Abstract
A number of large-scale clinical trials have demonstrated that using a combination of oxaliplatin and fluoropyrimidines as an adjuvant chemotherapy for stage II/III colon cancer improved the prognosis. However, there has only been experience in Japanese patients with using CapOX therapy, in which capecitabine and oxaliplatin are used in combination. Therefore, our objective was to evaluate the efficacy and safety of CapOX in Japanese patients as an adjuvant chemotherapy for colon cancer in a single institute retrospective study. The efficacy and safety of CapOX as an adjuvant chemotherapy for patients with stage III colon cancer and stage II patients who had a signature for high risk of recurrence were evaluated in patients who had undergone surgery at our institution between December 1, 2009 and March 31, 2013. Forty-one patients received CapOX therapy during the study period: 23 men and 18 women with median age of 68.0 years (35–79 years). Performance status was 0 for 33 patients, and PS 1 for eight patients. The clinical stages were stage II in 14 patients, stage IIIA in 15 patients, and stage IIIB in 12 patients. The median number of CapOX cycles was eight (two to eight courses). The treatment completion rate was 82.9%. Five-year DFS rates were 63.8%. Five-year OS rates were 71.0%. In terms of adverse events, the serious adverse events of grade 3 or higher seen among all patients were neutropenia in four patients, thrombocytopenia in one patient, and peripheral sensory neuropathy in seven patients. However, hand–foot syndrome, which is characteristic of capecitabine, was not observed. Efficacy and tolerability of CapOX in Japanese patients as an adjuvant chemotherapy after colon cancer surgery was demonstrated.
Key words: Capecitabine, Oxaliplatin, Adjuvant chemotherapy, Colon cancer
INTRODUCTION
Based on clinical studies, such as the National Surgical Adjuvant Breast and Bowel Project (NSABP), C04, 5-fluorouracil plus leucovorin (5-FU/LV) has long been used as the standard therapy for adjuvant chemotherapy for stage II/III colon cancer (1). In addition, several clinical trials have been done with two-agent combined therapy using oxaliplatin or irinotecan with 5-FU/LV. In contrast to the irinotecan combination regimen, which did not show efficacy for stage II/III colon cancer (2,3), oxaliplatin prolonged disease-free survival (DFS) and overall survival (OS) in comparison with 5-FU/LV therapy (LV5FU2) in the MOSAIC trial and is widely used in clinical practice as the standard therapy for adjuvant chemotherapy after stage III colon cancer surgery (4–7). On the other hand, for stage II, although no clear evidence exists, in some cases adjuvant chemotherapy has also been administered to groups at high risk for recurrence as described in the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) guidelines. According to Twelves et al., the noninferiority of capecitabine was demonstrated by the X-ACT trial (8) that studied the noninferiority of capecitabine, an oral prodrug of 5-FU, to 5-FU/LV. Because of its efficacy and convenience, capecitabine is also widely used in adjuvant chemotherapy that includes an oral anticancer drug. In addition, the NO16968 trial demonstrated that CapOX therapy, in which the intravenous 5-FU/LV in FOLFOX (5-FU/LV plus oxaliplatin) therapy is replaced with capecitabine as a regime containing oxaliplatin, significantly extended DFS, relapse-free survival (RFS), and OS in comparison with 5-FU/LV therapy [Mayo regimen or Roswell Park Memorial Institute (RPMI) regimen] (9). However, there have been few reports concerning the safety and tolerability of CapOX therapy in Japanese patients as an adjuvant therapy. Therefore, we administered adjuvant CapOX therapy to 41 Japanese patients in a high-risk group for recurrence of stage II or stage III colon cancer and studied its efficacy and safety, as reported below.
This study was approved by the institutional review board. Informed consents were obtained from all patients.
MATERIALS AND METHODS
Eligibility
The subjects were patients who had achieved histological R0 resection (no residual tumor) after surgery, were in a high-risk group for recurrence of stage II or stage III colon cancer, and who met the following inclusion criteria: 1) no age restrictions; 2) major organ function preserved [(i) leukocyte count: ≥4,000/mm3; (ii) blood platelet count: ≥100,000/mm3; (iii) total bilirubin value: ≤1.5 mg/dl; (iv) aspartate aminotransferase (AST), alanine aminotransferase (ALT): <2.5 times upper limit of normal; (v) creatinine clearance: ≥60 ml/min]; 3) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score: 0–2; 4) no active multiple primary cancers seen; 5) recovered from postoperative complications; 6) no serious complications (intestinal obstruction, diarrhea, fever); 7) able to begin within 4–8 weeks after surgery; and 8) provided written consent based on informed consent. In addition, the following were required to meet the definition of “high-risk group for recurrence of stage II”: T4 lesion, bowel obstruction or bowel perforation, poorly differentiated adenocarcinoma, number of dissected lymph nodes <12.
Administration Method
In principle, the first cycle was given as an in-patient treatment. After the oxaliplatin was dissolved to 130 mg/m2 in 500 ml saline, it was intravenously infused for 120–180 min on the first day. Capecitabine, 2,000 mg/m2, was orally administered twice per day, from the evening of day 1 through morning of day 15. The above was, in principle, repeated at 3-week intervals for a total of eight cycles. All patients were pretreated with 5-HT3 receptor antagonists and dexamethasone.
Evaluation
The evaluation items were, for safety, the incidence of adverse events and their severity. DFS was also evaluated.
Evaluation method: Evaluation was done via imaging diagnosis with CT at the start of the adjuvant, and after the fourth cycle and eighth cycle, and also with colonoscopy imaging diagnosis at the conclusion of the eight cycles. Imaging diagnosis evaluation with computed tomography (CT) was done every 4 months after the conclusion of therapy. Adverse events were evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Follow-Up Period
CapOX therapy was administered to 41 patients from December 1, 2009 to March 31, 2013; the final date of the follow-up period was December 31, 2014.
RESULTS
Patient Characteristics
Patient backgrounds of the 41 patients were as enrolled (Table 1).
Table 1.
Patients Characteristics (n = 41)
| Characteristic | No. of Patients |
|---|---|
| Gender | |
| Male | 23 |
| Female | 18 |
| Age (years) | |
| Median | 68.0 |
| Average | 65.6 |
| Range | 35–79 |
| Primary23 | |
| Acending | 23 |
| Transverse | 5 |
| Descending | 1 |
| Sigmoid | 4 |
| Rectosigmoid | 7 |
| PS | |
| 0 | 33 |
| 1 | 8 |
| Stage | |
| II | 14 |
| IIIA | 15 |
| IIIB | 12 |
| Pathological tumor stage | |
| T1 | 4 |
| T2 | 31 |
| T3 | 6 |
| T4 | 0 |
| Pathological nodal stage | |
| N0 | 14 |
| N1 | 19 |
| N2 | 8 |
| Lymphatic invasion | |
| Ly0 | 7 |
| Ly1 | 26 |
| ly2 | 7 |
| ly3 | 1 |
| Venous invasion | |
| v0 | 14 |
| v1 | 15 |
| v2 | 12 |
| v3 | 0 |
| Pathology | |
| Tub1 | 18 |
| Tub2 | 16 |
| Por1 | 6 |
| Muc | 1 |
| CEA level (0-5.0 ng/ml) | |
| Below normal range | 17 |
| Over normal range | 23 |
| C19-9A level (0-37.0 ng/ml) | |
| Below normal range | 32 |
| Over normal range | 9 |
Treatment Outcomes
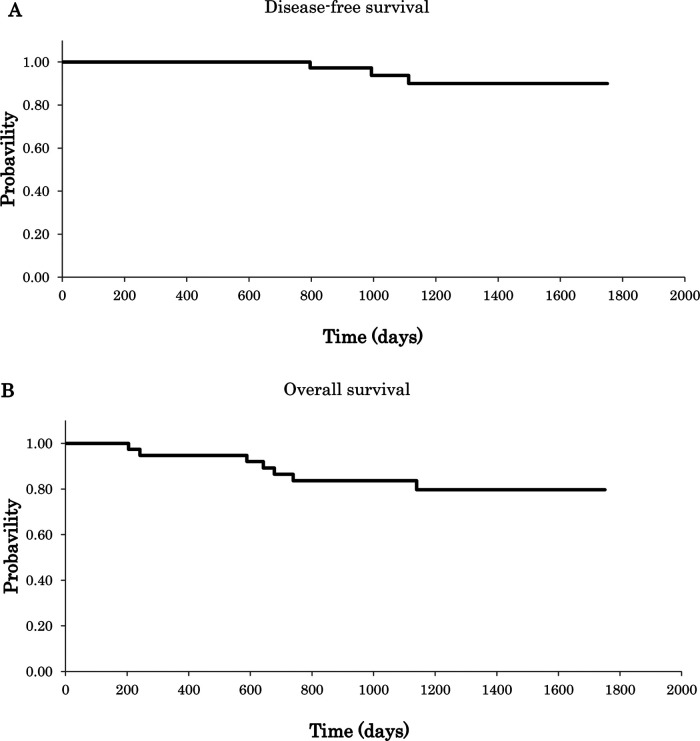
Treatment results are summarized in Table 2. The median number of cycles of CapOX therapy administered to the 41 patients evaluated was 8 (range: 2–8). The treatment completion rate was 82.9% (34 patients/41 patients). One patient terminated therapy after the sixth cycle, three patients converted CapOX into other regimen, and one patient died of another illness. Recurrence was observed in two subjects after completion of adjuvant chemotherapy. The median follow-up period was 1,385 days. Five-year DFS rates were 63.8% (Fig. 1A). Five-year OS rates were 71.0% (Fig. 1B).
Table 2.
Exposure of Treatment (n = 41)
| Time Between Operation and Treatment (range) | 40 Days (26–93) |
|---|---|
| Median number of cycles (range) | 8 (2–8) |
| Treatment completion | 34 (82.9%) |
| Discontinuation of treatment | 7 (17.1%) |
| Adverse event | 6 |
| Gastrointestinal disorder | 3 |
| Thrombocytopenia | 2 |
| Death of other diseases | 1 |
| Reccurence | 8 |
| Dose reduction | |
| Oxaliplatin | 20 (48.8%) |
| Capecitabine | 23 (56.1%) |
| Relative dose intensity | |
| Oxaliplatin | 76.5% (17.2–98.8) |
| Capecitabine | 75.8 (55.4–97.2) |
Figure 1.
(A) Disease-free survival (DFS); (B) overall survival (OS) (N = 41).
Safety
CapOX therapy was administered a total of 295 times to a total of 41 patients. One or more adverse events were seen during the administration period in all patients. Adverse events in all grades were, for hematological toxicity: leukocytopenia, 43.9%; neutropenia, 46.3%; anemia 58.5%; and thrombocytopenia, 53.7% (Table 3). Other abnormal laboratory test values were elevated liver enzymes, elevated lactate dehydrogenase (LDH), 68.3%, and elevated alkaline phosphatase (ALP), 48.8%. In nonhematological toxicity, gastrointestinal disorders were seen in 23 patients (56.1%), including nausea and anorexia (Table 4). All adverse events, both hematological and nonhematological, were mild to moderate and could be controlled. Moreover, hand–foot syndrome characteristic of capecitabine and allergic reactions due to oxaliplatin were not seen. However, one patient died of another illness, myocardial infarction, during the cycle of treatment.
Table 3.
Incidence of Hematological Adverse Events (n = 41, Total = 308)
| Grade | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Investigations | ||||
| Leukocytopenia | 16 (39.0%) | 2 (4.9%) | ||
| Neutropenia | 11 (26.8%) | 4 (9.8%) | 4 (9.8%) | |
| Anemia | 22 (53.7%) | 2 (4.9%) | ||
| Thrombocytopenia | 20 (48.8%) | 1 (2.4%) | 1 (2.4%) | |
| AST | 28 (68.3%) | |||
| ALT | 20 (48.8%) | |||
| γ-GTP | 13 (31.7%) | 1 (2.4%) | ||
| Blood bilirubin increased | 5 (12.2%) | |||
| LDH | 28 (68.3%) | |||
| ALP | 20 (48.8%) | |||
| BUN | 2 (4.9%) | |||
| Cr | 2 (4.9%) | |||
| Metabolism and nutrition disorders | ||||
| Hypokalemia | 7 (17.1%) | |||
| Hypoproteinemia | 4 (9.8%) | |||
Table 4.
Incidence of Nonhematological Adverse Events (n = 41, Total = 308)
| Grade | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Gastrointestinal disorder | ||||
| Nausea/vomit | 13 (31.7%) | 5 (12.2%) | ||
| Anorexia | 9 (22.0%) | 6 (14.6%) | ||
| Diarrhea | 2 (4.9%) | 2 (4.9%) | ||
| General disorders | ||||
| General fatigue | 7 (17.1%) | 4 (9.8%) | ||
| Alopecia | 5 (12.2%) | |||
| Skin and subcutaneous disorders | ||||
| Pigmentation | 12 (29.3%) | 1 (2.4%) | ||
| Skin induration | 7 (17.1%) | |||
| Nervous system disorders | ||||
| Peripheral sensory neuropathy | 15 (36.6%) | 19 (46.3%) | 7 (17.1%) | |
| Paresthesia | 24 (58.5%) | 7 (17.1%) | ||
| Dizziness | 8 (19.5%) | |||
| Dysgeusia | 13 (31.7%) | 2 (4.9%) | ||
DISCUSSION
In Japan, the recommended adjuvant chemotherapies are 5-FU/LV therapy, UFT plus LV therapy, capecitabine monotherapy, and FOLFOX therapy (10). Particularly in stage III, an added effect to inhibition of recurrence and survival time has been reported (4–7) when oxaliplatin is used with fluoropyrimidine-based therapy, and therefore FOLFOX therapy has been frequently used in Japan since its approval in August 2009. On the other hand, the oral fluoropyrimidine formulation, capecitabine, which is an alternative to continuous intravenous infusion in metastatic colorectal cancer, when used with oxaliplatin as CapOX therapy, is reported to have outcomes equivalent to those of FOLFOX therapy (11). In recent years, oral anticancer drugs have drawn attention as chemotherapies for colon cancer that are superior to intravenous 5-FU in terms of hygiene, management, and convenience, and so there have also been expectations that CapOX, which has obtained results equivalent to those of FOLFOX, will also be an adjuvant chemotherapy. However, there have been few reports concerning the safety and tolerability of CapOX therapy in Japanese patients, and so we administered adjuvant CapOX therapy to 41 patients at high risk for recurrence of stage II or stage III colon cancer and studied its efficacy and safety.
With CapOX therapy being administered a median of eight cycles (range: 2–8), the treatment completion rate was 82.9% (34 patients/41 patients), and 5-year DFS rates were 63.8% (Fig. 1A). Five-year OS rates were 71.0% (Fig. 1B). The average dosage of oxaliplatin was 76.5% (range: 17.2–98.8%); the average dosage of capecitabine was 75.8% (range: 55.4–97.2%).
In a study of capecitabine total oral dosage, it was reported that, in a subanalysis of the X-ACT study, no difference was seen in OS and RFS between the capecitabine total oral dosage ≥90% group and the 60–90% group (8). Moreover, the NO16968 trial and other studies have also reported that effects were maintained even when the amount of capecitabine was reduced or the drug stopped temporarily (12). The total oral dosage of capecitabine in this study was over the therapeutic threshold, and so was considered to be an adequate dosage. When it is necessary to reduce the amount of capecitabine in clinical practice because of hand–foot syndrome or gastrointestinal symptoms, it is preferable to continue capecitabine reduction in accordance with the reduction standards.
Seven patients were unable to complete CapOX therapy for eight cycles. One patient with stage IIIA who was unable to continue CapOX therapy because of thrombocytopenia thought to be caused by oxaliplatin was subsequently given capecitabine monotherapy and received 79% of the capecitabine total oral dosage. One patient with stage IIIB who discontinued therapy of six cycles because of gastrointestinal disorder received 64% of both the capecitabine oral dosage and the oxaliplatin average dosage. However, therapy was stopped because the dosage of the capecitabine was sufficient, considered from the results of the subanalysis of X-ACT and at the insistence of the patient. Of the two patients with stage IIIB who did not receive the complete amount of the capecitabine total oral dosage because gastrointestinal disorders were seen as a nonhematological adverse event, two subjects had the hematological adverse event of grade 1 thrombocytopenia. However, because even though both oxaliplatin and capecitabine were reduced in two stages it was 6 weeks until the platelet count returned to ≥100,000/mm3, and because the subject was an older patient (age 68 years) and the report with Japanese subjects by Sakamoto et al. (13), CapOX therapy was stopped after three cycles and 5-FU/LV therapy subsequently selected. The other subject, because of a report (14) demonstrating the noninferiority of S-1 plus oxaliplatin (SOX) therapy to CapOX therapy based on a phase III clinical trial conducted in South Korea and the young age (35 years) of the patient, selected SOX therapy rather than 5-FU/LV therapy after giving thorough informed consent. No recurrence was seen in these four subjects. Moreover, one patient died from myocardial infarction. From the result of autopsy, the cause appeared to be arteriosclerosis, and we judged that there was no relation to treated drugs. In one patient with stage IIIB, recurrence was seen after adjuvant chemotherapy, and so we started chemotherapy for metastatic colon cancer. The eight patients in whom recurrence was seen shared the following traits: ascending colon cancer; high CEA levels before surgery; venous invasion, 2; and large bowel obstruction. We next considered whether uniformly implementing adjuvant chemotherapy was appropriate as adaptation criteria for adjuvant chemotherapy in the case of stage II patients. In this study the stage II group at high risk for recurrence was defined as T4 lesions, bowel obstruction or bowel perforation, poorly differentiated adenocarcinoma, number of dissected lymph nodes <12. Yothers et al. (15) reported on the efficacy of oxaliplatin for stage II patients as the 2011 ASCO meeting. When the stage II group at high risk for recurrence was defined as T4 lesions, bowel obstruction or bowel perforation, poorly differentiated adenocarcinoma, number of dissected lymph nodes <12, the hazard ratio for all of the evaluation items including OS, DFS, and time to recurrence was less than 1. With reports of an increase in 5-year estimated survival of 3.5%, the combined use of oxaliplatin has been shown to improve prognoses. The ASCO guidelines (16) additionally include signet-ring cell carcinoma and mucinous carcinoma, and the ESMO guidelines (17) cite vascular infiltration, lymphatic infiltration, and paraneural infiltration. From our study of patients with recurrence in this study also, we think that preoperative CEA levels, preoperative CA19-9 levels, depth of wall invasion, vascular infiltration, venous invasion, etc., should be noted. The present study suggests that adjuvant chemotherapy is useful for such patients, even in stage II.
In terms of the adverse events of CapOX therapy (Table 4), in this study, hand–foot syndrome, a characteristic side effect of capecitabine, was not seen, but gastrointestinal symptoms such as nausea and anorexia were observed in 23 patients, suggesting the importance of controlling gastrointestinal symptoms. Moreover, no allergic reactions were seen with oxaliplatin, but peripheral neuropathy of the hands and feet continued after treatment in 17 patients. Therefore, the problems in terms of managing the adverse events of CapOX therapy are: (i) demonstrate decrease of motivation due to the 2-week oral administration period; (ii) appearance of gastrointestinal symptoms; (iii) difficulty of drug compliance (in the case of FOLFOX therapy, treatment can be confirmed with the infusion pump, but compliance in taking medication depends on the patient); (iv) appearance of hand–foot syndrome (this was not a problem in this study, but we think that general measures are important and maintain skin reactions by humectants or steroid-based ointments); and (v) the continuation of peripheral neuropathy. Regarding peripheral neuropathy in particular, the MOSAIC trial also reported that even 4 years after the end of adjuvant chemotherapy, peripheral neurotoxicity remained in 40% of patients (4). Viewed from the perspective of adjuvant chemotherapy in particular, the continuation of peripheral neuropathy in the limbs after treatment must be avoided.
When compared with the results from the XELOXA study (9), the dose intensity in our patient population is lower (Table 5), while the overall completion rate of treatment is higher. Meanwhile, both the DFS and OS rates are similar compared with MOSAIC study (4,5). We did not observe any incidence of hand–foot syndrome in our study, which presumably resulted from implementation of more aggressive skin care. Recently, Schmoll et al. reported that combination therapy with oxaliplatin provided improved outcomes without adversely affecting postrelapse survival in the adjuvant treatment of stage III colon cancer (18). On the other hand, in clinical situation, administration of oxaliplatin for adjuvant setting may be avoided because of increasing adverse events related to oxaliplatin. In the present study, adverse events were controllable because of emphasis on care of capecitabine-related hand–foot syndrome and oxaliplatin-related peripheral neuropathy on a routine basis. Therefore, CapOX therapy prevented early recurrence with high completion rate.
Table 5.
The Comparison With the Other Clinical Study of Oxaliplatin Based Regimen as Adjuvant Chemotherapy
| CapOX | mFOLFOX6 | |||
|---|---|---|---|---|
| Hospital visits | Every 3 weeks | Every 2 weeks | ||
| Port-catheter | Not require | Require | ||
| Our study | XELOXA | MOSAIC | JOIN | |
| Dose intensity | ||||
| Oxaliplatin | 76.5% | 87.0% | >80.0% | 78.2% |
| Capesitabine | 75.8% | 84.0% | – | – |
| 5-FU | – | – | 84.4% | 87.7% (bolus), 78.1% (infusion) |
| Treatment completion | 82.9% | 69.0% | 74.7% | 67.0% |
| DFS rate | 63.8% (five-year) | 66.1% (five-year) | 73.3% (five-year) | – |
| OS rate | 71.0% (five-year) | 77.6% (five-year) | 78.5% (six-year) | – |
| HFS (>grade 3) | 0% | 5.0% | – | – |
| PSN (>grade 3) | 17.1% | 9.0% | 12.4% | 5.8% |
HFS, hand–foot syndrome; PSN, peripheral sensory neuropathy.
In conclusion, CapOX therapy as an adjuvant chemotherapy for groups at high risk of recurrence of stage II and stage III colon cancer has been demonstrated to be excellent in terms of hygiene and safety, to require fewer hospital visits, to have convenient administration, and to have therapeutic effects not inferior to those of FOLFOX therapy. In addition, we have shown that the adjuvant CapOX therapy is safe and well-tolerated in Japanese patients. In the future, we plan to further evaluate DFS, RFS, and OS in patients treated with this combination regimen.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Wolmark N.; Rockette H.; Mamounas E.; Jones J.; Wieand S.; Wickerham D. L.; Bear H. D.; Atkins J. N.; Dimitrov N. V.; Glass A.; Fisher E. R.; Fisher B. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J. Clin. Oncol. 17:3553–3559; 1999. [DOI] [PubMed] [Google Scholar]
- 2. Saltz L. B.; Niedzwiecki D.; Hollis D.; Goldberg R. M.; Hantel A.; Thomas J. P.; Fields A. L.; Mayer R. J. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J. Clin. Oncol. 25:3456–3461; 2007. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E.; Labianca R.; Bodoky G.; Barone C.; Aranda E.; Nordlinger B.; Topham C.; Tabernero J.; André T.; Sobrero A. F.; Mini E.; Greil R.; Di Costanzo F.; Collette L.; Cisar L.; Zhang X.; Khayat D.; Bokemeyer C.; Roth A. D.; Cunningham D. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J. Clin. Oncol. 27:3117–3125; 2009. [DOI] [PubMed] [Google Scholar]
- 4. André T.; Boni C.; Mounedji-Boudiaf L.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Zaninelli M.; Clingan P.; Bridgewater J.; Tabah-Fisch I.; de Gramont A.; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350:2343–2351; 2004. [DOI] [PubMed] [Google Scholar]
- 5. André T.; Boni C.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Bonetti A.; Clingan P.; Bridgewater J.; Rivera F.; de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II and III colon cancer in the MOSAIC trial. J. Clin. Oncol. 27:3109–3116; 2009. [DOI] [PubMed] [Google Scholar]
- 6. Kuebler J. P.; Wieand H. S.; O’Connell M. J.; Smith R. E.; Colangelo L. H.; Yothers G.; Petrelli N. J.; Findlay M. P.; Seay T. E.; Atkins J. N.; Zapas J. L.; Goodwin J. W.; Fehrenbacher L.; Ramanathan R. K.; Conley B. A.; Flynn P. J.; Soori G.; Colman L. K.; Levine E. A.; Lanier K. S.; Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J. Clin. Oncol. 25:2198–2204; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Abrams T. A.; Brightly R.; Mao J.; Kirkner G.; Meyerhardt J. A.; Schrag D.; Fuchs C. S. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. J. Clin. Oncol. 29:3255–3262; 2011. [DOI] [PubMed] [Google Scholar]
- 8. Twelves C.; Wong A.; Nowacki M. P.; Abt M.; Burris H. 3rd; Carrato A.; Cassidy J.; Cervantes A.; Fagerberg J.; Georgoulias V.; Husseini F.; Jodrell D.; Koralewski P.; Kröning H.; Maroun J.; Marschner N.; McKendrick J.; Pawlicki M.; Rosso R.; Schüller J.; Seitz J. F.; Stabuc B.; Tujakowski J.; Van Hazel G.; Zaluski J.; Scheithauer W. Capecitabine as adjuvant treatment for stage III colon cancer. N. Engl. J. Med. 352:2696–2704; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Haller D. G.; Tabernero J.; Maroun J.; de Braud F.; Price T.; Van Cutsem E.; Hill M.; Gilberg F.; Rittweger K.; Schmoll H. J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol. 29:1–9; 2011. [DOI] [PubMed] [Google Scholar]
- 10. JSCCR Guidelines 2010 for the Treatment of Colorectal Cancer. Japanese Society for Cancer of the Colon and Rectum; 2010. [Google Scholar]
- 11. Cassidy J.; Clarke S.; Díaz-Rubio E.; Scheithauer W.; Figer A.; Wong R.; Koski S.; Lichinitser M.; Yang T. S.; Rivera F.; Couture F.; Sirzén F.; Saltz L. Randomized phase iii study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 26:2016-12; 2008. [DOI] [PubMed] [Google Scholar]
- 12. Cassidy J.; Cox J. V.; Scotto N.; Schmoll H. Effective management of patients receiving XELOX: Evaluation of the impact of dose modifications on outcome in patients from the NO16966, NO16967 and NO16968 trials. Gastrointestinal Cancer Symposium 497 (Poster Session); 2011. [Google Scholar]
- 13. Sakamoto J.; Hamada C.; Kodaira S.; Nakazato H.; Ohashi Y. Adjuvant therapy with oral fluoropyrimidines as main chemotherapeutic agents after curative resection for colorectal cancer. Individual patients data meta-analysis of andomized trials. Jpn. J. Clin. Oncol. 29:78–86, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Kim S. T.; Hong Y. S.; Lim H. Y.; Lee J.; Kim T. W.; Kim K. P.; Kim S. Y.; Baek J. Y.; Kim J. H.; Lee K. W.; Chung I. J.; Cho S. H.; Lee K. H.; Shin S. J.; Kang H. J.; Shin D. B.; Lee J. W.; Jo S. J.; Park Y. S. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: Updated results from a phase 3 trial. BMC Cancer 14:883; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yothers G. A.; Allegra C. J.; O’Connell M. J.; George T. J.; Sharif S.; Petrelli N. J.; Lopa S.; Wolmark N. The efficacy of oxaliplatin (Ox) when added to 5-fluorouracil/leucovorin (FU/L) in stage II colon cancer. ASCO, 3507 (Oral Abstract Session); 2011. [Google Scholar]
- 16. Benson A. B. 3rd; Schrag D.; Somerfield M. R.; Cohen A. M.; Figueredo A. T.; Flynn P. J.; Krzyzanowska M. K.; Maroun J.; McAllister P.; Van Cutsem E.; Brouwers M.; Charette M.; Haller D. G. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J. Clin. Oncol. 22:3408; 2004. [DOI] [PubMed] [Google Scholar]
- 17. Labianca R.; Nordlinger B.; Beretta G. D.; Mosconi S.; Mandalà M.; Cervantes A.; Arnold D. ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21(Suppl. 6):vi64–vi72; 2013. [DOI] [PubMed] [Google Scholar]
- 18. Schmoll H. J.; Twelves C.; Sun W.; O’Connell M. J.; Cartwright T.; McKenna E.; Saif M.; Lee S.; Yothers G.; Haller D. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: A pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 15:1481–1492; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]