOI] [PubMed] [Google Scholar]
- 11. Sakata N.; Ikeno S.; Hori M.; Hamada M.; Otani T. Cloning and nucleotide sequencing of the antitumor antibiotic C-1027 apoprotein gene. Biosci. Biotechnol. Biochem. 56:1592–1595; 1992. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T.; Hirama M.; Otani T. Solution structures of C-1027 apoprotein and its complex with the aromatized chromophore. J. Mol. Biol. 309:267–283; 2001. [DOI] [PubMed] [Google Scholar]
- 13. Xu Y. J.; Zhen Y. S.; Goldberg I. H. C-1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage. Biochemistry 33:5947–5954; 1994. [DOI] [PubMed] [Google Scholar]
- 14. Chen J.; Ou-Yang Z. G.; Zhang S. H.; Zhen Y. S. Down-regulation of NF-κB by lidamycin in association with inducing apoptosis in human pancreatic cancer cells and inhibiting xenograft growth. Oncol. Rep. 17:1445–1451; 2007. [PubMed] [Google Scholar]
- 15. Zhen Y. Z.; Lin Y. J.; Shang B. Y.; Zhen Y. S. Enediyne lidamycin induces apoptosis in human multiple myeloma cells through activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. Int. J. Hematol. 90:44–51; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Q.; Liu X. J.; Hu L.; Liao D. S.; Zheng Y. B.; Zhen Y. S.; Song X. Factor VII light chain targeted lidamycin targets tissue factor-overexpressing tumor cells for cancer therapy. Int. J. Mol. Med. 29:409–415; 2012. [DOI] [PubMed] [Google Scholar]
- 17. Zhen Y. Z.; Zhao Y. F.; Luo G. L.; Zhang G. L.; Liu X. J.; Li R.; Kan Q. Lidamycin inhibits mouse myeloma SP2/0 in vivo and in vitro. Basic Clin. Med. 33:992–997; 2013. [Google Scholar]
- 18. Pledgie-Tracy A.; Billam M.; Hacker A.; Sobolewski M. D.; Woster P. M.; Zhang Z.; Casero R. A.; Davidson N. E. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemocher. Pharmacol. 65:1067–1081; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao C.; Han Y.; Ren Y.; Wang Y. Apoptotic B16-F1 cells coated with recombinant calreticulin mediated antitumor immune response in mice. Chin. J. Cancer Res. 22:253–259; 2010. [Google Scholar]
- 20. Wang X.; Wu S. Y.; Zhen Y. S. Lidamycin inhibits proliferation and induces apoptosis in endothelial cells. Chin. J. Antibiot. 28:605–612; 2003. [Google Scholar]
- 21. Chen J.; Wu S. Y.; OU-Yang Z. G.; Zhen Y. S. Synergy of gemcitabine and lidamycin associated with NF-κB down regulation in pancreatic carcinoma cells. Acta Pharmacol. Sin. 29:614–619; 2008. [DOI] [PubMed] [Google Scholar]
- 22. Kennedy D. R.; Beerman T. A. The radiomimetic enediyne C-1027 induces unusual DNA damage responses to double-strand breaks. Biochemistry 45:3747–3754; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X.; He H.; Feng Y.; Zhang M.; Ren K.; Shao R. Difference of cell cycle arrests induced by lidamycin in human breast cancer cells. Anticancer Drugs 17:73–79; 2006. [DOI] [PubMed] [Google Scholar]
- 24. McHugh M. M.; Gawron L. S.; Matsui S.; Beerman T. A. The antitumor enediyne C-1027 alters cell cycle progression and induces chromosomal aberrations and telomere dysfunction. Cancer Res. 65:5344–5351; 2005. [DOI] [PubMed] [Google Scholar]
- 25. Huang Y. H.; Shang B. Y.; Zhen Y. S. Antitumor efficacy of lidamycin on hepatoma and active moiety of its molecule. World J. Gastroenterol. 11:3980–3984; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brusa D.; Garetto S.; Chiorino G.; Scatolini M.; Miqliore E.; Camussi G.; Matera L. Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine 26:6422–6432; 2008. [DOI] [PubMed] [Google Scholar]
- 27. Qin Y.; Han Y.; Cao C. Y.; Ren Y. S.; Li C. H.; Wang Y. L. Melanoma B16-F1 cells coated with fusion protein of mouse calreticulin and virus G-protein coupled receptor induced the antitumor immune response in Balb/C mice. Cancer Biol. Ther. 11:574–580; 2011. [DOI] [PubMed] [Google Scholar]
- 28. Wu H. Y.; Han Y.; Qin Y.; Cao C. Y.; Xia Y.; Liu C. B.; Wang Y. L. Whole-cell vaccine coated with recombinant calreticulin enhances activation of dendritic cells and induces tumour-specific immune responses. Oncol. Rep. 29:529–534; 2013. [DOI] [PubMed] [Google Scholar]